Abstract
AIM: To determine the distribution of Hepatitis B virus (HBV) genotypes in Benin, and to clarify the virological characteristics of the dominant genotype.
METHODS: Among 500 blood donors in Benin, 21 HBsAg-positive donors were enrolled in the study. HBV genotypes were determined by enzyme immunoassay and restriction fragment length polymorphism. Complete genome sequences were determined by PCR and direct sequencing.
RESULTS: HBV genotype E (HBV/E) was detected in 20/21 (95.2%), and HBV/A in 1/21 (4.8%). From the age-specific prevalence of HBeAg to anti-HBe seroconversion (SC) in 19 HBV/E subjects, SC was estimated to occur frequently in late teens in HBV/E. The comparison of four complete HBV/E genomes from HBeAg-positive subjects in this study and five HBV/E sequences recruited from the database revealed that HBV/E was distributed throughout West Africa with very low genetic diversity (nucleotide homology 96.7-99.2%). Based on the sequences in the basic core promoter (BCP) to precore region of the nine HBV/E isolates compared to those of the other genotypes, a nucleotide substitution in the BCP, G1757A, was observed in HBV/E.
CONCLUSION: HBV/E is predominant in the Republic of Benin, and SC is estimated to occur in late teens in HBV/E. The specific nucleotide substitution G1757A in BCP, which might influence the virological characteristics, is observed in HBV/E.
Keywords: HBV genotype, West Africa, Basic core promoter
INTRODUCTION
Hepatitis B virus(HBV) is a member of the family Hepadnaviridae. This virus is a small DNA virus with a partially double-stranded 3.2-kb genome. HBV has been classified into seven genotypes based on the sequence divergence over the entire genome exceeding 8%[1,2]. The seven genotypes show a distinctive geographical distribution. HBV genotype A (HBV/A) is prevalent in Northwestern Europe, North America, and Africa[3]. HBV/B and HBV/C are characteristics of Asia. HBV/D is predominant in the Mediterranean area, HBV/E in West Africa, and HBV/F in South America.
Recently, virological and clinical differences in genotypes have been reported[4,5]. In addition, subtypes of HBV/B, HBV/Ba, ‘a’ indicating Asia, and HBV/Bj, ‘j’ indicating Japan, have been reported[6]. The former has recombination with HBV/C, but the latter does not, and clinical differences have been shown between them[7]. Furthermore, HBV/A is phylogenetically classified into subtypes, namely HBV/Aa, ‘a’ indicating Asia/Africa, which is distributed in Asia and Africa, and HBV/Ae, ‘e’ indicating Europe, which is distributed in Europe[8].
It is now recognized that mutations in basic core promoter (BCP) and precore region regulate hepatitis B e antigen (HBeAg) expression. It was shown in an in vitro study that the double mutations in BCP, A1762T and G1764A, downregulate precore mRNA and slightly increase the efficiency of pregenome mRNA and core mRNA[9]. Additionally, the mechanism of HBeAg reduction through subtype-specific nucleotide substitutions just prior to the start of the precore open reading frame (ORF), known as “Kozak sequence” in the African subtype of HBV/A, has been shown in an in vitro study[10].
In Africa, the distribution of HBV genotypes is HBV/A in the South, HBV/D in the North, and HBV/E in the West. For HBV/E, only four human HBV/E full genomes have been reported so far, and the virological and clinical characteristics have not yet been sufficiently clarified.
In this study, the distribution of HBV genotypes in the Republic of Benin was investigated for the first time, and the virological and clinical characteristics of the dominant genotype were analyzed.
MATERIALS AND METHODS
Patients
Five hundred blood donors, who visited the National Blood Transfusion Center in the Republic of Benin in 2002, were screened for hepatitis B surface antigen (HBsAg). Twenty-four were positive for HBsAg (4.8%). Sera from 21 donors were available in this study (17 males, 3 females, unknown in 1; mean age, 23.1±6.7 years). All the donors were negative for the antibody to HIV-1 (anti-HIV) and the antibody to human adult leukemia virus 1 (anti-HTLV-1). The antibody to hepatitis C virus (anti-HCV) was positive in one donor. Informed consent was obtained from each patient.
Serological testing
HBsAg, anti-HCV, and anti-HTLV-1 were tested by counting immunoassay (PAMIA, Sysmex, Kobe, Japan). HBeAg and the antibody to HBeAg (anti-HBe) were determined using a commercially available chemiluminescent enzyme immunoassay (EIA, Lumipulse f, Fujirebio Inc., Tokyo, Japan). Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (T-Bil) were measured in all the samples.
Genotyping
The HBV genotypes were determined in the sera using an EIA with pre-S2 specific mAb[12,13]. When the result of the EIA method was indeterminate, HBV genotypes were detected by restriction fragment length polymorphism, as previously described[14].
PCR amplification and sequencing of HBV
The serum samples were stored at -80 °C until use. Total DNA was extracted from 100 µL of serum using microspin columns (QIAamp Blood kit, Qiagen KK, Tokyo, Japan). Purified DNA was resuspended in 80 µL of distilled water. PCR was carried out by the same protocol as described previously[15]. Nucleotide sequences of the amplified products were determined directly by the dideoxy method, using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit with a fluorescent 3100 DNA Sequencer (Applied Biosystems, Foster City, CA, USA).
Real-time detection polymerase chain reaction (RTD-PCR)
serum HBV DNA was quantitatively detected by RTD-PCR based on Taqman chemistry as reported previously[16]. Amplification was performed using primers corresponding to conserved sequences of the surface region. A 10-µL aliquot of DNA solution was used for RTD-PCR. A portion of the HBV surface region was amplified using primers: a forward primer HBSF2 (5’-CTTCATCCTGCTGCTATGCCT-3’, nucleotide position (nt) 406-426) and a reverse primer HBSR2 (5’-AAAGCCCAGGATGGGAT-3’, nt 646-627). A taqman probe was designed as HBSP2 (5’-ATGTTGCCCTTTGTCCTCCTCTAATTCCAG-3’, nt 461-488), with an additional G at the 3’-end of HBSP2 in the original method, the detection limit of this system was as low as five DNA copies/assay, and linear standard curve was obtained from 5 to 106 DNA copies/assay.
Phylogenetic analysis
Complete sequences of 21 HBV isolates were aligned with the CLUSTAL W software program[17], and the alignment was confirmed by visual inspection. Genetic distances were estimated by the six-parameter method, and phylogenetic trees were constructed by the neighbor-joining method using ODEN program of the National Institutes of Genetics (Mishima, Japan)[18]. To confirm the reliability of the phylogenetic trees, bootstrap resampling tests were performed 1 000 times.
RESULTS
HBV genotypes
Distribution of HBV genotypes among 21 HBV carriers in the Republic of Benin was 20/21 (95.2%) for HBV/E, and 1/21 (4.8%) for HBV/A. HBV/B, C, D, F, and G were not found. HBV/E was the predominant genotype in the Republic of Benin.
Clinical characteristics of asymptomatic HBV carriers in the Republic of Benin
HBeAg was positive for 4/21 (19.0%) subjects, and anti-HBe was positive for 17/21 (81.0%) subjects. The titers of four HBeAg-positive subjects were beyond the upper detection limit. In order to clarify the clinical characteristics of asymptomatic HBV carriers in this country, the clinical and laboratory data between HBeAg-positive patients and anti-HBe-positive patients were compared (Table 1).
Table 1.
Comparison of clinical characteristics between HBeAg-positive and anti-HBe-positive subjects
| HBeAg-positive (n = 4) | Anti-HBe-positive (n = 17) | P | |
| Age (yr) | 21.25 ± 2.21 | 23.5 ± 7.421 | NS2 |
| Sex (M/F) | 2/2 | 1/151 | NS3 |
| Genotype (A/E) | 0/4 | 1/16 | NS3 |
| AST (IU/L) | 25.8 ± 8.3 | 18.0 ± 5.4 | NS2 |
| ALT (IU/L) | 11.5 ± 10.3 | 5.7 ± 1.5 | NS2 |
| T-Bil (mg/dL) | 0.50 ± 0.35 | 0.57 ± 0.41 | NS2 |
| HBV DNA | 7.76 ± 0.29 | < 2.6 | < 0.00052 |
| (log (copies/mL) |
Sex and age unknown in one subject.
P values were calculated by Welch’s t-test.
P values were calculated by Fisher’s exact test. AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-Bil, total bilirubin.
The mean age of the HBeAg-positive group was not lower than that of the anti-HBe-positive group. As a matter of course, the difference in the mean HBV DNA titer between the two groups was statistically significant (P<0.0005). HBV DNA titers in anti-HBe-positive subjects were quite low. To examine the correlation between age and HBeAg/anti-HBe status in detail, the age-specific prevalence of the HBeAg/anti-HBe status in 19 HBV/E subjects was analyzed. Seroconversion (SC) during the late teens occurred in 7 of 19 subjects (36.8%, Figure 1). This was the reason why the comparison of age between the two groups was not statistically significant.
Figure 1.
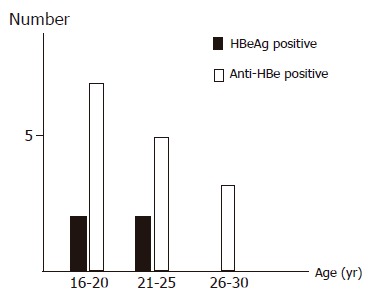
Age-specific prevalence of HBeAg/anti-HBe status in 19 HBV genotype E strains.
Phylogenetic analysis of four strains
HBV full genomes of four HBeAg-positive patients were determined to have a nucleotide length of 3 212 bp. Phylogenetic analysis of the complete genome sequences of these four strains compared to those of 22 HBV strains from the DDBJ/GenBank/EMBL database showed that four strains clustered with five other HBV/E strains (accession numbers: X75657, X75664[2]; and AB032431[19]; AB091255, AB091256[11]) (Figure 2). The nucleotide homology within the HBV/E cluster ranged from 96.7% to 99.2%. In addition, the phylogenetic analysis of four ORFs showed no recombination of HBV/E strains in the Republic of Benin (Figure 3). Genetic diversity among HBV/E strains was very low compared to the other genotypes, excluding HBV/G. The nucleotide sequence data reported in this article will appear in the DDBJ/GenBank/EMBL nucleotide sequence databases with the accession numbers AB201287-90.
Figure 2.
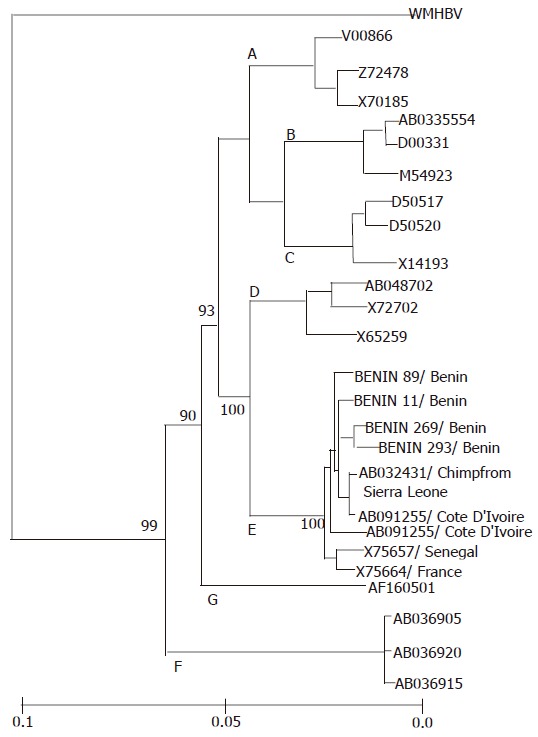
Phylogenetic tree constructed on complete nucleotide sequences of 26 HBV isolates. The nine HBV genotype E isolates were compared along with 16 HBV isolates representative of the other six genotypes (A–D, F and G), along with woolly monkey hepatitis virus (WMHBV) as an outgroup. The four HBV genotype E isolates determined in this study are indicated in boldface, and the other 22 HBV isolates are specified by accession numbers. The country of origin is indicated after the slash for each HBV isolate of genotype E.
Figure 3.
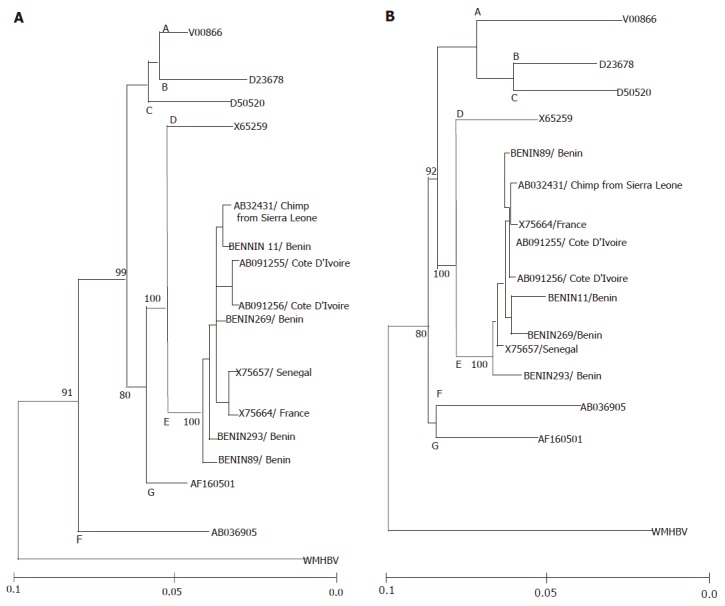
Phylogenetic trees representing the large S gene (A), and precore region plus core gene (B) along with the strains of the other six genotypes and WMHBV.
Characteristics of nucleotide substitutions in BCP and precore region
In order to find out the HBV/E-specific nucleotide substitution associated with HBe protein production, the BCP to the precore region of nine HBV/E strains and the strains of six genotypes were compared (Figure 4). The representative nucleotide sequences of HBV/A (Aa- Ae), B, C, D, F, and G were determined by aligning five sequences of each genotype, and the consensus nucleotide was deduced, if the identical nucleotide was detected in 60% or more of the sequences. In the BCP, the nucleotide substitution at A1757 was observed in all the strains of HBV/E. This substitution was not found in HBV/A-C, HBV/F, and HBV/G, but was found in more than half of HBV/D[20]. BCP double mutation (T1762/A1764) was not found in the HBV/E strains; however, T1772C substitution was found in all the HBV/E strains as well as in HBV/B, C and F. Other sequences in the BCP were conserved irrespective of genotypes, except for the above mutations. One HBV/E isolate had T1809 substitution prior to the start of precore ORF, which is recognized as the Kozak sequence frequently found in HBV/Aa. Precore stop mutation (A1896) was not found in the HBV/E.
Figure 4.
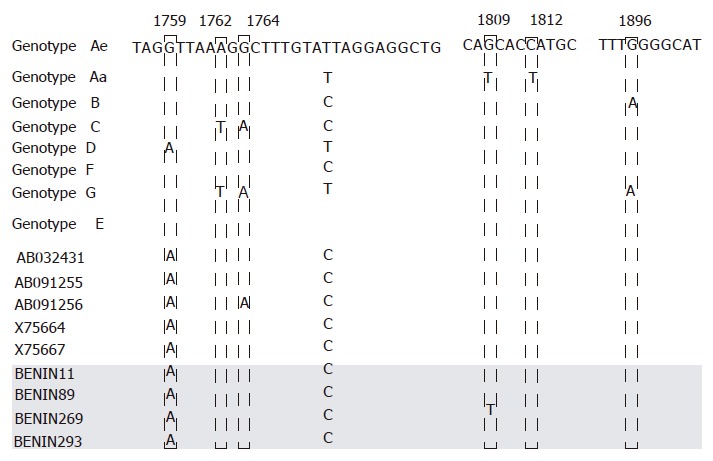
Nucleotide sequences constituting parts of the BCP to the precore of nine HBV genotype E isolates with the consensus sequences of the other six genotypes. The four Benin isolates determined in this study are shaded in gray.
DISCUSSION
In this study, the distribution of HBV genotypes among blood donors in the Republic of Benin was analyzed for the first time. In Africa, HBV/D is dominant in the Northern region[21], with HBV/A found in South Africa. HBV/E is the major genotype in the Republic of Benin. This agrees with previous reports that HBV/E is prevalent mainly in West Africa[2,11,22]. In addition, the distribution of HBV/E is restricted to West Africa, unlike other genotypes. Though slaves have migrated from West Africa to North America, HBV/E is not found in USA, suggesting that genotype E emerged from the mid to late 19th century[22,23].
East Asia and Sub-Saharan Africa are the two most HBV endemic areas; however, clinical differences have been shown between the two areas. Vertical transmission is the main route in Asia, whereas horizontal transmission is the main route in Africa, and SC occurs much earlier in Africa than in Asia[24,25]. It is important to examine the clinical characteristics of asymptomatic HBV carriers in Benin, which reflect the characteristics of HBV/E, because there are only a few reports concerning the clinical characteristics of HBV/E. As shown in Figure 1, SC is thought to occur at a young age in subjects with HBV/E in Benin. These data might explain one of the reasons why vertical transmission does not occur in West Africa. Recently, the difference in SC between HBV/B and HBV/C in Taiwan was reported[5]. SC occurs a decade earlier in HBV/B patients[26,27] and the mean SC age in HBV/B is approximately 30 years. In comparison with HBV/B, the mean SC age in HBV/E occurs even earlier. These data lead us to speculate that HBV/E has a better prognosis than other genotypes in terms of chronic liver disease.
Regarding hepatocellular carcinoma (HCC), a report from Gambia, where HBV/E is the most prevalent, revealed that chronic HBV infection is associated with HCC development at a younger age[28]. Infection with HBV/B is associated with HCC development at a young age in Taiwan[5]. In this sense, HBV/E in West Africa is clinically more similar to HBV/B than HBV/C, and virologically, HBV/E and HBV/B have a high frequency of a precore mutation, G1896A, in common[11,29]. Further information about the clinical characteristics of HBV genotypes might reveal the reason why the age of SC and the age-related HCC incidence differ among genotypes.
So far, only five complete genomes of HBV/E (one from a chimpanzee) have been reported. By adding the four complete genomes reported here, phylogenetic analyses of the full genome and ORFs show much more clearly that HBV/E has a low genetic diversity, as previously reported[11,22,23]. Although subtypes of HBV/B, which have recombination in the BCP to the core region[7] and subtypes of HBV/A[8,30] have been reported, the analysis of nine HBV/E genomes shows neither the possibility of subtypes nor the possibility of recombination.
As mentioned above, HBV/A is divided into two subtypes: HBV/Aa, ‘Aa’ indicates Asia and Africa, and HBV/Ae, ‘Ae’ indicates Europe[8,30]. Subtype HBV/Aa has subtype-specific substitutions just before precore start codons, known as the “Kozak sequence”. Substitutions reduce the HBeAg expression[10,31]. Hence, genotype- and subtype-specific nucleotide substitutions in the BCP region are crucial, not only in viral replication but also in HBe SC. In this study, we identified the mutation in the BCP, A1757, in HBV/E. Other genotypes have G1757 except for HBV/D, in which A1757 is observed in about 70%[20]. BCP has been identified as a sequence that regulates both precore and pregenomic messages[32]. The core promoter A1762T and G1764A double mutation is known to downregulate precore mRNA but does not seriously affect pregenome mRNA[33]. The double mutation (1762/1764) occurs over time, as the viral load and HBeAg/anti-HBe status change, and the sequences around the double mutations are conserved irrespective of the genotype so far. However, HBV/E seems to possess 1757A from the early stage of the infection, similar to Kozak sequence substitutions in genotype Aa. Thus, the different functions in relation to nuclear receptor binding between 1757G and 1757A was shown in an in vitro study, when the mechanism of suppression of HBV precore RNA transcription by core promoter double mutation was sought. The double mutation in 1762 and 1764 changes the nucleotide sequences of the nuclear-factor binding site of BCP. When the mutation occurs, the nuclear factor known as COUP TF I cannot bind to BCP. Instead, a different nuclear factor, HNF1, binds, and this alters the efficiency of precore and core mRNA transcription. The artificial mutation was created with the intention to create a sequence that neither COUP TF I nor HNF-1 can bind. This mutation is G1757A, and the mutation abolishes both the nuclear factors (COUP TFI and HNF1) binding to BCP in the in vitro experiment[34]. This unique mutation and its alteration of nuclear factor binding capacity to the BCP region might influence the clinical characteristics of HBV/E. Further studies, including in vitro studies, are expected.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69(Pt 10):2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 2.Norder H, Couroucé AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489–503. doi: 10.1006/viro.1994.1060. [DOI] [PubMed] [Google Scholar]
- 3.Norder H, Couroucé AM, Magnius LO. Complete nucleotide sequences of six hepatitis B viral genomes encoding the surface antigen subtypes ayw4, adw4q-, and adrq- and their phylogenetic classification. Arch Virol Suppl. 1993;8:189–199. doi: 10.1007/978-3-7091-9312-9_19. [DOI] [PubMed] [Google Scholar]
- 4.Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E, et al. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology. 2001;34:590–594. doi: 10.1053/jhep.2001.27221. [DOI] [PubMed] [Google Scholar]
- 5.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554–559. doi: 10.1016/s0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 6.Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Ueda R, et al. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J Virol. 2002;76:5985–5992. doi: 10.1128/JVI.76.12.5985-5992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Gish RG, et al. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology. 2003;124:925–932. doi: 10.1053/gast.2003.50140. [DOI] [PubMed] [Google Scholar]
- 8.Sugauchi F, Kumada H, Acharya SA, Shrestha SM, Gamutan MT, Khan M, Gish RG, Tanaka Y, Kato T, Orito E, et al. Epidemiological and sequence differences between two subtypes (Ae and Aa) of hepatitis B virus genotype A. J Gen Virol. 2004;85:811–820. doi: 10.1099/vir.0.79811-0. [DOI] [PubMed] [Google Scholar]
- 9.Buckwold VE, Xu Z, Chen M, Yen TS, Ou JH. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J Virol. 1996;70:5845–5851. doi: 10.1128/jvi.70.9.5845-5851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn SH, Kramvis A, Kawai S, Spangenberg H, Li J, Kimbi G, Kew MC, Wands J, Tong S. Sequence variation upstream of precore translation initiation codon reduces hepatitis B virus e antigen production. Gastroenterology. 2003;125:1370–1378. doi: 10.1016/j.gastro.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Sugauchi F, Orito E, Kato H, Usuda S, Siransy L, Arita I, Sakamoto Y, Yoshihara N, El-Gohary A, et al. Distribution of hepatitis B virus (HBV) genotypes among HBV carriers in the Cote d'Ivoire: complete genome sequence and phylogenetic relatedness of HBV genotype E. J Med Virol. 2003;69:459–465. doi: 10.1002/jmv.10331. [DOI] [PubMed] [Google Scholar]
- 12.Usuda S, Okamoto H, Iwanari H, Baba K, Tsuda F, Miyakawa Y, Mayumi M. Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods. 1999;80:97–112. doi: 10.1016/s0166-0934(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 13.Usuda S, Okamoto H, Tanaka T, Kidd-Ljunggren K, Holland PV, Miyakawa Y, Mayumi M. Differentiation of hepatitis B virus genotypes D and E by ELISA using monoclonal antibodies to epitopes on the preS2-region product. J Virol Methods. 2000;87:81–89. doi: 10.1016/s0166-0934(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 14.Mizokami M, Nakano T, Orito E, Tanaka Y, Sakugawa H, Mukaide M, Robertson BH. Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett. 1999;450:66–71. doi: 10.1016/s0014-5793(99)00471-8. [DOI] [PubMed] [Google Scholar]
- 15.Sugauchi F, Mizokami M, Orito E, Ohno T, Kato H, Suzuki S, Kimura Y, Ueda R, Butterworth LA, Cooksley WG. A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: complete genome sequence and phylogenetic relatedness. J Gen Virol. 2001;82:883–892. doi: 10.1099/0022-1317-82-4-883. [DOI] [PubMed] [Google Scholar]
- 16.Abe A, Inoue K, Tanaka T, Kato J, Kajiyama N, Kawaguchi R, Tanaka S, Yoshiba M, Kohara M. Quantitation of hepatitis B virus genomic DNA by real-time detection PCR. J Clin Microbiol. 1999;37:2899–2903. doi: 10.1128/jcm.37.9.2899-2903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ina Y. ODEN: a program package for molecular evolutionary analysis and database search of DNA and amino acid sequences. Comput Appl Biosci. 1994;10:11–12. doi: 10.1093/bioinformatics/10.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Brotman B, Usuda S, Mishiro S, Prince AM. Full-genome sequence analyses of hepatitis B virus (HBV) strains recovered from chimpanzees infected in the wild: implications for an origin of HBV. Virology. 2000;267:58–64. doi: 10.1006/viro.1999.0102. [DOI] [PubMed] [Google Scholar]
- 20.Kidd-Ljunggren K, Oberg M, Kidd AH. Hepatitis B virus X gene 1751 to 1764 mutations: implications for HBeAg status and disease. J Gen Virol. 1997;78(Pt 6):1469–1478. doi: 10.1099/0022-1317-78-6-1469. [DOI] [PubMed] [Google Scholar]
- 21.Saudy N, Sugauchi F, Tanaka Y, Suzuki S, Aal AA, Zaid MA, Agha S, Mizokami M. Genotypes and phylogenetic characterization of hepatitis B and delta viruses in Egypt. J Med Virol. 2003;70:529–536. doi: 10.1002/jmv.10427. [DOI] [PubMed] [Google Scholar]
- 22.Odemuyiwa SO, Mulders MN, Oyedele OI, Ola SO, Odaibo GN, Olaleye DO, Muller CP. Phylogenetic analysis of new hepatitis B virus isolates from Nigeria supports endemicity of genotype E in West Africa. J Med Virol. 2001;65:463–469. [PubMed] [Google Scholar]
- 23.Mulders MN, Venard V, Njayou M, Edorh AP, Bola Oyefolu AO, Kehinde MO, Muyembe Tamfum JJ, Nebie YK, Maiga I, Ammerlaan W, et al. Low genetic diversity despite hyperendemicity of hepatitis B virus genotype E throughout West Africa. J Infect Dis. 2004;190:400–408. doi: 10.1086/421502. [DOI] [PubMed] [Google Scholar]
- 24.Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–774. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 25.Botha JF, Ritchie MJ, Dusheiko GM, Mouton HW, Kew MC. Hepatitis B virus carrier state in black children in Ovamboland: role of perinatal and horizontal infection. Lancet. 1984;1:1210–1212. doi: 10.1016/s0140-6736(84)91694-5. [DOI] [PubMed] [Google Scholar]
- 26.Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology. 2002;122:1756–1762. doi: 10.1053/gast.2002.33588. [DOI] [PubMed] [Google Scholar]
- 27.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J Med Virol. 2004;72:363–369. doi: 10.1002/jmv.10534. [DOI] [PubMed] [Google Scholar]
- 28.Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, Hall AJ, Whittle H, Montesano R. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211–219. doi: 10.1002/hep.20027. [DOI] [PubMed] [Google Scholar]
- 29.Orito E, Mizokami M, Sakugawa H, Michitaka K, Ishikawa K, Ichida T, Okanoue T, Yotsuyanagi H, Iino S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology. 2001;33:218–223. doi: 10.1053/jhep.2001.20532. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa I, Tanaka Y, Kramvis A, Kato T, Sugauchi F, Acharya SK, Orito E, Ueda R, Kew MC, Mizokami M. Novel hepatitis B virus genotype a subtyping assay that distinguishes subtype Aa from Ae and its application in epidemiological studies. J Virol. 2004;78:7575–7581. doi: 10.1128/JVI.78.14.7575-7581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka Y, Hasegawa I, Kato T, Orito E, Hirashima N, Acharya SK, Gish RG, Kramvis A, Kew MC, Yoshihara N, et al. A case-control study for differences among hepatitis B virus infections of genotypes A (subtypes Aa and Ae) and D. Hepatology. 2004;40:747–755. doi: 10.1002/hep.20365. [DOI] [PubMed] [Google Scholar]
- 32.Yuh CH, Chang YL, Ting LP. Transcriptional regulation of precore and pregenomic RNAs of hepatitis B virus. J Virol. 1992;66:4073–4084. doi: 10.1128/jvi.66.7.4073-4084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto H, Tsuda F, Akahane Y, Sugai Y, Yoshiba M, Moriyama K, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J Virol. 1994;68:8102–8110. doi: 10.1128/jvi.68.12.8102-8110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Buckwold VE, Hon MW, Ou JH. Mechanism of suppression of hepatitis B virus precore RNA transcription by a frequent double mutation. J Virol. 1999;73:1239–1244. doi: 10.1128/jvi.73.2.1239-1244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


