Abstract
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway has been identified as an important pathway in renal cell carcinoma (RCC). We have reported a nonsense mutation in PIK3R1, which encodes the regulatory subunit of PI3K, in a metastatic RCC (mRCC), while the mutation was absent in the corresponding primary RCC (pRCC). To identify the function of PIK3R1 in RCC, we examined its expression in normal kidney, pRCC and mRCC by immunohistochemistry and real-time polymerase chain reaction. The expression of PIK3R1 significantly decreased in pRCC and was further reduced in mRCC compared with normal tissue. Besides, its expression levels were negatively correlated with T-category of tumor stage. Additionally, 786-O and A-704 cells with PIK3R1 depletion introduced by CRISPR/Cas9 system displayed enhanced proliferation, migration and epithelial-mesenchymal transition (EMT), and acquired a stem-like phenotype. Moreover, the PIK3R1 depletion promoted the phosphorylation of AKT in the cells. The knockdown of AKT by shRNA reduced p-GSK3β and CTNNB1 expression in the cells, while the depletion of CTNNB1 impaired stem-like phenotype of the cells. Overall, PIK3R1 down-regulation in RCC promotes propagation, migration, EMT and stem-like phenotype in renal cancer cells through the AKT/GSK3β/CTNNB1 pathway, and may contribute to progression and metastasis of RCC.
Renal cell carcinoma (RCC) is one of the most lethal types of urological cancer1. Recent studies have markedly increased the understanding of the cell molecular biology of RCC, dominated by the inactivation of VHL in ubiquitin-mediated proteolysis pathway (UMPP) and alteration of PBRM1 involved in chromatin regulation2,3,4. The increased understanding of RCC biological pathways has led to the development of molecularly targeted therapeutic agents that have improved patient outcomes1. However, the advanced and metastatic RCC (mRCC) remains incurable5, therefore further studies are highly needed to understand the mechanisms of the molecular basis of resistance and response, thus leading to the discovery of novel targets for the treatment of mRCC.
In addition to UMPP3, the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway has also been identified as an important pathway in RCC2,6. The PI3K/AKT pathway begins with the involvement of growth factors binding to the receptor tyrosine kinases7. PI3K is activated through attachment to receptors anchored on plasma membrane and generates phosphatidylinositol-3-phosphate (PIP3) by phosphorylating phosphatidylinositol 4, 5-bisphosphate8. Through a pleckstrin homology domain, AKT binds to PIP3 and is phosphorylated to pAKT8. Class IA PI3Ks are heterodimers that consist of a catalytic subunit (p110α, p110β and p110δ) and a regulatory subunit (p85α, p55α, p50α, p85β, and p55γ)9. The catalytic subunit p110α is encoded by PIK3CA, while the regulatory subunit p85α is encoded by PIK3R19. The PI3K/AKT pathway regulates many aspects of cellular functions, including cell growth, proliferation, translation and survival, and is also involved in pathological conditions6. Deregulation of the pathway can lead to disturbance of cell growth, proliferation and survival, thus resulting in growth advantage and metastatic competence of cancer cells10.
The alteration of PI3K/AKT pathway has been found in a broad spectrum of cancers9. Members of the pathway such as PIK3CA and PTEN are frequently altered in RCC2. Since the pathway plays an important role in RCC pathogenesis2, it has been showing a great promise for molecularly targeted treatment of RCC6,9. However, only a small number of patients benefit from single-agent PI3K targeted therapy11. The related mechanism of unsatisfied effect of PI3K targeted therapy remains to be clarified11. Can, in addition to PIK3CA and PTEN, other member of the pathway play a role and be a target of therapy in RCC?
Accumulating studies have showed an important role of PIK3R1 in human carcinogenesis8,12,13,14. PIK3R1 has been reported as an oncogene in ovarian and colon tumors15, whereas it has been shown as a tumor suppressor in hepatocellular carcinomas16. The underexpression of PIK3R1 has been reported to be associated with poor prognosis of breast cancers17. A missense mutation of PIK3R1 which resulted in decrease of PIK3R1 expression has also been strongly linked with colon cancers18. We have reported a nonsense mutation in PIK3R1 in an mRCC, while the mutation was absent in the corresponding primary renal cell carcinoma (pRCC)14. Therefore, we hypothesize that the downregulation of PIK3R1 may confer renal cancer cells a selective advantage to translocate, colonize and develop as mRCC.
We speculate that ectopic expression of PIK3R1 may be associated with progression and metastasis of RCC. To examine our hypothesis, we firstly analyzed the expression of PIK3R1 in RCC including both pRCC and mRCC by immunohistochemistry (IHC) and real-time polymerase chain reaction (RT-PCR). We discovered that the expression of PIK3R1 in RCC negatively correlated with tumor progression and metastasis. In addition, we induced deletion mutations of PIK3R1 in renal cancer cell lines (786-O and A-704 cell lines) using a CRISPR/Cas9 system to achieve haploid knockout of PIK3R1 which significantly decreased the expression of P85α. The mutated renal cancer cells displayed increased abilities of colony formation, tumor formation, migration, epithelial-mesenchymal transition and oncosphere formation. Thus, our current study demonstrates that the downregulation of PIK3R1 contributes to progression and metastasis of RCC.
Results
Downregulation of PIK3R1 correlates with progression and metastasis of RCC
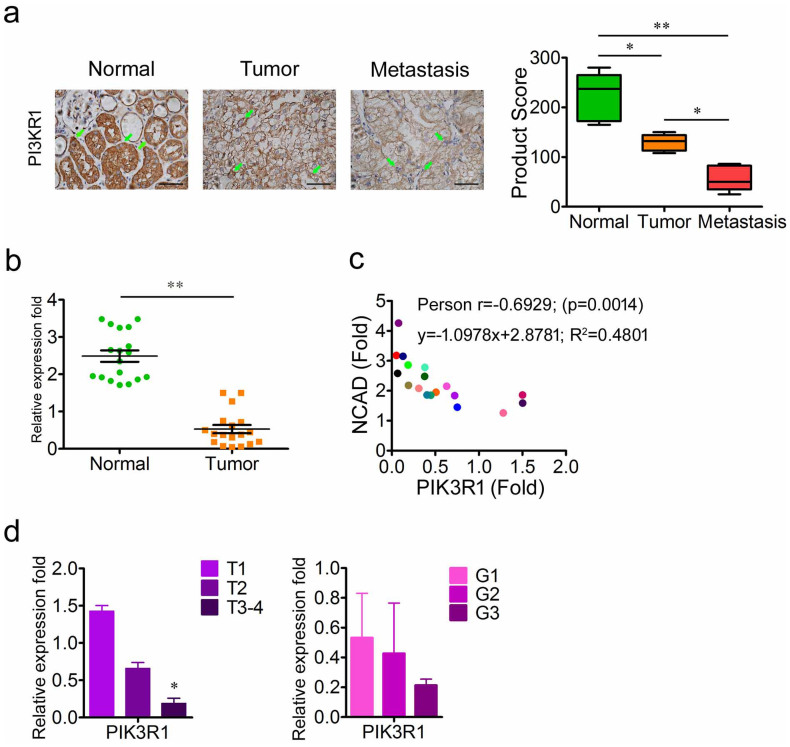
In order to examine the expression of PIK3R1 in RCC, the protein expression of PIK3R1 in normal kidney (n = 13), pRCC (n = 13) and mRCC (n = 21) was determined by IHC. As shown in Fig. 1a, normal kidney tissues displayed high level of PIK3R1 expression, whereas the expression of PIK3R1 was decreased in pRCC and was further reduced to a lower level in mRCC. The mRNA expression of PIK3R1 was then determined by real-time polymerase chain reaction (RT-PCR). Compared with normal kidney tissue group, the mRNA expression of PIK3R1 was significantly decreased in RCC group (n = 18) (Fig. 1b). The epithelial-mesenchymal transition (EMT) is considered to be crucial to tumor progression and metastasis, in which NCAD is the hallmark of EMT19,20. To determine whether the downregulation of PIK3R1 could affect the expression of NCAD, the mRNA expression of NCAD was examined, and data showed that the expression of NCAD had a negative correlation with the mRNA expression of PIK3R1 (Correlation = 0.6929, P = 0.0014) (Fig. 1c). Additionally, the mRNA expression of PIK3R1 negatively correlated with the T category of tumor, although there was no significant difference among different grades (Fig. 1d). These data suggest that the downregulation of PIK3R1 in RCCs correlates with their progression and metastasis.
Figure 1. Downregulation of PIK3R1 correlates with progression and metastasis in RCCs.
(a), The representative immunohistochemistry photographs of PIK3R1 expression in normal kidney tissues (n = 13), pRCCs (n = 13) and mRCCs (n = 21). Scale bar = 25 μm. (b), PIK3R1 mRNA levels in the pairs of pRCCs and corresponding normal renal tissues (n = 18). (c), PIK3R1 mRNA expression is negatively correlated with NCAD mRNA expression across a cohort of the pairs of normal renal tissues and pRCCs (n = 18). (d), PIK3R1 mRNA expression in pRCCs according to T category and grade. The TNM cancer staging system was designed to gauge the extent of cancer in a patient's body. T describes the size of the tumor and whether it has invaded nearby tissue, N describes regional lymph nodes that are involved, and M describes distant metastasis (spread of cancer from one body part to another). Grading classification for RCC was based on the Fuhrman grading system. Data are presented as mean ± SD. * P < 0.05, ** P < 0.01.
Haploid knockout of PIK3R1 by CRISPR/Cas9 promotes tumor proliferation
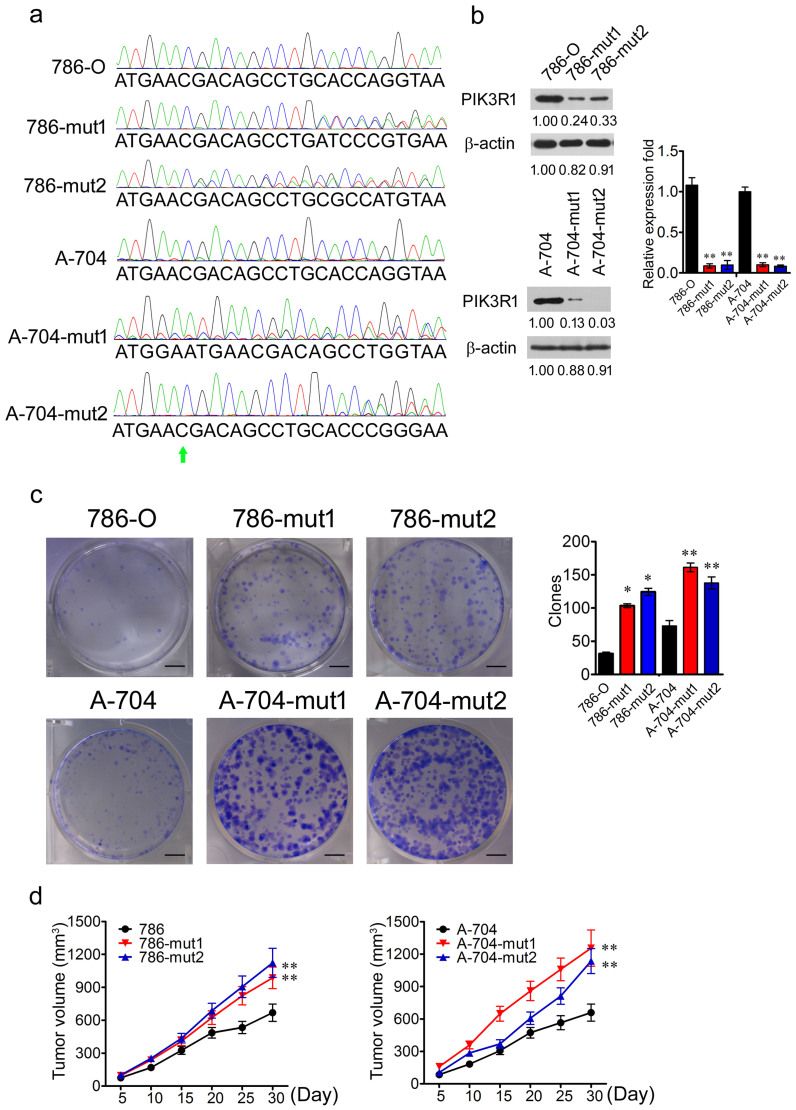
In order to reveal the functional influence of PIK3R1 depletion, a CRISPR/Cas9 strategy which was designed according to the nonsense mutation reported previously14, was applied to induce a haploid deletion mutation in order to achieve haploid knockout of PIK3R1. Firstly, we analyzed the expression levels of PIK3R1 in normal renal cell line (HK2) and RCC cell lines (786-O, A-498, A-704, and ACHN) by using RT-PCR. 786-O and A-704 cell lines showed higher expression of PIK3R1 compared with that of HK2 cell line (Supplementary Fig. 1). Therefore, we performed depletion of PIK3R1 in 786-O and A-704 cell lines. We also confirmed the sequence character of chr5: 67576819 in PIK3R1 in 786-O and A-704 cell lines with Sanger sequencing. The 786-O and A-704 cell lines harbored the wild type (WT) sequence of chr5: 67576819C in PIK3R1 (Fig. 2a).
Figure 2. Haploid knockout of PIK3R1 promotes tumor proliferation.
(a), The Sanger sequencing of PCR product including chr5: 67576819C in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Arrowhead indicates chr5: 67576819C. (b), The RT-PCR and WB analysis of PIK3R1 expression in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. β-actin served as control. (c), The colony formation assay of 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Colonies were stained with crystal violet and measured after two weeks cultivation. (d), The tumor formation assays of 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Scale bar = 50 mm. * P < 0.05, ** P < 0.01.
We then applied the CRISPR/Cas9 technology21 to screen 786-O cells and A-704 cells with haploid deletion mutation of PIK3R1. After Sanger sequencing validation, we obtained muted 786-O cells (786-mut1 and 786-mut2) and A-704 cells (A-704-mut1 and A-704-mut2) with haploid deletion mutation (Fig. 2a). To determine the knockout efficiency in the 786 and A-704 mutated cells, RT-PCR and western blot (WB) were carried out to examine PIK3R1 expression in 786-O WT cells (786-O), mutated 786-O cells, A-704 WT cells (A-704), and mutated A-704 cells. The mRNA expression of PIK3R1 was significantly decreased in 786 and A-704 mutated cells compared with 786-O and A-704 WT cells (Fig. 2b). The protein levels of PIK3R1 were also significantly decreased in 786 and A-704 mutated cells respectively (P < 0.01; P < 0.01) (Fig. 2b).
To determine the effects of haploid knockout of PIK3R1 on the growth of renal cancer cells, we carried out colony formation assays for the wild type and mutant cells. 786 and A-704 mutated cells displayed enhanced colony formation capability under the condition of separated single cells than that of 786-O and A-704 WT cells (Fig. 2c). In order to evaluate the tumor formation capability of the WT and muted cells, they were respectively inoculated subcutaneously into NOD/SCID mice to carry out tumor formation assays. The mutated cells formed bigger tumors than those of the wild type cells in NOD/SCID mice (Fig. 2d).
Haploid knockout of PIK3R1 promotes renal cancer cells migration and EMT in vitro
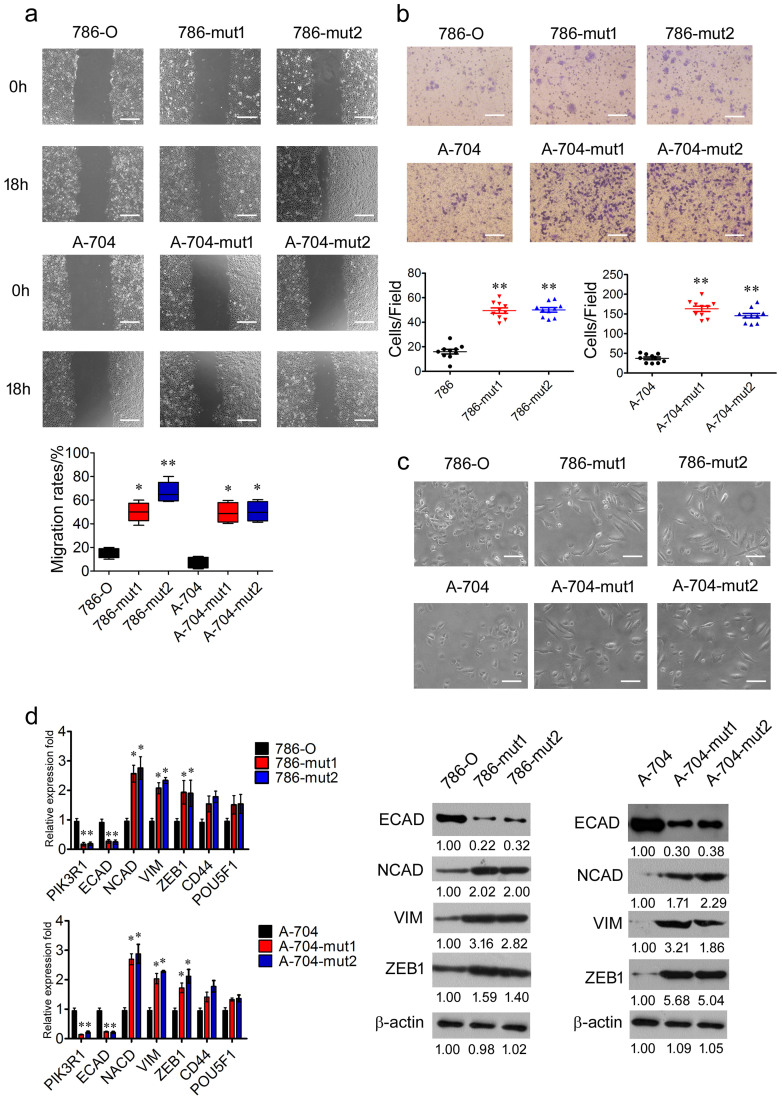
To confirm the effects of haploid knockout of PIK3R1 on the migration of renal cancer cells, wound-healing and transwell assays for 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells were carried out. Eighteen hours after artificial wounding, 786-O and A-704 WT cells demonstrated slight migration, whereas 786 and A-704 mutated cells showed enhanced migration ability and healed more than half of the wound (Fig. 3a). The 786 and A-704 mutated cells showed higher ability of migration in the transwell assay than that of 786-O and A-704 WT cells (Fig. 3b).
Figure 3. Haploid knockout of PIK3R1 promotes migration and EMT in renal cancer cells.
(a), The wound healing analysis of in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Migration rates were calculated by healing area/wound area. Scale bar = 50 μm. (b), Transwell of migration assays were applied to in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Scale bar = 25 μm. (c), Representative pictures of in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Scale bar = 25 μm. (d), The RT-PCR analysis of PIK3R1, ECAD, NCAD, VIM, ZEB1, CD44, and POU5F1 in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. The WB analysis of ECAD, NCAD, VIM, and ZEB1 expression in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. β-actin was applied as loading control. Data are expressed as mean ± SD. * P < 0.05, ** P < 0.01.
Additionally, 786 and A-704 mutated cells displayed a mesenchymal morphology21 that was different from the epithelial morphology of 786-O and A-704 WT cells, indicating that 786 and A-704 mutated cells might undergo EMT (Fig. 3c). In the wild type and mutant cells, the expression of EMT related gene was detected by RT-PCR and WB20. We found that, compared with 786-O and A-704 WT cells, in 786 and A-704 mutated cells with the downregulation of PIK3R1, the mRNA expression of ECAD was decreased, whereas the mRNA expression of NCAD, VIM and ZEB1 was increased (Fig. 3d). These observed changes were also found in the protein levels of ECAD (P < 0.01; P < 0.01), NCAD (P < 0.001; P < 0.05), VIM (P < 0.01; P < 0.05) and ZEB1 (P < 0.05; P < 0.05) in 786 and A-704 mutated cells respectively (Fig. 3d). However, no differences of CD44 and POU5F1 were observed between the wild type and mutant cells (Fig. 3d).
In order to confirm the function of PIK3R1 in renal cancer cells, we analyzed the expression of ECAD, NCAD, VIM, SNAIL, and TWIST in normal renal cell (HK2) and RCC cell lines (786-O, A498, A704, and ACHN) with RT-PCR. ACHN cell line showed higher expression of NCAD and VIM, compared to HK2 cell line (Supplementary Fig. 1). Therefore, we performed overexpression of PIK3R1 in ACHN cells. Compared with ACHN-vec cells, the mRNA expression of PIK3R1 and ECAD was increased in ACHN-PIK3R1 cells, whereas the expression of NCAD, VIM and ZEB1 was decreased (Supplementary Fig. 3a). These changes were also observed in the protein levels of PIK3R1 (P < 0.01), ECAD (P < 0.05), NCAD (P < 0.01), VIM (P < 0.05), and ZEB1 (P < 0.05) in ACHN-PIK3R1 cells (Supplementary Fig. 3b). In addition, ACHN-PIK3R1 cells displayed a epithelial morphology21 that was different from the mesenchymal morphology of ACHN-vec cells (Supplementary Fig. 3c). These results indicated that ACHN-PIK3R1 cells might undergo mesenchymal-epithelial transition (MET). Taken together, PIK3R1 negatively regulated renal cancer cell migration and EMT in vitro.
Haploid knockout of PIK3R1 promotes a cancer stem cell phenotype
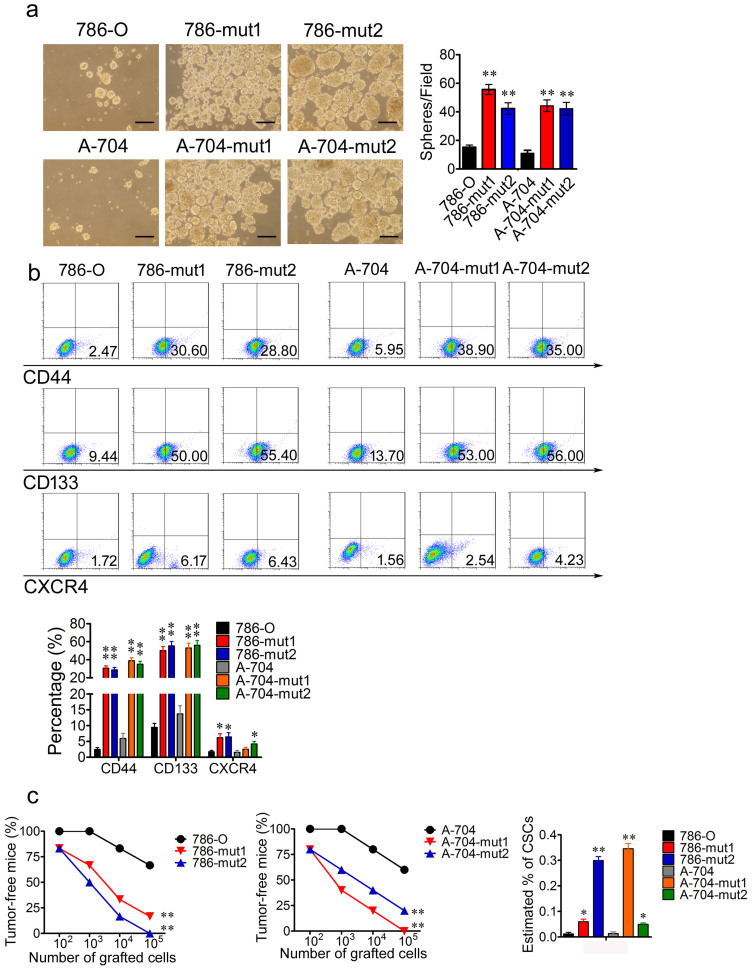
Cancer stem cells (CSCs) are defined as a subpopulation in tumors which harbors selfrenew, differentiation and serial tumor formation ability22. Cancer cell lines contain cancer stem-like cells which can form cell spheres under sphere-forming conditions23. To evaluate whether the reduction of PIK3R1 influences the cancer stem cell phenotype of renal cancer cells, cell sphere formation assays of 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells were performed. After two weeks of cultivation, 786 and A-704 mutated cells developed more and bigger spheres than that of 786-O and A-704 WT cells under the medium supplemented with 20 ng/mL EGF, FGF, N2, and B27 (Fig. 4a). CSCs have been identified based on expression of various markers such as CD44, CD133 and CXCR424,25,26. By using anti-CD44 antibodies, anti-CD133 antibodies and anti-CXCR4 antibodies we observed that 786 and A-704 mutated cells comprised more CD44+, CD133+ and CXCR4+ subset, respectively, compared with 786-O and A-704 WT cells (Fig. 4b).
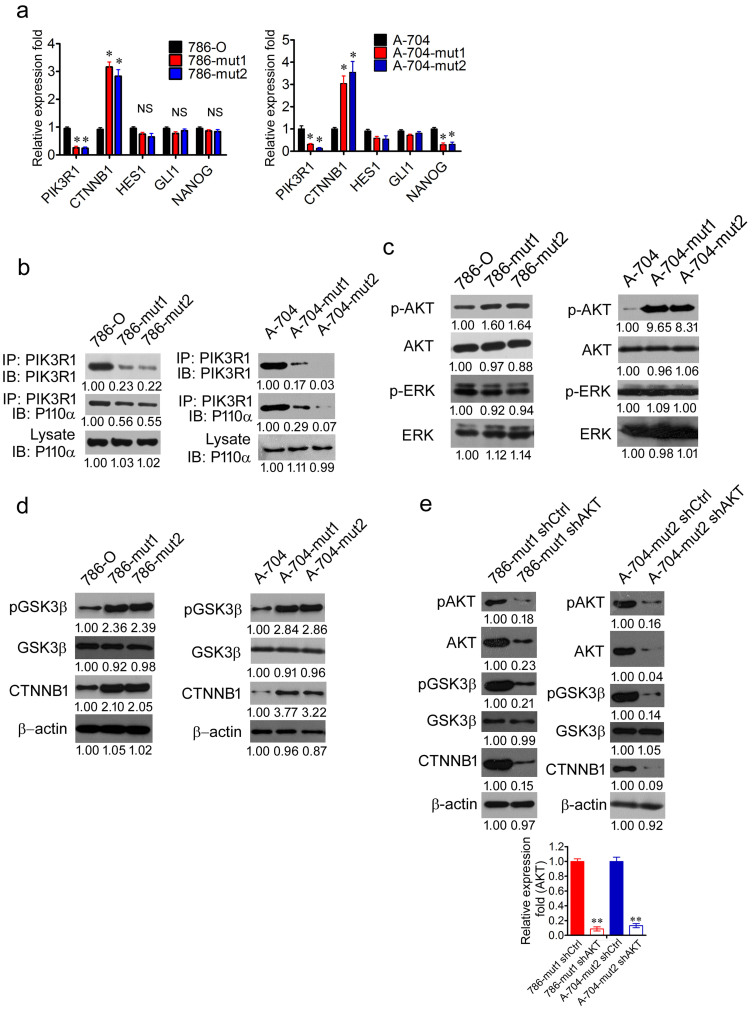
Figure 4. Haploid knockout of PIK3R1 promotes cancer stem cell phenotype through activating CTNNB1.
(a), The representative photographs of cell spheres formed by 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. Spheres were calculated after two weeks cultivation at five independent fields/well. Scale bar = 50 μm. (b), 786-O and A-704 WT cells comprise fewer CD44+, CD133+, and CXCR4+ subset, respectively, compared with 786-O and A-704 WT cells. (c), The percentage of tumor-free mice 1 month after the subcutaneous injection of the different dilutions of 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells into immunodeficient mice. The estimated percentage of renal cancer stem cells (RCSCs) in 786-O WT, 786-O Mut, A-704 WT, and A-704 Mut after the first transplantation using the extreme limiting dilution analysis. * P < 0.05, ** P < 0.01.
The gold standard assay to assess CSCs potential is the transplantation of limiting dilutions of highly purified prospectively identified cancer cell populations into immunodeficient mice to assess their ability to form tumors27,28. Transplantation of different dilutions of the wild type cells and PIK3R1 mutated cells showed that the mutated cells had a much greater ability to initiate tumors and increasing percentage of CSCs compared with the WT cells (Fig. 4c).
Haploid knockout of PIK3R1 activates WNT/β-catenin pathway dependent on the phosphorylation of AKT
In order to uncover the stem cell signaling pathway activated by the downregulation of PIK3R1, the mRNA level of CTNNB1, HES1, GLI1, and NANOG were analyzed in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells by RT-PCR. With the downregulation of PIK3R1 in 786 and A-704 mutated cells, the mRNA expression of CTNNB1 was higher than that in 786-O and A-704 WT cells (Fig. 5a). The protein levels of CTNNB1 were also increased in 786 and A-704 mutated cells respectively (P < 0.01; P < 0.05) (Fig. 5d) There were no significant differences in the mRNA expression of HES1 and GLI1 between the wild type and mutated cells (Fig. 5a). Though there were no significant differences in the NANOG mRNA expression between 786-O WT and 786 mutated cells, significant difference was observed between A-704 WT and A-704 mutated cells (Fig. 5a).
Figure 5. Haploid knockout of PIK3R1 activates WNT/β-catenin pathway dependent on the phosphorylation of AKT.
(a), The RT-PCR analysis of the mRNA expression of PIK3R1, CTNNB1, HES1, GLI1, and NANOG in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. (b), 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cell lysates were immunoprecipitated by anti-PIK3R1 antibody and immunoblotted with anti-PIK3R1 (upper panel) and anti-P110 antibody (middle panel). Whole lysates immunobotted with anti-P110 antibody (lower panel) served as control. (c), The WB analysis of p-AKT and p-ERK in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. AKT and ERK served as control. (d), The WB analysis of CTNNB1, p-GSK3β, GSK3β in 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. (e), The WB analysis of AKT, pAKT, pGSK3β, GSK3β, and CTNNB1 in 786-mut1 shCtrl, 786-mut1 shAKT, A-704-mut2 sh Ctrl, and A-704-mut2 shAKT cells. The RT-PCR analysis of AKT in 786-mut1 shCtrl, 786-mut1 shAKT, A-704-mut2 shCtrl, and A-704-mut2 shAKT cells. Data are presented as mean ± SD. * P < 0.05, ** P < 0.01.
In order to determine the molecular mechanism underlying β-catenin (CTNNB1) activation mediated by PIK3R1 depletion, immunoprecipitation with PIK3R1 antibody was carried out by using lysates of 786-O, 786-mut1, 786-mut2, A-704, A-704-mut1, and A-704-mut2 cells. The quantity of p110α immunoprecipitated by PIK3R1 was decreased due to knockout of PIK3R1 in 786 and A-704 mutated cells respectively, compared with 786-O and A-704 WT cells (P < 0.05; P < 0.05) (Fig. 5b). Additionally, more AKT was phosphorylated in 786 and A-704 mutated cells (P < 0.05; P < 0.01), compared with 786-O and A-704 WT cells that had the amount of unchanged AKT (P = 0.54; P = 0.49) (Fig. 5c). However, there was no significant change in the quantity of ERK (P = 0.07; P = 0.45) and ERK with phosphorylation (P = 0.08; P = 0.30) in 786 and A-704 mutated cells respectively (Fig. 5c).
He, K. et al reported that cancer cells acquired cancer stem-like phenotype through alteration of the PI3K/AKT/β-catenin signaling29. According to a previous study30, the phosphorylation of AKT could activate the WNT/β-catenin pathway through phosphorylating and inhibiting GSK3β. Interestingly, more GSK3β was phosphorylated in 786 and A-704 mutated cells compared with 786-O and A-704 WT cells (P < 0.01; P < 0.001) (Fig. 5d). To determine the function of AKT in the mutated cells, depletion of AKT were performed in 786-mut1 and A-704-mut2 cells. After the depletion of AKT (P < 0.001; P < 0.001), the expression of pAKT (P < 0.01; P < 0.001), p-GSK3β (P < 0.001; P < 0.001) and CTNNB1 (P < 0.001; P < 0.05) was decreased in 786-mut1 shAKT and A-704-mut2 shAKT cells respectively, compared with 786-mut1 shCtrl and A-704-mut2 sh Ctrl cells (Fig. 5e). Taken together, the depletion of PIK3R1 activates the WNT/β-catenin pathway dependent on the phosphorylation of AKT.
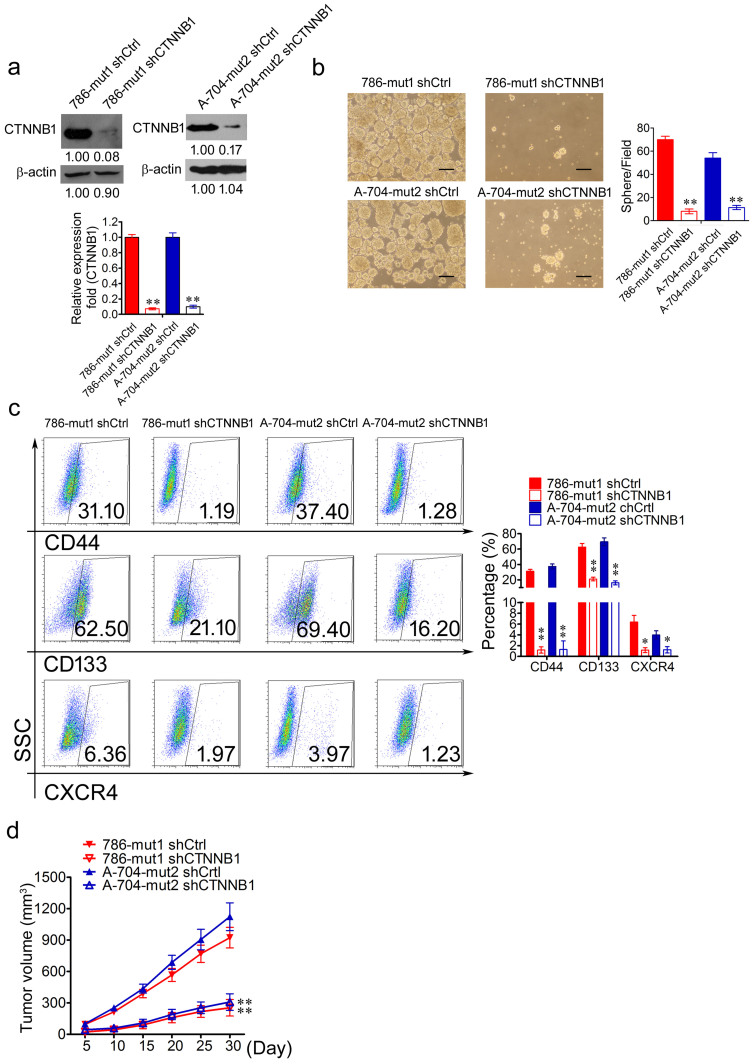
Haploid knockout of PIK3R1 promotes a cancer stem cell phenotype through WNT/β-catenin pathway
To determine the function of CTNNB1 in the mutated cells, depletion of CTNNB1 was performed in 786-mut1 and A-704-mut2 cells respectively. The mRNA expression of CTNNB1 was decreased in 786-mut1 shCTNNB1 and A-704-mut2 shCTNNB1 cells, compared to 786-mut1 and A-704-mut2 cells (Fig. 6a). The decrease of CTNNB1 protein was also observed in 786-mut1 shCTNNB1 and A-704-mut2 shCTNNB1 cells (P < 0.01; P < 0.01) (Fig. 6a). After two weeks of cultivation, 786-mut1 shCTNNB1 and A-704-mut2 shCTNNB1 cells developed fewer and smaller spheres than that of 786-mut1 shCtrl and A-704-mut2 shCtrl cells (Fig. 6b). By using anti-CD44 antibodies, anti-CD133 antibodies and anti-CXCR4 antibodies, we observed that 786-mut1 shCTNNB1 and A-704-mut2 shCTNNB1 cells comprised fewer CD44+, CD133+ and CXCR4+ subset, respectively, compared with 786-mut1 shCtrl and A-704-mut2 shCtrl cells (Fig. 6c). In order to evaluate the tumor formation capability of 786-mut1 shCTNNB1, A-704-mut2 shCTNNB1, 786-mut1 shCtrl, and A-704-mut2 shCtrl cells, they were respectively inoculated subcutaneously into NOD/SCID mice to carry out tumor formation assays. 786-mut1 shCTNNB1 and A-704-mut2 shCTNNB1 cells formed smaller tumors than those of 786-mut1 shCtrl and A-704-mut2 shCtrl cells in NOD/SCID mice (Fig. 6d). These results indicated that the haploid knockout of PIK3R1 enhanced the abilities of self-renewal and tumor formation dependent on the activation of the PI3K/AKT/CTNNB1 pathway in renal cancer cells.
Figure 6. Haploid knockout of PIK3R1 promotes cancer stem cell phenotype through activating CTNNB1.
(a), The WB and RT-PCR analysis of CTNNB1 in 786-mut1 shCtr, 786-mut1 shCTNNB1, A-704-mut2 shCtr, and A-704-mut2 shCTNNB1 cells. (b), The representative photographs of cell spheres formed by 786-mut1 shCtr, 786-mut1 shCTNNB1, A-704-mut2 shCtr, and A-704-mut2 shCTNNB1 cells. Scale bar = 50 μm. (c), 786-mut1 shCTNNB1 and A-704-mut2 shCTNNB1 cells comprised fewer CD44+, CD133+ and CXCR4+ subset, respectively, compared with 786-mut1 shCtr and A-704-mut2 shCtr cells. (d), The tumor formation assays of 786-mut1 shCtrl, 786-mut1 shCTNNB1, A-704-mut2 shCtrl, and A-704-mut2 shCTNNB1 cells. * P < 0.05, ** P < 0.01.
Discussion
Over the past 20 years, the functional role of the PI3K/AKT pathway in tumorigenesis has been reported by numerous studies31. Members of the pathway such as PIK3CA, AKT and PTEN, are frequently altered, and cause the activation of the pathway in RCC2,14. As a regulatory subunit of PI3Ks, PIK3R1 alteration is involved in carcinogenesis in a variety of cancers9, whereas the alteration of PIK3R1 in RCC has been rarely reported14. In this study, we report that PIK3R1 expression is reduced in RCC, and that the reduction of PIK3R1 can result in EMT, enhance migration ability, and promote colony formation, tumor formation and cancer stem cell phenotype in renal cancer cells.
Our data showed that PIK3R1 expression was reduced in RCC, especially in advanced and metastatic RCC, and the downregulation of PIK3R1 correlated with advanced or metastatic RCC. These results are in consistence with discoveries in hepatocellular and colon cancers16,18, indicating that the reduction of PIK3R1 expression may acquired tumorigenicity in RCC, and thus supporting the view that PIK3R1 may function as a potential cancer suppressor, and that the downregulation of PIK3R1 may promote progression and metastasis of RCC.
We then tested the role of PIK3R1 in renal cancer cell lines. We observed that the haploid deletion mutations of PIK3R1 by CRISPR/Cas9 system led to the haploid knockout and downregulation of PIK3R1, and generated cell lines with the insufficiency of PIK3R1. The binding of p110α to p85α was decreased in 786 and A704 mutated cells with the reduced expression of PIK3R1. We also showed that the downregulation of PIK3R1 functionally activated the PI3K/AKT pathway, as evidenced by the enhanced phosphorylation of AKT in Ser473 residue. The activation of the pathway may be due to the combined effect of several factors. The p85 regulatory subunit of PI3K is necessary to stabilize and recruit p110 catalytic subunit onto cellar membrane13. However, monomeric p85 is in competition with p85-p110 heterodimers for binding to insulin receptor substrate-1 (IRS-1) to form a non-signaling cytosolic protein complex32. The insufficiency of PIK3R1 expression would primarily result in the reduction of monomeric p85, which would suppress the negative regulation of IRS-1/PI3K pathway by p85, thus leading to the hyperactivation of PI3K signaling axis32. PIK3R1 has also been shown to have a positive regulatory effect on tumor suppressor PTEN, which negatively regulates the PI3K/AKT pathway16. Therefore, the downregulation of PIK3R1 leads to the inhibition of PTEN function that reduces the breakdown of PIP3, and activates the downstream of the PI3K/AKT signaling axis16.
Our data show that the reduction of PIK3R1 expression increases the motility and migration capacity of renal cancer cells. A growing body of evidence indicates that the PI3K/AKT pathway is involved in the migratory process of cells, including metastatic cancer cells33. Activated AKT mediates cell polarity and reorganizes cytoskeleton, thus regulates the contraction of the cellular body and facilitates the migration of cells33. AKT-mediated phosphorylation activates components closely related to the cellular filaments which are important for cytoskeleton dynamics34. In our present study, the expression of VIM was elated in cells with the downregulation of PIK3R1. Vimentin, which is phosphorylated and protected by AKT from degradation35, is substantially up-regulated when cells are highly motile, especially during EMT. It can promote the migration of different type of cells such as cancer cells35.
Interestingly, spindle-shaped morphology of mesenchymal cells has been observed in cells with the down-expression of PIK3R1. EMT is conducive to the survival and spread of cancer cells36. The activation of AKT induces EMT and contributes to migration of squamous cell carcinoma lines37. Evidences are emerging that the action of AKT is related with EMT-inducing transcription factor38. The hallmarks of EMT include decreased expression of epithelial marker, such as ECAD, and a simultaneous increased expression of mesenchymal markers, such as NCAD, VIM, CD44, ZEB1, and POU5F120. In our study, the expression of NCAD, VIM and ZEB1 was elated in the cells with the downregulation of PIK3R1, whereas the expression of ECAD was decreased in these cells. The activated AKT positively regulates the expression of NCAD33, and the inhibition of the PI3K/AKT signaling inhibits the expression of NCAD39. N-cadherin encoded by NCAD is enriched in cellular protrusions and plays a key role in cell migration33. Altogether, through the activation of the PI3K/AKT pathway which phosphorylates several cytoskeleton-regulating and EMT–activating proteins, the downregulation of PIK3R1 promotes migration and EMT in renal cancer cells.
CSCs are defined as cells which are able to produce a new tumor22, and regarded as a source of tumor progression and metastasis22. Cancer cell lines contain cancer stem-like cells23. In our current study, we observed that the down-expression of PIK3R1 promoted the colony formation, cell sphere and tumor formation of renal cancer cells and increased the expression of CTNNB1. Our data are consistent with the study by Zhou et al, who reported that the CSC viability and maintenance of breast cancer stem-like cells required the activation of the PI3K/AKT signaling axis40. It has been suggested that the PI3K/AKT pathway is critical to maintain prostate cancers stem-like cells41. Signaling pathways including the Hedgehog/GLI1, WNT/CTNNB1, NANOG/OCT4, and Notch/HES1 are activated in CSCs and contribute to the function of CSCs42. We performed RT-PCR to reckon the expression of CTNNB1, HES1, GLI1, and NANOG. Interestingly, no significant change was observed in the expression of HES1, GLI1 and NANOG, whereas the expression of CTNNB1 was markedly increased, suggesting that CTNNB1 may be involved in increased renal cancer stem-like phenotype caused by the down-expression of PIK3R1.
Our present study demonstrates that the downregulation of PIK3R1 in RCC enhances stem cells expansion as a result of the activated PI3K/AKT signaling, involving the WNT/CTNNB1 pathway. The PI3K/AKT pathway has always been ascribed to the proliferation and self-renewal of cancer stem-like cells29. However, under certain conditions, such as ectopic gene expression, cancer stem-like cells can also be transformed from cells lacking cancer stem-like phenotype43. The activation of the PI3K/AKT pathway has been reported to induce differentiated cells to transform into highly tumorigenic and cancer stem-like cells44. In our study, after the depletion of AKT, the expression of p-GSK3β and CTNNB1 was decreased in 786-mut1 shAKT and A-704-mut2 shAKT cells. Therefore, in consistence with previous studies, our study suggests that the PI3K-AKT and WNT/CTNNB1 pathways converge on GSK3β in renal cancer cells29. The WNT/CTNNB1 signaling is involved in regulating the various types of stem cells29. In present study, the depletion of CTNNB1 in renal cancer cells decreased the cancer stem-like phenotype of these cells. Activated AKT can block the kinase activity of GSK3β by phosphorylating GSK3β30, resulting in the accumulation of β-catenin which transforms differentiated cancer cells to cancer stem-like cells29,30. Therefore, the PI3K/AKT and WNT/CTNNB1 pathway work together as a PI3K/AKT/GSK3β/CTNNB1 pathway which induces differentiated renal cancer cells to transform into a more cancer stem-like cells29.
To our knowledge, this study is the first report of the functional identification of PIK3R1 in RCC. The important discovery of our study is that PIK3R1 is down expressed in RCC, especially in advanced and metastatic RCC. We observed that the downregulation of PIK3R1 resulted in EMT, increased migration, colony formation, cell sphere, and tumor formation in renal cancer cells, suggesting that the reduction of PIK3R1 expression confers renal cancer cells a selective advantage to translocate, colonize and develop as mRCC, and that PIK3R1 may be involved in the progression and metastasis of RCC through the PI3K/AKT/GSK3β/CTNNB1 pathway. Since the reduction of PIK3R1 expression and activating alterations in PIK3CA can simultaneously be involved in RCC, conformational changes may result from these alterations. These changes would eliminate the repression of PIK3CA caused by PIK3R132. Therefore, the exploitation of agents that can restore the repression regulation of PIK3R1 and inhibit the expression of PIK3CA, may be a new strategy to treat RCC.
In summary, our study suggests that PIK3R1 is down-regulated in RCC, especially in advanced and metastatic RCC. The downregulation of PIK3R1 promotes EMT, migration, and cancer stem cell phenotype in renal cancer cells and may contribute to the progression and metastasis of RCC by activating the PI3K/AKT/GSK3β/CTNNB1 pathway. Increasing or restoring expression of PIK3R1 may be a novel treatment strategy for RCC.
Methods
Reagent and antibody
Antibodies that recognize pAKT (Ser473) (Cell Signaling Technology, #9271), AKT (Cell Signaling Technology, #9272), pERK (Thr202/Tyr204) (Cell Signaling Technology, #4370), ERK (Cell Signaling Technology, #4695), p85α (Santa Cruz, sc-376112), p110α (Cell Signaling Technology, #4249), β-actin (Sigma, A1978), NCAD (Abcam, AB98952), ECAD (Cell Signaling Technology, #3195), VIM (RV202) (Abcam, AB8978), ZEB1 (D80D3) (Cell Signaling Technology, #3396), pGSK3β (Ser9) (Cell Signaling Technology, #9323), GSK3β (Y174) (Abcam, AB32391), and non-phospho CTNNB1 (Ser33/37/Thr41) (Cell Signaling Technology, #4270) were used in the study. Horseradish peroxidase (HRP) labelled secondary antibody (Beyotime Biotech) was used in western blots. Protein A-Sepharose 4B beads (Invitrogen, 10-1041) was used for immunoprecipitation studies.
Patients and tissue specimens
For the protein expression of PIK3R1, the formalin-fixed, paraffin-embedded (FFPE) tissue samples of patient-matched normal renal, pRCC and mRCC tissues from 13 patients who underwent nephrectomy between May 2008 and April 2013 were obtained from the archives of the Zhujiang Hospital of the Southern Medical University (Supplementary table 1). The biopsy samples of mRCCs from eight patients who underwent biopsies of metastases but not nephrectomy between May 2009 and September 2013 were also collected in the hospital (Supplementary table 1). Median age of these patients was 56 years (range: 16–71 years). Thirteen of patients were male and the rest were female. For the mRNA expression of identified genes, pRCCs tissues and corresponding normal renal tissues from 18 patients who underwent nephrectomy in the hospital between August 2012 and October 2013 were immediately collected following surgical resection (Supplementary table 2). The median age of these patients was 50 years (range: 29–65 years). Thirteen of these patients were male and the others were female. The disease stage of all patients was classified according to the American Joint Committee on Cancer staging system45. This study was reviewed and approved by the Ethics Committee of the Southern medical University, and was carried out according to the World Medical Association Declaration of Helsinki. The written informed consents were obtained from all patients or their guardians.
Immunohistochemistry
Sections (4 μm) were deparaffinized and rehydrated. After antigen retrieval, these sections were treated with 3% H2O2 solution, incubated with 8% bovine serum albumin for 30 min and primary antibody at 4°C overnight, then incubated with corresponding secondary antibody, and subsequently stained with DAB kit (ZSGB Bio). The nucleus was counterstained with hematoxylin. The overall staining for PIK3R1 was measured by the multiplication of staining percentage (0%–100%) and staining intensity on a numerical scale (none = 1, weak = 2, moderate = 3, strong = 4), resulting in an overall product score.
Real-Time PCR analysis of PIK3R1 expression
Total RNA was extracted using RNA isolation kit (Tiangen Biotech) and subjected to cDNA synthesis using PrimeScript RT reagent Kit (Takara Bio). The cDNA was then used for the evaluation of the relative mRNA levels of the indentified gene, running in an ABI 7300 analyzer (Applied Biosystems). SYBR Green I (Tiangen Biotech) was used as the fluorescent probe. Primer sequences for RT-PCR are listed in the supplementary table 3. The relative expression levels of the target genes were referred to as a housekeeping gene, GAPDH.
Cell line
HK-2 cells were cultured in DMEM/F12 medium (Gibco). Other cells were maintained in RPMI 1640 medium (Gibco). All mediums were supplemented with 10% fetal bovine serum (FBS) (Gibco), 100 U/mL penicillin (Gibco) and 100 mg/mL streptomycin (Gibco), and incubated at 37°C with 5% CO2 in a humidified atmosphere.
PIK3R1 knockout by CRISPR/Cas9
The knockout of PIK3R1 in 786-O and A-704 cell lines was achieved by CRISPR/Case9 system. One 23-base sgRNA (5′-AATGAACGACAGCCTGCACCAGG-3′) was designed to the target site (g.chr5: 67576819) in the PIK3R1 (Gene ID: 5295). The U6-gRNA expression cassette including U6 promoter and +83 bp sgRNA tails was synthesized (Invitrogen) and inserted into pCEP4 vector (Invitrogen) by KpnI and XhoI. The sgRNA was synthesized (Invitrogen) and inserted into two AarI site between U6 promoter and sgRNA tails to form the pCEP4-U6-gRNA expression vector (Supplementary Figure 2). The sequence of Cas9 was consistent with previous reports46,47. The T7 promoter was added upstream of the Cas9 sequence. All the sequences were synthesized (Invitrogen) and ligated to the pMD-18T-CMV-MCS-BGPA by NheI/AflII to form CMV-Cas9 expression vector (Supplementary Figure 2).
786-O and A-704 cells were transfected according to Lipo-fectamine 2000 (Invitrogen) protocol. Briefly, Cells were transfected at 60% confluency in 12 well with 500 ng of pCEP4-U6-gRNA expression vector, 500 ng of CMV-Cas9 expression vector and 3 μL Lipo-fectamine 2000. After G418 (500 ng/μL) selection for 10 days, G418-resistant colonies were picked and cultured in 24 well plates. Genomic DNA was extracted with E. Z.N.A. Tissue DNA Kit (OMEGA). Targeted cleavage was measured by PCR amplification (forward primer: 5′ CTCATCAGTATTGGCTTACGCTT 3′ and reverse primer: 5′ GGTTGTTTAGACTTTCCACGGTA 3′) and T7 E1 assay. The fragments were determined by Sanger sequencing. To better verify the mutations (insertions and deletions), the PCR product of mutated colonies was then ligated to pMD-18T vector (Takara Bio). Individual mutation and mutational spectra were obtained from Sanger sequence. Cell clones with haploid deletion mutation of PIK3R1 were selected for evaluating the function of PIK3R1 in renal cancer cells.
Western blot and immunoprecipitation (IP)
Cells were lysed with radioimmunoprecipitation assay buffer (Beyotime Biotech). Protein was determined, separated and transferred to polyvinylidene difluoride membranes (Pall Life Sciences). The membranes were blocked, then probed with corresponding primary antibody and HRP-conjugated secondary antibodies (Beyotime Biotech), and detected using BeyoECL PLUS Kit (Beyotime Biotech).
For immunoprecipitation assays, cell lysates were incubated with the PIK3R1 antibody overnight and precipitated with protein A-Sepharose 4B beads (Invitrogen) at 4°C for 4 h. The immunoprecipitated proteins were washed, separated on SDS-PAGE, transferred onto a PVDF membrane, and followed by western blot analysis.
Colony formation assay
Briefly, single cells were seeded onto 6-well plates (Corning) at a density of 1000 cells/well and then incubated at 37°C. After two weeks of culture, colonies were stained with 0.5% crystal violet solution for 30 min at room temperature. The colonies from three replicate wells were counted, and pictures were captured digitally.
Generation of xenografts
For generation of xenografts, different dilutions of cells were injected with Matrigel (BD Biosciences) subcutaneously into the backs of NOD/SCID mice. The volume of xenografts was serially measured. The mice were sacrificed after 30 days. Animal work was carried out in compliance with the ethical regulations approved by the Animal Care Committee, Southern medical University.
Wound healing assay
Briefly, 4 × 105 cells were seeded into a 24 well plate. After overnight incubation, monolayer cells grew to confluency. Artificial wounds were introduced with the P200 pipette tip. The pictures of the wounded area were taken immediately (time 0 h), and at 18 h with an inverted microscope (Olympus Corp). The whole assay was repeated three times.
Transwell Assay
Cells were respectively harvested, suspended and added to the upper compartment of transwell inserts (8 μm pore size; Corning). The lower chambers were filled with RPMI-1640 supplemented with 10% FBS. After incubation for 16 hr, cells that had not migrated from the upper chamber were scraped away. The cells that migrated to the lower surface of each membrane were stained and counted under a light microscope in in five fields/well. The whole assay was repeated three times.
Spheres Culture
Single cells were seeded at a density of 5 × 103 cells/well in 6-well plates with ultra-low attachment surface (Corning). Cells were maintained in serum-free 1:1 DMEM/F12 (Gibco) supplemented with 20 ng/mL EGF (Invitrogen), 20 ng/mL bFGF (Invitrogen), 2% B27 (Invitrogen), 1% N2 (Invitrogen), and 100 mg/mL streptomycin (Gibco). The number of tumor spheres was counted 14 days after seeding. The whole assay was repeated three times.
Cell labeling and flow cytometry analyzing
Cells were harvested and washed twice in ice-cold PBS. Then cancer cells were stained with FITC-conjugated anti-CD133 antibody, PE-conjugated anti-CD44 antibody and FITC-conjugated anti-CXCR4 antibody, respectively, for 30 min on ice. After washing with PBS, the labeled cells were analyzed by flow cytometry (BD FACSAria III).
Knockdown of AKT and CTNNB1 with shRNA
Four shRNA targeted against the different regions of AKT and CTNNB1 and scrambled shRNAs were designed, synthesized and cloned into pSicoR vector: ShCTNNB1: Ccgg CAGATGGTGTCTGCTATTGTACTCGAGTACAATAGCAGACACCATCTGTTTTT g; shCtrl: CcggTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGA ATTTTTg; shAKT: CcggCCTCAAGAATGATGGCACCCTCGAGGGTGCCATCAT TCTTGAGGTTTTTg; shAKT Ctrl: CcggAGTTCCAGTACGGCTCCAACTCGAGTT GGAGCCGTACTGGAACT TTTTTg. The resulting lentiviral vectors containing the CTNNB1 and AKT shRNA were named shCTNNB1 and shAKT lentivirus. Cells were transfected with the shCTNNB1 and shAKT lentivirus to obtain cell lines stably expressing the CTNNB1 and AKT shRNA.
Overexpression of PIK3R1
PIK3R1 (NM_181523.2) was amplified by PCR with the following primers (forward: 5'- CGG AAT TCC ATG CAT GGT GAT TAT ACT CTT ACA -3' and reverse: 5'- CGC GGA TCC TCA TCG CCT CTG CTG TGC ATA TAC -3'). The PCR product was purified and cloned into pSin-GFP-vector to obtain Psin-GFP-PIK3R1. ACHN cells were transfected with the pSin-GFP-PIK3R1 lentivirus to obtain a cell line stably expressing the PIK3R1 cDNA.
Statistical analysis
Student's t-test was used to comparing the means of two samples. Linear correlation analysis was used to determine relationship between PIK3R1 and NCAD mRNA expression. A value of p less than 0.05 (* P < 0.05, ** P < 0.01) was regarded statistically significant.
Author Contributions
Y.L., Z.Y., H.L. (Huan Liu), F.L., D.S., X.Z. and X.W. performed the experiments and analyzed the data. C.L. and S.W. designed the project and wrote the main manuscript text. A.X., P.D., Y.H., D.Z., H.L. (Hongjie Liu), M.Z. and Y.F. edited various parts of the manuscript text. A.X., H.W., Q.X., Y.W. (Yongqiang Wang), Y.Z., Y.W. (Yu Wang) and Z.C. helped with the experimental design. C.L. and S.W. supervised the data analysis and edited text for the manuscript. All authors reviewed the manuscript.
Supplementary Material
Supplementary data
Acknowledgments
The authors thank all the faculties and staffs of BGI-Shenzhen, Zhujiang Hospital of Southern Medical University and Shenzhen Second People's Hospital whose names were not included in the author list, but who contributed to this work.
Financial Support: This project was supported by theNational Natural Science Foundation of China (Grant No. 81301740) and Innovation Program of Shenzhen (Grant No. JCYJ20130401114715714; No. JCYJ20130401114928183; No. JSGG20130411091246833; No. CXZZ2013051653248144).
References
- Rini B. I., Campbell S. C. & Escudier B. Renal cell carcinoma. Lancet 373, 1119–32 (2009). [DOI] [PubMed] [Google Scholar]
- Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G. et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet 44, 17–9 (2012). [DOI] [PubMed] [Google Scholar]
- Varela I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ather M. H., Masood N. & Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urol J 7, 1–9 (2010). [PubMed] [Google Scholar]
- Porta C. & Figlin R. A. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol 182, 2569–77 (2009). [DOI] [PubMed] [Google Scholar]
- Yan W. et al. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun 382, 631–6 (2009). [DOI] [PubMed] [Google Scholar]
- Miller T. W., Rexer B. N., Garrett J. T. & Arteaga C. L. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 13, 224 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T. L. & Cantley L. C. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27, 5497–510 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. H. et al. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science 318, 1744–8 (2007). [DOI] [PubMed] [Google Scholar]
- Fruman D. A. & Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 13, 140–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urick M. E. et al. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res 71, 4061–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal B. S. et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16, 463–74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. et al. Multilayered molecular profiling supported the monoclonal origin of metastatic renal cell carcinoma. Int J Cancer 135, 78–87 (2014). [DOI] [PubMed] [Google Scholar]
- Philp A. J. et al. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res 61, 7426–9 (2001). [PubMed] [Google Scholar]
- Taniguchi C. M. et al. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res 70, 5305–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizkova M. et al. PIK3R1 underexpression is an independent prognostic marker in breast cancer. BMC Cancer 13, 545 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Plummer S. J., Thompson C. L., Tucker T. C. & Casey G. Association between phosphatidylinositol 3-kinase regulatory subunit p85alpha Met326Ile genetic polymorphism and colon cancer risk. Clin Cancer Res 14, 633–7 (2008). [DOI] [PubMed] [Google Scholar]
- Qian X. et al. N-cadherin/FGFR promotes metastasis through epithelial-to-mesenchymal transition and stem/progenitor cell-like properties. Oncogene 33, 3411–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Dedhar S., Kalluri R. & Thompson E. W. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172, 973–81 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D., Li Y., Wang Z. & Sarkar F. H. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins? Cancers (Basel) 3, 716–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. T. Cancer stem cell biology: from leukemia to solid tumors. Curr Opin Cell Biol 16, 708–12 (2004). [DOI] [PubMed] [Google Scholar]
- Kondo T., Setoguchi T. & Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A 101, 781–6 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli I. & Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol 198, 281–93 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 17, 313–9 (2011). [DOI] [PubMed] [Google Scholar]
- Gassenmaier M. et al. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem Cells 31, 1467–76 (2013). [DOI] [PubMed] [Google Scholar]
- Boumahdi S. et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246–50 (2014). [DOI] [PubMed] [Google Scholar]
- Beck B. & Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer 13, 727–38 (2013). [DOI] [PubMed] [Google Scholar]
- He K. et al. Cancer cells acquire a drug resistant, highly tumorigenic, cancer stem-like phenotype through modulation of the PI3K/Akt/beta-catenin/CBP pathway. Int J Cancer 134, 43–54 (2014). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–47 (2002). [DOI] [PubMed] [Google Scholar]
- Engelman J. A., Luo J. & Cantley L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7, 606–19 (2006). [DOI] [PubMed] [Google Scholar]
- Luo J. & Cantley L. C. The negative regulation of phosphoinositide 3-kinase signaling by p85 and it's implication in cancer. Cell Cycle 4, 1309–12 (2005). [DOI] [PubMed] [Google Scholar]
- Xue G. & Hemmings B. A. PKB/Akt-dependent regulation of cell motility. J Natl Cancer Inst 105, 393–404 (2013). [DOI] [PubMed] [Google Scholar]
- Bugyi B. & Carlier M. F. Control of actin filament treadmilling in cell motility. Annu Rev Biophys 39, 449–70 (2010). [DOI] [PubMed] [Google Scholar]
- Zhu Q. S. et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 30, 457–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Acloque H., Huang R. Y. & Nieto M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–90 (2009). [DOI] [PubMed] [Google Scholar]
- Grille S. J. et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res 63, 2172–8 (2003). [PubMed] [Google Scholar]
- Tsukamoto T., Hama S., Kogure K. & Tsuchiya H. Selenate induces epithelial-mesenchymal transition in a colorectal carcinoma cell line by AKT activation. Exp Cell Res 319, 1913–21 (2013). [DOI] [PubMed] [Google Scholar]
- Yang X., Chrisman H. & Weijer C. J. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development 135, 3521–30 (2008). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A 104, 16158–63 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A. et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A 106, 268–73 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz P., Iliou M. S. & Esteller M. Epigenetic alterations involved in cancer stem cell reprogramming. Mol Oncol 6, 620–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–15 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Xu T. & Goldkorn A. Cancer cells cyclically lose and regain drug-resistant highly tumorigenic features characteristic of a cancer stem-like phenotype. Mol Cancer Ther 10, 938–48 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge S. B. & Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17, 1471–4 (2010). [DOI] [PubMed] [Google Scholar]
- Cong L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–83 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data