Abstract
Objectives. We examined electronic health records (EHRs) to assess the impact of systems change on tobacco use screening, treatment, and quit rates among low-income primary care patients in Louisiana.
Methods. We examined EHR data on 79 777 patients with more than 1.2 million adult primary care encounters from January 1, 2009, through January 31, 2012, for evidence of systems change. We adapted a definition of “systems change” to evaluate a tobacco screening and treatment protocol used by medical staff during primary care visits at 7 sites in a public hospital system.
Results. Six of 7 sites met the definition of systems change, with routine screening rates for tobacco use higher than 50%. Within the first year, a 99.7% screening rate was reached. Sites had a 9.5% relative decrease in prevalence over the study period. Patients were 1.03 times more likely to sustain quit with each additional intervention (95% confidence interval = 1.02, 1.04).
Conclusions. EHRs can be used to demonstrate that routine clinical interventions with low-income primary care patients result in reductions in tobacco use and sustained quits.
Despite reductions in prevalence, tobacco use or tobacco smoke remains 1 of the major modifiable risk factors for 4 of the 5 leading causes of death in the United States.1 Estimated direct medical costs from tobacco use have reached $133 billion annually.2 Until evidence-based interventions to increase population quit attempts and quit rates become routine, especially in health care settings, national objectives to reduce tobacco use will be difficult to achieve.3
Brief tobacco cessation interventions are effective in helping smokers quit.4,5 Clinical protocols such as the 5 A’s (ask, advise, assess, assist, arrange),6 2 A’s and a C (ask, advise, connect),7 and 2 A’s (ask, act)8 promote tobacco use screening and treatment. However, in addition to clinician interventions, the US Public Health Service guideline for treating tobacco use and dependence advocates that health care organizations adopt systems change supporting delivery of tobacco dependence treatments.6
The use of health care delivery systems has the potential to reach a large number of smokers, 80% of whom see a doctor at least once a year.2 Systems change enhances the identification and treatment of tobacco users within health care settings. The guideline recommends systems strategies to facilitate integration of evidence-based treatments for tobacco use, including electronic documentation of screening and intervention.6
Use of electronic health records (EHRs) improves adherence to clinical protocols.9 Legislation incentivizing nationwide adoption of EHRs will accelerate integration of this technology into diverse delivery systems.10 Still, long-term follow-up and measurements are needed to verify changes in patterns of care and to determine whether such changes result in improved health outcomes.
By examining EHR data, Land et al.11 demonstrated the ability to measure the effect of systems change on smoking prevalence and health care utilization. Their results illustrated the importance of consistent exposure to evidence-based interventions and showed that a system-wide adoption of standardized evidence-based tobacco measures decreased smoking prevalence in a relatively affluent, primarily White suburban population with lower than average smoking prevalence. Because people in lower socioeconomic classes have difficulty sustaining quits,12,13 it is important to show that routine clinical interventions with low-income patients promote successful quit attempts and help recent quitters remain so.
In 2002, the Louisiana legislature enacted a cigarette excise tax as part of a statewide tobacco control program,14 including the Tobacco Control Initiative (TCI). The TCI, an early adopter of EHR-supported, clinic-based interventions for tobacco use, offers standardized cessation services, with a focus on implementation in ambulatory care settings. The TCI’s goal is to identify tobacco users and deliver evidence-based treatments. It provides designated personnel, clinician training, behavioral counseling, free or low-cost pharmacotherapy, quit line referral, and bedside consultation, along with performance appraisal and feedback. Systems thinking was used as the framework for program conceptualization and development. The TCI program is described in greater detail elsewhere.15
Over several years, the TCI integrated treatment of tobacco use into routine care for all patients in the Health Care Services Division of Louisiana State University Health New Orleans (LSUHNO) public hospital system using US Public Health Service guidelines. Therefore, the impact of this systems change on quit attempts, quits, and health outcomes should reflect what can be achieved when evidence-based tobacco measures are evaluated for their impact on population health.
In this article, we examine the impact of systems change on a public health care network and the capacity of EHR data to capture these changes. We evaluate the effectiveness of the system-wide implementation of tobacco interventions to promote quitting.
METHODS
Seven LSUHNO facilities, varying in size, currently participate in the electronically supported TCI program; all are located in the southern region of the state. These facilities manage more than 30 000 admissions annually, along with 500 000 outpatient and emergency department (ED) visits for more than 230 000 Louisiana residents (mean age = 40 years; 51% African American; 57% female; 61% uninsured). Three facilities (Medical Center of Louisiana, Earl K. Long Medical Center, and University Medical Center) are in urban centers, and 4 (Bogalusa Medical Center, Lallie Kemp Medical Center, Leonard J. Chabert Medical Center, and Walter O. Moss Medical Center) are in rural areas. All facilities provide inpatient, emergency, primary, and some specialty care. Those treated at the 3 urban facilities, serving 56% of the total Louisiana State University patient population, are similar to those at the 4 rural facilities with regard to patient age, sex, and insurance distributions. However, urban facilities have a greater percentage of African American patients (62% vs 38%). Across sites, self-reported smoking prevalence ranged from 26% to 40% (R. H., written communication, July 2013).
Data Collection
LSUHNO uses CliQ, an internally developed EHR.16 CliQ stores patient demographics, clinical notes, electronic orders, lab results, prescriptions, and medication information and has a reminder system for interventions based on evidence-based guidelines. CliQ presents a 5 A's–based clinical protocol for brief intervention (< 10 min). Although inclusion of each element of the 5 A’s within the EHR was completed in December 2008, allowing for electronic tracking of implementation of the TCI intervention, we used only 2 A’s (ask and advise) in this analysis. Screening for tobacco use occurs during the nursing assessment and may be conducted by nurses, medical assistants, or health professionals in training who enter the assessment information into the EHR. Figure 1 depicts the workflow for identifying and treating tobacco users at LSUHNO facilities.
FIGURE 1—
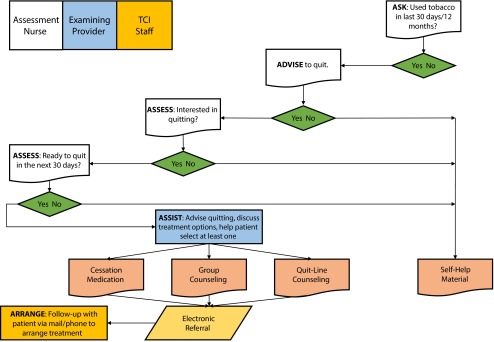
The Tobacco Control Initiative 5 A’s (ask, advise, assess, assess, assist, and arrange) protocol for tobacco intervention and parties responsible for each component: Louisiana State University Health New Orleans Health Care Services Division.
Note. TCI = Tobacco Control Initiative.
Study Population
The data set from EHR records included inpatient and outpatient encounters at all sites between January 1, 2002, and January 31, 2012 (7 329 716 visits for 311 517 patients). We extracted 2 different study populations from the EHR data.
We used the first population to examine the implementation of the TCI across the LSUHNO system. This population included all primary care encounters with adults between January 1, 2009, and January 31, 2012, at the 7 sites (n = 1 215 183). To assess the trend in tobacco use in the past 30 days, we restricted this analysis to 79 777 patients who had at least 1 primary care visit from January 1, 2009, to June 30, 2009.
We used the second population, restricted to patients who entered the TCI as current smokers, to examine the association and impact between the TCI and smoking cessation. We restricted these analyses to patients who reported current tobacco use in the past 30 days at their first 2 visits after January 1, 2009 (n = 18 162). Because the TCI focused on implementation in the primary care setting, 2 of the 4 unique visits required to establish a sustained quit had to have taken place in the primary care setting. We dropped eligible patients from the analyses if they (1) had missing demographic information, (2) were not aged 18 years or older at the time of the first intervention, or (3) could not be assigned to a primary facility or payer. Because people with exacerbated health problems are more likely to quit,17 we further restricted the analysis to patients who were not hospitalized during the study period. (See Figure A, available as a supplement to this article at http://www.ajph.org, for a flow diagram of the data set.)
Among current tobacco users, patients were identified as having a sustained quit, attempted quit, or nonquit, depending on their screenings at the last 2 primary care visits within the study period. We considered patients as having (1) a sustained quit if they reported not using tobacco in the past 30 days at 2 consecutive primary care visits at least 90 days apart, (2) an attempted quit if they reported no tobacco use within the past 30 days but could not meet the criteria for a sustained quit, or (3) a nonquit if they reported using tobacco in the past 30 days at both of their last 2 visits. Because LSUHNO used a 90-day criterion to prompt providers to screen for tobacco use, we adapted the definition of systems change used by Land et al.11 The Land et al. study examined clinical sites that adopted an every-patient, every-visit model for tobacco interventions. The operational definition of systems change was linked to the month in which more than 50% of all patients at all visits were asked about smoking as long as the rate did not drop below 50% for the succeeding 12 months. In this study, the date that systems change occurred at a LSUHNO site was defined as the first month in which more than half of all office visit patients at the site were asked about tobacco use if 90 days or more had elapsed since the previous screening for tobacco use. Furthermore, the rate of identification of tobacco users had to remain at 50% or more for at least 12 consecutive months after the initial month when the 50% threshold was reached.
Number of Interventions and Potential Confounders
Patients were advised to quit smoking on 95.8% of visits in which a smoker was identified. Because the vast majority of these encounters included advice to quit, we counted all visits during which a smoker was identified as interventions.
We extracted patient demographic information, including age (18–29, 30–39, 40–49, 50–64, and ≥ 65 years), sex (male, female), and race (African American, White, other) from the EHRs for use in analyses as potential confounders. Information on the payer was collected each time a patient visited a medical provider. Payers were listed as commercial, free, Medicaid, Medicare, prisoner, and self-pay, and we categorized them into commercial, public (free, Medicaid, Medicare, prisoner), and self-pay.
The analyses also included the facility because the intervention was more fully implemented at some facilities than at others. We assigned patients a primary facility on the basis of the site at which they received most of their ambulatory care (e.g., if a patient had 9 visits total, 5 at site A and 4 at site B, site A was assigned as primary facility). Finally, because the number of visits was positively skewed, we created visit categories that reduced the impact of outliers. We coded emergency department (ED) visits as 0, 1 to 2, 3 to 4, and 5 or greater during the 37-month study period. Other ambulatory care visits (specialty and primary care) were summed and coded as 2 to 4, 5 to 14, and 15 or greater during the study period.
Analyses
We analyzed data with SAS version 9.3 (SAS Institute, Cary, NC). Three sets of analyses were conducted. In the first set, we investigated the frequency of interventions over time across the 7 sites by creating ratios of the number of smoking interventions to the total primary care encounters among patients aged 18 years or older. To assess site-specific systems change in the LSUHNO, we also mapped a cohort of patients with at least 1 primary care visit in the first 6 months of the intervention throughout the study period to examine responses to the question regarding 30-day smoking. In a second set of analyses, we examined associations between demographic characteristics, payer, LSUHNO facility, and success of quitting at the level of the encounter. In the final set of analyses, we examined the relationship between the number of smoking intervention encounters and the likelihood of quitting by means of logistic regressions, adjusting for age, race, facility, sex, payer, total number of ED visits, and total number of primary care and specialty visits.
RESULTS
Current analyses used a two-phased process. We assessed the extent to which tobacco interventions were implemented and determined the impact of those interventions on smoking behavior.
Implementation of Site-Based Tobacco Interventions
Within the first year of the study, the tobacco use status of 99.7% of the adult population had been assessed within the past 12 months. Of the 7 participating centers, all but 1 had primary care providers recording smoking status at 50% or more of their visits by the end of the study period, even though the LSUHNO protocol for assessing smoking status only required asking every 90 days (Figure 2). The recording of tobacco status increased within 6 months after implementation of the TCI, with the greatest increases in screening occurring at the Earl K. Long Medical Center, Medical Center of Louisiana, University Medical Center, and Walter O. Moss Medical Center sites.
FIGURE 2—
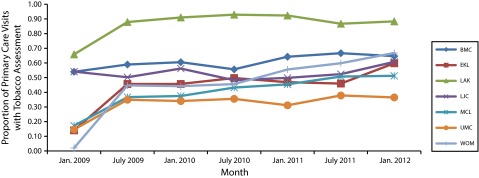
Percentage of primary care visits with recorded tobacco assessment: Louisiana State University Health New Orleans Health Care Services Division intervention sites, January 1, 2009–January 31, 2012.
Note. BMC = Bogalusa Medical Center; EKL = Earl K. Long Medical Center; LAK = Lallie Kemp Medical Center; LJC = Leonard J. Chabert Medical Center; MCL = Medical Center of Louisiana; UMC = University Medical Center; WOM = Walter O. Moss Medical Center.
Land et al.11 developed an operational definition of systems change that required a sustained identification rate of 50% or more to demonstrate that a site had achieved systems change. Land et al.’s definition was based on an assumption that clinical sites would record tobacco use status for every patient at every visit. Given the high visit rate of patients to multiple clinics within an LSUHNO facility, electronic prompts were presented when 90 or more days had elapsed since tobacco use status was documented in the EHR. Using the 90-day rule as the foundation of the LSUHNO reminder system, 6 of 7 sites met the definition of having achieved systems change, and overall the system maintained monthly identification rates of 50% or more for at least 1 year after the threshold was reached.
Along with an increase in implementation of the intervention over the 3-year period, the number of patients reporting tobacco use in the past 30 days decreased among the population who had at least 1 primary care visit in the first 6 months of the study period (n = 79 777 patients). From January 2009 through June 2009, 27.5% of the visits made by this group had a recorded positive response to tobacco use in the past 30 days. For the same time period in 2011, only 24.9% of visits by the same group reported tobacco use within the past 30 days. This 9.5% relative decrease in prevalence was significant (P < .001).
Impact of Tobacco Interventions on Quit Rates
Among eligible patients, 18 162 were classified as current tobacco users. Of these, 82.7% were nonquits (n = 15 013), 10.3% were attempted quits (n = 1867), and 7.1% were sustained quits (n = 1282) at the end of the study period. Among those who were tobacco users at the beginning of the study, a greater proportion of patients who had sustained quits were older, were White, and had either commercial or public insurance as their primary payer when compared with the total population (Table 1).
TABLE 1—
Demographics Among Tobacco Users by Final Status: Louisiana State University Health New Orleans Health Care Services Division Intervention Sites, January 1, 2009–January 31, 2012
| Characteristic | Total (n = 18 162), % | Sustained Quits (n = 1282), % | Attempted Quits (n = 1867), % | Nonquit (n = 15 013), % |
| Age,* y | ||||
| 18–29 | 10.5 | 11.4 | 12.9 | 10.2 |
| 30–39 | 13.6 | 12.2 | 15.3 | 13.6 |
| 40–49 | 29.8 | 26.2 | 28.3 | 30.3 |
| 50–64 | 41.9 | 43.1 | 39.4 | 42.1 |
| ≥ 65 | 4.2 | 7.1 | 4.1 | 4.0 |
| Sex | ||||
| Female | 63.0 | 62.2 | 64.4 | 62.9 |
| Male | 37.0 | 37.8 | 35.6 | 37.1 |
| Facility* | ||||
| BMC | 13.9 | 13.4 | 12.5 | 14.1 |
| EKL | 12.6 | 12.8 | 14.4 | 12.4 |
| LAK | 11.0 | 14.0 | 16.2 | 10.1 |
| LJC | 12.5 | 11.4 | 9.5 | 13.0 |
| MCL | 17.0 | 16.9 | 19.7 | 16.6 |
| UMC | 16.7 | 13.8 | 14.6 | 17.3 |
| WOM | 16.3 | 17.6 | 13.0 | 16.6 |
| Payer* | ||||
| Commercial | 4.6 | 6.4 | 4.1 | 4.5 |
| Public | 86.8 | 88.3 | 86.8 | 86.7 |
| Self-paid | 8.6 | 5.3 | 9.1 | 8.9 |
| Race* | ||||
| African American | 42.7 | 47.7 | 46.0 | 41.8 |
| White | 55.8 | 50.0 | 52.1 | 56.7 |
| Other | 1.6 | 2.3 | 1.9 | 1.5 |
Note. BMC = Bogalusa Medical Center; EKL = Earl K. Long Medical Center; LAK = Lallie Kemp Medical Center; LJC = Leonard J. Chabert Medical Center; MCL = Medical Center of Louisiana; UMC = University Medical Center; WOM = Walter O. Moss Medical Center. Percentages may not add to 100 because of rounding.
*χ2 P < .001.
As determined by logistic regression, the number of smoking interventions in the outpatient setting predicted the likelihood of a sustained quit, even after controlling for demographics, payer status, number of ED visits, and number of specialty and primary care visits (Table 2). For each additional intervention after adjusting for potential confounders, patients were 1.03 times more likely to have a sustained quit (95% confidence interval = 1.02, 1.04). The impact of these interventions was also seen when examining both attempted and sustained quits together; patients increased their odds of either a sustained quit or an attempted quit, even after adjusting for demographics and number of visits (adjusted odds ratio = 1.02; 95% confidence interval = 1.01, 1.02). (See Table A, available as a supplement to this article at http://www.ajph.org, for descriptive statistics on the number of ED, primary care, and specialty care visits during the study period.)
TABLE 2—
Logistic Regression Results Predicting Sustained Quit Among Patients Receiving the Tobacco Intervention: Louisiana State University Health New Orleans Health Care Services Division Intervention Sites, January 1, 2009–January 31, 2012
| Characteristic | OR (95% CI) |
| No. of interventions | 1.03 (1.02, 1.04) |
| Race | |
| African American | 1.33 (1.17, 1.51) |
| Other | 2.07 (1.39, 3.08) |
| White (Ref) | 1.00 |
| Sex | |
| Male | 1.05 (0.93, 1.18) |
| Female (Ref) | 1.00 |
| Age, y | |
| 18–29 | 1.03 (0.76, 1.38) |
| 30–39 | 0.71 (0.53, 0.94) |
| 40–49 | 0.60 (0.46, 0.77) |
| 50–64 | 0.65 (0.51, 0.83) |
| ≥ 65 (Ref) | 1.00 |
| Payer | |
| Commercial | 1.40 (1.10, 1.79) |
| Self-paid | 0.77 (0.59, 0.99) |
| Public (Ref) | 1.00 |
| Facility | |
| BMC | 0.88 (0.72, 1.09) |
| EKL | 0.84 (0.68, 1.05) |
| LAK | 1.39 (1.13, 1.71) |
| LJC | 0.81 (0.65, 1.01) |
| MCL | 0.81 (0.66, 0.99) |
| UMC | 0.64 (0.52, 0.79) |
| WOM (Ref) | 1.00 |
| ED visits, no. | |
| 0 | 0.93 (0.78, 1.10) |
| 1–2 | 0.80 (0.68, 0.94) |
| 3–4 | 0.92 (0.76, 1.10) |
| ≥ 5 (Ref) | 1.00 |
| PCSC visits, no. | |
| 2–4 (Ref) | 1.00 |
| 5–14 | 1.46 (0.97, 2.21) |
| ≥ 15 | 2.52 (1.65, 3.84) |
Note. BMC = Bogalusa Medical Center; CI = confidence interval; ED = emergency department; EKL = Earl K. Long Medical Center; LAK = Lallie Kemp Medical Center; LJC = Leonard J. Chabert Medical Center MCL = Medical Center of Louisiana; OR = odds ratio; PCSC = primary care and specialty care; UMC = University Medical Center; WOM = Walter O. Moss Medical Center. The results of this logistic regression included only sustained quits (n = 1282) and nonquits (n = 15 013). These analyses did not include attempted quits.
DISCUSSION
The 5 A's–based tobacco intervention implemented by LSUHNO was effective in lowering the prevalence of tobacco use among its patient population. This is notable in light of studies that show the difficulty of maintaining quit status among those in lower socioeconomic populations.12,13 After 3 years, a cohort of 79 777 primarily low-income patients had a relative reduction of tobacco users of 9.5%. Examination of the prevalence rates from Louisiana’s Behavioral Risk Factor Surveillance System showed no comparable decreases in smoking prevalence. As determined with these data, smoking prevalence among adults 18 and older was essentially stable, with rates of 20.5% in 2008, 22.1% in 2009, and 22.1% in 2010.18
Furthermore, each intervention increased the odds that a patient would sustain a quit (2.8% per intervention) for at least 90 days. This rate is comparable with that found previously among a relatively affluent population in suburban Boston, Massachusetts (2.6%),11 whereas more than half the patient population in this study was uninsured or had public insurance coverage. Finally, we found site-specific effects, indicating that higher intervention rates had a greater impact on reducing tobacco use. Differences in program implementation, including staff turnover, administrative support, and uptake and adoption of EHR use may have contributed to site variation in intervention rates.14 We should note that the facility failing to reach criteria for systems change was the only facility that had not adopted a smoke-free policy restricting tobacco use on its campus during the study period.
The TCI used system-level strategies recommended by the guideline, integrating comprehensive tobacco cessation services into a health care system serving high-risk, medically vulnerable patients. A key element in this process involved developing the capacity to identify and track tobacco users electronically. Systems that allow for structured intervention and data collection improve adherence to clinical guidelines for treatment of tobacco dependency and enhance knowledge of what works in real-world clinical settings.19,20 Given that structured data were captured for each visit, it also allowed an evaluation of changes in provider and patient behavior, confirming the impact of brief tobacco interventions and elucidating what happens when effective systems change occurs.
The LSUHNO system was unable to gain universal acceptance of screening for tobacco use at every clinical encounter. Some clinicians and staff found the process burdensome and disruptive and reported that patients were annoyed by the repeated queries. Although clinical trials have pointed to the effectiveness of an every patient, every visit screening protocol for tobacco use,6 practical considerations may make that difficult. Data obtained from EHRs could be used to determine whether interval-based screening such as that at LSUHNO is as effective and whether there is an optimal screening interval that ensures adherence to the protocol.
Limitations and Strengths
This study has several potential limitations. First, there was no independent method for determining the quality of the EHR data collected at LSUHNO. Nevertheless, more than 90% of the records for patients had the same tobacco use status from 1 visit to the next. As these data are examined further, one would expect health improvements for patients who report no longer using tobacco. Such clinical data would confirm the quality of the LSUHNO data, but they were beyond the scope of this analysis.
Self-reports of tobacco use may not be entirely accurate. Patients may present a more positive assessment of risky behavior to circumvent uncomfortable discussions about a persistent habit. Although pregnant women underreport smoking,21,22 studies of other populations have shown that self-reports are generally accurate.23 Less than 1% of the EHR records indicated that a patient was pregnant, and for these we found no discernible pattern of underreporting of tobacco use. Moreover, a change in 1% of the records evaluated would not alter the conclusions drawn.
The nonrandomized study has no true comparison population. We examined statewide trends using the Behavioral Risk Factor Surveillance System to make comparisons, but these statewide trends may not be representative of the population served by LSUHNO. Although our comparison may have been imperfect, it is unlikely that the LSUHNO patient population would have shown decreases in smoking prevalence that the general population did not.
There was also no way to determine whether patients had sought care at facilities other than LSUHNO. In August 2005, Hurricane Katrina displaced large numbers of Louisiana residents. Because residents returned to the state over the course of years, gaps could exist in the histories of some patients, who may have obtained care elsewhere. If so, effective tobacco use interventions could have been delivered at these locations, and LSUHNO would have no record of those encounters. To address this possibility, we looked at data 2.5 years after Hurricane Katrina, including only patients who had at least 4 visits at LSUHNO in the study period. We concluded that patients who were seen at LSUHNO sites more than once per year were likely to have had most of their care at these sites.
A major strength of this study is the large, mature data set for a high-risk, vulnerable population. The data set includes all inpatient and outpatient encounters, including ED visits. The breadth and depth of these data will allow future work to look for changes in clinical outcomes, such as reductions in blood pressure, which would be expected for patients who have quit smoking. Reductions in tobacco use should also lead to decreases in ED visits and hospitalizations, especially among patients with coronary heart disease.
Conclusions
In this study, we demonstrated that EHR data can be used to track systems change and the subsequent impact of routine clinic-based intervention on quit rates and prevalence of tobacco use in a health care delivery system serving low-income smokers. Data from systems such as that at LSUHNO can be used by analysts, researchers, and clinicians to learn how to deliver effective clinical interventions. As more clinical sites make their encounter records available, measures that tie clinical encounters to quitting behavior and health outcomes will enhance the meaningful use of the EHR in real-world settings.
Acknowledgments
This study was supported by a contract (Contract Financial Management System 599454) from the Louisiana Cancer Research Consortium (http://www.louisianacancercenter.org).
We acknowledge the contributions made by the local Tobacco Control Initiative field staff and the cooperation of the Louisiana State University Health New Orleans Health Care Services Division.
Note. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Human Participant Protection
The Louisiana State University Health Sciences Center New Orleans institutional review board approved this study, granting waiver of Health Insurance Portability and Accountability Act authorization and written informed consent. We used anonymized and de-identified patient records.
References
- 1.Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death—United States, 2008-2010. MMWR Morb Mortal Wkly Rep. 2014;63(17):369–374. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 3.US Department of Health and Human Services. Healthy People 2020. Washington, DC: US Department of Health and Human Service; 2013. Available at: http://www.healthypeople.gov/2020. Accessed May 13, 2014. [Google Scholar]
- 4.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;(5):CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aveyard P, Begh R, Parsons AC, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction. 2012;107(6):1066–1073. doi: 10.1111/j.1360-0443.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med. 2008;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidrine JI, Shete S, Cao Y et al. Ask-advise-connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Family Physicians. Ask and act. Available at: http://www.aafp.org/about/initiatives/ask-act.html. Accessed May 7, 2014.
- 9.Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2011;(12):CD008743. doi: 10.1002/14651858.CD008743.pub2. [DOI] [PubMed] [Google Scholar]
- 10.DesRoches CM, Charles D, Furukawa MF et al. Adoption of electronic health records grows rapidly, but fewer than half of US hospitals had at least a basic system in 2012. Health Aff (Millwood) 2013;32(8):1478–1485. doi: 10.1377/hlthaff.2013.0308. [DOI] [PubMed] [Google Scholar]
- 11.Land TG, Rigotti NA, Levy DE, Schilling T, Warner D, Li W. The effect of systematic clinical interventions with cigarette smokers on quit status and the rates of smoking-related primary care office visits. PLoS One. 2012;7(7):e41649. doi: 10.1371/journal.pone.0041649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffer CE, Stitzer M, Landes R, Brackman L, Munn T, Moore P. Socioeconomic disparities in community-based treatment of tobacco dependence. Am J Public Health. 2012;102(3):e8–e16. doi: 10.2105/AJPH.2011.300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiscock R, Bauld L, Amos A, Fidler JA, Munaf M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- 14.House Legislative Services. Highlights of the 2002 first extraordinary and regular sessions of the Louisiana legislature. Available at: http://wwwhouselouisianagov/H_Highlights/HighlightsOf2002Sessionspdf. Accessed December 24, 2002.
- 15.Moody-Thomas S, Celestin MD, Jr, Horswell R. Use of systems change and health information technology to integrate comprehensive tobacco cessation services in a statewide system for delivery of healthcare. Open J Prev Med. 2013;3(1):75–83. [Google Scholar]
- 16.Horswell R, Butler MK, Kaiser M et al. Disease management programs for the underserved. Dis Manag. 2008;11(3):145–152. doi: 10.1089/dis.2007.0011. [DOI] [PubMed] [Google Scholar]
- 17.Ockene J, Kristeller JL, Goldberg R et al. Smoking cessation and severity of disease: the Coronary Artery Smoking Intervention Study. Health Psychol. 1992;11(2):119–126. doi: 10.1037//0278-6133.11.2.119. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. Available at http://www.cdc.gov/brfss. Accessed April 2, 2014.
- 19.Hesse BW. Time to reboot: resetting health care to support tobacco dependency treatment. Am J Prev Med. 2010;39(6 suppl 1):S85–S87. doi: 10.1016/j.amepre.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse GR, Kelley JH, Linder JA, Park ER, Rigotti NA. Implementation of an electronic health record-based care management system to improve tobacco treatment. J Gen Intern Med. 2012;27(12):1690–1696. doi: 10.1007/s11606-012-2174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burstyn I, Kapur N, Shalapay C et al. Evaluation of the accuracy of self-reported smoking in pregnancy when the biomarker level in an active smoker is uncertain. Nicotine Tob Res. 2009;11(6):670–678. doi: 10.1093/ntr/ntp048. [DOI] [PubMed] [Google Scholar]
- 22.Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship. Nicotine Tob Res. 2009;11(1):12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]


