Abstract
Ganoderma lucidum is a basidiomycete white rot fungus that has been used for medicinal purposes worldwide. Although information concerning its genome and transcriptome has recently been reported, relatively little information is available for G. lucidum at the proteomic level. In this study, protein fractions from G. lucidum at three developmental stages (16-day mycelia, and fruiting bodies at 60 and 90 days) were prepared and subjected to LC-MS/MS analysis. A search against the G. lucidum genome database identified 803 proteins. Among these proteins, 61 lignocellulose degrading proteins were detected, most of which (49 proteins) were found in the 90-day fruiting bodies. Fourteen TCA-cycle related proteins, 17 peptidases, two argonaute-like proteins, and two immunomodulatory proteins were also detected. A majority (470) of the 803 proteins had GO annotations and were classified into 36 GO terms, with “binding”, “catalytic activity”, and “hydrolase activity” having high percentages. Additionally, 357 out of the 803 proteins were assigned to at least one COG functional category and grouped into 22 COG classifications. Based on the results from the proteomic and sequence alignment analyses, a potentially new immunomodulatory protein (GL18769) was expressed and shown to have high immunomodulatory activity. In this study, proteomic and biochemical analyses of G. lucidum were performed for the first time, revealing that proteins from this fungus can play significant bioactive roles and providing a new foundation for the further functional investigations that this fungus merits.
Introduction
Ganoderma lucidum is a basidiomycete white rot fungus and has been one of the best-known medicinal macrofungi for many years [1, 2]. This fungus’s pharmacological activities are widely recognized, as evidenced by its inclusion in the American Herbal Pharmacopoeia and Therapeutic Compendium [2–7]. Many different types of active ingredients have been identified (e.g., polysaccharides, triterpenoids, and lignocellulose degrading enzymes), indicating that this mushroom is a cellular reservoir for biologically useful compounds [8–16].
With the rapid development of next-generation sequencing technology [17, 18], the genome and transcriptome of G. lucidum of different strains and at different developmental stages have recently been sequenced [19–24]. By analyzing the G. lucidum genome, 417 genes have been assigned to carbohydrate-active enzyme (CAZymes) families [19, 25]. However, the actual existence of these lignocellulose degrading enzymes has yet to be experimentally verified by, for example, mass spectrometry sequencing technology [26–28].
After genomics, proteomics is thought to be the next most powerful approach for the study of complex biological systems [29, 30]. The recent evolution of rapid protein identification technologies has made large-scale proteome analysis possible [31]. One of the most common methods in proteomic analysis is the use of one-dimensional or two-dimensional gel electrophoresis (1-DGE or 2-DGE) followed by enzymatic hydrolysis and mass spectrometry analysis [32–38]. Using this method, several proteomic studies have been conducted with fungi and mushrooms, including Termitomyces heimii [39], Sparassis crispa [35], Hericium erinaceum [35], Arthrobotrys oligospora [40], Metarhizium acridum [30], Agrocybe aegerita [41] and Cordyceps militaris [42]. These studies provided a useful informational resource for proteins. However, because comparable genomic information for these organisms was lacking at the time that the proteomic investigations were done, the proteomic investigations were mostly based on the non-redundant protein database of the National Center for Biotechnology Information (NCBInr).
Many proteins in macrofungi are bioactive. The examples include Fip-vvo from Volvariella volvacea [43], GMI from Ganoderma microsporum [44, 45], IPAF from Anoectochilus formosanus [46], AAL and AAL-2 from Agrocybe aegerita [47, 48], and immunomodulatory protein LZ-8 from G. lucidum [49, 50]. Considering the availability of G. lucidum genome, we assume there should be many undiscovered proteins in G. lucidum which remain to be identified by proteomic methods. Although several secretome proteins were analyzed [51], and a class of natural glycopeptides with sugar moiety-dependent antioxidant activities was published [52], comprehensive proteomic studies were still missing for G. lucidum.
In this study, we report the first proteomic characterization of G. lucidum. A total of 803 proteins were identified from G. lucidum by LC-MS/MS. Many significant proteins were detected, such as lignocellulose degrading proteins, tricarboxylic acid cycle related proteins, peptidases, argonaute-like proteins, and immunomodulatory proteins. In addition, based on the results of proteomic analysis, we cloned and expressed a new immunomodulatory protein named GL18769, which exhibited high immunomodulating activity.
Materials and Methods
Sources of G. lucidum
The 16-day mycelia and fruiting bodies (at 60, 90 days) of G. lucidum (yw-1 strain) were obtained from the Guangdong Institute of Microbiology (Guangdong, China, Fig. 1). As described previously [21], vegetative mycelia were grown on potato dextrose agar plates in the dark at 25°C, and the fruiting bodies were cultured on basswood medium bags (from Guangdong Institute of Microbiology). Three independent samples at each growth stage were used for this study.
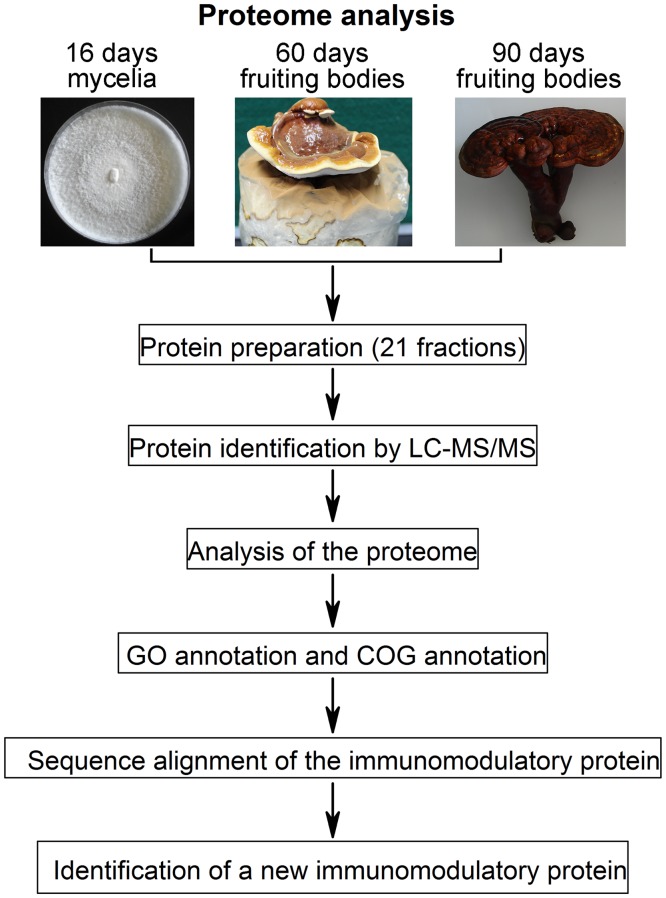
Fig 1. Analysis pipeline of the G. lucidum proteome.
Preparation of protein samples from G. lucidum at three developmental stages
Proteins were prepared from 16-day mycelia (16dM) and 60-day fruiting bodies (60dF) using the same method, and the procedure is as follows. Fresh mycelia (1 g) or 60dF (5 g) were ground into a fine powder with the use of liquid nitrogen. A protein mixture was obtained using the trichloroacetic acid/acetone (TCA/acetone) method as previously described [53]. Briefly, 10 ml of cold TCA/acetone solution (10% TCA (w/v) and 0.07% β-mercaptoethanol in acetone) was added to 1 g of fungal powder, and the suspension was thoroughly vortexed for 1 h at -20°C and were centrifuged at 16,000 × g for 15 min at 4°C. The supernatant was discarded and the pellet was washed three times with pre-chilled washing solution (0.07% β-ME, 2 mM EDTA, and EDTA-free proteinase inhibitor cocktail tablets (Roche) in 100% acetone) followed by the removal of all the residual acetone. The pellet was dried and solubilized in 100 μl homogenization buffer (0.2 M Tris-HCl buffer, pH 7.8, containing 5 mM EDTA·2Na, 14 mM β-ME, 10% (v/v) glycerol and 2 EDTA-free proteinase inhibitor tablets (Roche) per 100 ml of buffer solution in MQ H2O). To solubilize the protein pellet further, 60 μl SDS-sample buffer (2.5×, 62 mM Tris (pH 6.8) containing 10% (v/v) glycerol, 2.5% (w/v) SDS, and 5% (v/v) 2-ME, pH 6.8) was added to the mixture, followed by vortexing and sonication. After centrifugation at 16,000×g for 10 min at 4°C, the supernatant was collected and subjected to protein quantification using the Pierce BCA method.
Approximately 100 g of the 90-day fruiting bodies (90dF) were crushed into a fine powder and extracted twice with 1.5 L cold 0.01 M PBS (pH 8.5) and 10 EDTA-free proteinase inhibitor cocktail tablets at 4°C for 24 hours. The supernatant was collected by centrifugation at 12,000 × g for 20 min at 4°C and loaded onto a DEAE Sepharose Fast Flow (GE Healthcare) column equilibrated with 10 mM PBS (Fig. 2B). The bound materials were eluted with the same buffer containing 1 M NaCl. Both the flow through fraction and the eluate were collected. The flow through fraction was further separated by reverse phase high-performance liquid chromatography (RP-HPLC) using an RP-HPLC column (Flexar, PerkinElmer, C18 column, 10 × 250 mm). The elution was carried out with a 0% to 30% gradient of acetonitrile in 0.1% (v/v) trifluoroacetic acid (TFA) at 2 ml/min for 35 min, and then with a 30% to 40% gradient of acetonitrile in 0.1% TFA at 0.8 ml/min for 85 min. Nine fractions were collected (Fig. 2D), and each was dialyzed extensively against distilled water and lyophilized. The DEAE column eluate (with 1 mM NaCl) and nine HPLC fractions were separately concentrated by the TCA/acetone method described above.
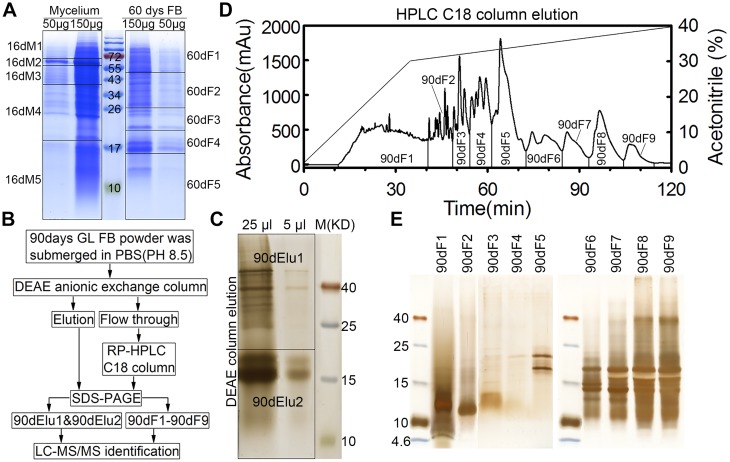
Fig 2. Protein preparation from three different developmental stages of G. lucidum.
(A) Protein preparation of mycelium (16 days) and fruiting bodies at 60 days (60dF). The grids indicate how the SDS-PAGE gel bands (16dM1–16dM5, 60dF1–60dF5) were cut for MS identification. The middle lane represents the molecular weight of the markers (kDa). (B) Workflow of protein separation from fruiting bodies at 90 days (90dF). (C) SDS-PAGE of the DEAE column elution fractions of the 90dF total proteins. The gel was silver stained and prepared as two fractions (90dElu1 and 90dElu2) before mass spectrometry. (D) The DEAE flow through fraction of total proteins from 90dF (90dF1–90dF9) was separated by HPLC. The x-axis represents the run time of HPLC method, the left y-axis shows the absorbance value of proteins at 280 nm and the right y-axis indicates the acetonitrile concentration. (E) The 9 HPLC fractions were dialyzed, lyophilized and subjected to SDS-PAGE and silver staining.
One-Dimensional Gel Electrophoresis (1-DGE) and Mass Spectrometry Analysis
The proteins prepared from the 16-day mycelium and the 60dF (200 μg per sample) were separated on a 15% SDS-PAGE gel. The gel was stained with Coomassie brilliant blue (CBB) R-250, and divided into 10 sections (Fig. 2A). For the 90dF, the protein pellet of the DEAE eluate, originating from 100 g of 90dF, was dissolved in 500 μl of SDS-PAGE loading buffer, from which 30 μl was taken and resolved on a 15% SDS-PAGE gel. The gel was stained with a SilverSNAP Stain Kit II (PIERCE, Thermo scientific) and divided into 2 sections (Fig. 2C). Each of the 9 HPLC fractions was dissolved in 100 μl of SDS-PAGE loading buffer and 10 μl was applied to a 15% SDS-PAGE gel. The gel was stained with a SilverSNAP Stain Kit II (Fig. 2E). These 21 gel slices were ‘in-gel’ reduced, S-alkylated and digested with trypsin [42]. The tryptic peptides derived from the gel bands were subsequently separated by a C18 reverse-phase column and analyzed on a nanoelectrospray ionization mass spectrometer (microTOF-Q II ESI-Q-ToF LC/MS/MS, Bruker Daltonics, Germany) operated in the positive ion mode. After sample loading and desalting at 4 μl/min, a Switchos II column switching device transferred the flow paths to the analytical column. The nanoflow was eluted at 400 nl/min using a 90 min gradient from 90% solvent A (0.1% formic acid in H2O) to 90% solvent B (0.1% formic acid in ACN). The ESI-MS was operated in a data-dependent MS/MS mode in which each full MS scan was followed by five MS/MS scans. The nanospray voltage was 1.5 kV and the MS data acquisition time was set to 3 s per spectrum over a m/z range of 300–1500 Da.
The MS/MS data were processed further by Flex Analysis software (Bruker Daltronics) using the recommended parameters (mass window for precursor ion selection: 2; relative collision energy: 27%; parameters for dynamic exclusion: 15 seconds). Protein identification was performed by searching against a virtual protein database (16,495 sequences) translated from the G. lucidum genome using the Mascot program (http://www.matrixscience.com). The search parameters were set to 15 ppm and 0.6 Da for peptide and fragment mass tolerance, respectively. The fixed modification was carbamidomethyl (C) and the variable modification was Gln->pyro-Glu (N-term Q), Oxidation (M). For individual data analysis, the significance threshold p < 0.05 and the Mascot score ≥ 25 were considered to be the standards for assigning a positive match to a protein in the database. The false discovery rates (FDR) were tested for all experimental runs using the Decoy option in Mascot and were 1%, both at the peptide and protein levels (FDR < 0.01).
Functional annotations of the proteome
The proteins identified by LC-MS/MS were searched against the functional annotation database of G. lucidum genome [19]. GO annotation and COG annotation of our proteome were extracted from the functional annotation database of G. lucidum and subjected to classification using the GO (http://geneontology.org/) and COG websites (http://www.ncbi.nlm.nih.gov/COG/), respectively [54, 55].
Sequence analysis of immunomodulatory proteins
The amino acid sequences of 11 published immunomodulatory proteins (gi|636613877, gi|597978919, gi|636613749, gi|729544, gi|597981577, gi|283488736, gi|597978931, gi|187961980, gi|126657, gi|348167218 and gi|62739082) were downloaded from NCBI website. The sequences of GL18769 and GL18770 were extracted from the G. lucidum genome data [19]. The sequence alignment of these 13 proteins was performed using ClustalX and Jalview software.
Cloning and expression of GL18769 gene in Escherichia coli
Total RNA from the 60-day fruiting bodies of G. lucidum was extracted using TriZol reagent (Promega). Full-length cDNAs were synthesized from 1 μg of total RNA using MMLV (Moloney murine leukaemia virus) reverse transcriptase (Promega) after RQ1 RNase-free DNase treatment (Promega) according to the manufacturer’s instructions. According to the current proteomic study and the published G. lucidum genome data, the nucleotide sequence of GL18769 was extracted from G. lucidum genome [19]. To clone the GL18769 coding sequence, the following primer pair was used: sense primer, 5'-CATGCCATGGATGCCCTCCAACACCGCTCT-3'; anti-sense primer, 5'-CCCAAGCTTGTTCCACTGGGCGATGAGGT-3'. PCR was performed using KOD-Plus-Neo (KOD-401) DNA polymerase (TOYOBO) with the following temperature profile: 5 min at 94°C, 20 s at 94°C, 30 s at 55°C, 11 s at 68°C and 5 min at 68°C, for 28 cycles. The PCR product was isolated and cloned into pET-28a vector (Novagen). The resulting plasmid containing the GL18769 coding sequence was transformed into E. coli BL21(DE3) cells (TransGen Biotech, Lot#G301130). Induction of protein expression was performed with 0.5 mM IPTG (isopropylβ-D-thiogalactopyranoside) at the mid-exponential phase (D 600 of 0.4–0.6), and the bacteria were grown for an additional 4 h at 37°C on a shaker at 220 rev/min. The bacteria were harvested and lysed, and the supernatant of the lysate was loaded on to a His Trap FF column (GE Healthcare) using ÄKTAprime plus (General Electric Company). The GL18769 protein was eluted with elution buffer (20 mM sodium phosphate, 0.5 M NaCl, 500 mM imidazole, pH 7.4) at a flow rate of 1 ml/min and detecting the absorbance at 280 nm.
Blast-formation stimulatory activity
Six-week-old male C57BL/6 mice were purchased from Wuhan University Center for Animal Experiment/Animal Biosafety Level III Lab (A3Lab) and housed in a specific pyrogen-free room until used in the experiment. All study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Wuhan University School of Medicine (Wuhan, China) in accordance with the regulations of the National Institute of Health “Guide for the Care and Use of Laboratory Animals” and all details of animal welfare and steps taken to ameliorate suffering were in accordance with the recommendations of the Weatherall report. Three mice were killed by cervical dislocation and their spleens were aseptically removed. The spleen cells were collected by passage through a wire screen (300 mesh) using EZ-Sep Mouse 1 x Lymphocyte Separation Medium (Dakewe Biotech Co., Ltd.) according to the manufacturer’s protocol. The red blood cells in the cell suspension were hemolyzed with 0.17 M Tris-HC1 (pH 7.7) containing 0.16M NH4Cl. After washing the cells with 15 ml of RPMI 1640 and centrifuging at 800 × g for 10 min, the cells were re-suspended in 8 ml of RPMI 1640 supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 pg/ml streptomycin. The cell density was then adjusted to 3 × 106/ml. Splenic lymphocytes (0.1 ml, 3 × 105 cells/well) were seeded onto a 96-well microtiter plate and incubated with various concentrations of GL18769 protein (2.5, 5, 10 μg/ml) or with Concanavalin A (ConA, 2 μg/ml). The cells were maintained at 37°C under 5% CO2 in air for 36 hours, and 10 μl of CCK-8 was then added to the cells (Cell Counting Kit-8, Dojindo, http://dojindo.cn/products/C/cck-8.htm), followed by an additional incubation for 1–2 h. The absorbance at 450 nm was measured for each well and the ‘relative fold change’ of the treated condition was calculated by following the manufacturer’s protocol [56]. The cell morphology was also monitored microscopically (LEICA DM IRB) at a 50 X magnification.
Statistical analysis
The two-sample Student’s t test was used for comparisons between groups of the "Blast-formation Stimulatory Activity" assay. Statistical analysis was performed using GraphPad Prism 5 and Origin 7 software. The results were expressed as means ± S.E.M., and the statistical significance was defined as P<0.05.
Results and Discussion
Preparation of protein from G. lucidum
Fig. 1 presents the workflow outline for the G. lucidum proteome analysis carried out in this study. G. lucidum is a macrofungus which undergoes tremendous changes during the developmental stages from mycelium to mature fruiting body (Fig. 1). To study the proteome of G. lucidum comprehensively and identify as many proteins as possible, proteins from three different developmental stages of G. lucidum were extracted. Based on the previous studies on G. lucidum [19, 21], we chose G. lucidum mycelia at approximately 16 days and fruiting bodies at approximately 60 days. In addition, because fruiting bodies at approximately 90 days have been widely used as a traditional herbal medicine and the commercial products are readily available from the market [1], we also chose the 90-day G. lucidum an experimental material.
The proteins of 16-day mycelia (16dM) and 60-day fruiting bodies (60dF) were extracted by the same TCA/acetone method and subsequently resolved by SDS-PAGE (Fig. 2A). As expected, the protein profiles were different between 16dM and 60dF, especially in the regions corresponding to 17–26 and 43–72 kDa. The gels were cut horizontally into 5 slices (16dM1–16dM5, 60dF1–60dF5) for each sample.
Because 90-day fruiting bodies (90dF) of G. lucidum are highly lignified, we needed to use a different method to extract the proteins (see Materials and methods). The protein mixtures extracted by PBS (pH 8.5) were further separated by DEAE anion-exchange and HPLC reverse-phase column chromatography (Fig. 2B). The eluate from the DEAE column was resolved by SDS-PAGE and the gel was cut into two slices (90dElu1 and 90dElu2) (Fig. 2C). The DEAE flow through was loaded onto a HPLC C18 column and separated into 9 fractions (90dF1–90dF9) (Fig. 2D), which were then subjected to SDS-PAGE followed by silver staining (Fig. 2E). Compared with the protein profiles of 16-day mycelium and the 60-day fruiting bodies, most proteins of the 90-day fruiting body were distributed in the 4.6–40kDa region, especially in the 10–25 kDa area (Fig. 2C and Fig. 2E).
In total, 21 protein fractions (16dM1–16dM5, 60dF1–60dF5, 90dElu1, 90dElu2 and 90dF1–90dF9) from three different developmental stages of G. lucidum were produced for the following MS identification.
Proteomic characterization of G. lucidum
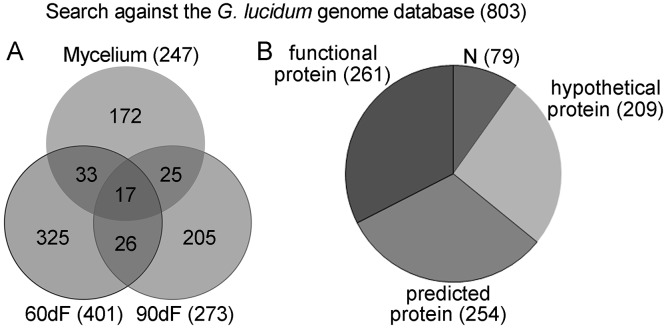
The 21 protein fractions were digested with trypsin and subjected to proteomic characterization by LC-MS/MS. The peptides identified by mass spectrometry were analyzed using the Mascot program and searched against the G. lucidum genome database. A total of 803 non-redundant proteins was identified from all the samples combined (S1 Table, FDR < 0.01 for protein identifications). Fig. 3A shows the distribution of proteins among the different developmental stages, as revealed by SDS-PAGE and LC-MS/MS analysis. Respectively, 247, 401 and 273 non-redundant proteins were detected from the samples at 16dM, 60dF and 90dF stages. Among these proteins, 17 were found in samples from all three stages, including an immunomodulatory protein Ling Zhi-8 (GL18770), α-galactosidase (GL30909), exo-1,3-β-glucanase (GL30087), translation elongation factor (GL29943), glycoside hydrolase (GL29873 and GL23600), aspergillopepsin (GL26523), and glutathione reductase (GL22863). Respectively, 172, 325 and 205 proteins could only be found in 16dM, 60dF and 90dF samples (Fig. 3A).
Fig 3. Proteomic results from combined SDS-PAGE/LC-MS/MS analysis.
(A) Venn diagrams of proteomes from the three developmental stages of G. lucidum. By searching against genome database, 803 proteins were identified with 247, 401 and 273 proteins detected from 16dM, 60dF and 90dF stages, respectively. (B) Distribution of the 803 proteins identified. Among the 803 proteins, 79, 209, 254 and 261 proteins were grouped into ‘N’, ‘hypothetical protein’, ‘predicted protein’ and ‘functional protein’ classes, respectively. ‘N’ represents proteins with no particular annotations, while ‘functional protein’ represents proteins that have a clear functional annotation.
Compared to human and model organisms, functional proteome studies of non-model organisms, including macroscopic fungi, are not as advanced. Thus among the 803 proteins identified in the G. lucidum genome, 79 proteins were defined as ‘N’ (no annotation), 209 as ‘hypothetical protein’, and 254 as ‘predicted protein’ (Fig. 3B and S1 Table); only 261 out of 803 proteins had particular functions annotated. Although the remaining 542 proteins had no specific functional annotations available at this time, the current proteomic analysis at least confirmed the physical existence of these proteins in G. lucidum, enriching the protein database for this fungus. Further investigation should be performed to discover the function of those proteins not annotated to date.
1. Identification of wood-degrading enzymes
At present, the limitation of fossil fuel reserves and its negative impact on the environment have spurred an urgent need for the development of alternative energy resources that could meet future demands. The wood-degrading enzyme family has been considered as a valuable resource because of its potential to produce sustainable biofuels from lignocellulose substrates. We detected 61 lignocellulose degrading proteins with high MS scores in the G. lucidum proteome (Table 1). These proteins included catalase, laccase, cellobiose dehydrogenase, endoglucanase, cellobiohydrolase, alginate lyase, chitinase, carbohydrate esterase, glycoside hydrolase, and exo-1,3-beta-glucanase (Table 1). Searching against the CAZy database [25, 57–59] allowed the classification of these 61 wood degrading enzymes into the families of Auxiliary Activities (AAs, 15 proteins), Carbohydrate-Binding Modules (CBMs, 13), Carbohydrate Esterases (CEs, 3), Glycoside Hydrolases (GHs, 28), GlycosylTransferases (GTs, 1) and Polysaccharide Lyases (PLs, 1). Many of them (28 proteins) were thus grouped into the GHs family. The relative abundance of GHs enzymes observed in our proteome is consistent with a previous genomic study of G. lucidum which showed that this fungus contained the largest number of GH enzymes among the six CAZy families (GHs, CEs, GTs, PLs, CBAs and AAs) [19]. The detailed sub-categories of the 61 proteins are shown in Table 1. Among the 61 lignocellulose degrading proteins, 15, 13, and 49 proteins were found in the extracts from mycelium, 60dF, and 90dF, respectively, indicating that the fruiting bodies at 90 days may contain more related enzymes (Table 1).
Table 1. Detailed information of 61 identified lignocellulose degrading proteins.
| Protein ID a | Protein Description | Protein MW(Da) | Mascot score | Protein coverge(%) | Matched peptides b | CAZy families c | Stage d |
|---|---|---|---|---|---|---|---|
| GL22189-R1_1 | catalase | 59605 | 15218 | 48.1 | 18 | AA1 | 16dM,60dF |
| GL16398-R1_1 | laccase | 56868 | 118 | 7.3 | 2 | AA1 | 90dF |
| GL21497-R1_1 | laccase | 59332 | 37 | 2.8 | 1 | AA1 | 90dF |
| GL22256-R1_1 | cellobiose dehydrogenase | 84146 | 25 | 6.9 | 1 | AA3 | 60dF |
| GL25348-R1_1 | copper radical oxidase | 70424 | 51 | 5.6 | 1 | AA5 | 16dM |
| GL27858-R1_1 | copper radical oxidase | 100390 | 108 | 7.7 | 3 | AA5 | 90dF |
| GL21192-R1_1 | copper radical oxidase variant A | 82336 | 423 | 17.6 | 5 | AA5 | 90dF |
| GL24805-R1_1 | glucooligosaccharide oxidase | 53907 | 366 | 17.6 | 6 | AA7 | 90dF |
| GL24807-R1_1 | glucooligosaccharide oxidase | 59940 | 582 | 24.2 | 9 | AA7 | 90dF |
| GL23615-R1_1 | glucooligosaccharide oxidase | 35311 | 165 | 18.2 | 2 | AA7 | 90dF |
| GL24786-R1_1 | glucooligosaccharide oxidase | 57385 | 111 | 7.9 | 1 | AA7 | 90dF |
| GL24789-R1_1 | glucooligosaccharide oxidase | 58094 | 28 | 8.8 | 2 | AA7 | 90dF |
| GL30499-R1_1 | cytochrome b2 | 54647 | 32 | 11.8 | 1 | AA8 | 16dM |
| GL28634-R1_1 | endo-beta-glucanase | 32410 | 209 | 16.2 | 3 | AA9 | 60dF,90dF |
| GL24196-R1_1 | endoglucanase | 41497 | 91 | 5.4 | 2 | AA9 | 90dF |
| GL18725-R1_1 | cellobiohydrolase I | 50008 | 709 | 37.6 | 10 | CBM1 | 90dF |
| GL29727-R1_1 | cellobiohydrolase I | 51017 | 466 | 26.1 | 7 | CBM1 | 90dF |
| GL30351-R1_1 | cellobiohydrolase I | 50138 | 212 | 18.8 | 7 | CBM1 | 90dF |
| GL24712-R1_1 | cellobiohydrolase II | 47213 | 237 | 16.3 | 4 | CBM1 | 90dF |
| GL26036-R1_1 | endo-1,4-beta-xylanase C precursor | 50246 | 108 | 7.4 | 2 | CBM1 | 90dF |
| GL20229-R1_1 | endo-1,4-B-xylanase A | 44982 | 539 | 29.6 | 9 | CBM1 | 90dF |
| GL25283-R1_1 | mannanase | 49168 | 36 | 3.7 | 1 | CBM1 | 90dF |
| GL16814-R1_1 | carbohydrate-binding module family 12 protein | 35744 | 538 | 10.6 | 1 | CBM12 | 16dM |
| GL22047-R1_1 | carbohydrate-binding module family 13 protein | 15848 | 116 | 33.8 | 2 | CBM13 | 16dM,90dF |
| GL16341-R1_1 | alginate lyase | 33672 | 168 | 11.9 | 3 | CBM2 | 90dF |
| GL25627-R1_1 | chitinase | 46320 | 62 | 3.5 | 1 | CBM2 | 90dF |
| GL30108-R1_1 | carbohydrate binding domain-containing protein from family CBM21 | 86164 | 28 | 1.5 | 1 | CBM21 | 16dM |
| GL21331-R1_1 | putative laminarinase | 35134 | 35 | 3.8 | 1 | CBM4 | 90dF |
| GL26613-R1_1 | candidate lipase/esterase from carbohydrate esterase family CE10 | 93064 | 34 | 3.1 | 1 | CE10 | 16dM |
| GL29877-R1_1 | candidate polysaccharide deacetylase from carbohydrate esterase family CE10 | 54773 | 204 | 3.8 | 1 | CE10 | 60dF |
| GL28882-R1_1 | carbohydrate esterase family 15 protein | 42513 | 73 | 7.3 | 2 | CE15 | 90dF |
| GL24039-R1_1 | candidate beta-glucosidase from glycoside hydrolase family 1 | 55862 | 46 | 3.7 | 1 | GH1 | 16dM |
| GL30087-R1_1 | exo-1,3-beta-glucanase | 47029 | 174 | 12.5 | 4 | GH132 | 16dM,60dF,90dF |
| GL23395-R1_1 | exo-beta-1,3-glucanase | 82884 | 95 | 2.6 | 1 | GH132 | 90dF |
| GL31059-R1_1 | glycoside hydrolase family 13 protein | 59698 | 516 | 19.5 | 8 | GH132 | 90dF |
| GL23580-R1_1 | glycoside hydrolase family 15 protein | 61201 | 185 | 20.2 | 4 | GH15 | 60dF,90dF |
| GL23600-R1_1 | glycoside hydrolase family 15 protein | 57540 | 347 | 16.5 | 5 | GH15 | 16dM,60dF,90dF |
| GL25075-R1_1 | glycoside hydrolase family 16 protein | 71204 | 127 | 5.5 | 2 | GH16 | 90dF |
| GL24376-R1_1 | glycoside hydrolase family 18 protein | 67342 | 43 | 1 | 1 | GH18 | 90dF |
| GL21024-R1_1 | glycoside hydrolase family 27 protein | 34228 | 55 | 4.2 | 1 | GH27 | 90dF |
| GL27011-R1_1 | glycoside hydrolase family 27 protein | 19926 | 56 | 6.3 | 1 | GH27 | 90dF |
| GL20947-R1_1 | endo-polygalacturonase PG1 | 37820 | 204 | 22.7 | 3 | GH28 | 90dF |
| GL19093-R1_1 | beta-xylosidase | 88017 | 85 | 4.6 | 3 | GH3 | 60dF,90dF |
| GL22886-R1_1 | beta-xylosidase | 87234 | 70 | 5.9 | 3 | GH3 | 90dF |
| GL27550-R1_1 | glycoside hydrolase family 3 protein | 85637 | 58 | 4.8 | 3 | GH3 | 90dF |
| GL21973-R1_1 | endo-arabinase | 30804 | 28 | 1.8 | 1 | GH43 | 90dF |
| GL15164-R1_1 | glycoside hydrolase family 43 protein | 35013 | 69 | 14.3 | 2 | GH43 | 90dF |
| GL15780-R1_1 | glycoside hydrolase family 43 protein | 36021 | 100 | 13 | 3 | GH43 | 90dF |
| GL20698-R1_1 | glycoside hydrolase family 47 protein | 59518 | 808 | 26.8 | 9 | GH47 | 90dF |
| GL21451-R1_1 | glycoside hydrolase family 55 protein | 93018 | 397 | 12.8 | 7 | GH55 | 90dF |
| GL29873-R1_1 | glycoside hydrolase family 72 protein | 94656 | 243 | 4.2 | 3 | GH72 | 16dM,60dF,90dF |
| GL30540-R1_1 | glycoside hydrolase family 74 protein | 76162 | 73 | 5.4 | 2 | GH74 | 90dF |
| GL26459-R1_1 | glycoside hydrolase family 79 protein | 53522 | 92 | 6.4 | 1 | GH79 | 16dM,90dF |
| GL29728-R1_1 | D-xylose reductase | 35040 | 1202 | 38.3 | 6 | GH8 | 16dM,60dF |
| GL18249-R1_1 | glycoside hydrolase family 92 protein | 93316 | 53 | 10.9 | 2 | GH92 | 60dF,90dF |
| GL23422-R1_1 | glycoside hydrolase family 92 protein | 93686 | 181 | 10 | 4 | GH92 | 16dM,90dF |
| GL29257-R1_1 | glycoside hydrolase family 92 protein | 78244 | 120 | 1.4 | 1 | GH92 | 60dF |
| GL29258-R1_1 | glycoside hydrolase family 92 protein | 84221 | 252 | 19.3 | 7 | GH92 | 90dF |
| GL21099-R1_1 | glycoside hydrolase family 95 protein | 90612 | 507 | 16 | 10 | GH95 | 60dF,90dF |
| GL21375-R1_1 | glycogen phosphorylase | 114415 | 2759 | 16.5 | 11 | GT35 | 16dM |
| GL23979-R1_1 | polysaccharide lyase family 8 protein | 79492 | 186 | 8.5 | 3 | PL8 | 90dF |
a, matched protein ID was derived from the G. lucidum genome database.
b, the details of matched peptides was shown in S1 Table.
c, these wood-degrading enzymes were classified into CAZy sub-families.
d, the identified proteins were from three developmental stages of G. lucidum (16dM, 60dF, 90dF).
2. Characterization of other proteins
In addition to the 61 identified wood degrading proteins, other important proteins were also characterized (Table 2). Fourteen proteins are involved in the tricarboxylic acid (TCA) cycle [60, 61], including aconitate hydratase [62], citrate synthase [63], fumarate reductase [64], glyceraldehyde-3-phosphate dehydrogenase, phosphoglucomutase [65], phosphopyruvate hydratase, pyruvate kinase [66], succinate semialdehyde dehydrogenase [67], succinate-CoA ligase, acetyl CoA carboxylase, glucose-6-phosphate 1-dehydrogenase, and mannose-6-phosphatase. Most of these proteins (10) were found in the 16-day mycelium (Table 2), suggesting the significance of TCA cycle during G. lucidum development. Two N-acetylhexosaminidases and one UTP-glucose-1-phosphate uridylyltransferase were found [68, 69]. These three proteins are involved in the hexosamine biosynthesis pathway [70]. Of 17 different peptidases detected in the proteome [71, 72], most (12) were found in the fruiting bodies at 90 days. This probably explains the SDS-PAGE protein profile of 90dF (see above) in which most protein bands occurred in the 10–25 kDa region (Fig. 2C and Fig. 2E). Aflatoxin-detoxifizyme is a protein that detoxifies aflatoxins [73]. Aldehyde dehydrogenase plays an important role in acetaldehyde detoxification [74]. Argonaute-like protein is involved in the expression of miRNA-like RNAs in fungi [75]. The identification of two argonaute-like proteins suggests the potential existence of miRNA-like RNAs in G. lucidum. Farnesyl-diphosphate synthase and beta-glucan synthesis-associated proteins are involved in triterpenoid biosynthesis and polysaccharide biosynthesis, respectively [76, 77]. Two immunomodulatory proteins (GL18770 and GL18769) were also detected from the proteome of this medicinal fungus.
Table 2. A list of proteins involved in tricarboxylic acid cycle, hexosamine biosynthesis pathway, peptidase and other interesting bioactivities.
| Protein ID | Protein Description | Protein MW(Da) | Mascot score | Protein coverge(%) | Matched peptides a | Stage |
|---|---|---|---|---|---|---|
| Tricarboxylic acid cycle | ||||||
| GL21959-R1_1 | acetyl CoA carboxylase | 252793 | 61 | 2.5 | 1 | 16dM |
| GL18572-R1_1 | aconitate hydratase | 85821 | 54 | 8.1 | 2 | 16dM |
| GL24555-R1_1 | citrate synthase | 51487 | 30 | 3.3 | 1 | 90dF |
| GL20259-R1_1 | citrate synthase | 55510 | 35 | 2.2 | 1 | 16dM |
| GL17816-R1_1 | fumarate reductase | 67459 | 77 | 11.9 | 5 | 90dF |
| GL31587-R1_1 | glucose-6-phosphate 1-dehydrogenase | 56070 | 299 | 9.1 | 2 | 16dM |
| GL21313-R1_1 | glyceraldehyde-3-phosphate dehydrogenase | 34528 | 1910 | 22.3 | 4 | 60dF |
| GL20532-R1_1 | mannose-6-phosphatase | 37754 | 436 | 32.8 | 10 | 16dM,90dF |
| GL24763-R1_1 | mannose-6-phosphatase | 39883 | 114 | 7.4 | 2 | 90dF |
| GL24280-R1_1 | phosphoglucomutase | 61444 | 554 | 15.9 | 5 | 16dM,60dF |
| GL30114-R1_1 | phosphopyruvate hydratase | 46944 | 9340 | 69.5 | 18 | 16dM,60dF |
| GL30680-R1_1 | pyruvate kinase | 61232 | 8782 | 47.2 | 18 | 16dM |
| GL23356-R1_1 | succinate semialdehyde dehydrogenase | 53519 | 102 | 9.4 | 2 | 16dM |
| GL29894-R1_1 | succinate-CoA ligase | 44637 | 27 | 5.5 | 1 | 16dM |
| Hexosamine biothesis pathway | ||||||
| GL24346-R1_1 | N-acetylhexosaminidase | 60350 | 200 | 10.8 | 5 | 16dM,90dF |
| GL24347-R1_1 | N-acetylhexosaminidase | 81223 | 192 | 8.3 | 4 | 90dF |
| GL25739-R1_1 | UTP-glucose-1-phosphate uridylyltransferase | 58901 | 255 | 27.8 | 5 | 16dM |
| Peptidase | ||||||
| GL18792-R1_1 | aspartic peptidase A1 | 47176 | 190 | 8.5 | 3 | 60dF,90dF |
| GL19589-R1_1 | aspartic peptidase A1 | 45789 | 110 | 8.3 | 2 | 90dF |
| GL26523-R1_1 | aspergillopepsin | 27828 | 1104 | 4.5 | 1 | 16dM,60dF,90dF |
| GL18287-R1_1 | aspergillopepsin A | 43958 | 1784 | 15.4 | 2 | 16dM,60dF |
| GL23283-R1_1 | endopeptidase | 94366 | 37 | 5.5 | 1 | 16dM,60dF |
| GL31420-R1_1 | endopeptidase | 46824 | 2238 | 34.4 | 11 | 16dM |
| GL28218-R1_1 | metallopeptidase MepB | 86674 | 719 | 12.4 | 5 | 16dM |
| GL20779-R1_1 | peptidase M28 | 52988 | 349 | 18.5 | 5 | 90dF |
| GL24319-R1_1 | peptidyl-Lys metalloendopeptidase | 37080 | 95 | 19.3 | 3 | 90dF |
| GL24399-R1_1 | peptidyl-Lys metalloendopeptidase | 38070 | 128 | 13.5 | 2 | 90dF |
| GL24396-R1_1 | Peptidyl-Lys metalloendopeptidase | 38694 | 432 | 12.7 | 2 | 90dF |
| GL24402-R1_1 | Peptidyl-Lys metalloendopeptidase | 36457 | 25 | 3.5 | 1 | 90dF |
| GL24406-R1_1 | Peptidyl-Lys metalloendopeptidase | 36648 | 28 | 8.5 | 1 | 90dF |
| GL24410-R1_1 | Peptidyl-Lys metalloendopeptidase | 13937 | 62 | 9.4 | 1 | 90dF |
| GL26638-R1_1 | serine carboxypeptidase | 53487 | 66 | 2.6 | 1 | 90dF |
| GL29874-R1_1 | serine carboxypeptidase | 56049 | 53 | 3.3 | 1 | 90dF |
| GL31396-R1_1 | tripeptidyl peptidase A | 66494 | 28 | 7 | 1 | 60dF |
| Other interesting proteins | ||||||
| GL31548-R1_1 | aflatoxin-detoxifizyme | 77614 | 472 | 4.3 | 2 | 16dM,60dF |
| GL30174-R1_1 | aldehyde dehydrogenase | 58137 | 5806 | 32.9 | 12 | 16dM |
| GL15827-R1_1 | argonaute-like protein | 112897 | 31 | 1.2 | 1 | 90dF |
| GL31293-R1_1 | argonaute-like protein | 79522 | 108 | 8.8 | 1 | 60dF |
| GL29980-R1_1 | beta-glucan synthesis-associated protein | 118511 | 55 | 1.2 | 1 | 90dF |
| GL25499-R1_1 | farnesyl-diphosphate synthase | 41202 | 66 | 3.3 | 1 | 16dM |
| GL23374-R1_1 | high nitrogen upregulated cytochrome P450 monooxygenase 2 | 65111 | 30 | 5.9 | 1 | 60dF |
| GL24810-R1_1 | high nitrogen upregulated cytochrome P450 monooxygenase 2 | 56857 | 45 | 2 | 1 | 60dF |
| GL18770-R1_1 | immunomodulatory protein | 17501 | 828 | 11 | 1 | 16dM,60dF,90dF |
| GL18769-R1_1 | immunomodulatory protein | 12534 | 682 | 12.5 | 1 | 60dF,90dF |
| GL25550-R1_1 | manganese peroxidase | 38810 | 115 | 2.5 | 1 | 16dM |
| GL15091-R1_1 | PAH-inducible cytochrome P450 monooxygenase PC-PAH 3 | 63420 | 160 | 2.7 | 1 | 16dM |
| GL28943-R1_1 | PAH-inducible cytochrome P450 monooxygenase PC-PAH 4 | 59857 | 35 | 2.8 | 1 | 16dM |
| GL20529-R1_1 | superoxide dismutase | 27197 | 3190 | 18.1 | 3 | 16dM,90dF |
a, the details of matched peptides was shown in S1 Table.
In summary, the 803 proteins identified by this study confirmed the existence of many putative proteins predicted from the G. lucidum genome and thus greatly enriched the G. lucidum protein database.
Functional annotation of the G. lucidum proteome
As shown in Fig. 1, to analyze the G. lucidum proteome further, the 803 non-redundant proteins were subjected to annotation by GO and COG [54, 55].
1. Gene ontology annotation
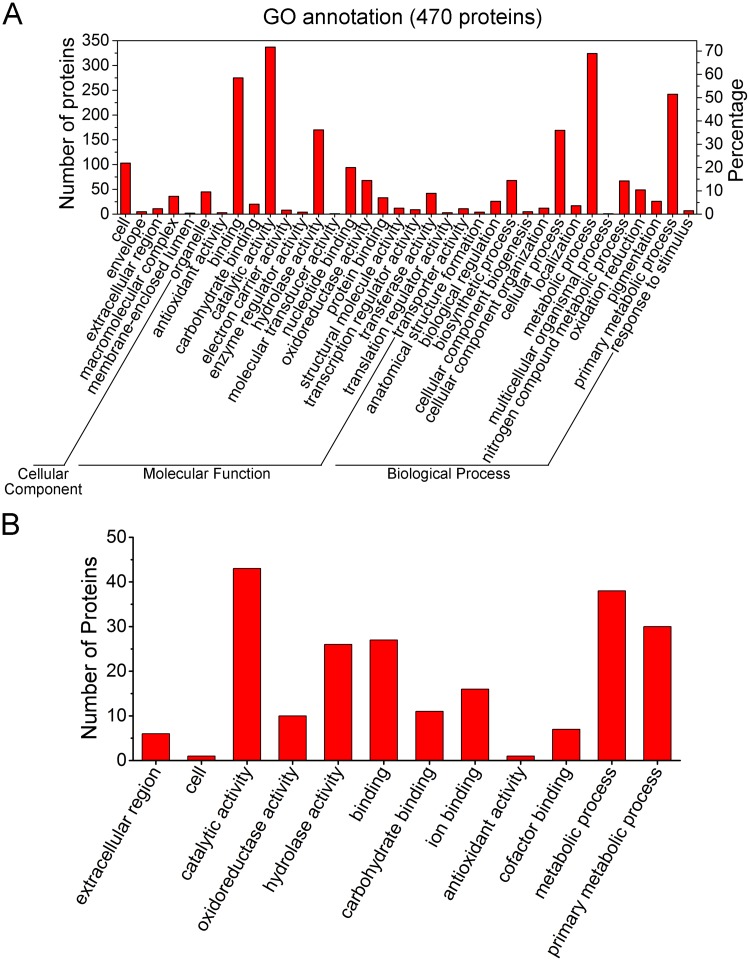
A total of 470 proteins were annotated across the GO sub-categories (S2 Table) and classified into 36 functional groups with 6 involved in cellular component, 16 in molecular function and 14 in biological processe (Fig. 4A). Among these GO classifications, ‘binding’ (275 proteins, 58.5%), ‘catalytic activity’ (337, 71.7%), ‘hydrolase activity’ (170, 36.2%), ‘cellular process’ (169, 36%), ‘metabolic process’ (324, 68.9%), and ‘primary metabolic process’ (242, 51.5) were dominant. In contrast, a small number of proteins were assigned to ‘membrane-enclosed lumen’ (2 proteins, 0.4%), ‘molecular transducer activity’ (1, 0.2%), and ‘multicellular organismal process’ (1, 0.2%).
Fig 4. GO annotation of the G. lucidum proteome.
(A) GO annotation of the total identified 803 proteins. GO annotations for 470 proteins were classified into 36 GO sub-categories. The results were summarized in three main GO categories: cellular component, molecular function and biological process. The left y-axis indicates the number of proteins in a sub-category. The right y-axis indicates the percentage of a specific sub-category of proteins in that main category. (B) GO annotation of the 61 wood-degrading proteins. GO annotations for 43 out of the 61 proteins were classified into 12 GO sub-categories. One protein could be annotated into more than one GO term.
Among the 61 identified wood degrading proteins, 43 of them received annotations across GO sub-categories, grouped into 12 functional groups (Fig. 4B). Among these GO categories, ‘catalytic activity’ (43 proteins, 100%), ‘hydrolase activity’ (26, 60.5%), ‘binding’ (27, 72.8%), ‘metabolic process’ (38, 88.4%), and ‘primary metabolic process’ (30, 69.8%) were dominant. All of these GO categories were related to the lignocellulose degradation, suggesting that the current proteome analysis is reasonably accurate.
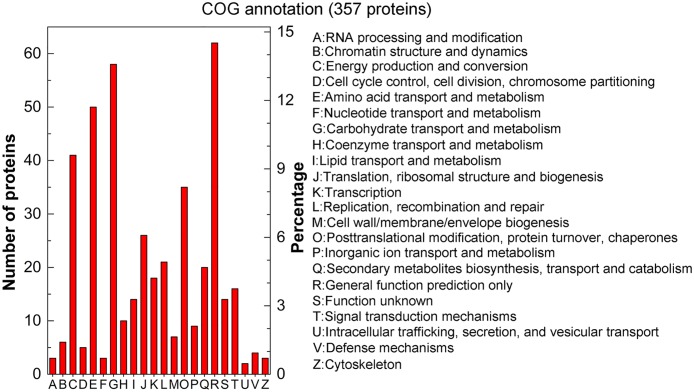
2. COG annotation
To further evaluate the effectiveness of our annotation process and the accuracy of our proteome analysis, we searched the identified proteins against COG classifications. A total of 357 proteins were assigned to at least one COG functional category, grouped into 22 classifications (Fig. 5 and S3 Table). Of the 22 COG categories, ‘General function prediction only’ (14.52%) contained the most proteins, followed by ‘Carbohydrate transport and metabolism’ (13.58%), ‘Amino acid transport and metabolism’ (11.71%), and ‘Energy production and conversion’ (9.6%). These COG classifications are consistent with the results described above (Table 1) in which many wood-degrading proteins were detected in our proteome. A few proteins related to ‘Intracellular trafficking, secretion, and vesicular transport’ (0.47%), ‘Cytoskeleton’ (0.7%), ‘Nucleotide transport and metabolism’ (0.7%), and ‘RNA processing and modification’ (0.7%) were found. These annotations provide new resources for further exploring this macrofungus.
Fig 5. COG annotation of the total identified 803 proteins.
COG annotations for 357 proteins were grouped into 22 categories. The left y-axis indicates the number of proteins in a particular category. The right y-axis indicates the percentage of a specific category of proteins in that main category.
Cloning and expression of the gene for a new immunomodulatory protein (GL18769)
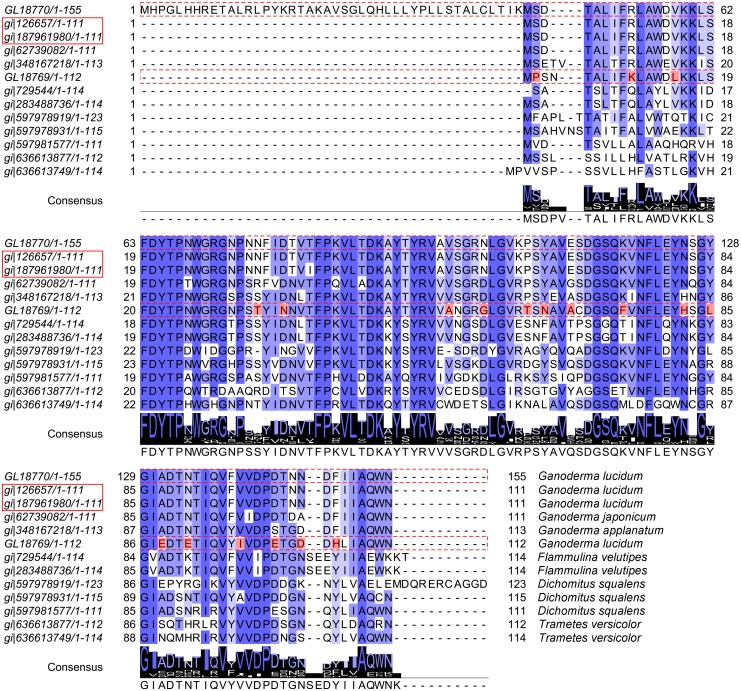
Previous studies reported the existence of immunomodulating activities in G. lucidum [3, 4, 6, 49]. Many studies have been published on immunomodulatory proteins, indicating the pharmacological significance of this activity [49, 78–80]. From the G. lucidum proteomic analysis, two immunomodulatory proteins were identified in the fruiting bodies at both 60 and 90 days (Table 2). Previous studies reported that fungal immunomodulatory proteins (FIPs) belonged to a new protein family with high sequence and structural similarities [81]. Therefore, we downloaded 11 different immunomodulatory proteins from the NCBI website and performed a sequence alignment between them and the two proteins, GL18769 and GL18770, which we identified as likely immunomodulatory proteins (Fig. 6) from our proteome. Both GL18769 and GL18770 showed a high similarity with the 11 proteins from Ganoderma lucidum (gi|126657 and gi|187961980), Ganoderma japonicum (gi|62739082), Ganoderma applanatum (gi|348167218), Flammulina velutipes (gi|283488736 and gi|729544), Dichomitus squalens (gi|597981577, gi|597978931 and gi|597978931), and Trametes versicolor (gi|636613877 and gi|636613749). From this alignment, we could see that these proteins shared the same conserved amino acid sequence. Moreover, the recognition of this consensus sequence could aid in immunomodulatory protein engineering in the future (Fig. 6).
Fig 6. Sequence analysis of 13 immunomodulatory proteins.
GL18770 and GL18769 were identified from the G. lucidum proteome. The immunomodulatory protein sequences of Ganoderma lucidum (gi|126657 and gi|187961980), Ganoderma japonicum (gi|62739082), Ganoderma applanatum (gi|348167218), Flammulina velutipes (gi|283488736 and gi|729544), Dichomitus squalens (gi|597981577, gi|597978931 and gi|597978931) and Trametes versicolor (gi|636613877 and gi|636613749) were all available on the NCBI website. The protein ID in the red rectangle represents the LZ-8 protein ID. Strictly conserved residues are indicated by blue shadows and are also displayed in the ‘Consensus’ sequence. The residues in red shadow are the significant amino acid differences of GL18769 from the other proteins. GL18770 had an identical amino acid sequence to LZ-8protein (gi|126657 and gi|187961980), except for the redundant 44 amino acids at its N-terminus. GL18769 had a 72.32% identity to LZ-8 protein.
Except for a redundant 44 amino acid sequence at the N-terminus, GL18770 is essentially identical to LZ-8 protein (gi|126657 and gi|187961980). GL18769, on the other hand, only has a 72.32% similarity to LZ-8 (gi|126657 and gi|187961980) (Fig. 6). GL18770 is the LZ-8 protein and the minor differences between them might result from different G. lucidum strains used in the respective studies. Nevertheless, as shown in Fig. 6, GL18769 had 19 amino acid differences from the other immunomodulatory proteins, suggesting that GL18769 could be a new immunomodulatory protein. To assess the reliability of our proteome analysis, we decided to clone the gene for GL18769, and express and purify the protein in E. coli, to test whether the protein indeed had immunomodulatory activity.
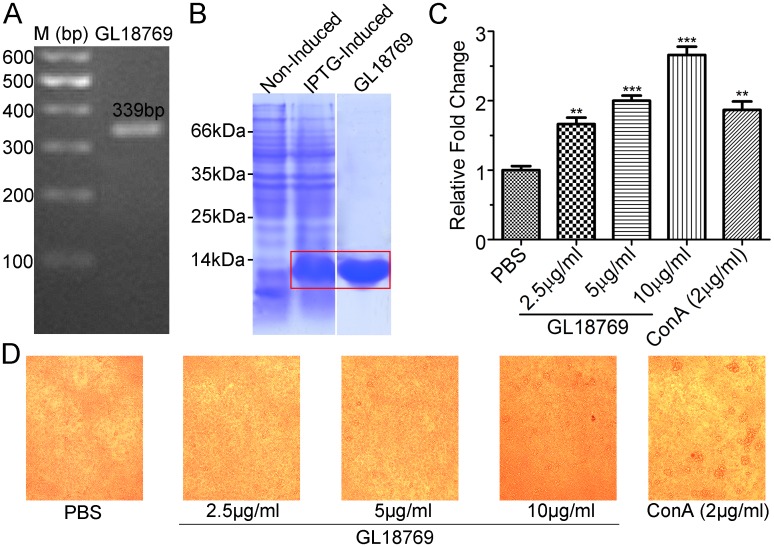
Because 90dF is highly lignified, it was difficult to extract intact RNA from the 90dF; therefore, we cloned the putative GL18769 coding sequence using a total cDNA library from G. lucidum 60dF (Fig. 7A), and determined that the sequence of PCR product was identical with that of GL18769. The PCR product was cloned into a pET-28a vector with a His-tag at its C-terminus to produce the recombinant protein. After separating the induced bacterial lysates by SDS-PAGE, a strong band of approximately 13 kDa corresponding to the recombinant GL18769 protein was produced (Fig. 7B). The supernatant of the lysate was loaded onto a His Trap FF column from which the recombinant GL18769 protein was eluted to produce a single band of 13 kDa on SDS-PAGE (Fig. 7B). Unlike GL18769 described above, GL18770 is essentially the same as LZ-8, whose immunoregulatory activities have already been studied in some detail. We therefore did not pursue a functional analysis of GL18770 in this study.
Fig 7. Cloning and bioactivity determination of the GL18769 protein.
(A) The PCR product of GL18769 coding sequence. Total RNA was isolated from G. lucidum fruiting bodies (60 days) and amplified by reverse transcription-PCR. The GL18769 coding sequence (339bp) was amplified by PCR. (B) Expression and purification of GL18769 protein. A strong protein band appeared at approximately 13 kDa after IPTG-induction for 4 h compared with non-induction. This protein was purified using a His Trap FF column as indicated in the SDS-PAGE. (C) Stimulatory effect of GL18769 on mouse spleen lymphocytes. Compared to the ConA (2 μg/ml) treatment, GL18769 could significantly enhance the proliferation of mouse spleen lymphocytes (MSLs) in a dose-dependent manner (2.5, 5 and 10 μg/ml). (D) Representative morphology images of the MSLs treated with different dose of GL18769 (2.5, 5 and 10 μg/ml) after 36 hours. The results are means ± S.E.M. (n = 3); * P<0.05, ** P<0.01, *** P<0.001.
Determination of immunomodulatory activity of GL18769 protein
To determine whether GL18769 protein possess any immunomodulatory activity similar to other immunomodulatory proteins [49, 81], a system of mouse splenic lymphocytes (MSLs) was employed with Concanavalin A (ConA, 2 μg/ml) as the positive control. Compared to the ConA protein, GL18769 indeed stimulated the MSLs proliferation in a dose-dependent manner (Fig. 7C). Incubated at 2.5 μg/ml for 36 hours, GL18769 had a stimulating effect similar to that of 2 μg/ml ConA. Incubated at 10 μg/ml for 36 hours, GL18769 significantly stimulated the lymphocyte proliferation 2.7-fold relative to the negative control (PBS treatment). Imaging analysis by microscopy showed that GL18769 treatment induced an increase in the cell density of splenic lymphocytes, but unlike with ConA treatment, did not cause cell aggregation (Fig. 7D). This difference in effect between ConA and GL18769 implies that GL18769 might involve a lymphocyte stimulation mechanism different from that of ConA.
Conclusions
In this study, we provided the first comprehensive attempt to elucidate the G. lucidum proteome. To date, only a limited number of studies on lignocellulolytic enzymes have been conducted in G. lucidum [51, 82, 83]. By searching against the G. lucidum genome, 61 proteins were identified as wood degrading enzymes, greatly broadening the database of lignocellulose degrading proteins of G. lucidum. Most of these enzymes (49) were identified in the fruiting bodies at 90 days. The differences in developmental stages, methods used to extract proteins, and culture conditions of G. lucidum may explain this result. Many TCA-cycle related enzymes and peptidases were detected in our proteome, suggesting their importance in the development of G. lucidum. For the first time, two argonaute-like proteins that participate in the expression of miRNA-like RNAs in fungi [75] were identified from G. lucidum. This finding implies that the miRNA-like RNAs may exist in G. lucidum.
In keeping with previous proteomic studies in other fungi, most proteins in the G. lucidum proteome were grouped into the COG categories ‘Carbohydrate transport and metabolism’, ‘Amino acid transport and metabolism’ and ‘Energy production and conversion’ [35, 40, 42]. Perhaps proteins in these COG classifications are particularly important for fungal development in general.
GL18770 is the LZ-8 protein. A small difference exists between GL18770 and LZ-8, but the difference is likely attributable to the use of different Ganoderma strains in the published genome report [19] and the current proteome study. Based on the current proteome data and the published genome, the GL18769 was successfully cloned, expressed and proved to be a new immunomodulatory protein. Differently than ConA, which induced aggregation and proliferation of MSLs, GL18769 increased the density of splenic lymphocytes with little cell aggregation observed. Further study should be done in the future to elucidate the mechanism for stimulating the MSLs proliferation.
G. lucidum is a macrofungus which undergoes tremendous changes from mycelium to mature fruiting body (Fig. 1). The high concentration of interfering compounds (e.g., lignin, pigments, polysaccharides, and terpenoids) makes it technically difficult to use the same protein extraction method for all the different developmental stages, especially the 90dF stage, which is highly lignified. Different methods have been tried to extract proteins from 90dF, and the method used in this study offers the best results. For mycelium and 60dF, it is easy to obtain proteins using the same TCA/acetone method. Performing a quantitative proteomic analysis for samples prepared by different methods is unreasonable; therefore, it is currently impractical to perform a quantitative proteomic study of the three developmental stages for G. lucidum. However, in this study, the qualitative proteomic analysis of this macrofungus is still significant. Further quantitative proteomic study is required to characterize G. lucidum extensively.
Supporting Information
By searching against the G. lucidum genome database, 803 proteins from the three developmental stages of G. lucidum (16 days mycelium, 60dF and 90dF) were identified by LC-MS/MS. 247, 401 and 273 proteins were identified from these three developmental stages, respectively.
(XLSX)
GO annotations for 470 proteins were classified into 39 functional groups.
(XLSX)
COG annotations for 357 proteins were classified into 22 sub-categories.
(XLSX)
Acknowledgments
We would like to thank Professor Fu Xiang Dong (University of California, San Diego, USA) for his advice and technical assistance. We also would like to thank Professor Du Hai Ning (Department of Biochemistry, College of Life Sciences, Wu Han University) for his instrumental help. Genome data of G. lucidum was obtained at http://www.herbalgenomics.org/galu/. The G. lucidum sample was obtained from the Guangdong Institute of Microbiology (Guangdong, P.R China).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present research project was funded by the National Science Foundation of China (NSFC) program [31301426, 31370849] (http://www.nsfc.gov.cn/), the Research Fund for the Doctoral Program of Higher Education of China program [20130146120019], the National Key Technologies R&D Program [2009ZX09103–676, 2012ZX10001-006] (http://www.nmp.gov.cn/), the Chinese 111 project [B06018], and Wuhan University project [No. 2042014kf0226]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Paterson RR. Ganoderma—a therapeutic fungal biofactory. Phytochemistry. 2006;67(18):1985–2001. [DOI] [PubMed] [Google Scholar]
- 2. Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Current pharmaceutical biotechnology. 2009;10(8):717–42. [DOI] [PubMed] [Google Scholar]
- 3. Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnology annual review. 2007;13:265–301. [DOI] [PubMed] [Google Scholar]
- 4. Yoon HM, Jang KJ, Han MS, Jeong JW, Kim GY, Lee JH, et al. Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-kappaB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells. Experimental and therapeutic medicine. 2013;5(3):957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu GS, Guo JJ, Bao JL, Li XW, Chen XP, Lu JJ, et al. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—a review. Expert opinion on investigational drugs. 2013;22(8):981–92. 10.1517/13543784.2013.805202 [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Wang Y, Liu X, Yuan Y, Yue T. Free radical scavenging and immunomodulatory activities of Ganoderma lucidum polysaccharides derivatives. Carbohydrate polymers. 2013;91(1):33–8. 10.1016/j.carbpol.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 7. Zhou X, Lin J, Yin Y, Zhao J, Sun X, Tang K. Ganodermataceae: natural products and their related pharmacological functions. The American journal of Chinese medicine. 2007;35(4):559–74. [DOI] [PubMed] [Google Scholar]
- 8. Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chemical record. 2003;3(3):172–80. [DOI] [PubMed] [Google Scholar]
- 9. Dudhgaonkar S, Thyagarajan A, Sliva D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. International immunopharmacology. 2009;9(11):1272–80. 10.1016/j.intimp.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 10. Kimura Y, Taniguchi M, Baba K. Antitumor and antimetastatic effects on liver of triterpenoid fractions of Ganoderma lucidum: mechanism of action and isolation of an active substance. Anticancer research. 2002;22(6A):3309–18. [PubMed] [Google Scholar]
- 11. Wu X, Zeng J, Hu J, Liao Q, Zhou R, Zhang P, et al. Hepatoprotective effects of aqueous extract from Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher basidiomycetes) on alpha-amanitin-induced liver injury in mice. International journal of medicinal mushrooms. 2013;15(4):383–91. [DOI] [PubMed] [Google Scholar]
- 12. Li P, Deng YP, Wei XX, Xu JH. Triterpenoids from Ganoderma lucidum and their cytotoxic activities. Natural product research. 2013;27(1):17–22. 10.1080/14786419.2011.652961 [DOI] [PubMed] [Google Scholar]
- 13. Bao X, Liu C, Fang J, Li X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydrate research. 2001;332(1):67–74. [DOI] [PubMed] [Google Scholar]
- 14. Zhang CR, Yang SP, Yue JM. Sterols and triterpenoids from the spores of Ganoderma lucidum. Natural product research. 2008;22(13):1137–42. 10.1080/14786410601129721 [DOI] [PubMed] [Google Scholar]
- 15. Ko EM, Leem YE, Choi HT. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Applied microbiology and biotechnology. 2001;57(1–2):98–102. [DOI] [PubMed] [Google Scholar]
- 16. D'Souza TM, Merritt CS, Reddy CA. Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Applied and environmental microbiology. 1999;65(12):5307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shendure J, Ji H. Next-generation DNA sequencing. Nature biotechnology. 2008;26(10):1135–45. 10.1038/nbt1486 [DOI] [PubMed] [Google Scholar]
- 18. Ansorge WJ. Next-generation DNA sequencing techniques. New biotechnology. 2009;25(4):195–203. 10.1016/j.nbt.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 19. Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nature communications. 2012;3:913 10.1038/ncomms1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu D, Gong J, Dai W, Kang X, Huang Z, Zhang HM, et al. The genome of Ganoderma lucidum provides insights into triterpenes biosynthesis and wood degradation [corrected]. PloS one. 2012;7(5):e36146 10.1371/journal.pone.0036146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu GJ, Wang M, Huang J, Yin YL, Chen YJ, Jiang S, et al. Deep insight into the Ganoderma lucidum by comprehensive analysis of its transcriptome. PloS one. 2012;7(8):e44031 10.1371/journal.pone.0044031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang YH, Wu HY, Wu KM, Liu TT, Liou RF, Tsai SF, et al. Generation and analysis of the expressed sequence tags from the mycelium of Ganoderma lucidum. PloS one. 2013;8(5):e61127 10.1371/journal.pone.0061127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo H, Sun C, Song J, Lan J, Li Y, Li X, et al. Generation and analysis of expressed sequence tags from a cDNA library of the fruiting body of Ganoderma lucidum. Chinese medicine. 2010;5:9 10.1186/1749-8546-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ren A, Li MJ, Shi L, Mu DS, Jiang AL, Han Q, et al. Profiling and quantifying differential gene transcription provide insights into ganoderic acid biosynthesis in Ganoderma lucidum in response to methyl jasmonate. PloS one. 2013;8(6):e65027 10.1371/journal.pone.0065027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park BH, Karpinets TV, Syed MH, Leuze MR, Uberbacher EC. CAZymes Analysis Toolkit (CAT): web service for searching and analyzing carbohydrate-active enzymes in a newly sequenced organism using CAZy database. Glycobiology. 2010;20(12):1574–84. 10.1093/glycob/cwq106 [DOI] [PubMed] [Google Scholar]
- 26. Steen H, Mann M. The ABC's (and XYZ's) of peptide sequencing. Nature reviews Molecular cell biology. 2004;5(9):699–711. [DOI] [PubMed] [Google Scholar]
- 27. Sickmann A, Mreyen M, Meyer HE. Mass spectrometry—a key technology in proteome research. Advances in biochemical engineering/biotechnology. 2003;83:141–76. [DOI] [PubMed] [Google Scholar]
- 28. de Oliveira JM, de Graaff LH. Proteomics of industrial fungi: trends and insights for biotechnology. Applied microbiology and biotechnology. 2011;89(2):225–37. 10.1007/s00253-010-2900-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monteiro R, Vitorino R, Domingues P, Radhouani H, Carvalho C, Poeta P, et al. Proteome of a methicillin-resistant Staphylococcus aureus clinical strain of sequence type ST398. Journal of proteomics. 2012;75(10):2892–915. 10.1016/j.jprot.2011.12.036 [DOI] [PubMed] [Google Scholar]
- 30. Barros BH, da Silva SH, dos ReisMarques Edos R, Rosa JC, Yatsuda AP, Roberts DW, et al. A proteomic approach to identifying proteins differentially expressed in conidia and mycelium of the entomopathogenic fungus Metarhizium acridum. Fungal biology. 2010;114(7):572–9. 10.1016/j.funbio.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 31. Scherl A, Francois P, Bento M, Deshusses JM, Charbonnier Y, Converset V, et al. Correlation of proteomic and transcriptomic profiles of Staphylococcus aureus during the post-exponential phase of growth. Journal of microbiological methods. 2005;60(2):247–57. [DOI] [PubMed] [Google Scholar]
- 32. Matallana-Surget S, Derock J, Leroy B, Badri H, Deschoenmaeker F, Wattiez R. Proteome-wide analysis and diel proteomic profiling of the cyanobacterium Arthrospira platensis PCC 8005. PloS one. 2014;9(6):e99076 10.1371/journal.pone.0099076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D'Ambrosio C, Arena S, Rocco M, Verrillo F, Novi G, Viscosi V, et al. Proteomic analysis of apricot fruit during ripening. Journal of proteomics. 2013;78:39–57. 10.1016/j.jprot.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 34. Bharti RK, Srivastava S, Thakur IS. Proteomic analysis of carbon concentrating chemolithotrophic bacteria Serratia sp. for sequestration of carbon dioxide. PloS one. 2014;9(3):e91300 10.1371/journal.pone.0091300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horie K, Rakwal R, Hirano M, Shibato J, Nam HW, Kim YS, et al. Proteomics of two cultivated mushrooms Sparassis crispa and Hericium erinaceum provides insight into their numerous functional protein components and diversity. Journal of proteome research. 2008;7(5):1819–35. 10.1021/pr070369o [DOI] [PubMed] [Google Scholar]
- 36. Johnson SL, Villarroel M, Rosengrave P, Carne A, Kleffmann T, Lokman PM, et al. Proteomic analysis of chinook salmon (Oncorhynchus tshawytscha) ovarian fluid. PloS one. 2014;9(8):e104155 10.1371/journal.pone.0104155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. Journal of proteomics. 2012;75(5):1486–92. 10.1016/j.jprot.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 38. Restelli L, Codrea MC, Savoini G, Ceciliani F, Bendixen E. LC-MS/MS analysis of visceral and subcutaneous adipose tissue proteomes in young goats with focus on innate immunity and inflammation related proteins. Journal of proteomics. 2014;108:295–305. 10.1016/j.jprot.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 39. Rahmad N, Al-Obaidi JR, Nor Rashid NM, Zean NB, Mohd Yusoff MH, Shaharuddin NS, et al. Comparative proteomic analysis of different developmental stages of the edible mushroom Termitomyces heimii. Biological research. 2014;47(1):30 10.1186/0717-6287-47-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang J, Wang L, Ji X, Feng Y, Li X, Zou C, et al. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS pathogens. 2011;7(9):e1002179 10.1371/journal.ppat.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang M, Gu B, Huang J, Jiang S, Chen Y, Yin Y, et al. Transcriptome and proteome exploration to provide a resource for the study of Agrocybe aegerita. PloS one. 2013;8(2):e56686 10.1371/journal.pone.0056686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin Y, Yu G, Chen Y, Jiang S, Wang M, Jin Y, et al. Genome-wide transcriptome and proteome analysis on different developmental stages of Cordyceps militaris. PloS one. 2012;7(12):e51853 10.1371/journal.pone.0051853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu HC, Hsu CI, Lin RH, Kao CL, Lin JY. Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. The Biochemical journal. 1997;323 (Pt 2):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsin IL, Ou CC, Wu TC, Jan MS, Wu MF, Chiu LY, et al. GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy. 2011;7(8):873–82. [DOI] [PubMed] [Google Scholar]
- 45. Lin CH, Hsiao YM, Ou CC, Lin YW, Chiu YL, Lue KH, et al. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor alpha-induced expression of matrix metalloproteinase 9 via NF-kappaB pathway in human alveolar epithelial A549 cells. Journal of agricultural and food chemistry. 2010;58(22):12014–21. 10.1021/jf103068w [DOI] [PubMed] [Google Scholar]
- 46. Kuan YC, Wu TJ, Kuo CY, Hsu JC, Chang WY, Sheu F. Molecular cloning of a new immunomodulatory protein from Anoectochilus formosanus which induces B cell IgM secretion through a T-independent mechanism. PloS one. 2011;6(6):e21004 10.1371/journal.pone.0021004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang S, Chen Y, Wang M, Yin Y, Pan Y, Gu B, et al. A novel lectin from Agrocybe aegerita shows high binding selectivity for terminal N-acetylglucosamine. The Biochemical journal. 2012;443(2):369–78. 10.1042/BJ20112061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao C, Sun H, Tong X, Qi Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. The Biochemical journal. 2003;374(Pt 2):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kino K, Yamashita A, Yamaoka K, Watanabe J, Tanaka S, Ko K, et al. Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (LZ-8), from Ganoderma lucidium. The Journal of biological chemistry. 1989;264(1):472–8. [PubMed] [Google Scholar]
- 50. Tanaka S, Ko K, Kino K, Tsuchiya K, Yamashita A, Murasugi A, et al. Complete amino acid sequence of an immunomodulatory protein, ling zhi-8 (LZ-8). An immunomodulator from a fungus, Ganoderma lucidium, having similarity to immunoglobulin variable regions. The Journal of biological chemistry. 1989;264(28):16372–7. [PubMed] [Google Scholar]
- 51. Manavalan T, Manavalan A, Thangavelu KP, Heese K. Secretome analysis of Ganoderma lucidum cultivated in sugarcane bagasse. Journal of proteomics. 2012;77:298–309. 10.1016/j.jprot.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 52. Wu Y, Wang D. A new class of natural glycopeptides with sugar moiety-dependent antioxidant activities derived from Ganoderma lucidum fruiting bodies. Journal of proteome research. 2009;8(2):436–42. 10.1021/pr800554w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen YY, Lin SY, Yeh YY, Hsiao HH, Wu CY, Chen ST, et al. A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis. 2005;26(11):2117–27. [DOI] [PubMed] [Google Scholar]
- 54. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature genetics. 2000;25(1):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, et al. The COG database: an updated version includes eukaryotes. BMC bioinformatics. 2003;4:41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44(7):1299–305. [DOI] [PubMed] [Google Scholar]
- 57. Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic acids research. 2014;42(Database issue):D490–5. 10.1093/nar/gkt1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic acids research. 2009;37(Database issue):D233–8. 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Levasseur A, Drula E, Lombard V, Coutinho PM, Henrissat B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnology for biofuels. 2013;6(1):41 10.1186/1754-6834-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aoshima M. Novel enzyme reactions related to the tricarboxylic acid cycle: phylogenetic/functional implications and biotechnological applications. Applied microbiology and biotechnology. 2007;75(2):249–55. [DOI] [PubMed] [Google Scholar]
- 61. Nunes-Nesi A, Araujo WL, Obata T, Fernie AR. Regulation of the mitochondrial tricarboxylic acid cycle. Current opinion in plant biology. 2013;16(3):335–43. 10.1016/j.pbi.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 62. Terol J, Soler G, Talon M, Cercos M. The aconitate hydratase family from Citrus. BMC plant biology. 2010;10:222 10.1186/1471-2229-10-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aleksandrov A, Zvereva E, Field M. The mechanism of citryl-coenzyme A formation catalyzed by citrate synthase. The journal of physical chemistry B. 2014;118(17):4505–13. 10.1021/jp412346g [DOI] [PubMed] [Google Scholar]
- 64. McNeil MB, Hampton HG, Hards KJ, Watson BN, Cook GM, Fineran PC. The succinate dehydrogenase assembly factor, SdhE, is required for the flavinylation and activation of fumarate reductase in bacteria. FEBS letters. 2014;588(3):414–21. 10.1016/j.febslet.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 65. Sanfelix-Haywood N, Coll-Marques JM, Yebra MJ. Role of alpha-phosphoglucomutase and phosphoglucose isomerase activities at the branching point between sugar catabolism and anabolism in Lactobacillus casei. Journal of applied microbiology. 2011;111(2):433–42. 10.1111/j.1365-2672.2011.05045.x [DOI] [PubMed] [Google Scholar]
- 66. Tomm JM, Krause C, Simon JC, Treudler R, von Bergen M, Averbeck M. Pyruvate kinase and phosphopyruvate hydratase as novel IgE reactive proteins in prawn. Journal of investigational allergology & clinical immunology. 2013;23(6):443–5. 10.1111/ddg.12103 [DOI] [PubMed] [Google Scholar]
- 67. Zheng H, Beliavsky A, Tchigvintsev A, Brunzelle JS, Brown G, Flick R, et al. Structure and activity of the NAD(P)+-dependent succinate semialdehyde dehydrogenase YneI from Salmonella typhimurium. Proteins. 2013;81(6):1031–41. 10.1002/prot.24227 [DOI] [PubMed] [Google Scholar]
- 68. Liebminger E, Veit C, Pabst M, Batoux M, Zipfel C, Altmann F, et al. Beta-N-acetylhexosaminidases HEXO1 and HEXO3 are responsible for the formation of paucimannosidic N-glycans in Arabidopsis thaliana. The Journal of biological chemistry. 2011;286(12):10793–802. 10.1074/jbc.M110.178020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guranowski A, de Diego A, Sillero A, Sillero MAG. Uridine 5 '-polyphosphates (p(4)U and p(5)U) and uridine(5 ')polyphospho(5 ')nucleosides (Up(n)Ns) can be synthesized by UTP: glucose-1-phosphate uridylyltransferase from Saccharomyces cerevisiae. FEBS letters. 2004;561(1–3):83–8. [DOI] [PubMed] [Google Scholar]
- 70. Johswich A, Longuet C, Pawling J, Rahman AA, Ryczko M, Drucker DJ, et al. N-glycan remodeling on glucagon receptor is an effector of nutrient sensing by the hexosamine biosynthesis pathway. The Journal of biological chemistry. 2014;289(23):15927–41. 10.1074/jbc.M114.563734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ohnuma K, Hosono O, Dang NH, Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Advances in clinical chemistry. 2011;53:51–84. [DOI] [PubMed] [Google Scholar]
- 72. Horimoto Y, Dee DR, Yada RY. Multifunctional aspartic peptidase prosegments. New biotechnology. 2009;25(5):318–24. 10.1016/j.nbt.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 73. Liu DL, Yao DS, Liang YQ, Zhou TH, Song YP, Zhao L, et al. Production, purification, and characterization of an intracellular aflatoxin-detoxifizyme from Armillariella tabescens (E-20). Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2001;39(5):461–6. [DOI] [PubMed] [Google Scholar]
- 74. Yao Z, Zhang C, Lu F, Bie X, Lu Z. Gene cloning, expression, and characterization of a novel acetaldehyde dehydrogenase from Issatchenkia terricola strain XJ-2. Applied microbiology and biotechnology. 2012;93(5):1999–2009. 10.1007/s00253-011-3541-7 [DOI] [PubMed] [Google Scholar]
- 75. Lau SK, Chow WN, Wong AY, Yeung JM, Bao J, Zhang N, et al. Identification of microRNA-like RNAs in mycelial and yeast phases of the thermal dimorphic fungus Penicillium marneffei. PLoS neglected tropical diseases. 2013;7(8):e2398 10.1371/journal.pntd.0002398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Douglas CM. Fungal beta(1,3)-D-glucan synthesis. Medical mycology: official publication of the International Society for Human and Animal Mycology. 2001;39 Suppl 1:55–66. [DOI] [PubMed] [Google Scholar]
- 77. Shahinian S, Bussey H. beta-1,6-Glucan synthesis in Saccharomyces cerevisiae. Molecular microbiology. 2000;35(3):477–89. [DOI] [PubMed] [Google Scholar]
- 78. Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, et al. An anti-infective peptide that selectively modulates the innate immune response. Nature biotechnology. 2007;25(4):465–72. [DOI] [PubMed] [Google Scholar]
- 79. Li S, Nie Y, Ding Y, Shi L, Tang X. Recombinant Expression of a Novel Fungal Immunomodulatory Protein with Human Tumor Cell Antiproliferative Activity from Nectria haematococca. International journal of molecular sciences. 2014;15(10):17751–64. 10.3390/ijms151017751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li F, Wen H, Liu X, Zhou F, Chen G. Gene cloning and recombinant expression of a novel fungal immunomodulatory protein from Trametes versicolor. Protein expression and purification. 2012;82(2):339–44. 10.1016/j.pep.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 81. Ko JL, Hsu CI, Lin RH, Kao CL, Lin JY. A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence. European journal of biochemistry / FEBS. 1995;228(2):244–9. [PubMed] [Google Scholar]
- 82. Jakucs E, Racz I, Lasztity D. Some characteristics and partial purification of the Ganoderma lucidum cellulase system. Acta microbiologica et immunologica Hungarica. 1994;41(1):23–31. [PubMed] [Google Scholar]
- 83. Sripuan T, Aoki K, Yamamoto K, Tongkao D, Kumagai H. Purification and characterization of thermostable alpha-galactosidase from Ganoderma lucidum. Bioscience, biotechnology, and biochemistry. 2003;67(7):1485–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
By searching against the G. lucidum genome database, 803 proteins from the three developmental stages of G. lucidum (16 days mycelium, 60dF and 90dF) were identified by LC-MS/MS. 247, 401 and 273 proteins were identified from these three developmental stages, respectively.
(XLSX)
GO annotations for 470 proteins were classified into 39 functional groups.
(XLSX)
COG annotations for 357 proteins were classified into 22 sub-categories.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.