Abstract
AIM: To study the role of hepatic sinusoidal capillarization and perisinusoidal fibrosis in rats with alcohol-induced portal hypertension and to discuss the pathological mechanisms of alcohol-induced hepatic portal hypertension.
METHODS: Fifty SD rats were divided into control group (n=20) and model group (n=30). Alcoholic liver fibrosis rat model was induced by intragastric infusion of a mixture containing alcohol, corn oil and pyrazole (1 000:250:3). Fifteen rats in each group were killed at wk 16. The diameter and pressure of portal vein were measured. Plasma hyaluronic acid (HA), type IV collagen (CoIV) and laminin (LN) were determined by radioimmunoassay. Liver tissue was fixed in formalin (10%) and 6-μm thick sections were routinely stained with Mallory and Sirius Red. Liver tissue was treated with rabbit polyclonal antibody against LN and ColIV. Hepatic non-parenchymal cells were isolated, total protein was extracted and separated by SDS-PAGE. MMP-2 and TIMP-1 protein expression was estimated by Western blotting.
RESULTS: The diameter (2.207 ± 0.096 vs 1.528 ± 0.054 mm, P<0.01) and pressure (11.014±0.395 vs 8.533±0.274 mmHg, P<0.01) of portal vein were significantly higher in model group than those in the control group. Plasma HA (129.97±16.10 vs 73.09±2.38 ng/mL, P<0.01), ColIV (210.49±4.36 vs 89.65±4.42 ng/mL, P<0.01) and LN (105.00±7.29 vs 55.70±4.32 ng/mL, P<0.01) were upregulated in model group. Abundant collagen deposited around the central vein of lobules, hepatic sinusoids and hepatocytes in model group. ColI and ColIII increased remarkably and perisinusoids were almost surrounded by ColIII. Immunohistochemical staining showed that ColIV protein level (0.130±0.007 vs 0.032±0.004, P<0.01) and LN protein level (0.152±0.005 vs 0.029±0.005, P<0.01) were up-regulated remarkably in model group. MMP-2 protein expression (2.306±1.089 vs 0.612±0.081, P<0.01) and TIMP-1 protein expression (3.015±1.364 vs 0.446±0.009, P<0.01) in freshly isolated hepatic non-parenchymal cells were up-regulated in model group and TIMP-1 protein expression was evidently higher than MMP-2 protein expression (2.669±0.170 vs 1.695±0.008, P<0.05).
CONCLUSION: Hepatic sinusoidal capillarization and peri-sinusoidal fibrosis are responsible for alcohol-induced portal hypertension in rats.
Keywords: Alcoholic liver fibrosis, Portal hypertension, Hepatic sinusoidal capillarization, Perisinusoidal fibrosis
INTRODUCTION
In China, the number of patients with alcoholic liver fibrosis is increasing. It has been reported that about 20% of people with 10-20 years of alcohol drinking might develop alcoholic liver fibrosis[1]. Portal hypertension is a major complication of liver fibrosis and cirrhosis, which can lead to variceal bleeding, fluid retention and reduced renal blood flow, etc. being a major cause of death in patients with liver fibrosis. The pathological mechanisms of portal hypertension remain unclear. Recently, it has been found that pathological changes of hepatic microvasculature play a major role in the formation of portal hypertension[2]. Wang et al[3]. pointed out that alcohol might cause microvasculature obstruction and capillarization of hepatic sinusoids. Capillarization of the sinusoids is an early event in liver fibrogenesis, which is gradually formed by deposition of type IV collagen and LN in the perisinusoidal space and finally forms a complete and thick basement membrane[4]. When liver fibrosis occurs, it affects normal material exchange between blood and hepatocytes while the blood flow resistance and portal hypertension ensue. Studies on the relation between alcoholic liver injury and portal hypertension are rarely available and the pathological mechanisms are still controversial. One of the popular hypotheses is that hepatic non-parenchymal cells might synthesize basement membrane proteins and take part in the formation of extra-cellular matrix (ECM)[5].
In order to understand the pathological mechanisms of alcohol-induced portal hypertension, we explored the possible changing rule by which ECM of perisinusoid basement membrane (types І, III and IV collagen and LN) abides. Moreover, the synthesis of MMP-2 and TIMP-1 protein in freshly isolated hepatic non-parenchymal cells and their correlation with portal hypertension were also investigated. Hopefully, this may provide new insights for the prevention of alcoholic liver fibrosis.
MATERIALS AND METHODS
Materials
Male SD rats weighing 160±10 g, were purchased from Vital River Experimental Animal Company (eligible certificate number: SCXK2002-2003). Pyrazole was purchased from Aldrich Chemical Company. Alcohol was purchased from Beijing Red Star Alcohol Ltd (concentration: 52%). Conventional corn oil was from the supermarket. Rabbit immunoglobulins directed against mouse ColIV, LN, MMP-2, and TIMP-1 were from Santa Cruz (USA). Secondary antibody (PV-6001) and DAB kit were purchased from Beijing Zhongshan Company. Unless specifically indicated, all other reagents were purchased from Beijing Chemical Agent Company.
Modeling alcoholic liver fibrosis
Fifty rats were divided into control group (n = 20) and model group (n = 30). Alcoholic liver fibrosis model was induced by intra-gastric infusion of a mixture containing alcohol, corn oil and pyrazole (1 000:250:3) as previously described[6,7]. The control rats received intra-gastric infusion of saline solution and had normal diet. Fifteen animals in each group were killed at wk 16.
Measurement of portal vein diameter and pressure
Rats were anesthetized with sodium pentobarbital (40 mg/kg, ip) and the abdomen was opened to expose the portal vein. Portal vein diameter and pressure were measured. The numerical value was recorded as previously described8].
Radioimmunoassay of HA, ColIV and LN
Blood samples were obtained by abdominal aortic artery puncture for radioimmunoassay of HA, ColIV and LN using a standardized and optimized commercial radioimmunoassay kit.
Liver tissue collagen staining
Liver tissue was fixed in buffered formalin (10%) and embedded in paraffin. Sections (6-μm thick) were routinely stained with Mallory and Sirius Red, then observed under ordinary optical and polarization microscope.
Liver tissue immunohistochemical staining
Liver tissue sections were treated with 1% hydrogen peroxide, 0.1% proteinase K and 1% Triton X-100. The sections were reacted with 1:150 diluted rabbit polyclonal antibody against LN and ColIV overnight at 4 ˚C, then with PV-6001 kits for 2 h at room temperature (SP method).
Isolation of hepatic nonparenchymal cells
Hepatic non-parenchymal cells were isolated from male SD rats with proteinase E and collagenase digestion as described previously[9]. Following perfusion, hepatocytes were removed by low-speed centrifugation. Hepatic non-parenchymal cells were separated by density centrifugation in a Nycodenz (33%) gradient. Hepatic sinusoidal cells, Kupffer cells and hepatic stellate cells were harvested.
Western blotting
Hepatic non-parenchymal cells were treated with RIPA (50 mmol/L TrisHCl, 150mmol/L NaCl, 0.025% NaN3, 0.1% SDS, 100 μg/mL PMSF, 1 mmol/L NaF, 1% NP-40, 2 mmol/L Na3VO4, 50mmol/L Hepes, 1% Triton X-100) to extract total protein. Total protein was detected with the BCA protein assay method. Total protein(12μg) was loaded into each lane. Cell extracts were separated by SDS-PAGE using a 12% gel and transferred onto a PVDF membrane. The membranes were blocked for 1 h at room temperature in 10 g/L BSA and incubated overnight at 4˚C with surviving antibody(MMP-2 and TIMP-1), then with sheep anti-rabbit second antibody conjugated to horseradish peroxidase for 2 h. Finally the membranes were developed with DAB and incubated until color developed sufficiently. An equal amount of proteins was loaded onto the stocking gel, β-actin expression was simultaneously estimated in each sample as the internal marker by Western blotting using anti-actin monoclonal antibody.
Image analysis of immunohistochemical staining and Western blot results
Immunohistochemical staining results were analyzed by “5-point sampling” method using the Motic patho-image analysis system on 7 sections for each group. Under the same magnification (10×20), we collected 35 samples from each group, then measured the average facio-density (average facio-density = whole area of positive reaction grains/whole area of visual field). Western blotting results were analyzed by the Motic gel analysis system. We compared the gray scale value of target protein and internal marker to gain the relative optical density value.
Statistical analysis
SPSS version 10.0 was used. Data were analyzed using test and one-way ANOVA. All values were expressed as mean±SE. P<0.05 or <0.01 was considered statistically significant.
RESULTS
Changes of portal vein diameter and pressure
All data of portal vein diameter and pressure were collected and analyzed by a computer. The results are shown in Table 1. Portal vein diameter and pressure were significantly higher in model group than in control group (bP<0.01)
Table 1.
Comparison of portal vein diameter and pressure in control and model groups (mean±SE)
| Group | n | Portal vein diameter (mm) | Portal vein pressure (mmHg) |
| Control | 10 | 1.528 ± 0.054 | 8.533 ± 0.274 b |
| Model | 10 | 2.207 ± 0.096 | 11.014 ± 0.395b |
P<0.01 vs control.
Plasma HA, ColIV and LN assay
Plasma HA, ColIV and LN levels were detectable by radioimmunoassay. The levels of plasma HA(129.97±16.10 vs73.09±2.38ng/mL), ColIV(210.49±4.36 vs 89.65±4.42 ng/mL) and LN(105.00±7.29 vs 55.70±4.32 ng/mL) were all up-regulated in model group compared to control group(P<0.01, Figure 1).
Figure 1.
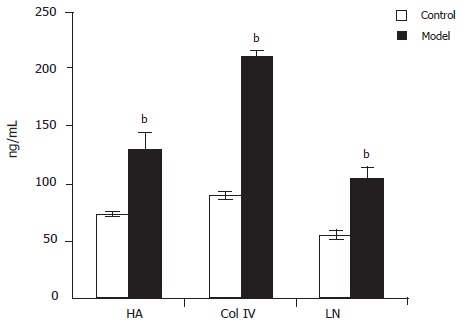
Levels of plasma HA, ColIV and LN in model and control groups bP<0.01 vs control.
Results of Mallory and Sirius Red staining
More collagen was stained with Mallory in model group than in control group. Optical microscopy showed that there was only a little collagen around the central vein of lobules in control rats (Figure 2A). On the contrary, abundant collagen deposited around the central vein of lobules, hepatic sinusoids and hepatocytes in model group, which interweaved to form a net-shape pattern and connected with each other (Figure 2B). Polarization microscopy showed that ColIV was red or yellow while ColIV was green, suggesting that there was a small quantity of ColIV around the central vein of lobules in control rats (Figure 2C). But in model rats, ColIV and ColIV increased remarkably and perisinusoids were almost surrounded by increased ColIV (Figure 2D).
Figure 2.
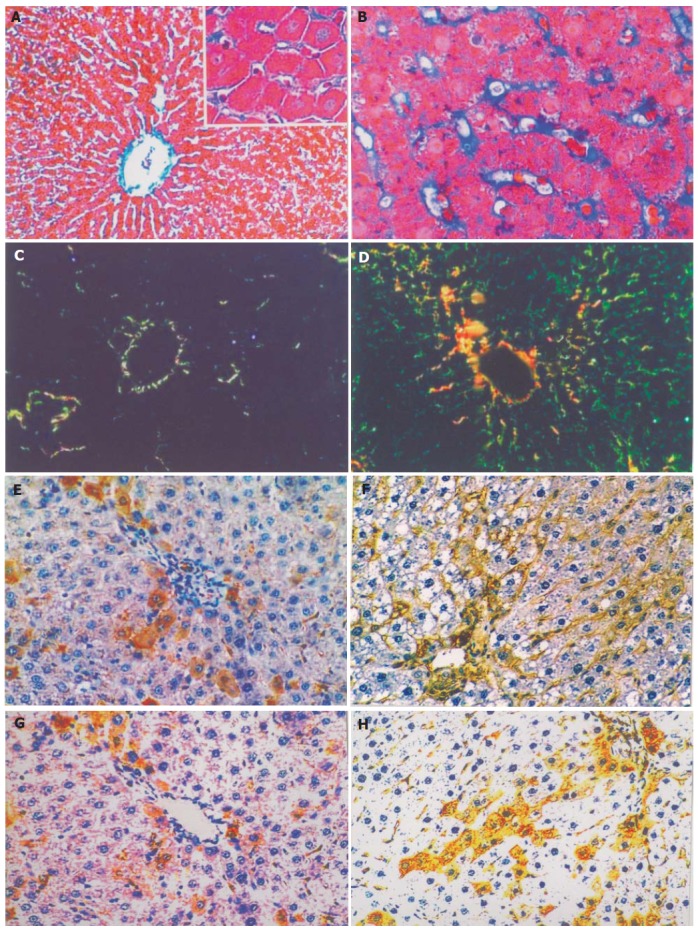
Mallory, Sirius Red and immunohistochemical staining of liver tissue. A: Mallory staining of normal rat liver tissue, ×200 B: Mallory staining of rat alcoholic fibrosis liver tissue, ×400 C: Sirius Red staining of normal rat liver tissue and polarization microscopy, ×200 D: Sirius Red staining of rat alcoholic fibrosis liver tissue and polarization microscopy, ×200 E: Immunohistochemical staining of the first antibody of ColIV of normal rat liver tissue, ×200 F: Immunohistochemical staining of the first antibody of ColIV rat alcoholic liver fibrosis tissue, ×200 G: Immunohistochemistry staining of the first antibody of LN in normal rat liver tissue, ×200 H: Immunohistochemical staining of the first antibody of LN in rat alcoholic liver fibrosis tissue, ×200.
Immunohistochemical staining
The immune reaction for ColIV in normal liver was detected only at the level of portal tract and around the major central vein of lobules (Figure 2E). In alcoholic liver fibrosis tissue, positive ColIV was detected at the level of portal tract and in the central vein of lobules or in the Disse space between the sinusoid cells and hepatocytes (Figure 2F). Image analysis showed that the average facio-density of positive granules in ColIV of alcoholic liver fibrosis tissue was upregulated remarkably compared to the normal liver tissue (0.130±0.007 vs 0.032±0.004, P<0.01, Figure 3).
Figure 3.
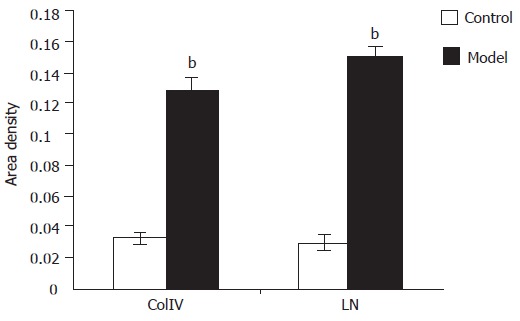
Density of ColIV and LN in positive granule area bP<0.01 vs control in model and co.
In normal liver tissue, a few LN positive cells were detected (Figure 2G). However, the LN positivity increased significantly in alcoholic liver fibrosis tissue. The LN positive granules deposited not only in the cytoplasm but also in the perisinusoidal area (Figure 2H). Image analysis showed that the average facio-density of positive granules in LN was upregulated remarkably compared to the normal liver tissue (0.1518±0.0058 vs 0.0299±0.0058, P<0.01, Figure 3).
Western blotting analysis
Western blotting analysis showed that MMP-2 and TIMP-1 proteins were significantly up-regulated in rat fibrosis liver tissue compared to control group (2.306±1.089 vs 0.612±0.081 and 3.015±1.364 vs 0.446±0.009, P<0.01).Image analysis of rat fibrosis liver tissue showed that protein expression was higher in TIMP-1 than in MMP-2 (2.669±0.170 vs 1.695±0.008, P<0.05, Figure 4).
Figure 4.
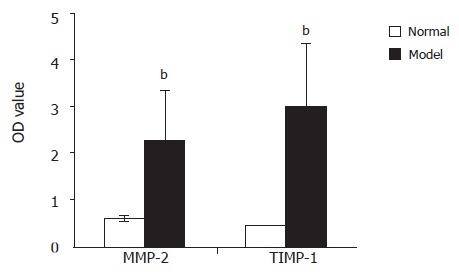
Comparison of MMP-2 and TIMP-1 protein expression in hepatic non-parenchymal cells between model and control groupsbP<0.01, model group vs control group; cP<0.05, TIMP-1 vs MMP-2.
DISCUSSION
In this study, portal vein pressure increased significantly in the early stage of alcoholic liver fibrosis. ColI, ColIII, ColIV and LN levels were increased in the ECM of basement membrane. Furthermore, TIMP-1 protein level was increased in hepatic non-parenchymal cells isolated from alcoholic liver fibrotic tissue, suggesting that the major pathological changes are capillarization of hepatic sinusoids and perisinusoidal fibrosis in early stage of alcoholic liver fibrosis.
Some studies demonstrated that kupffer cells, hepatic stellate cells (HSC) and sinusoidal endothelial cells (SEC) play a key role in the synthesis or decomposition of extra cellular matrix (ECM)[10,11]. Others believe that the levels of serum hyaluronate are strongly related to SEC injury and hepatic microcirculation disorders[12]. Serum hyaluronate is a better index of morphological and functional changes in SECs. Our results showed that the level of rat serum hyaluronate increased remarkably in the early stage of alcoholic liver fibrosis, indicating that SECs are severely injured. When SECs get injured, a large number of inflammatory leucocytes move to the injured sinusoids and trigger the self-recovery process. Therefore, the ECM of basement membrane increases and enwraps the injured sinusoids in order to defend against inflammatory leucocytes. After repeated injury, over-recovery emerges and the ECM of basement membrane including type I, III, IV collagen and LN is over-deposited in perisinusoid[13,14]. Deposition of type I, III, IV collagen and LN reduces hepatic sinusoidal penetration and causes sinusoidal capillarization[15].
Hepatic sinusoids are special capillaries that are limited by fenestrated endothelial cells without a genuine basement membrane and surrounded by perisinusoidal cells storing vitamin A and Kupffer cells as well as pit cells, resident macrophages and large granular lymphocytes[16]. Hepatic sinusoidal capillarization includes a series of pathological changes, in which SECs lose fenestrae, basement membrane develops and sinusoidal outlets collapse[17]. All these pathological changes disable the normal sinusoid intrinsic functions[18]. Capillarization of the sinusoid may cause a disturbance in the exchange of many bioactive substances between the sinusoidal blood and hepatocytes across the Disse space, contributing to the pathogenesis of alcoholic liver fibrosis. On the other hand, with the occurrence of sinusoidal capillarization, the sinusoidal space is significantly narrowed and the sinusoidal endothelial fenestrations are decreased in size and number or disappear in the end. All these pathological changes increase resistance of the hepatic vascular bed and pressure of hepatic portal vein.
Additionally, the breakdown of ECM is essential for tissue remodeling. The matrix metalloproteinases (MMPs), also called matrixins, are thought to play a central role in these processes[19]. The activities of MMPs are precisely controlled during the activation from their precursors and inhibited by tissue inhibitors of metalloproteinases (TIMPs)[20]. MMP-2 is secreted from Kupffer cells and HSCs as they become activated. Also, the activation of MMP-2 is in turn inhibited by TIMP-1. Generally, TIMP-1 and MMP-2 affect biological functions by non-covalent bond [20]. At the early stage of alcoholic liver fibrosis, with the activation of Kupffer cells and HSCs, the level of MMP-2 expression increases. MMP-2 can decompose type I, III, IV collagen and assist Kupffer cells, HSCs and SECs to activate and transfer. Emmanouil et al[21]. showed that increased MMP-2 could decompose the basal membrane of neighboring SECs. The signal of injured SECs triggers the mechanisms of TIMP synthesis to balance the levels of MMPs. However, while repeated injury occurs, the synthesis of ECM becomes stronger than decomposition[22,23]. We examined the expression of MMP-2 and TIMP-1 protein in freshly isolated hepatic non-parenchymal cells of normal and fibrotic rats by Western blotting. The results showed that the levels of MMP-2 and TIMP-1 protein expression were upregulated in the early stage of alcoholic liver fibrosis, the level of TIMP-1 was significantly higher than that of MMP-2, suggesting that the synthesis of basal membrane collagen is stronger than decomposition and more and more ECMs of the basement membrane are synthesized due to the inhibition deposit around SECs and hepatocytes of MMPS[24]. This in turn induces perisinusoidal fibrosis and causes aggravating portal hypertension[25,26]. The therapy of alcohol-induced liver disease varies according to the severity of histological liver damage and clinically overt portal hypertension and hepatic dysfunction[27,28]. Only a few studies have been conducted on alcohol-induced portal hypertension in the past decades[29,30]. Our experimental results showed that hepatic portal hypertension occurred earlier than alcohol-induced cirrhosis, suggesting that hepatic portal hypertension plays a potential role in cirrhosis.
In conclusion, hepatic sinusoidal capillarization and perisinusoidal fibrosis are the vital causes for alcohol-induced portal hypertension in rats. A thorough evaluation of hepatic microcirculation and anti-capillarization may provide the basis for new therapeutic avenues.
Footnotes
Supported by National Natural Science Foundation of China, No. 30130220 and Program for Changjiang Scholars and Innovative Research Team in University (2004)
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Lu XL, Luo JY, Tao M, Gen Y, Zhao P, Zhao HL, Zhang XD, Dong N. Risk factors for alcoholic liver disease in China. World J Gastroenterol. 2004;10:2423–2426. doi: 10.3748/wjg.v10.i16.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Xb, Liu P, Tang ZP, Lu X, Liu CH, Hu YY, Xu LM, Gu H-T, Liu CH. Study on the mechanisms of development of hepatic sinusoidal capillarization. Zhonghua Xiaohua Zazhi. 2004;24:289–292. [Google Scholar]
- 3.Wang BY, Fu BY, Zhang J, Ju XH, Cao YX. The influence of alcohol on the liver sinusoids endothelial cell fenestrae of rats. Zhonghua GanZangBing ZaZhi. 2004;12:479–481. [PubMed] [Google Scholar]
- 4.Xu GF, Wang XY, Ge GL, Li PT, Jia X, Tian DL, Jiang LD, Yang JX. Dynamic changes of capillarization and peri-sinusoid fibrosis in alcoholic liver diseases. World J Gastroenterol. 2004;10:238–243. doi: 10.3748/wjg.v10.i2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neubauer K, Kruger M, Quondamatteo F, Knittel T, Saile B, Ramadori G. Transforming growth factor-beta1 stimulates the synthesis of basement membrane proteins laminin, collagen type IV and entactin in rat liver sinusoidal endothelial cells. J Hepatol. 1999;31:692–702. doi: 10.1016/s0168-8278(99)80350-x. [DOI] [PubMed] [Google Scholar]
- 6.Lin H, Lu M, Zhang YX, Wang BY, Fu BY. Induction of a rat model of alcoholic liver diseases. Shijie Huaren Xiaohua Zazhi. 2001;9:24–28. [Google Scholar]
- 7.Ding X, Meng YC, Ben CE, Tian DL. Induction of alcoholic liver fibrosis animal model. Zhongguo Zhongyi Jichu Yixue Zazhi. 1999;5:47–48. [Google Scholar]
- 8.Scorticati C, Prestifilippo JP, Eizayaga FX, Castro JL, Romay S, Fernández MA, Lemberg A, Perazzo JC. Hyperammonemia, brain edema and blood-brain barrier alterations in prehepatic portal hypertensive rats and paracetamol intoxication. World J Gastroenterol. 2004;10:1321–1324. doi: 10.3748/wjg.v10.i9.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Yu WP, Gao L, Wei KB, Ju JL, Xu JZ. Effects of lipopolysaccharides stimulated Kupffer cells on activation of rat hepatic stellate cells. World J Gastroenterol. 2004;10:610–613. doi: 10.3748/wjg.v10.i4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann A, Zhao D, Reichen J. Myofibroblasts in the cirrhotic rat liver reflect hepatic remodeling and correlate with fibrosis and sinusoidal capillarization. J Hepatol. 1999;30:646–652. doi: 10.1016/s0168-8278(99)80195-0. [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Mochida S, Inao M, Matsui A, Fujiwara K. Angiopoietin/tie receptors system may play a role during reconstruction and capillarization of the hepatic sinusoids after partial hepatectomy and liver necrosis in rats. Hepatol Res. 2004;29:51–59. doi: 10.1016/j.hepres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Hao JH, Shi J, Ren WH, Han GQ, Zhang JR, Wang WZ, Wang SY, Wang YH. The relationship between serum hyaluronate level and hepatic microcirculation status. Linchuang Ganbing Zazhi. 2002;18:35–37. [Google Scholar]
- 13.Xu GF, Tian DL. Progressive of hepatic sinusoidal capillarization. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;10:314–316. [Google Scholar]
- 14.Jia YT, Zeng MD. Progressive on hepatic sinusoidal capillarization. Vol. 20. Guowai Yixue: Xiaohuadao Jibing; 2000. pp. 86–90. [Google Scholar]
- 15.Reichen J. The Role of the Sinusoidal Endothelium in Liver Function. News Physiol Sci. 1999;14:117–121. doi: 10.1152/physiologyonline.1999.14.3.117. [DOI] [PubMed] [Google Scholar]
- 16.Paolo Onori, Sergio Morini, Antonio Franchitto, Robert Sferra, Domenico Alvaro, Eugenio Gaudio. Hepatic microvascular features in experimental cirrhosis: a structural and morphometrical study in CCl4-treated rats. J Hepatol. 2000;33:555–563. doi: 10.1034/j.1600-0641.2000.033004555.x. [DOI] [PubMed] [Google Scholar]
- 17.Xiao W, Wang Y, Liu X. [The coordinated expression of laminin and its integrin receptor in hepatic sinusoidal capillarization] Zhonghua Nei Ke Za Zhi. 2001;40:618–620. [PubMed] [Google Scholar]
- 18.Nakashima O, Kurogi M, Yamaguchi R, Miyaaki H, Fujimoto M, Yano H, Kumabe T, Hayabuchi N, Hisatomi J, Sata M, et al. Unique hypervascular nodules in alcoholic liver cirrhosis: identical to focal nodular hyperplasia-like nodules? J Hepatol. 2004;41:992–998. doi: 10.1016/j.jhep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–G249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 20.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 21.Karagiannis ED, Popel AS. A theoretical model of type I collagen proteolysis by matrix metalloproteinase (MMP) 2 and membrane type 1 MMP in the presence of tissue inhibitor of metalloproteinase 2. J Biol Chem. 2004;279:39105–39114. doi: 10.1074/jbc.M403627200. [DOI] [PubMed] [Google Scholar]
- 22.Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017–7026. doi: 10.4049/jimmunol.167.12.7017. [DOI] [PubMed] [Google Scholar]
- 23.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Niu JZ, Zeng Y, Yang MJ, Tao XH, Wang JF. Effects of FFBJRGP on collagen deposition in hepatic Disse space in alcohol induced liver fibrosis in rat. Jiefangjun Yixue Zazhi. 2004;29:567–570. [Google Scholar]
- 25.Lu X, Liu P, Xu GF, Liu CH, Li FH, Liu C. The role of hepatic sinusoid capillarization during the formation of portal hypertension in fibrotic rats induced by dimethylnitrosamine. Zhonghua Gan Zang Bing Za Zhi. 2003;11:595–598. [PubMed] [Google Scholar]
- 26.Zhang RP, Zhang WH, Xue DB, Wei YW. Morphology of portal hypertension at the early stage of liver damage induced by CCl4: an experimental study with dogs. Zhonghua Yi Xue Za Zhi. 2004;84:1118–1121. [PubMed] [Google Scholar]
- 27.Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, Christensson B, Ericzon BG, Holgersson J, et al. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163:1275–1289. doi: 10.1016/S0002-9440(10)63487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patch D, Armonis A, Sabin C, Christopoulou K, Greenslade L, McCormick A, Dick R, Burroughs AK. Single portal pressure measurement predicts survival in cirrhotic patients with recent bleeding. Gut. 1999;44:264–269. doi: 10.1136/gut.44.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mookerjee RP, Sen S, Davies NA, Hodges SJ, Williams R, Jalan R. Tumor necrosis factorα is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182–1187. doi: 10.1136/gut.52.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diehl AM. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/s0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]


