Abstract
AIM: To evaluate endoscopic mucosal resection (EMR) in patients with high-grade dysplasia (HGD) and/or intramucosal cancer (IMC) in Barrett’s esophagus (BE).
METHODS: Between June 2000 and December 2003, 39 consecutive patients with HGD (35) and/or IMC (4) underwent EMR. BE >30 mm was present in 27 patients. In three patients with short segment BE (25.0%), HGD was detected in a normal appearing BE. Lesions had a mean diameter of 14.8±10.3 mm. Mucosal resection was carried out using the cap method.
RESULTS: The average size of resections was 19.7±9.4×14.6±8.2 mm. Histopathologic assessment post-resection revealed 5 low-grade dysplasia (LGD) (12.8%), 27 HGD (69.2%), 2 IMC (5.1%), and 5 SMC (-12.8%). EMR changed the pre-treatment diagnosis in 10 patients (25.6%). Three patients with SMC underwent surgery. Histology of the surgical specimen revealed 1 T0N0 and 2 T1N0 lesions. The remaining two patients were cancer free at 32.5 and 45.6 mo, respectively. A metachronous lesion was detected after 25 mo in one patient with HGD. Intra-procedural bleeding, controlled at endoscopy, occurred in four patients (10.3%). After a median follow-up of 34.9 mo, all patients remained in remission.
CONCLUSION: In the medium term, EMR is effective and safe to treat HGD and/or IMC within BE and is a valuable staging method. It could become an alternative to surgery.
Keywords: Endoscopic mucosal resection, Barrett’s esophagus, High-grade dysplasia, Intramucosal cancer
INTRODUCTION
The incidence of adenocarcinoma (AC) of the esophagus has increased in the last three decades in the Western world[1-3]. Most esophageal adenocarcinomas arise in a precursor lesion, Barrett’s esophagus (BE). The esophageal cancer risk in BE patients is about 1 cancer per 200 patient-years, or 0.5% per year[4,5]. The prognosis is poor for the typical patient who presents with invasive cancer, with a 5-year survival rate of under 10%[6].
Dysplasia arising in BE is a marker of progression toward invasive cancer. High-grade dysplasia (HGD) is an uncommon but a serious problem. In 16-60% of patients found to have HGD, invasive cancer was diagnosed in the next 5-7 years[6,7], although spontaneous regression of HGD can also occur[8]. Following surgical esophagectomy for HGD, 10-50% of cases had previously undetected foci of invasive cancer found in the resected specimen[9,10].
When HGD is detected, the three options that are available are: endoscopic surveillance, esophagectomy, and endotherapy.
Surveillance in patients with HGD is controversial. The uncertainty of natural history of HGD and its slow progression rate, could justify a contemplative attitude. Schnell et al[7] evaluated the long-term outcome of 75 patients with HGD who were enrolled in an endoscopic surveillance program. After a mean follow-up of 7 years, AC occurred in 12 (16%) patients.
Esophagectomy has been the standard treatment of HGD and early cancer in BE. However esophagectomy is associated with surgical morbidity of 20-50% and mortality of about 3%, even at high-volume centers. In patients older than 70 years, mortality was 11%[11,12]. A less invasive treatment would be desirable. A newer alternative to esophagectomy is endoscopic mucosal resection (EMR). Improved diagnosis of early malignancy in BE, including endoscopic ultrasound (EUS), may change the therapeutic approach. The superficial lesions of HGD and intramucosal cancer (IMC), with minimal risk of lymph node metastasis, can be removed by EMR. This procedure allows adequate histologic assessment and definitive treatment.
The use of EMR to treat HGD and IMC in BE is increasing, but the number of published series remains less. Our aim was to evaluate EMR in the treatment of HGD and IMC in Barrett’s patients in terms of complications and recurrence rate.
MATERIALS AND METHODS
Patients
Between June 2000 and December 2003, 39 consecutive patients (mean age 62.8±11.4 years) with histologically confirmed HGD (35) or IMC (4) in BE underwent EMR in three Departments of Gastroenterology acting as regional referral centers. All patients were previously identified by endoscopic examination performed in our centers or referred from other hospitals. In this case, the original histologic slides were re-evaluated by two expert pathologists (G.L., V.V.) on BE before EMR.
This study was approved by our institutional review board. All patients gave written informed consent to endoscopic therapy. Patients were evaluated and treated using the same protocol by one of the authors representing each of the three institutions.
At endoscopy, superficial lesions were defined using the following classification: slightly elevated (0-IIa), flat (0-IIb), and slightly depressed (0-IIc)[13]. Endoscopy was performed with standard diagnostic videoendoscopes (GIF-Q145, Olympus Optical Co. Ltd., Tokyo, Japan). Patients with ulcerated lesions seen on endoscopy were excluded.
Before EMR, EUS with mechanical rotating transducer, using the water-filling method (GF-UMQ130, 7.5-20 MHz, Olympus Optical Co. Ltd.), was routinely performed to assess lesion depth and mediastinal lymph node status. All patients had a CT scan of the thorax. Only lesions confined to the mucosal layer with no apparent lymph node metastases were considered for EMR.
Deep sedation with propofol was used. EMR was performed using a plastic cap (MH-594, Olympus Optical Co. Ltd, Tokyo, Japan) preloaded on the tip of a standard diagnostic forward-viewing endoscope. The cap had an outer diameter of 13 mm and a length of 15 mm. Inside the distal end of the cap was a gutter, which positions the opened polypectomy snare. A 2-mm segment of the gutter was removed with a scalpel before placement on the endoscope tip. This modification was then aligned with the operative channel to avoid interference by the injection needle or other devices (hemoclips, biopsy forceps). Submucosal injection of epinephrine solution (1:60.000-1:100.000) to create a fluid cushion was performed using variceal injection needles (VIN-23, Wilson Cook Medical Inc; Variject Contrast Injection Needle, Boston Scientific) in all patients. Injected volume ranged between 8 and 30 mL, depending on the lesion diameter. Methylene blue was added to the solution for visual enhancement of the fluid cushion in contrast to the lesion. The cap was next applied against the lesion, which was aspirated into it. A monofilament polypectomy snare (SD-221U-25, Olympus Optical Co. Ltd, Tokyo, Japan) was then firmly secured around the tissue and resection was performed. Resection was performed by endocut mode only, using the ERBE-ICC 200 cautery device (ERBE Elektromedizin GmbH, Tubingen, Germany). The output setting predefined by the manufacturer was adopted: cut 120 W, coagulation 60 W. To minimize interobserver variability among the three endoscopists performing EMR, the diameter of the lesion and the resected areas were estimated by placing an open polypectomy snare around the lesion. In patients with lesions ≤12 mm wide “en-bloc” resection was performed. For larger lesions, piecemeal resection was completed by applying the cap close to the previously resected area. Specimens were aspirated into the cap and all materials were retrieved for histopathologic assessment. To complete EMR, multiple withdrawals and re-intubations were needed. No overtube was used.
Intra-procedural bleeding (during the EMR) was controlled by epinephrine-saline injections (1:10.000) and, when required, by placing hemoclips (HX-600-090L; rotatable clip fixing device HX-6UR-1, Olympus Optical Co., Ltd).
All patients were hospitalized for 48 h. They were kept fasting for 24 h, then a soft diet was advised for the next two weeks. After EMR, an intravenous PPI (omeprazole, 40 mg/d) was administered for 24 h, followed by a maintenance oral dose of 40 mg/d.
Surveillance
Following EMR, patients were contacted by phone weekly in the first fifteen days, then monthly, to monitor for symptoms such as dysphagia. Endoscopy was repeated at 3, 6, and 12 mo and then yearly, with multiple biopsies from the EMR site, and four-quadrant biopsies from the residual BE.
Complete remission was defined, when well demarcated areas of squamous re-epithelialization without mucosal irregularities, were observed. A lesion was considered metachronous, when diagnosed more than 12 mo from the EMR, irrespective of its location.
Histology
Following the WHO guidelines, we defined HGD by cytologic and architectural changes confined to the mucosa. IMC was defined by cytologic and architectural changes confined to the lamina propria. Invasive cancers were considered to be those infiltrating the submucosa (SMC)[14,15].
Statistical analysis
Statistical data were expressed as mean±SD. The Kruskal-Wallis test was used to compare histologic severity with lesion size. A P value of 0.05 or less was considered statistically significant.
RESULTS
EMR was performed in 39 patients, 34 males and 5 females. Their mean age was 62.8±11.4 years. Thirty-six patients had type 0-IIa mucosal abnormalities and three had HGD detected by random biopsies in a normal appearing BE.
Mean Barrett’s length was 4.3±2.5 cm. Long segment BE (LSBE, ≥3 cm) was present in 27 patients. The three patients with non-visible lesions had short segment BE (SSBE). These patients underwent EMR with the aim of completely removing the metaplastic epithelium.
Lesions had a mean diameter of 14.8±10.3 mm. Histologic severity did not correlate with lesion size. The average size of reconstructed resected specimens was 19.7±9.4×14.6±8.2 mm. In all patients the EMR was completed in one session. “En-bloc” resection was performed in 19 cases with lesions of ≤12 mm. The size of the first resected specimen by EMR-C method ranged between 8 and 12 mm.
Following EMR, the pre-treatment histology was re-classified in 10/39 patients (25.6%). Among the 35 initially diagnosed as HGD, five were found to have only LGD, but three had SMC. Of the four patients initially diagnosed as IMC, two were re-classified as SMC. EUS did not help to identify submucosal infiltration.
Table 1 shows the characteristics of patients and lesions according to the histology found in the EMR resection specimens.
Table 1.
Characteristics of patients and lesions according to histology of mucosa resected at EMR (mean±SD)
| LGD | HGD | IMC | SMC | |
| (no surgery) | ||||
| Age (yr) | 60.6±8.8 | 61.76±11.8 | 56±4.2 | 73.6±8.3 |
| BE length (cm) | 6.6±4.1 | 3.6±1.8 | 3.7±1.8 | 5.8±3.1 |
| Size of lesion (mm) | 22.5±8.7 | 13±10.2 | 16±5.7 | 17.6±11.9 |
| Metachronous lesion (%) | ||||
| No | 5 (100) | 24 (88.9) | 2 (100) | 2 (100) |
| Yes | - | 1 (3.7) | - | - |
| No follow-up endoscopy (%) | - | 2 (7.4) | - | - |
| Total | 5 | 27 | 2 | 2 |
LGD: low-grade dysplasia; HGD: high-grade dysplasia; IMC: intramucosal cancer; SMC: submucosal cancer; BE: Barrett’s esophagus.
Cancers
The five patients with SMC had a mean age of 73.6±8.3 years. The mean BE length was 5.8±3.1 cm, and lesion size ranged from 5 to 30 mm. The lesions were type IIa (superficial elevated). Histologic assessment detected tiny areas of low-grade differentiation, and in two of them, lymphatic permeation. Three (7.7% of all patients) underwent esophagectomy and the histopathologic assessment showed one T0N0 and two T1N0. The remaining two patients were considered unfit for surgery due to advanced age (81 and 84 years) and/or comorbidities (cardiovascular disease). They were included in the surveillance program.
Complications
Intra-procedural bleeding occurred in four patients (10.3%), and was controlled with epinephrine injections in two, and with epinephrine plus clipping in the other two. Delayed bleeding was not seen. No patient needed blood transfusion. No perforations occurred. Retrosternal pain was present in one patient. An esophageal stenosis developed 8 mo later in a patient with LSBE (7 cm). He had a 30 mm HGD lesion and the diameter of the EMR area was 40 mm. He was successfully treated by a single bougienage.
Surveillance
Follow-up endoscopy was performed in 32 of the 34 patients without invasive cancer (94.1%), two patients declining repeat examination. The follow-up ranged from 16.3 to 72.1 mo (median 34.9 mo). In one patient (3.1%), with an original lesion of 20 mm (HGD), a metachronous lesion was detected after 25 mo. It was easily removed by EMR, and the histology showed HGD. One of the two patients with SMC, who did not undergo surgery died of cardiovascular disease 45.6 mo later, and the other was alive and cancer free at a 32.5 mo surveillance.
EUS was repeated at 3 and 6 mo after EMR. CT of the thorax and upper abdomen were also performed after 6 mo, and then after 1 year to evaluate the lymph node status and the presence of metastases. Figures 1A and 1B show endoscopic appearance of a SSBE with HGD before and three months after EMR.
Figure 1.
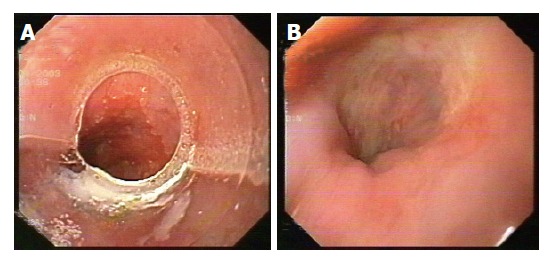
Endoscopic appearance of SSBE with HGD before (A) and 3 mo after EMR (B).
DISCUSSION
The use of EMR for EC in the digestive tract was described by Inoue et al[16]. We employed EMR to remove 39 focal esophageal HGD and IMC lesions of 3-30 mm size in BE, with few complications. Most lesions were flat mucosal abnormalities. Systematic biopsies were taken from the remainder of the BE to exclude non-visible multifocal lesions.
The data reported in the present study represent the first experience of EMR in BE, in Italy. Three major referral centers joined in the effort of evaluating the clinical outcome of EMR. In a period of three years a relatively small number of patients has been included in our study, especially when compared with the series reported by German authors[17]. This difference is attributable to the still limited number of patients with early neoplastic lesions in BE, detected in endoscopic centers. Furthermore, as surgery is still the gold standard treatment for HGD and IM, the majority of these patients is referred for esophagectomy.
EMR could become a management option for HGD, and also for IMC, where the risk of lymph node involvement is from 0% to 4%[18,19]. Unfortunately, in cases with SMC, the incidence of regional lymph-node metastasis is 15-50%[20-22]. Current data support surgical resection in the setting of submucosal infiltration by AC, unless comorbidity or advanced patient age precluded it.
Table 2 displays data from selected studies on EMR in BE since 2000. Most published studies report EMR of endoscopically visible areas of HGD. Some of these studies differ in methodology and it is difficult to compare them. However, our results are in accordance with the data reported by other authors. EMR provides greater diagnostic precision than endoscopic biopsy, despite endoscopy with biopsies and standard EUS before EMR in all patients. In five of 39 cases, undetected SMC was found on histological examination of the resected specimen. Reclassification of the histology after EMR occurred in 26% of our patients. Other authors have reported reclassification in 0% to 75% of cases after EMR (Table 2). Causes may include biopsy sampling error and observer interpretation variability.
Table 2.
Selected studies on EMR in Barrett’s esophagus
| Author | Number of patiens | Size of lesions cm (mean) | Technique | Histology pre-EMR | Histology post-EMR | Change in diagnosis (%) | Complications | Follow-up months (mean) | Recurrence |
| Seewald et al 2003 Germany[24] | 12 | Median 5 | EUS Snare | 5 HGD/ IMC (visible) 7 IMC | 2 BE 1 LGD 5 HGD | 75% | Bl: 33% Stricture:17% | Median: 9 | 0 |
| (non-visible) | |||||||||
| AC 4 | |||||||||
| Ahmad et al 2002 | 19/101 | 0.5-3 | EUS | AC 6 | AC 8 | 58% | Bl: 11% | >=24 | 0 |
| USA[33] | EMR-C | HGD 6 | HGD 4 | ||||||
| Snare | NOS 7 | LGD 1 | |||||||
| injection | Benign 6 | ||||||||
| May et al 2002 | 80 | Nos | EUS | HGD 7 | AC: 11/80 | Nos | Bl: 6% | 34 | 24/78 |
| Germany[17] | EMR±PDT | EC 73 | Stricture: 4% |
Bleeding is the most frequent adverse event with EMR, reported in a median 10% of patients. Intra-procedural bleeding also occurred in 10% of our patients and was managed endoscopically without transfusion. Esophageal stenosis is a late complication of EMR, reported in 0-30% of cases (Table 3). In our study, one patient (2.5%) developed stenosis. Larger EMR resections may increase the risk; in a study of 137 patients, stenosis was seen only when EMR involved more than two-thirds of the esophageal circumference[23]. However, in one report of circumferential EMR, only two of 12 patients developed stenosis[24]. The perforation risk is generally less than 1%. No perforations occurred in our series. Overall, complications seem fewer for EMR than for surgical resection. In one study, complications occurred in 48% of esophagectomies vs 16% for EMR combined with photodynamic therapy (PDT)[25].
Table 3.
Selected studies on EMR in Barrett’s esophagus
| Author | Number of pts | Size of lesions cm (mean) | Technique | Histology pre-EMR | Histology post-EMR | Change in diagnosis (%) | Complications | Follow-up Months (mean) | Recurrence |
| Buttar et al 2001 USA[34] | 17 | 8 | EUS VLD-PDT | IMC: 7 AC: 10 | IMC: 7 AC: 10 | 47% | Bl: 6% Stricture: 30% | 13 | HGD (1)1 AC (1) |
| Injection | |||||||||
| Nijhawan et al 2000 USA[35] | 25 | 7 | EUS Lift-and-cut VLD | 2 BE 8 LGD 5 HGD | 2 BE 3 LGD 5 HGD | 48% | 0 | 14.6 | 0 |
| Injection | 9 AC | 13 AC | |||||||
| 1 other | 2 other | ||||||||
| Ell et al 2000 | 35 | 0.9 | EUS | HGD: 3 | HGD: 3 | 0 | Bl: 20% | 12 | 11% |
| Germany[36] | EMR± | EC: 32 | EC: 32 | ||||||
| injection |
Pts: patients; EMR: endoscopic mucosal resection; EUS: endoscopic ultrasonography; HGD: high-grade dysplasia; IMC: intramucosal carcinoma; BE: Barrett's esophagus; LGD: low-grade dysplasia; AC: invasive adenocarcinoma; Bl: bleeding; EMR-C: EMR with cap; PDT: photodynamic therapy; Nos: not otherwise specified; VLD: variceal ligator device; EC: early cancer;
persistence of HGD.
A recent controlled study of 100 mucosectomies compared the cap method and a ligation method for suction EMR. The diameter of the removed specimen, the diameter of the resected area, and the complication rate showed no significant differences between the two groups, and no severe complications occurred[26].
There is limited information on the long-term effec-tiveness of EMR. May et al[17] followed 70 patients with HGD or early AC for a mean of 34 mo after EMR. Ten percent had minor complications. During follow-up, 21/70 patients were found to have locally recurrent or metachronous disease, treated endoscopically with success in all but one case. The only death from Barrett's AC was in a patient who had surgery for SMC. In our series, follow-up for a median 35 mo was available in 94.1% of patients. One of the 39 patients (2.6%) had a metachronous lesion after 25 mo, successfully treated with another EMR. According to other authors, malignant transformation of HGD is about 34% in 6-54 mo[27], corresponding to our follow-up period. In this time range we did not find invasive AC. In our two patients with SMC who did not undergo surgery, no histologic evidence of disease was detected.
We used EMR to remove focal lesions, but did not attempt to resect long circumferential segments of Barrett mucosa. This might seem a logical extension of the use of EMR. Removing both focal lesions and also the remainder of the BE might give greater assurance that no neoplasia or columnar mucosa remained than with PDT or thermal methods, as well as providing complete histological assessment of the mucosa. Potential risks and technical difficulties have so far limited the use of circumferential EMR, but this has now been tried. Experimentally, we assessed the feasibility of 3 cm circumferential EMR in a porcine model using EMRC. One out of four pigs developed a severe stenosis[28]. This work was advanced by Rajan et al[29] who performed more extensive EMR without complications. In the clinical setting, Satodate et al[30] resected an entire 5 cm circumferential BE, together with 2 cm of gastric mucosa. The patient had early multifocal AC, with one small area of submucosal invasion. EMR was performed using the cap method in a single session, 30 separate pieces of the mucosa being removed. Following dilatations for esophageal stenosis, at 10 mo he was asymptomatic and endoscopy showed no stenosis, no recurrent cancer, and no remaining BE. Seewald et al[24] performed circumferential EMR in 12 patients with HGD and IMC. Seven had no visible lesions. A monofilament polypectomy snare without a cap was used. In each case, the entire BE (median length 5 cm) was completely removed in 1-5 sessions, with a median number of 5 snare resections per endoscopic session. Four patients had minor bleeding, and two required esophageal dilations.
Recent advances in techniques as chromoendoscopy with methylene-blue and high-magnification endoscopy may help in identifying non-visible dysplastic lesions and in recognizing their width in Barrett’s esophagus. Chromoendoscopy with methylene-blue may be useful to detect dysplastic mucosal areas. In fact, about 90% of these areas are unstained[31]. High-magnification endoscopy allows the identification of specific pit-patterns of the esophageal epithelium. Dysplasia seems to distort this pattern[32]. In our three patients with non-visible lesion in SSBE, we did not use these techniques because we performed EMR with the aim to completely remove the metaplastic epithelium.
Our study confirms that EMR is a feasible, low risk procedure to treat focal HGD and IMC within BE. Given encouraging short and medium-term results, endoscopic therapy is more often being considered as primary treatment[17]. However, controlled studies comparing EMR and esophagectomy are not available. Further experience is needed to determine the place of total removal of Barrett’s mucosa by a more extensive EMR.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 2.Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–555. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Conio M, Lapertosa G, Blanchi S, Filiberti R. Barrett's esophagus: an update. Crit Rev Oncol Hematol. 2003;46:187–206. doi: 10.1016/s1040-8428(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 4.Conio M, Blanchi S, Lapertosa G, Ferraris R, Sablich R, Marchi S, D'Onofrio V, Lacchin T, Iaquinto G, Missale G, et al. Long-term endoscopic surveillance of patients with Barrett's esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–1939. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 6.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O'Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 8.Weston AP, Sharma P, Topalovski M, Richards R, Cherian R, Dixon A. Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–1893. doi: 10.1111/j.1572-0241.2000.02234.x. [DOI] [PubMed] [Google Scholar]
- 9.Cameron AJ, Carpenter HA. Barrett's esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586–591. [PubMed] [Google Scholar]
- 10.Korst RJ, Altorki NK. High grade dysplasia: surveillance, mucosal ablation, or resection? World J Surg. 2003;27:1030–1034. doi: 10.1007/s00268-003-7057-x. [DOI] [PubMed] [Google Scholar]
- 11.Collard JM. High-grade dysplasia in Barrett's esophagus. The case for esophagectomy. Chest Surg Clin N Am. 2002;12:77–92. doi: 10.1016/s1052-3359(03)00067-x. [DOI] [PubMed] [Google Scholar]
- 12.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–1197. doi: 10.1136/bmj.327.7425.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002;34:163–168. doi: 10.1055/s-2002-19855. [DOI] [PubMed] [Google Scholar]
- 14.Jass JR, Sobin LH, Watanabe H. The World Health Organization's histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162–2167. doi: 10.1002/1097-0142(19901115)66:10<2162::aid-cncr2820661020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 17.May A, Gossner L, Pech O, Fritz A, Gunter E, Mayer G, Muller H, Seitz G, Vieth M, Stolte M, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol. 2002;14:1085–1091. doi: 10.1097/00042737-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Baba H, Maehara Y, Okuyama T, Orita H, Anai H, Akazawa K, Sugimachi K. Lymph node metastasis and macroscopic features in early gastric cancer. Hepatogastroenterology. 1994;41:380–383. [PubMed] [Google Scholar]
- 19.Nigro JJ, Hagen JA, DeMeester TR, DeMeester SR, Theisen J, Peters JH, Kiyabu M. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg. 1999;230:433–48; discussion 433-48;. doi: 10.1097/00000658-199909000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein HJ, Feith M, Mueller J, Werner M, Siewert JR. Limited resection for early adenocarcinoma in Barrett's esophagus. Ann Surg. 2000;232:733–742. doi: 10.1097/00000658-200012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hölscher AH, Bollschweiler E, Schneider PM, Siewert JR. Early adenocarcinoma in Barrett's oesophagus. Br J Surg. 1997;84:1470–1473. [PubMed] [Google Scholar]
- 22.Nigro JJ, Hagen JA, DeMeester TR, DeMeester SR, Peters JH, Oberg S, Theisen J, Kiyabu M, Crookes PF, Bremner CG. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg. 1999;117:16–23; discussion 23-25. doi: 10.1016/s0022-5223(99)70464-2. [DOI] [PubMed] [Google Scholar]
- 23.Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc. 2003;57:165–169. doi: 10.1067/mge.2003.73. [DOI] [PubMed] [Google Scholar]
- 24.Seewald S, Akaraviputh T, Seitz U, Brand B, Groth S, Mendoza G, He X, Thonke F, Stolte M, Schroeder S, et al. Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854–859. doi: 10.1016/s0016-5107(03)70020-0. [DOI] [PubMed] [Google Scholar]
- 25.Pacifico RJ, Wang KK, Wongkeesong LM, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett's esophagus. Clin Gastroenterol Hepatol. 2003;1:252–257. [PubMed] [Google Scholar]
- 26.May A, Gossner L, Behrens A, Kohnen R, Vieth M, Stolte M, Ell C. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167–175. doi: 10.1067/mge.2003.339. [DOI] [PubMed] [Google Scholar]
- 27.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett's esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–1032. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 28.Conio M, Sorbi D, Batts KP, Gostout CJ. Endoscopic circumferential esophageal mucosectomy in a porcine model: an assessment of technical feasibility, safety, and outcome. Endoscopy. 2001;33:791–794. doi: 10.1055/s-2001-16516. [DOI] [PubMed] [Google Scholar]
- 29.Rajan E, Gostout CJ. Widespread endoscopic mucosal resection. Gastrointest Endosc Clin N Am. 2001;11:489–497, vi. [PubMed] [Google Scholar]
- 30.Satodate H, Inoue H, Yoshida T, Usui S, Iwashita M, Fukami N, Shiokawa A, Kudo SE. Circumferential EMR of carcinoma arising in Barrett's esophagus: case report. Gastrointest Endosc. 2003;58:288–292. doi: 10.1067/mge.2003.361. [DOI] [PubMed] [Google Scholar]
- 31.Canto MI, Setrakian S, Willis JE, Chak A, Petras RE, Sivak MV. Methylene blue staining of dysplastic and nondysplastic Barrett's esophagus: an in vivo and ex vivo study. Endoscopy. 2001;33:391–400. doi: 10.1055/s-2001-14427. [DOI] [PubMed] [Google Scholar]
- 32.Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett's oesophagus. Gut. 2003;52:24–27. doi: 10.1136/gut.52.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 34.Buttar NS, Wang KK, Lutzke LS, Krishnadath KK, Anderson MA. Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett's esophagus. Gastrointest Endosc. 2001;54:682–688. doi: 10.1067/gien.2001.0003. [DOI] [PubMed] [Google Scholar]
- 35.Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc. 2000;52:328–332. doi: 10.1067/mge.2000.105777. [DOI] [PubMed] [Google Scholar]
- 36.Ell C, May A, Gossner L, Pech O, Günter E, Mayer G, Henrich R, Vieth M, Müller H, Seitz G, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]


