Abstract
AIM: To investigate the clinical significance of KL-6 as a tumor marker of HCC in two different ethnic groups with chronic liver disease consecutively encountered at outpatient clinics.
METHODS: Serum KL-6 was measured by the sandwich enzyme immunoassay method using the KL-6 antibody (Ab) as both the capture and tracer Ab according to the manufacturer’s instructions (Eisai, Tokyo, Japan). Assessment of alpha fetoprotein (AFP) and protein induced vitamin K deficiency or absence (PIVKA-II) was performed in both groups using commercially available kits.
RESULTS: A significantly higher mean serum KL-6 (556±467 U/L) was found in HCC in comparison with non-HCC groups either with (391±176 U/L; P<0.001) or without (361±161 U/L; P<0.001) liver cirrhosis (LC). Serum KL-6 level did not correlate with either AFP or PIVKA-II serU/Levels. Using receiver operating curve analysis for KL-6 as a predictor for HCC showed that the area under the curve was 0.574 (95%CI = 0.50-0.64) and the KL-6 level that gave the best sensitivity (61%) was found to be 334 U/L but according to the manufacturer’s instructions; a cut-off point of 500 U/L was used that showed the highest specificity (80%) in comparison with AFP and PIVKA-II (78% vs 72% respectively). Combining the values of the three markers improved specificity of AFP for HCC diagnosis from 78% for AFP alone; 93% for AFP plus PIVKA-II to 99% for both plus KL-6 value (P<0.001). Mean serum alkaline phosphatase level was significantly higher in KL-6 positive (564±475) in comparison with KL-6 negative (505±469) HCC patients (P = 0.021), but such a difference was not found among non-HCC corresponding groups.
CONCLUSION: KL-6 is suggested as a tumor for HCC. Its positivity may reflect HCC-associated cholestasis and/or local tumor invasion.
Keywords: Tumor markers, Liver disease, Hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 4th most common cancer worldwide, and it is a well-known complication of chronic hepatitis[1,2]. Asymptomatic patients diagnosed as HCC through screening programs are more likely to be candidates for curative treatment and have improved short- and medium-term survival[3,4]. Although serum alpha-fetoprotein (AFP) had been shown to be associated with HCC since 1963[5], unfortunately it is also elevated in a wide variety of non-hepatic malignancies[6,7] and benign hepatic conditions[8,9]. Moreover, it is uncertain whether serum AFP is a useful marker for HCV-related HCC in some ethnic groups e.g., North American patients of African origin[10]. Thus, searching another tumor marker, that together with AFP could improve the diagnostic utility of the later, seemed to be justified. KL-6 was originally found using a murine monoclonal antibody that recognized an undefined sialylated carbohydrate chain on a mucin-like glycoprotein[11] which was also defined as MUC1[12]. The cell membrane MUC1 was found to regulate cell adhesion properties[13]. KL-6 has been first shown to be elevated in patients with interstitial pneumonia[14]. It was also reported to have a high positive rate in different non-hepatic malignancies and its expression was also correlated with metastatic potential of the primary tumor in some of them[15-17]. It has also been studied as a fibrosis marker in patients with HCV-related chronic liver disease[18] and was found to correlate with the degree of irregular regeneration of hepatocytes[19]. A recent study addressed its clinical significance as a tumor marker in HCV-related HCC[20]. However, all these studies investigated KL-6 in HCV-related disease only so that its actual significance as a marker for screening HCC in patients with different chronic liver disease is not yet fully understood. In this study, we aimed to investigate KL-6 as a tumor marker in consecutive patients with chronic liver disease seen at outpatient settings in two different ethnic groups of possible different risk factors for HCC, so that we could get a wider spectrum of disease in order to assess KL-6 validity for HCC screening.
MATERIALS AND METHODS
Study population
We conducted a cross-sectional study between October 2001 and November 2002. Data were gathered from two Affiliations; Shinshu University (Japan) and Suez Canal University (Egypt) Hospitals. A total of 334 consecutive patients with chronic liver disease seen at outpatient liver clinics in the two settings (who met our inclusion/exclusion criteria) were included; of them: 110 patients were diagnosed as HCC with a mean age of 61±11 years and M:F (4:1). Sixty-five were Egyptians and 45 Japanese with viral-related liver disease accounting for 94% and 98% of them respectively. Non-HCC patients were 234 with a mean age of 56±13 years; M:F (7/3). One hundred and six were Egyptians and 128 Japanese with viral-related liver disease accounting for 91% and 84% of them respectively (Table 1).
Table 1.
Background data of the study groups
|
Egyptian |
Japanese |
|||||
| HCC (+) | HCC (–) | P | HCC (+) | HCC (–) | P | |
| n = 65 | n = 106 | n = 45 | n = 128 | |||
| Mean age (SD, yr) | 57±11b | 47±9 | <0.001 | 66±10b | 63±10 | NS |
| Age <50 yr | 16 (25)d | 65 (61) | <0.001 | 3 (7)d | 17 (13) | NS |
| Male | 50 (77) | 82 (77) | NS | 38 (84) | 87 (68) | 0.024 |
| Liver disease | ||||||
| Viral | 61 (94) | 96 (91) | 44 (98) | 107 (84) | ||
| HCV-related | 59 (91) | 92 (87) | NS | 36 (80) | 81 (63) | 0.031 |
| HBV-related | 2 (3)1 | 4 (4) | NS | 8 (18)1 | 28 (22) | NS |
| Non-viral | 4 (6) | 10 (9) | NS | 1 (2) | 20 (16) | 0.010 |
| Cirrhosis | 46 (71) | 45 (42) | <0.001 | 40 (89) | 40 (31) | <0.001 |
| Child’s C | 25 (38)f | 17 (16) | 0.001 | 4 (9)f | 1 (1) | 0.017 |
| Mean±(SD) | ||||||
| ALT (IU/L) | 73±95 | 66±45 | 0.08 | 55±35 | 50±39 | NS |
| Serum Albumin (g/L) | 3.0±0.7 | 3.0±0.5 | NS | 3.6 ±0.5 | 4.2±0.4 | <0.001 |
| Platelet count×1 000/mL3 | 186±107h | 89±53 | 0.001 | 130±51h | 170±71 | <0.001 |
| AFP >10 ng/mL (+) | 64 (99) | 28 (26) | <0.001 | 30 (67) | 23 (18) | <0.001 |
| PIVIKA>40 mAU/L (+) | 51 (79) | 38 (36) | <0.001 | 16 (36) | 27 (21) | 0.047 |
P<0.001,
P<0.001,
P<0.001,
P<0.001 vs Japannese,
P = 0.001.
Chronic liver disease and cirrhosis were identified and diagnosed according to liver biopsy findings, clinical and/or radiological evidence of portal hypertension. HCC was excluded by imaging studies (abdominal ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and/or hepatic angiography), one of which must have been performed at least 6 months following the measurement of AFP.
HCC was diagnosed when meeting our inclusion criteria of positive cytology and/or histology or by the presence of characteristic hepatic masses on liver CT, MRI and/or hepatic angiography (i.e., enlarging tumors and/or tumors with typical arterial vascularization).
We excluded patients with alcoholic and schistosomal liver diseases from our study populations. We had also excluded patients known from their medical history to have interstitial lung fibrosis or any other lung disease from our study population.
Tumor markers measurement
Serum KL-6 was measured by the sandwich enzyme immunoassay method using the KL-6 antibody (Ab) as both the capture and tracer Ab (14) according to the manufacturer’s instructions (Eisai, Tokyo, Japan). KL-6 cut-off point was set at 500 U/L for this study. Assessment of alpha fetoprotein (AFP) and protein-induced vitamin K deficiency or absence (PIVKA-II) was performed using commercially available kits. Cut-off points were set at 10 ng/mL for AFP and 40 mAU/L for PIVKA-II.
Statistical analysis
Univariate statistical analysis was performed using Student's t-test for quantitative and χ2 test with Yates’ correction for qualitative data. Fisher’s exact test was used for comparison of small numbers; statistical significant level was set at P<0.05. Statistical analysis was performed using a computer software (SPSS, version 6.0).
RESULTS
Population background
A difference in mean age, prevalence of advanced Child class and HBV infection was observed between Egyptian and Japanese patients with HCC (Table 1). However, no difference in tumor characteristics was found between the two studied populations (Table 2).
Table 2.
Comparison of background tumor characteristics between Egyptian and Japanese HCC patients
| Tumor characteristic | Egyptian | Japanese | P |
| (n = 65) | (n = 45) | ||
| Tumor multiplicity | |||
| Solitary | 25 (38) | 22 (49) | >0.2 |
| Multiple | 40 (62) | 23 (51) | 0.06 |
| Tumor size | |||
| <3 cm | 32 (49) | 20 (44) | >0.2 |
| 3:< 5cm | 15 (23) | 14 (31) | >0.2 |
| ≥5 cm | 18 (28) | 11 (25) | 0.13 |
| Metastases | 01 (2) >0.2 | ||
| Tumor grade1 | |||
| Well differentiated | 5 (16) | 2 (8) | >0.2 |
| Poorly differentiated | 5 (16) | 4 (16) | >0.2 |
Tumor grade is analyzed in 32 of the Egyptian and 22 of the Japanese groups who passed HCC resection operation during the study period.
KL-6 and other tumor markers in HCC
A significantly higher mean serum KL-6 (556±467) was found in HCC in comparison with non-HCC groups of patients with (391±176; P<0.001) and without (361±161; P<0.001) liver cirrhosis (LC). Serum KL-6 level did not correlate with either AFP (Figure 1) or PIVKA-II (Figure 1) serU/Levels. Using receiver operation characteristic (ROC) curve, the KL-6 level that gave the best sensitivity (61%) was found to be 334 U/L with a specificity of 50%, while PIVKA-II and AFP showed a sensitivity/specificity of (60/72)% and (80/78)% respectively. However, according to the manufacturer’s instructions; a cut-off point of 500 U/L was used in this study that showed the highest specificity (80%) for KL-6 in comparison with the other two markers. Combining the values of KL-6; AFP and PIVKA-II resulted in improvement in the specificity of AFP for HCC diagnosis from 78% for AFP alone; 93% for AFP plus PIVKA-II to 99% for both plus KL-6 (P<0.001) (Table 3).
Figure 1.
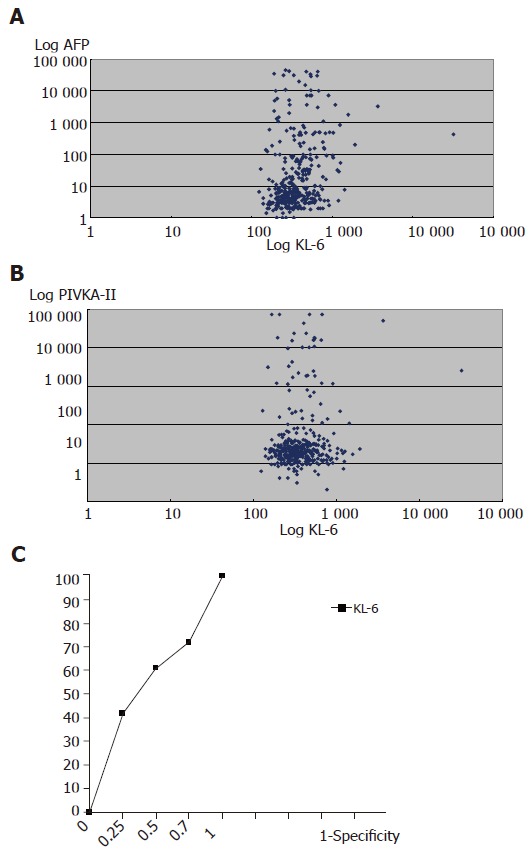
A: Correlation between KL-6 U/L and AFP ng/ml serU/Levels in the study population. C = 0.04, P>0.1. The Log values of both markers are shown; B: Correlation between KL-6 U/L and PIVKA-II mAU/L serU/Levels in the study population. C = 0.03, P>0.5. The Log values of both markers are shown; C: Receiver operating characteristic curves for KL-6 as predictors of HCC. The area under the ROC was found to be 0.574 (95%CI = 0.50–0.64). The best KL-6 sensitivity was obtained at a cut-off point = 334 U/L.
Table 3.
Comparison of the result of different tumor markers between HCC and non-HCC group
| Tumor marker (cut-off point) | Sensitivity | Specificity |
| % | % | |
| AFP (10 ng/mL) | 86 | 78 |
| PIVKA-II (35 mAU/L) | 61 | 72 |
| KL-6 (500 U/L) | 34 | 80 |
| AFP+PIVKA-II | 861 | 93 |
| AFP+KL-6 | 872 | 94 |
| AFP+PIVKA-II+KL-6 | 87 | 99 |
All PIVKA-II (+) HCC patients are AFP (+).
One KL-6 (+) HCC patient is AFP (–).
Factors associated with positive KL-6 in the study population
Univariate analysis (Table 4) of possible factors that could be associated with elevated serum KL-6 in our study group showed that elevated AFP (P<0.001), Child's class C (P = 0.002), Egyptian race (P = 0.003) and HCC (P = 0.008) were significantly associated with positive serum KL-6. Also mean serum alkaline phosphatase level was significantly higher in KL-6 positive (564±475) in comparison with KL-6 negative (505±469) HCC patients (P = 0.021), but such a difference was not found among non-HCC corresponding group (Table 5). Mean serum bilirubin was found to be higher in KL-6 positive subgroups in both HCC and non-HCC (P = 0.077, 0.023) respectively, while mean serum albumin was significantly lower in both groups (P = 0.029, 0.041), respectively (Table 5).
Table 4.
Factors associated with KL-6 positivity in the study population
| Factors | Total | KL-6 (+) | KL-6 (–) |
| Age (yr) | |||
| >=50 | 244 | 60 (24) | 184 (75) |
| <50 | 100 | 24(24) | 76 (76) |
| P | NS | ||
| Sex | |||
| Male | 257 | 61 (24) | 196 (76) |
| Female | 87 | 23 (26) | 64 (73) |
| P | NS | ||
| Ethnicity | |||
| Egyptian | 171 | 53 (31) | 118 (69) |
| Japanese | 173 | 31 (18) | 142 (82) |
| P | 0.003 | ||
| Underlying liver | |||
| disease | |||
| HCV-related | 267 | 71 (27) | 196 (73) |
| HBV-related | 42 | 5 (12) | 37 (88) |
| Non-viral | 35 | 8 (23) | 27 (77) |
| P | NS | ||
| Cirrhosis | |||
| (+) | 169 | 46 (27) | 123 (73) |
| (–) | 175 | 38 (22) | 137 (78) |
| P | NS | ||
| Child’s class | |||
| C | 47 | 20 (43) | 27 (57) |
| A&B | 297 | 64 (21) | 233 (78) |
| P | 0.002 | ||
| HCC: | |||
| (+) | 110 | 37 (34) | 73 (66) |
| (–) | 234 | 47 (20) | 187 (80) |
| P | 0.008 | ||
| AFP | |||
| (+) | 145 | 49 (34) | 96 (66) |
| (–) | 199 | 35 (17) | 164 (82) |
| P | <0.001 |
Table 5.
Comparison of the clinical profile of KL-6 positive and negative patients with and without HCC
|
HCC (+) |
HCC (–) |
|||||
| KL-6 (+) | KL-6 (–) | P | KL-6 (+) | KL-6 (–) | P | |
| n = 37 | n = 73 | n = 47 | n = 187 | |||
| Mean age (yr)1 | 59±12 | 62±10 | NS | 57±12 | 56±13 | NS |
| Cirrhosis | 39 (81) | 55 (76) | NS | 16 (34) | 65 (35) | NS |
| Child’s C | 13 (35) | 15 (21) | NS | 7 (15) | 11 (6) | 0.045 |
| Mean ALT | 74±101 | 62±61 | NS | 59±33 | 57±45 | NS |
| Serum | ||||||
| Albumin (g/L)1 | 2.9±0.7 | 3.3±0.7 | 0.029 | 3.5±0.9 | 3.8±0.8 | 0.041 |
| Bilirubin (mmol/L)1 | 2.7±2.8 | 2.5±3.0 | 0.077 | 2.4±2.9 | 1.4±1.9 | 0.023 |
| ALP (IU/L)1 | 564±475 | 505±469 | 0.021 | 316±139 | 299±152 | NS |
| AFP (+) | 36 (97) | 58 (80) | 0.013 | 13 (28) | 37 (20) | NS |
| PIVIKA (+) | 23 (62) | 44 (60) | NS | 14 (30) | 50 (27) | NS |
Data is shown as mean±SD. Other data is shown as n (%).
KL-6 in Egyptian vs Japanese
Mean KL-6 was significantly higher in Egyptians (576±522) in comparison with Japanese (510±300) HCC patients (P = 0.041) (Table 6). Although a significant difference in mean KL-6 level between HCC and non-HCC was observed in both Egyptian and Japanese patients with chronic liver disease (P<0.001 respectively), the difference was not statistically significant among Japanese patients with HCV-related disease (Table 6). No difference in mean KL-6 level was found between cirrhotic and non-cirrhotic in either HCC or non-HCC patients.
Table 6.
Comparison of mean serum KL-6 level among different study sub-groups
| Egyptian | Japanese | |||||
| HCC (+) | HCC (-) | P | HCC (+) | HCC (-) | P | |
| Chronic liver disease1: | 576 (522) | 398 (185) | 0.001 | 510 (300) | 350 (147) | <0.001 |
| HCV-related | 558 (524) | 400 (172) | 0.008 | 356 (290) | 382 (209) | >0.2 |
| HBV-related | 778 (663) | 246 (72) | >0.2 | 877 (292) | 340 (163) | <0.001 |
| Non-viral | 729 (538) | 446 (309) | >0.2 | 2622 () | 357 (160) | – |
| Cirrhotics | 599 (586) | 406 (159) | 0.035 | 510 (350) | 374 (196) | 0.035 |
| Non-cirrhotics | 518 (325) | 398 (185) | 0.045 | 225 (73) | 349 (222) | <0.001 |
The KL-6 values are shown as mean (SD) U/L.
Only one patient’s data.
KL-6 and tumor characteristics
In the HCC groups of both Egyptian and Japanese patients; KL-6 showed no significant association with tumor site, echogenicity or multiplicity. However, a significantly lower mean KL-6 (Table 7) was noticed in larger size tumors of >5 cm (371±168 U/L) in comparison with tumors of less than or equal to 5 cm (537±323) (P<0.05 in the Japanese group).
Table 7.
Difference in mean KL-6 level according to HCC size
| Egyptian | P1 value | Japanese | P value | |
| (n = 65) | (n = 45) | |||
| Tumor size | ||||
| <3 cm | 485±227 | 618±361 | ||
| 3:<5 cm | 643±685 | >0.1 | 456±285 | 0.17 |
| ≥5 cm | 581±420 | >0.1 | 371±168 | 0.04 |
1P value is shown for the difference group (<3 cm) and the other two groups.
DISCUSSION
KL-6 was studied as a tumor marker in different mali-gnancies like breast, lung and pancreatic cancer and it was reported to be elevated in up to 50% of these mali-gnancies[14]. Two previous studies by Moriyama et al[19,20] addressed KL-6 as a tumor marker for HCC in patients with HCV-related chronic liver disease, and his results showed that the estimated cumulative incidence of HCC development in HCV-related chronic liver disease patients was significantly greater in patients with positive KL-6[19] and suggested KL-6 to be used as a serological marker for HCC development in HCV-positive patients[20]. In our study, we included consecutive patients with chronic liver disease seen at outpatient settings in two different ethnic groups of possible different risk factors for HCC[22,23] in order to have a wider spectrum of disease to judge KL-6 validity as a diagnostic test for HCC; however, one limitation was that most of the encountered patients in the two settings were actually with HCV-related disease with low proportion of HBV and non-viral-related disease. Our results showed a significantly higher mean KL-6 in HCC compared with non-HCC; either with or without LC; in addition no difference in mean KL-6 was found among HCC patients with and without LC; such findings together point to KL-6 association with HCC independent on the presence or absence of LC. A significantly higher mean KL-6 level was found in HBV-related in comparison with HCV-related HCC in both Egyptian and Japanese populations; a finding that deserves future study on a larger population of HBV-related disease. Our results also showed a significantly higher mean KL-6 level in HCC patients of Egyptian compared with Japanese race. The finding of a difference in the clinical background between both in terms of lower mean age and lower prevalence of HBV-related HCC could reflect a difference in the risk factors for HCC in both groups. Also, a higher prevalence of advanced Child class in the HCC Egyptian patients was observed that could stand behind the finding of higher mean KL-6 level in this group compared to their corresponding Japanese group. Although we excluded patients with overt schistosomal from this study, still some Egyptian patients had a past history of schistosomiasis with US evidence of hepatic periportal fibrosis (denoting a background of schistosomal liver disease) that could also explain the finding of higher mean KL-6 level in Egyptian HCC patients, if we consider the possibility that KL-6 could be a fibrosis marker too[21]. This topic is highly suggested for future study.
We used a cut-off point of 500 U/L for KL-6 posi-tivity in this study; however, applying the ROC analysis showed that a cut-off point of 334 U/L would give the best sensitivity in our study population of 60% compared with only 32% for a cut-off (500 U/L); however, the best specificity was obtained using the later. Moriyama et al[19,20] used a cut-off point of 300 U/L in his analysis of KL-6 in HCV-related disease. KL-6 serU/Level did not correlate with either serum AFP or PIVKA-II levels, which points to its behavior independently from either of them and this may justify its clinical significance as an independent tumor marker for HCC diagnosis when considered with both AFP and PIVKA-II. Our results also supported this finding as AFP specificity for HCC diagnosis improved from 78% for AFP alone and 93% of both AFP and PIVKA-II to 99% when combined with KL-6. Univariate analysis showed that low serum albumin, hyperbilirubinemia and elevated ALP were significantly associated with positive KL-6 in HCC patients, while KL-6 showed no association with LC in turn, and this denotes a possible association between positive KL-6 and deterioration of hepatic condition in HCC patients independent from their cirrhotic status; a finding that might point to KL-6 as a predictor of tumor aggression and/or local or systemic metastasizing potential. A follow-up study is needed to confirm its exact role in this regard.
ACKNOWLEDGMENTS
We would like to thank Takeda Foundation, Osaka, Japan for their financial support. We also thank Dr. Alla Sad, Dr. Essam Abd Alla, Dr. Khaled Gad for their help with various laboratory techniques.
Footnotes
Supported by the Takeda Foundation, Osaka, Japan
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 2.Szilagyi A, Alpert L. Clinical and histopathological variation in hepatocellular carcinoma. Am J Gastroenterol. 1995;90:15–23. [PubMed] [Google Scholar]
- 3.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 4.Wong LL, Limm WM, Severino R, Wong LM. Improved survival with screening for hepatocellular carcinoma. Liver Transpl. 2000;6:320–325. doi: 10.1053/lv.2000.4875. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 6.Iwai M, Kashiwadani M, Takino T, Ibata Y. Demonstration by light and ultrastructural immunoperoxidase study of alpha-fetoprotein-positive non-hepatoma cells and hepatoma cells during 3'-methyl-4-dimethylaminoazobenzene hepatocarcinogenesis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55:117–123. doi: 10.1007/BF02896568. [DOI] [PubMed] [Google Scholar]
- 7.McIntire KR, Waldmann TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975;35:991–996. [PubMed] [Google Scholar]
- 8.Gallo V, Cerutti E, Riberi A, Re M, Petrino R, Pecchio F. Alpha-fetoprotein and tissue polypeptide antigen in non neoplastic hepatic disorders. J Nucl Med Allied Sci. 1989;33:89–93. [PubMed] [Google Scholar]
- 9.Alpert E, Feller ER. Alpha-fetoprotein (AFP) in benign liver disease. Evidence that normal liver regeneration does not induce AFP synthesis. Gastroenterology. 1978;74:856–858. [PubMed] [Google Scholar]
- 10.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–417. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 11.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest. 1989;96:68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- 12.Stahel RA, Gilks WR, Lehmann HP, Schenker T. Third International Workshop on Lung Tumor and Differentiation Antigens: overview of the results of the central data analysis. Int J Cancer Suppl. 1994;8:6–26. doi: 10.1002/ijc.2910570704. [DOI] [PubMed] [Google Scholar]
- 13.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest. 1999;46:151–158. [PubMed] [Google Scholar]
- 15.Sagara M, Yonezawa S, Nagata K, Tezuka Y, Natsugoe S, Xing PX, McKenzie IF, Aikou T, Sato E. Expression of mucin 1 (MUC1) in esophageal squamous-cell carcinoma: its relationship with prognosis. Int J Cancer. 1999;84:251–257. doi: 10.1002/(sici)1097-0215(19990621)84:3<251::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, Tanaka S, Irimura T, Kim YS, Sato E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998;4:2605–2614. [PubMed] [Google Scholar]
- 17.Tanimoto T, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N. MUC1 expression in intramucosal colorectal neoplasms. Possible involvement in histogenesis and progression. Oncology. 1999;56:223–231. doi: 10.1159/000011969. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Takada H, Oka S, Kanouzawa S, Iimuro M, Kitazumi Y, Arima T, Ohyama R, Kuwayama H. Clinical significance of KL-6, a marker of interstitial pneumonia, in cases of HCV-associated chronic liver disease. Intern Med. 2003;42:650–654. doi: 10.2169/internalmedicine.42.650. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama M, Matsumura H, Mikuni M, Arkawa Y, Ohshiro S, Aoki H, Yamagami H, Kaneko M, Shioda A, Saito H, et al. The clinical significance of serum KL-6 levels in patients with type C liver diseases. Hepatol Res. 2003;25:385–395. doi: 10.1016/s1386-6346(02)00307-8. [DOI] [PubMed] [Google Scholar]
- 20.Moriyama M, Matsumura H, Watanabe A, Nakamura H, Arakawa Y, Oshiro S, Aoki H, Shimizu T, Yamagami H, Kaneko M, et al. Detection of serum and intrahepatic KL-6 in anti-HCV positive patients with hepatocellular carcinoma. Hepatol Res. 2004;30:24–33. doi: 10.1016/j.hepres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Hirasawa Y, Kohno N, Yokoyama A, Inoue Y, Abe M, Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol. 1997;17:501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- 22.Hassan MM, Zaghloul AS, El-Serag HB, Soliman O, Patt YZ, Chappell CL, Beasley RP, Hwang LY. The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J Clin Gastroenterol. 2001;33:123–126. doi: 10.1097/00004836-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Sakai H, Hashizume M, Hirohata T. A long-term follow-up study on risk factors for hepatocellular carcinoma among Japanese patients with liver cirrhosis. Jpn J Cancer Res. 1998;89:1241–1250. doi: 10.1111/j.1349-7006.1998.tb00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


