Abstract
AIM: To examine the serum from black African patients with acute hepatitis B to ascertain if integrants of viral DNA can be detected in fragments of cellular DNA leaking from damaged hepatocytes into the circulation.
METHODS: DNA was extracted from the sera of five patients with uncomplicated acute hepatitis B and one with fulminant disease. Two subgenomic PCRs designed to amplify the complete genome of HBV were used and the resulting amplicons were cloned and sequenced.
RESULTS: HBV and chromosomal DNA were amplified from the sera of all the patients. In one patient with uncomplicated disease, HBV DNA was integrated into host chromosome 7 q11.23 in the WBSCR1 gene. The viral DNA comprised 200 nucleotides covering the S and X genes in opposite orientation, with a 1 169 nucleotide deletion. The right virus/host junction was situated at nucleotide 1 774 in the cohesive overlap region of the viral genome, at a preferred topoisomerase I cleavage motif. The chromosomal DNA was not rearranged. The patient made a full recovery and seroconverted to anti-HBs- and anti-HBe-positivity. Neither HBV nor chromosomal DNA could be amplified from his serum at that time.
CONCLUSION: Integration of viral DNA into chromosomal DNA may occur rarely during acute hepatitis B and, with clonal propagation of the integrant, might play a role in hepatocarcinogenesis.
Keywords: Hepatocellular, Chronic hepatitis B infection, Clonal propagation
INTRODUCTION
Hepatitis B Virus (HBV), the prototype member of the family Hepadnaviridae, belongs to the genus Orthohepadnavirus. Compelling evidence supports a causal role for chronic HBV infection in hepatocellular carcinoma (HCC)[1,2]. Less certain is the pathogenesis of HBV-induced HCC. Direct and indirect carcinogenic mechanisms have been implicated, the latter mainly by the frequency with which this tumor co-exists with cirrhosis[3,4]. Evidence for a direct effect is provided by the development of HCC in an otherwise normal liver (in as many as 40% or more of black African patients[5]) and by observations in a variety of animal models. The latter include the development of HCC in the absence of cirrhosis, both in animals that were chronically infected with other members of the family Hepadnaviridae[6-8] and in transgenic mice with HBV DNA incorporated into the germline[9]. Further support comes from the finding of integrated hepadnaviral DNA in cellular DNA in HCCs in animal and human hosts[10-14]. The latter does not, however, prove that insertion of viral DNA is essential for hepatocarcinogenesis. If clonal expansion of integrated HBV DNA proves to be a pivotal step in hepatocarcinogenesis, the timing of integration becomes important. Because HCC typically develops in patients chronically infected with HBV, it has been assumed that DNA insertion occurs at some point during persistent infection. Integration during the short period of viral replication in acute hepatitis B had not been conclusively demonstrated[15-17]. However, in a recent study, integration of HBV DNA was demonstrated in 3 of 19 liver specimens from patients with acute hepatitis B, one of whom had subacute fulminant hepatitis[18]. Moreover, a study in acutely infected ducks showed that insertion of duck HBV (DHBV) DNA into host DNA may occur as early as d 6 after the infection[12]. HCC does not, however, develop in ducks chronically infected with DHBV. The time at which integration occurred was studied in the liver tissue and a similar study could not, for obvious ethical reasons, be performed in human beings with acute hepatitis B.
In a previous study, primers used to amplify the complete genome of HBV were found to amplify chromosomal DNA also. Taking advantage of this phenomenon and the leakage into the bloodstream of fragments of cellular DNA from damaged hepatocytes during acute liver injury[19,20], we examined the serum of patients with acute hepatitis B for integrated HBV DNA.
MATERIALS AND METHODS
Patients studied
Blood samples were drawn, with informed consent, from six South African blacks suffering from acute hepatitis B during the peak of the disease or shortly thereafter. Permission to undertake this study was given by the Human Ethics Committee of the University of Witwatersrand. In five patients, the illness was uncomplicated, and all recovered completely; the remaining patient had fulminant hepatitis B and died of liver failure. Alanine aminotransferase levels in the early stages of the illness ranged between 799 and 4 747 IU/L. The patients varied in age from 18 to 32 years: 3 were males and 3 females. Serum known to be HBsAg-positive and HBsAg-negative was used as positive- and negative-extraction controls, respectively. The sera were stored at -70 °C until analyzed.
DNA extraction, amplification, cloning, and sequencing
Total DNA was extracted from the sera using the QIAamp DNA Mini Kit (QIAGEN GmbH, Hilden, Germany), according to the manufacturer’s instructions. Two subgenomic PCRs designed to amplify the complete genome of HBV were used and the resulting amplicons were cloned and sequenced. A modification of the method of Takahashi K et al[21] was used, which involved the amplification of two overlapping fragments of HBV, fragment A (1.35 kb) and fragment B (2.2 kb). However, this amplification designed for amplification of viral DNA also fortuitously, randomly recognized chromosomal DNA, leading to the amplification of genomic DNA in addition to HBV DNA. The sequence of the primers is given in Table 1. The reaction mix for the amplification consisted of 2.25 μL 10× Ex Taq buffer with 20 mmol/L MgCl2 set as subscript, 2 μL 2.5 mmol/L dNTP mix, 1.25 μL each of the appropriate primers (Table 1), 2.5 μL DNA, made up to 22.5 μL with water (SABAX water for injection, Adcock Ingram, Johannesburg, South Africa). The enzyme mix was made up of 1.875 μL water, 0.25 μL 10× Ex Taq and 0.375 μL TaKaRa Ex Taq polymerase [TaKaRa Biotechnology (Dalian) Co., Ltd., Shiga, Japan]. The 22.5μL reaction mix was preheated to 94 °C for 2 min and 2.25 μL TaKaRa Ex Taq enzyme mix was added at the first annealing step. This was followed by 40 cycles of amplification with the cycling profile shown in Table 1. Both HBV-positive and HBV-negative controls were included. The latter consisted of water instead of DNA in the PCR mixture. To avoid cross-contamination and false-positive results, the precautions and procedures suggested by Kwok and Higuchi[22] were strictly adhered to. DNA extraction, PCR amplification, and electrophoresis were performed in physically separated venues.
Table 1.
Oligonucleotide primers and PCR cycling profiles used in the study
| Fragment | Primer | Position1 | Sequence | Denaturation | Annealing | Extension | Size2 |
| A | 455(+) | 455-474 | 5’-CAAGGTATGTTGCCCGTTTG-3 | 1 345 | |||
| 1 800(–) | 1 800-1 773 | 5’-AGACCAATTTATGCCTACAGCCTCCTA-3’ | 94 °C 30 s | 62 °C 30 s | 72 °C 90 s | ||
| B | 1 687(+) | 1 687-1 708 | 5’-CGACCGACCTTGAGGCATAC-3’ | ||||
| 685(-) | 704-685 | 5’-CGAACCACTGAACAAATGGC-3’ | 94 °C 30 s | 63 °C 30 s | 72 °C 30 S | 2 198 |
(+) sense (–) anti-sense.
Denotes the nucleotide position of HBV adw genome (GenBank accession no. V00866) where the EcoRI cleavage site is position 12. Size of the amplicons in base pairs.
Amplicons were cloned into a pPCR-ScriptTM Amp SK+ vector (Stratagene, La Jolla, CA, USA) according to the protocol provided by the manufacturer. The positive clones containing either inserts of the correct or shorter size were prepared for direct sequencing using the BigDye Terminator v3.0 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, USA) and sequenced on a 377 DNA automated sequencer (Applied Biosystems, Inc.) using primers T3 (5’-AATTAACCCTCACTAAAGGG-3’) and T7 (5’-GTAATACGACTCACTATAGGGC-3’). All sequences were analyzed in both forward and reverse directions.
RESULTS
In addition to yielding amplicons of the expected size, i.e., 1.35 kb for fragment A and 2.2 kb for fragment B, the two subgenomic PCRs designed to amplify the complete genome of HBV gave rise to smaller amplicons ranging in size from 100 to 750 bp. These amplicons were successfully cloned into a pPCR-ScriptTM Amp SK+ vector (Figure 1) and a number of clones from each of the six patients were sequenced (Table 2).
Figure 1.
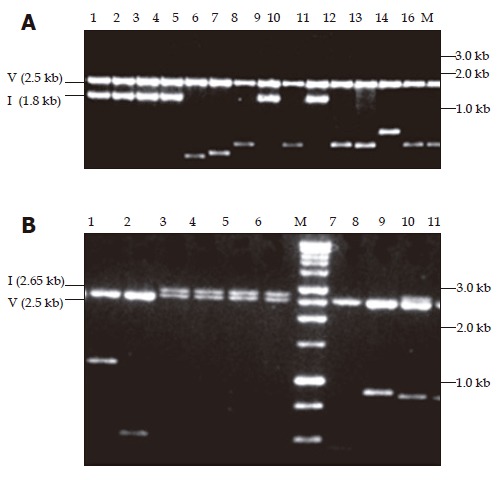
Ethidium bromide stained with 1% agarose gel showing pPCR-Script Amp SK(+) plasmid containing amplicons restricted with PvuII. In panel A, the fragments cloned were amplified with primers 455(+) and 1 800(–). Lanes 1-4, 8, and 10 show the expected size insert of -1.8 kb for fragment A amplicon (1.3 kb) whereas lanes 5-7, 9, and 11-16 show shorter inserts ranging in size 0.6-1.1 kb. In panel B, the fragments cloned were amplified with primers 1 687(+) and 685(–). Lanes 3-6 and 9 show the expected size insert of -2.65 kb for fragment B amplicon (2.2 kb), lane 7 is vector alone and lanes 1, 2, 8-11 show shorter inserts ranging in size 0.5-1.2 kb. M: Promega 1 kb molecular weight marker, V: vector, I: insert of expected size (A and B).
Table 2.
Summary of cloning and sequencing results
| Patient details | PCR fragment1 |
Number of clones |
Chromosome number and length | ||||||
| No. | Sex | Age | Sequenced | With vector | With HBV | WithHBV/ chromosomal | With chromosomal | ||
| (yr2) | DNA | DNA only | DNA | DNA only | |||||
| 355 | F | 18 | B | 5 | 1 | 3 | 0 | 1 | 7 (117 bp) |
| 8225 | M | 23 | A | 3 | 0 | 2 | 0 | 1 | 1 (278 bp) |
| B | 4 | 0 | 4 | 0 | 0 | – | |||
| 28 | M | 26 | A | 4 | 0 | 2 | 0 | 2 | 4 (512 bp) |
| 16 (140 bp) | |||||||||
| B | 4 | 0 | 4 | 0 | 0 | – | |||
| 4038 | F | 27 | A | 6 | 0 | 5 | 0 | 1 | 1 (97 bp) |
| B | 4 | 0 | 4 | 0 | 0 | – | |||
| 52833 | F | 32 | A | 12 | 2 | 6 | 0 | 4 | 4 (456 bp) |
| 10 (235 bp) | |||||||||
| 10 (330 bp) | |||||||||
| 2 (422 bp) | |||||||||
| B | 4 | 0 | 4 | 0 | 0 | – | |||
| 962 | M | 27 | A | 8 | 0 | 4 | 1 | 3 | 1 (300 bp) |
| 14 (410 bp) | |||||||||
| 17 (410 bp) | |||||||||
| B | 5 | 0 | 5 | 0 | 0 | – | |||
Fragment A amplified with primers 455 (+) and 1 800 (–); fragment B amplified with primers 1 687 (+) and 685 (–).
yr, years; bp, base pairs; 3Patient with fulminant hepatitis.
The majority of the clones contained HBV DNA only and the detailed analysis of these sequences will be published elsewhere. Fortuitously, the primers designed for the amplification of viral DNA amplified Chromosomal DNA was amplified in 5 patients using primers designed to amplify fragment A (Table 1) and in the remaining patient with primers designed to amplify fragment B. As shown in Table 2, the chromosome number and the lengths of the fragments varied, although in three cases the fragment amplified corresponded to the sequences from chromosome 1.
In one patient with uncomplicated hepatitis B, HBV DNA was found to be integrated into host chromosome 7q11.23 in the WBSCR1 gene using primers for fragment A. WBSCR1 corresponds to the eukaryotic initiation factor 4H identified in rabbits[23,24] and is within the region encompassing the elastin gene. This region is commonly deleted in patients with Williams–Beuren syndrome, giving rise to loss of heterozygosity[24,25]. The viral DNA comprised 200 nucleotides covering the S and X genes in opposite orientation, with an 1 169 nucleotide deletion (Figure 2). The right virus/host junction was situated at nucleotide 1 774 in the cohesive overlap region of the viral genome, at a preferred topoisomerase I cleavage motif (TAA). The left virus/host junction of the integrant was not identified and, therefore, the length of the integrant could not be determined. Following phylogenetic analysis of the 175 nucleotides of the S region (nucleotides 4-630), the sequence clustered with subgenotype A1 of genotype A, which is the predominant genotype in southern Africa[26-28] . When the 175 nucleotides of the insert covering the S region were translated, starting at nucleotide 3 of the fragment, the amino acid at position 122 was K, which is characteristic of serotype ad. Amino acid 160, which encodes for the w/r determinant, was not included in this fragment and, therefore, we cannot conclusively determine the serotype, although we can probably assume that the determinant is w because the predominant serotype in southern Africa is adw. The nucleotide sequence of the chromosomal DNA (262 bp) was completely conserved and not rearranged. The 3’ end was amplified by primer 1 800 (-) (Table 1). A BLAST search[29] revealed that in addition to binding to chromosome 7, the primer 1 800 (–) (Table 1) has identical sequences on human chromosomes 2, 3, 12, 13, 20, 22, and Y. The sequence of the integrated HBV DNA and flanking the chromosomal DNA has been deposited in GenBank (accession no. AY223548).
Figure 2.
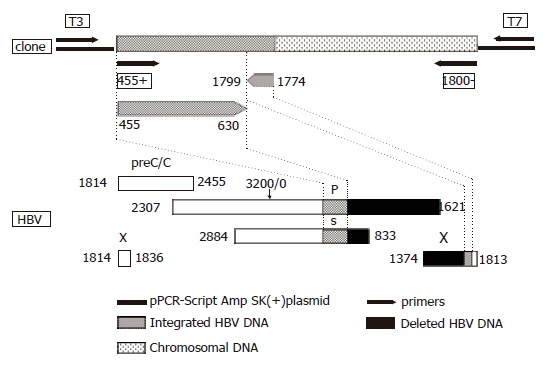
Schematic representation of the HBV DNA integrant amplified from the serum of acute hepatitis patient (#0962) using primers 455 (+) and 1 800 (–) and cloned into pPCR-Script Amp SK (+) plasmid relative to the HBV genome. The lower part of the figure represents the genetic organization of the four open reading frames of HBV, preC/C: precore/core, P: polymerase gene, S: surface gene and X: X gene. Numbering according to nucleotide position of HBV GenBank accession no. V00866 where the EcoRI cleavage site is position 1.
The patient with the integrant was a 27-year-old Xhosa gold-mine laborer, who was previously healthy and presented with the typical clinical features of uncomplicated acute viral hepatitis. When he was first tested, the tests of liver function showed a serum bilirubin concentration of 257 μmol/L (conjugated bilirubin 228 μmol/L), alkaline phosphatase 91 IU/L, γ-glutamyl transferase 94 IU/L, alanine aminotransferase 3 238 IU/L, aspartate aminotransferase 2 781 IU/L, and albumin 4 g/L. HBV surface antigen, e antigen, and IgM core antibody were present in the serum. The patient made an uneventful and complete recovery, with return of biochemical tests to normal and disappearance of surface and e antigens and the appearance of IgG core antibody and antibody to surface and e antigens in his serum. HBV DNA could not be detected in the serum. after he had recovered and cloning and whole genome sequencing were not attempted at that time.
DISCUSSION
Unlike retroviruses, in which insertion of viral DNA into host DNA is integral to the replication cycle, integration does not occur during normal hepadnaviral replication. Integration of hepadnaviral DNA may, however, take place as a result of an illegitimate recombination mechanism mediated by cellular enzymes[12,30]. Clonally propagated integrations have been detected in the majority of HCCs that develop in the presence of chronic hepadnaviral infection[6-10] and have been incriminated in the hepatocarcinogenesis that often complicates persistent infection. HBV DNA insertions are found at random sites in human cellular DNA, although some chromosomes are affected more often than others[31]. In the present study, we detected an integrant in chromosome 7 of an acute hepatitis B patient.
Although we cannot exclude the possibility that the HBV integrant amplified was the result of a PCR artefact, there are a number of observations that argue against this interpretation and support our conviction that this integrant was authentic.
Firstly, the site of integration in chromosome 7q11.23 is within the WBSCR1 gene. This gene corresponds to the eukaryotic initiation factor 4H identified in rabbits[23,24] and the integrant is within the region commonly deleted in patients with Williams-Beuren syndrome, giving rise to loss of heterozygosity[24,25]. Moreover, the WBSCR1 gene contains a high abundance of Alu repeats[24], repetitive elements that have been shown to be the preferred sites for recombination and for HBV DNA insertion[32]. Analogously the sites of integration in the three patients with acute hepatitis B reported by Murakami and coworkers[18] were the intronic sequence of the tumor necrosis factor-induced protein gene and a repetitive sequence in the two patients with a single integrant; and the intronic sequence of a hypothetical protein gene (LOC 169443) and a repetitive sequence in the patient with two integrants.
Secondly, the region of HBV integrated (position 1 774-1 799 from EcoRI site) is a highly preferred site of integration of hepadnaviral DNA, that is, close to the 11-bp direct repeat sequence DR1 (position 1 824-1 834) and within the cohesive overlap region between DR1 and DR2[33].
Thirdly, the one viral DNA/chromosomal DNA junction in the single integrant detected in our patient with acute hepatitis B was located at nucleotide 1 774 of the viral genome, a highly preferred topoisomerase I cleavage motif. Linear hepadnaviral DNA is the primary substrate for integration[12,30], and the circular DNA must first be linearized before integration can take place. Insertion is then accomplished by strand invasion of cellular DNA. Thus, integration of viral DNA into chromosomal DNA requires that the linear DNA be imported into the nucleus of the infected cell[30,34,35]. Wang and Rogler[30] have produced experimental evidence for a key role for the cellular enzyme topoisomerase I in the illegitimate recombination between hepadnaviral and host DNA. A role for this enzyme in illegitimate recombination is supported both by the finding of highly preferred topoisomerase I cleavage motifs in the immediate vicinity of almost all crossover sites in mammalian DNA, and by the observation that integrants in WHV-induced HCC in woodchucks occurred at preferred topoisomerase I cleavage sites[30].Fourthly, the integrated HBV DNA covered the S and X regions, the open reading frames most commonly integrated into chromosomal DNA[36].
Finally, the integrant detected in the serum of our patient with acute hepatitis B conformed in general to the integrants described in HCC developing in the presence of chronic HBV infection[31]. It contained a long deletion (1 169 nucleotides in length), and the viral DNA between the right end of the deletion and the right virus/chromosome junction was inverted X sequence (Figure 1). One or more deletions or other more complex rearrangements are invariably found in inserted hepadnaviral DNA[10,31], indicating that specific mechanisms for integration of functional viral DNA into chromosomal DNA are not encoded by the virus. Simple microdeletions are most common, probably because the single-stranded ends of viral DNA resulting from topoisomerase I cleavage are sensitive to cellular nucleases. The larger deletions and complex rearrangements of the viral DNA are generally believed to occur after the integration[37], although WHV with extensive rearrangements of DNA has been described in hepatocyte nuclei in woodchucks[38] and there is some evidence in these animals that DNA rearranged in this way may be preferentially integrated[39]. Similarly, Yaginuma et al[40] observed features of HBV integration to be common to HCC and patients with chronic active hepatitis and an analogous integrant to the one detected in this study has been described in a 9-year-old child with HCC[41].
Integration of hepadnaviral DNA into host DNA is normally rare, perhaps because recycling of viral DNA into the nucleus is at a low level during productive infection[12]. The frequency of integration of DHBV in experimentally infected ducklings was estimated to be one viral genome per 103-104 cells by 6 d after the infection[12]. It is not known if these recombination events occurred in many cells or if a few cells produced many such events. In keeping with the belief that insertion events are uncommon, we found only a single integrant among many clones in only one of 6 patients with acute hepatitis B. Our finding, and that of others[12,18], of integrated viral DNA in early infection is in accordance with the observation, that importation of linear DNA into the nucleus is necessary for insertion of viral DNA into chromosomal DNA, and that such importation is known to occur in the phase of cccDNA amplification during the initiation of infection[42].Opportunities for viral integration beyond the early phase of infection would require continued importation of viral DNA into the nucleus, depend on new rounds of infection or loss and replacement of cccDNA by cell turnover[12]. Thus, insertion of linear forms of viral DNA may occur preferentially during early phases of infection or during periods of extensive hepatocyte turnover and/or re-infection, at times when linear viral DNA is imported into the nuclei. Integration may be enhanced during these periods by the availability of DNA ends resulting from DNA replication or damage[43]. Alternatively, interference with the normal replication cycle at some stage in chronic infection may result in the accumulation of linear forms of DNA, and there is some evidence for defective replication of HBV and accumulation of replicative intermediates in patients with HBV-associated HCC[44].
The clinical, biochemical, and serological features of the acute hepatitis B in the patient with the integrant did not differ obviously from those in the other patients with uncomplicated disease. He made a complete clinical and biochemical recovery, and seroconverted to anti-HBs and IgG anti-HBc. At this stage, HBV DNA could no longer be detected in his serum by PCR amplification and further cloning was not attempted. Even if the integrated HBV DNA was still present in hepatocytes, it would not be detected in peripheral blood once healing of hepatocyte cell membranes had taken place. Thus, persistence of the integrated HBV DNA could only have been detected by analysis of liver tissue, and a liver biopsy was not felt to be justified. Although sources other than the liver for the chromosomal DNA amplified in the peripheral blood of the six patients with acute hepatitis B (including the one with fulminant hepatitis) are possible (for example, peripheral blood mononuclear cells), the finding of viral DNA integrated into host DNA in one of the patients favors leakage of chromosomal DNA fragments from damaged hepatocytes during the acute liver injury.
Our finding of an integrant in a patient with acute hepatitis B may have no significance in relation to subsequent HCC development. Clonal expansion of an integrant is required for tumor formation, and the finding of a viral insertion in a tumor may simply reflect the fact that the clonal progenitor of the tumor happened to carry a viral integration[10]. Moreover, integrants may be lost from successive generations of cells[45]. Nevertheless, it is in accordance with the observation made in ducks that hepadnaviral DNA insertion may occur at a very early stage of infection, and raises the possibility that in certain circumstances early integration of HBV DNA might play a role in HBV-induced hepatocarcinogenesis.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Jesse Summers and Dr. Charles Rogler for helpful discussions.
Footnotes
Supported by grants from the Poliomyelitis Research Foundation of South African and the HE Griffin Cancer Trust
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
References
- 1.Kew MC. Hepatitis B virus in the etiology of hepatocellular carcinoma. In: Tabor E, ed , editors. Viruses and iver cancer. Amsterdam: Elsevier; 2002. pp. 17–30. [Google Scholar]
- 2.Feitelson MA. Hepatitis B virus in hepatocarcinogenesis. J Cell Physiol. 1999;181:188–202. doi: 10.1002/(SICI)1097-4652(199911)181:2<188::AID-JCP2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Kew MC, Popper H. Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis. 1984;4:136–146. doi: 10.1055/s-2008-1040653. [DOI] [PubMed] [Google Scholar]
- 4.Shikata T. Primary liver carcinoma and cirrhosis. In: Okuda K PR, ed , editors. Hepatocellular carcinoma. New York: John Wiley; 1987. pp. 53–68. [Google Scholar]
- 5.Paterson AC, Kew MC, Herman AA, Becker PJ, Hodkinson J, Isaacson C. Liver morphology in southern African blacks with hepatocellular carcinoma: a study within the urban environment. Hepatology. 1985;5:72–78. doi: 10.1002/hep.1840050116. [DOI] [PubMed] [Google Scholar]
- 6.Popper H, Shih JW, Gerin JL, Wong DC, Hoyer BH, London WT, Sly DL, Purcell RH. Woodchuck hepatitis and hepatocellular carcinoma: correlation of histologic with virologic observations. Hepatology. 1981;1:91–98. doi: 10.1002/hep.1840010202. [DOI] [PubMed] [Google Scholar]
- 7.Marion PL, Knight SS, Salazar FH, Popper H, Robinson WS. Ground squirrel hepatitis virus infection. Hepatology. 1983;3:519–527. doi: 10.1002/hep.1840030408. [DOI] [PubMed] [Google Scholar]
- 8.Marion PL, Knight SS, Ho BK, Guo YY, Robinson WS, Popper H. Liver disease associated with duck hepatitis B virus infection of domestic ducks. Proc Natl Acad Sci USA. 1984;81:898–902. doi: 10.1073/pnas.81.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 10.Ogston CW, Jonak GJ, Rogler CE, Astrin SM, Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from hepatocellular carcinomas of woodchucks. Cell. 1982;29:385–394. doi: 10.1016/0092-8674(82)90155-6. [DOI] [PubMed] [Google Scholar]
- 11.Marion PL, Van Davelaar MJ, Knight SS, Salazar FH, Garcia G, Popper H, Robinson WS. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci USA. 1986;83:4543–4546. doi: 10.1073/pnas.83.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Summers J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J Virol. 1999;73:9710–9717. doi: 10.1128/jvi.73.12.9710-9717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bréchot C, Hadchouel M, Scotto J, Fonck M, Potet F, Vyas GN, Tiollais P. State of hepatitis B virus DNA in hepatocytes of patients with hepatitis B surface antigen-positive and -negative liver diseases. Proc Natl Acad Sci U S A. 1981;78:3906–3910. doi: 10.1073/pnas.78.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafritz DA, Kew MC. Identification of integrated hepatitis B virus DNA sequences in human hepatocellular carcinomas. Hepatology. 1981;1:1–8. doi: 10.1002/hep.1840010102. [DOI] [PubMed] [Google Scholar]
- 15.Marconi M, Scotto J, Laliam M, Hadchouel M, Dazza MC, Larouzé B. A study of liver HBV DNA during follow-up of acute viral hepatitis in children. J Pediatr Gastroenterol Nutr. 1988;7:507–510. doi: 10.1097/00005176-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Lugassy C, Bernuau J, Thiers V, Krosgaard K, Degott C, Wantzin P, Schalm SW, Rueff B, Benhamou JP, Tiollais P. Sequences of hepatitis B virus DNA in the serum and liver of patients with acute benign and fulminant hepatitis. J Infect Dis. 1987;155:64–71. doi: 10.1093/infdis/155.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Yoffe B, Burns DK, Bhatt HS, Combes B. Extrahepatic hepatitis B virus DNA sequences in patients with acute hepatitis B infection. Hepatology. 1990;12:187–192. doi: 10.1002/hep.1840120202. [DOI] [PubMed] [Google Scholar]
- 18.Murakami Y, Minami M, Daimon Y, Okanoue T. Hepatitis B virus DNA in liver, serum, and peripheral blood mononuclear cells after the clearance of serum hepatitis B virus surface antigen. J Med Virol. 2004;72:203–214. doi: 10.1002/jmv.10547. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Schur PH, Carr RI, Kunkel HG. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1966;45:1732–1740. doi: 10.1172/JCI105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 21.Takahashi K, Akahane Y, Hino K, Ohta Y, Mishiro S. Hepatitis B virus genomic sequence in the circulation of hepatocellular carcinoma patients: comparative analysis of 40 full-length isolates. Arch Virol. 1998;143:2313–2326. doi: 10.1007/s007050050463. [DOI] [PubMed] [Google Scholar]
- 22.Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- 23.Richter-Cook NJ, Dever TE, Hensold JO, Merrick WC. Purification and characterization of a new eukaryotic protein translation factor. Eukaryotic initiation factor 4H. J Biol Chem. 1998;273:7579–7587. doi: 10.1074/jbc.273.13.7579. [DOI] [PubMed] [Google Scholar]
- 24.Martindale DW, Wilson MD, Wang D, Burke RD, Chen X, Duronio V, Koop BF. Comparative genomic sequence analysis of the Williams syndrome region (LIMK1-RFC2) of human chromosome 7q11.23. Mamm Genome. 2000;11:890–898. doi: 10.1007/s003350010166. [DOI] [PubMed] [Google Scholar]
- 25.Osborne LR, Martindale D, Scherer SW, Shi XM, Huizenga J, Heng HH, Costa T, Pober B, Lew L, Brinkman J, et al. Identification of genes from a 500-kb region at 7q11.23 that is commonly deleted in Williams syndrome patients. Genomics. 1996;36:328–336. doi: 10.1006/geno.1996.0469. [DOI] [PubMed] [Google Scholar]
- 26.Bowyer SM, van Staden L, Kew MC, Sim JG. A unique segment of the hepatitis B virus group A genotype identified in isolates from South Africa. J Gen Virol. 1997;78(Pt 7):1719–1729. doi: 10.1099/0022-1317-78-7-1719. [DOI] [PubMed] [Google Scholar]
- 27.Kramvis A, Weitzmann L, Owiredu WK, Kew MC. Analysis of the complete genome of subgroup A' hepatitis B virus isolates from South Africa. J Gen Virol. 2002;83:835–839. doi: 10.1099/0022-1317-83-4-835. [DOI] [PubMed] [Google Scholar]
- 28.Kimbi GC, Kramvis A, Kew MC. Distinctive sequence characteristics of subgenotype A1 isolates of hepatitis B virus from South Africa. J Gen Virol. 2004;85:1211–1220. doi: 10.1099/vir.0.19749-0. [DOI] [PubMed] [Google Scholar]
- 29.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HP, Rogler CE. Topoisomerase I-mediated integration of hepadnavirus DNA in vitro. J Virol. 1991;65:2381–2392. doi: 10.1128/jvi.65.5.2381-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slagle BL, Lee TH, Butel JS. Hepatitis B virus and hepatocellular carcinoma. Prog Med Virol. 1992;39:167–203. [PubMed] [Google Scholar]
- 32.Shaul Y, Garcia PD, Schonberg S, Rutter WJ. Integration of hepatitis B virus DNA in chromosome-specific satellite sequences. J Virol. 1986;59:731–734. doi: 10.1128/jvi.59.3.731-734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih C, Burke K, Chou MJ, Zeldis JB, Yang CS, Lee CS, Isselbacher KJ, Wands JR, Goodman HM. Tight clustering of human hepatitis B virus integration sites in hepatomas near a triple-stranded region. J Virol. 1987;61:3491–3498. doi: 10.1128/jvi.61.11.3491-3498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong SS, Jensen AD, Wang H, Rogler CE. Duck hepatitis B virus integrations in LMH chicken hepatoma cells: identification and characterization of new episomally derived integrations. J Virol. 1995;69:8102–8108. doi: 10.1128/jvi.69.12.8102-8108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong SS, Jensen AD, Chang CJ, Rogler CE. Double-stranded linear duck hepatitis B virus (DHBV) stably integrates at a higher frequency than wild-type DHBV in LMH chicken hepatoma cells. J Virol. 1999;73:1492–1502. doi: 10.1128/jvi.73.2.1492-1502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unsal H, Yakicier C, Marçais C, Kew M, Volkmann M, Zentgraf H, Isselbacher KJ, Ozturk M. Genetic heterogeneity of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1994;91:822–826. doi: 10.1073/pnas.91.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogler CE, Summers J. Novel forms of woodchuck hepatitis virus DNA isolated from chronically infected woodchuck liver nuclei. J Virol. 1982;44:852–863. doi: 10.1128/jvi.44.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogler CE, Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from a chronically infected liver. J Virol. 1984;50:832–837. doi: 10.1128/jvi.50.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kew MC, Miller RH, Chen HS, Tennant BC, Purcell RH. Mutant woodchuck hepatitis virus genomes from virions resemble rearranged hepadnaviral integrants in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1993;90:10211–10215. doi: 10.1073/pnas.90.21.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaginuma K, Kobayashi H, Kobayashi M, Morishima T, Matsuyama K, Koike K. Multiple integration site of hepatitis B virus DNA in hepatocellular carcinoma and chronic active hepatitis tissues from children. J Virol. 1987;61:1808–1813. doi: 10.1128/jvi.61.6.1808-1813.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuei DJ, Hsu TY, Chen JY, Chang MH, Hsu HC, Yang CS. Analysis of integrated hepatitis B virus DNA and flanking cellular sequences in a childhood hepatocellular carcinoma. J Med Virol. 1994;42:287–293. doi: 10.1002/jmv.1890420316. [DOI] [PubMed] [Google Scholar]
- 42.Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 43.Petersen J, Dandri M, Bürkle A, Zhang L, Rogler CE. Increase in the frequency of hepadnavirus DNA integrations by oxidative DNA damage and inhibition of DNA repair. J Virol. 1997;71:5455–5463. doi: 10.1128/jvi.71.7.5455-5463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raimondo G, Burk RD, Lieberman HM, Muschel J, Hadziyannis SJ, Will H, Kew MC, Dusheiko GM, Shafritz DA. Interrupted replication of hepatitis B virus in liver tissue of HBsAg carriers with hepatocellular carcinoma. Virology. 1988;166:103–112. doi: 10.1016/0042-6822(88)90151-1. [DOI] [PubMed] [Google Scholar]
- 45.Gong SS, Jensen AD, Rogler CE. Loss and acquisition of duck hepatitis B virus integrations in lineages of LMH-D2 chicken hepatoma cells. J Virol. 1996;70:2000–2007. doi: 10.1128/jvi.70.3.2000-2007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


