Abstract
AIM: To investigate the effects of AT1 (Type 1 angiotensin II receptor) antagonist (Losartan) on the apoptosis, proliferation and migration of the human pancreatic stellate cells (hPSCs).
METHODS: hPSCs were isolated from pancreatic sample of patients with pancreatic carcinoma using radioimmunoassay (RIA) technique to detect the concentration of AngII in culture media and cell homogenate. Immunocytochemistry (ICC) and in situ hybridization (ISH) methods were utilized to test AT1 expression in hPSCs. Effects of Losartan on hPSCs proliferation, apoptosis and migration were investigated using BrdU incorporation, TUNEL, flow cytometry (FCM), and phase-contrast microscope separately when cells treated with Losartan. Immunofluorescence and Western blot were applied to quantify the expression of type I collagen in hPSCs.
RESULTS: There exists AT1 expression in hPSCs, while no AngII was detected in culture media and cell homogenate. Losartan induces cell apoptosis in a dose- and time-dependent manner (apparently at 10-5 mol/L), no pro-proliferative effect was observed in the same condition. Corresponding dosage of Losartan can also alleviate the motion capability and type I collagen content of hPSCs compared with AngII treatment and non-treatment control groups.
CONCLUSION: These findings suggest that paracrine not autocrine functions of AngII may have effects on hPSCs, which was mediated by AT1 expressed on cells, while Losartan may exert anti-fibrotic effects by inhibiting hPSCs motion and partly by inducing apoptosis.
Keywords: Pancreatic stellate cell, Angiotensin II receptor, Antagonist, Losartan
INTRODUCTION
Fibrosis is a dynamic process in which both altered matrix degradation and abnormal extracellular matrix (ECM) protein synthesis play key roles[1,2]. Chronic pancreatitis is accompanied by progressive fibrosis that is characterized by the loss of functional tissue and its replacement by ECM, while the molecular mechanisms of pancreatic fibrogenesis remain to be elucidated.
In 1998, star-shaped cells in the pancreas, namely PSCs, were identified and characterized[3,4]. These cells like their counterparts, hepatic stellate cells (HSCs) and pancreatic stellate cells (PSCs) are responsible for the development of pancreatic fibrosis. Meanwhile, many of the morphological and metabolic changes associated with the activation of PSCs in animal models of fibrosis also occur when these cells are grown in culture on plastics. Therefore, a culture of primary PSCs on plastics has been accepted as a model that mimics the in vivo process of PSC activation following pancreatic injury[5].
Numerous studies have emphasized the important role of the local Renin-angiotensin system (RAS) in the fibrogenesis that occurs in the setting of chronic kidney or cardiac diseases and the antifibrogenic effect of therapies with AT1 blockers or angiotensin-converting enzyme (ACE) inhibitors in this condition. Recent study has shown that AngII may induce apoptosis in some type of cells triggered by the interaction with AT1[6]. Also, AngII can promote cell migration even though the mechanism is still unclear. A local RAS has been described in the pancreas[7-9]. Though apoptosis, migration and proliferation of human pancreatic stellate cells (hPSCs) might affect the pancreatic fibrotic process, it has reason to hypothesize that AngII and AT1 may participate in this process by different mechanisms.
The present study attempts to investigate the effects of Losartan, an antagonist of AT1 on hPSCs, providing laboratory data for interfering pancreatic fibrosis.
MATERIALS AND METHODS
Cell isolation and culture
hPSCs were isolated from pancreatic sample of patients with pancreatic carcinoma (kindly gifted by Prof. Bachem MG)[4], culturing in a 95% air and 50 mL/L CO2 incubator at 37 °C with DMEM containing 10% FCS supplemented with 100 U/mL penicillin,100 µg/mL streptomycin and 2 mmol/L L-glutamine.
AngII level in culture media and cell homogenate
Cell homogenate and culture media were taken and kept at -30 °C for assays. The levels of AngII were determined by radioimmunoassay (RIA) method following the company directions (Northern Biot Co, China).
ICC Staining of AT1
For analysis of AT1 expression, cells were fixed with acetone for 5 min, exposed to a rabbit anti-human AT1 antibody (4 °C overnight, 1:200 in PBS, Boster, Wuhan, China), washing and incubating with an anti-rabbit secondary antibody (37 °C, 2 h, 1:200 in PBS, Dako, USA) and with the substrate solution (DAB in H2O2 in Tris buffer pH 7.4) for 15 min after repeating the above-mentioned steps. Pictures were visualized and taken with microscope (Olympus, Japan) and camera (Japan). For control staining, PBS was used instead of the primary antibody.
ISH for AT1
In situ hybridization (ISH) kit (Boster, Wuhan, China) was used to detect AT1 expression in hPSCs, Briefly, slides were incubated with the probe and a biotinylated secondary antibody subsequently. The bound antibody was visualized with an indirect biotin streptavidin system coupled to alkaline phosphatase and were detected by the DAB reagent. Human heart tissue was used as a positive control and the sense probes (included in kit) were used as a negative control.
Apoptosis evaluation of hPSCs
Immunocytochemistry (ICC) detection of apoptotic cells was carried out with the use of TUNEL, in which residues of digoxigenin-labeled dUTP are catalytically incorporated into the DNA by terminal deoxynucleotidyl transferase II. After treatment with Losartan (Merck & Co, New Jersey, USA) in different concentrations (10-9, 10-7, 10-5 and 10-3 mol/L), the slides were fixed and washed thrice in 0.01 mol/L PBS, the following procedures were performed according to the manufacturer’s guidance (Boster, Wuhan, China). The positive particles of DAB staining were viewed with a microscope (Olympus Japan). The number of apoptotic cells was counted and expressed as a percentage of total hPSCs population. Next, after a certain concentration of Losartan treatment, harvested cells were washed twice with PBS (0.01 mmol/L, pH 7.4), and the cells were resuspended in bind buffer to a final concentration of 1×106 cells/mL. Annexin-V FITC/PI solution (Roche, Germany) was added, which can stain the damaged DNA in apoptotic cells, and then the cells were kept in a dark place at room temperature for 15 min. Quantitative analysis for apoptosis was performed with regular flow cytometry (FCM)[10].
Cell proliferation assessment
The effect of Losartan on hPSCs proliferation was determined by BrdU incorporation and cell counting. After being cultured for 3 d on glass slides, hPSCs were incubated with Losartan (a series of final concentrations 10-9, 10-8, 10-7 mol/L) for 48 h. Each concentration included six wells, cells being cultured for 48 h firstly, and then supplemented with BrdU (Boster, Wuhan, China) at 1×10-5 mmol/L. After 24 h, slides were stained according to the protocol. Positive signals were visualized by DAB reagent plus substrate intensifier. Five random areas of the slides were chosen for counting stained and unstained hPSCs nuclei [positive cells/(negative+positive cells) ×100].
Migration study
Cell migration was assessed by an in vitro wound-healing assay. For the wound-healing assay, hPSCs were cultured until confluence on 35 mm-diameter culture dishes. Cells were serum-starved for 24 h, and a 2-mm wide linear wound was cleared. Cells were then incubated with AngII (10-8mol/L, Sigma, USA), AngII (10-8 mol/L) plus Losartan (10-7 mol/L) for 24 h[11]. Cells migrating into the wound were detected using a phase-contrast microscope.
Western blotting
Western blotting was performed under standard conditions. Briefly, hPSCs (1×106 ) were cultured in 60 mm culture dishes, and were stimulated with AngII (10-7 mol/L) and AngII plus Losartan (10-6 mol/L) separately for 24 h, 4 mL of the cell media as precipitated with 0.76 g of sodium sulfite at 4 °C for 4 h and then centrifuged at 12 000 g for 30 min. Pellets were resuspended in buffer (containing 50 mmoL Tris-Cl, 50 mmol DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol), protein concentrations were determined by BCA protein assay (Pierce, IL). Protein samples were heated for 5 min at 100 °C and were subjected to electrophoresis on a 8% acrylamide gel, and transferred to PVDF membrane (Schleicher&Schuell, Germany). The membrane was blocked in 50 mL/L non-fat dried milk in TBS buffer for 2 h at 20 °C, then membrane was incubated overnight at 4 °C with rabbit anti-human collagen type I antibody (diluted 1:200 in TBS buffer; Boster, Wuhan China) and for 60 min with corresponding horseradish-conjugated secondary antibodies (Santa Cruz, USA) at room temperature. Membrane was washed thrice in Tris-buffered saline containing 1% Tween-20 for 10 min after each of the steps. The protein bands were visualized with ECL chemiluminescence system.
Immunofluorescent staining for collagen type I
hPSCs grew in chamber slides fixed with 2% paraformaldehyde, permeabilized by 0.1% Triton X-100. For non-specific binding site blocking, 1:50 normal goat serum was applied for 10 min, and then incubated with primary antibody for type collagen I (Boster, Wuhan, China) at 4 °C overnight, biotinylated secondary antibody and streptavidin-conjugated FITC (Southern Biotechnology Association, Birmingham, USA) were used to intensify the images. Images were taken by fluorescent microscope (Olympus, Japan).
Statistical analysis
Data values we expressed as mean±SD, these experiments were performed in triplicate for each of the six samples. Statistical analysis was performed with a two-tailed Student’s t test, and P<0.05 was considered statistically significant.
RESULTS
AT1 expression and AngII concentration in hPSCs
hPSCs were cultured for 14 d (activated PSCs), immunostaining revealed that positive signals for AT1 were located in cell plasm as shown (in Figures 1A and B). ISH assay shows positive nuclei in hPSCs at mRNA level, and representative examples of ISH staining are shown (in Figures 1C and D). AngII was not detected in the culture media or cell homogenate by RIA.
Figure 1.
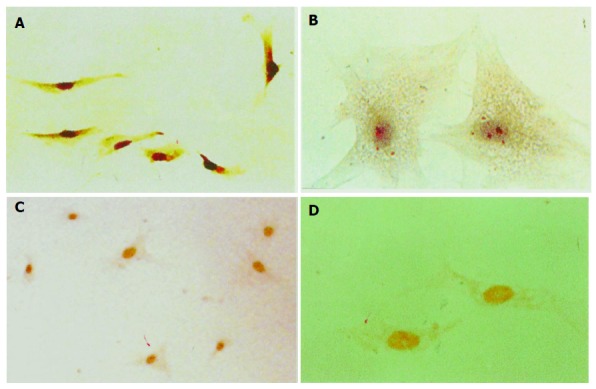
Immunocytochemistry and ISH detection of AT1 in hPSCs at protein and mRNA levels. Immunostaining positive particles were located in plasm, A (magnification ×100); B (magnification ×400). ISH positive signals were located in nuclei C (magnification ×100); D (magnification ×400).
Effects of Losartan on hPSCs
TUNEL staining results suggest a marked increase in apoptotic cell death in Losartan treatment groups in a dosage-dependent manner compared to PBS treatment group (Figure 2).
Figure 2.
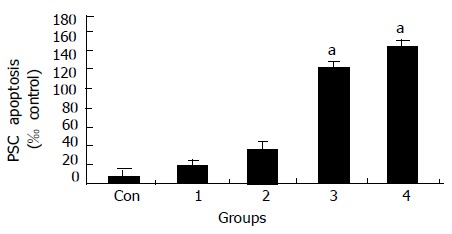
Dose-dependent induction of apoptosis by Losartan at different concentrations. (Con, 1 = 10-9, 2 = 10-7 , 3 = 10-5, 4 = 10-3 mol/L; aP < 0.05 vs different from all groups ).
The percentage of apoptotic cell induced by Losartan (Figure 3) was achieved using FCM (EpicsXL Coulter, USA). In the two-dimensional histogram the horizontal axis shows Annexin-V binding and the vertical axis shows PI uptake. The percentage of cells are indicated in the apoptotic quadrant (bottom right). Apoptosis began 24 h after certain dosage of Losartan (10-5 mol/L) treatment, and the percentage of apoptotic hPSCs rose from 3.7% (24 h) to 13.1% (72 h) during treatment.
Figure 3.
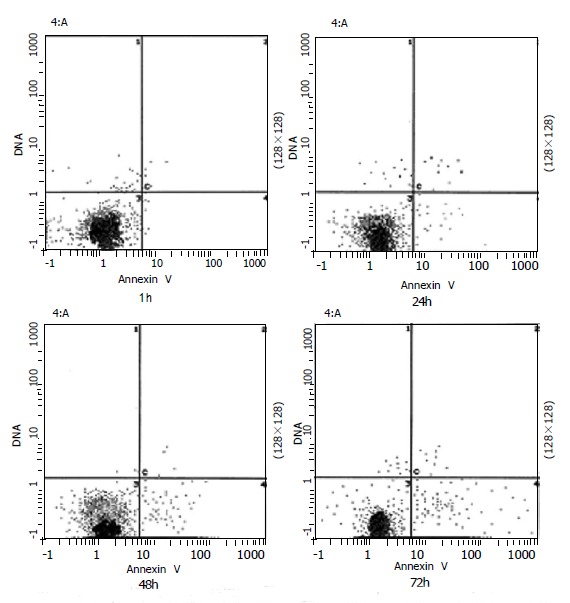
Time-course of Losartan-induced apoptosis. hPSCs in 0.1% FCS were exposed to 10-5 mol/L Losartan, and was assessd at 24, 48,72 h. Results indicate that Losartan induces apoptosis in a time-dependent manner.
The BrdU labeling index in control cells was 3.5×0.8%. Treatment with Losartan did not increase the labeling indices (4.7×1.1%, 3.6×0.7%, 4.3×0.5%, Figure 4).
Figure 4.
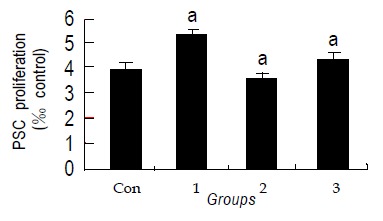
Effects of Losartan on hPSCs proliferation assessed with BrdU incorporation. Losartan itself did not affect cell proliferation compared to control group. (aP>0.05 different from all the groups).
Migration of hPSCs was assessed with an in vitro wound-healing assay. AngII (10-8 mol/L) and 10% FCS induced migration of hPSCs into the wound. This effect might be inhibited by Losartan (10-7 mol/L)[10], as demonstrated by the assay. Images are representative of this experiment (Figure 5).
Figure 5.
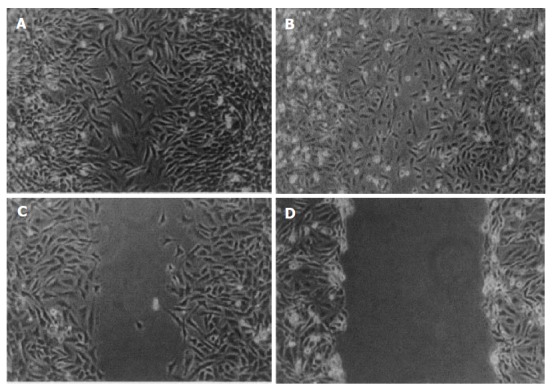
AngII accelerates in vitro wound healing confluent, culture-activated PSCs were serum deprived for 48 h. A wound was produced in the monolayer with a pipette tip, and the cells were exposed to (A) AngII (10-8 mol/L), (B) 10% fetal bovine serum, (C) AngII + Losartan, (D) serum-free medium along 24 h later, the cells were photographed.
Western blotting and immunofluorescence assay for collagen type I
The collagen type I level was substantially down-regulated after 10-6 mol/L Losartan treatment in hPSCs. In contrast, levels of collagen were higher in control groups, and the same results were shown by immunofluorescence assay (Figure 6).
Figure 6.
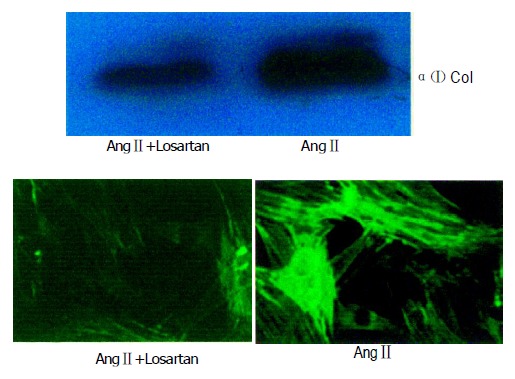
Losartan down-regulated collagen I protein expression by western blot and immunofluoresence. hPSCs were incubated with 10-6 mol/L Losartan. Samples were exposed to certain concentration of Losartan+AngII and AngII for 24 h.
DISCUSSION
Chronic pancreatitis is an irreversible progressive disease characterized by the destruction of acinar and ductule cells. Increased accumulation of ECM is a histologic characteristic of chronic pancreatitis that results in pancreatic fibrosis[12,13].
The molecular mechanisms resulting in the fibrosis in pancreas remain unknown. PSCs may participate in matrix remodeling through the regulation of ECM production and matrix degradation. Like his counterpart HSCs, it is now generally accepted that PSCs are major ECM producing cells in the pancreas[14,15]. On activation by different stimuli, such as growth factor, cytokines, ethanol, oxidative stress, PSCs produce a large amount of ECM proteins. Increasing synthesis of ECM results from the number of PSCs, determined by the balance of cell proliferation and apoptosis, or cells migrating from non-injured areas to injured areas. Apoptosis, proliferation and migration of PSCs must play a crucial role in pancreatic fibrotic process.
Recent evidence indicates that AngII plays a role in liver fibrogenesis[16]. The AngII is locally expressed in injured livers, and AngII inhibition attenuates experimental liver fibrosis[17]. AngII is able to induce contraction and proliferation of HSCs[18], and also increase the expression of collagen I in lung fibroblasts[19]. Recently, it was shown that ACE inhibitor suppressed progression of hepatic fibrosis in rats[18]. In addition, AngII and AT1 interaction results in hepatic fibrosis, which could be suppressed by AT1 antagonist in vivo and in vitro[16]. Since the pathophysiologic process of pancreatic fibrosis is similar to hepatic fibrosis, and the hPSCs are a counterpart of HSCs, we hypothesize that AngII participates in pancreatic fibrogenesis, though the mechanisms underlying the fibrogenic action of AngII in the pancreas merit further investigation. We speculated that AngII and AT1 also play an important role in the fibrogenesis of pancreas, so the expression of AT1 in PSCs was assessed in our study.
It is reported that increased AngII may affect activation and proliferation of PSCs[20]. Therefore, we propose a link between AngII and PSCs during pancreatic fibrosis. While the effects of AngII on cells are mediated by interaction with its receptor (mainly AT1), we here present evidence that hPSCs express AT1 at protein and mRNA level, this finding suggests that AngII could exert biological actions in these cells. Moreover we found that no AngII was detected by RIA either in culture media or in hPSCs homogenate, indicating that it is paracrine but not autocrine function of AngII interacting with AT1 in hPSCs, which plays a role in pancreatic fibrosis. In injured areas of pancreas, the level of AngII alleviate apparently. We observed that AngII derived from exogenous pathway could promote hPSCs migration, which is an important way for increasing hPSCs number in these areas, and this process can be blocked with certain concentration of Losartan. We know that AngII may modulate the re-organization of actin and vinculin through Paxillin and other downstream molecules such as small Rho family members after combining to a certain membrane receptor, leading to cell polarity and migration[21,22]. hPSCs migrating from uninjured areas accumulate in the injured area, and participate in ECM secretion and fibrotic formation[23,24]. The inhibitory effect of Losartan might be attributed to AngII receptor intervention. However, the precise modulating mechanisms still need to be elucidated.
It is well accepted that AngII can induce apoptosis through AT1 in some cell types, and this apoptotic process could be alleviated with Losartan[25]. AT1 and AT2 are two subtypes receptor of AngII. The AT1 regulates vasoconstriction and sodium and water re-absorption, as well as promote cell growth, proliferation, and collagen matrix deposition. The AT2 appears to counterbalance the AT1 by increasing the production of bradykinin, nitric oxide, and cyclic guanosine monophosphate-mediating vasodilation and by promoting cell differentiation, anti-proliferation, and apoptosis. It is meaningful that our results suggest that Losartan itself can induce hPSCs apoptosis in a dose- and time-dependent manner clarified by TUNEL and FCM assays, while there are no marked effects on hPSCs proliferation. We made a serial concentration of Losartan firstly in order to ascertain the adaptable pro-apoptotic dosage, and found that 10-5 mol/L had significant pro-apoptotic effect. Also we chose 0.1% FCS in time-course apoptotic test in order to decrease the serum’s effect on anti-apoptosis. Based on this result, we used 10-6 mol/L in pro-proliferation study in order to avoid pro-apoptotic effect. Apoptosis is a physiological mechanism of deleting cells that regulates cell mass and architecture in many tissues. Inducing PSCs apoptosis or inhibiting PSCs proliferation are always hope-giving methods for complete treatment of pancreatic fibrosis. The regulatory mechanisms and signaling pathways in this process are poorly defined. However, how can we explain this paradox? Actually, AngII is also a potent anti-apoptotic factor, capable of reversing the effects of NO as well as the response to serum withdrawal-induced apoptosis was noted at concentrations as low as 10 nmol/L[26]. The observation that AngII inhibits cGMP analog-induced apoptosis suggests that the survival signal is downstream from the activation of cGMP protein kinase[26]. Given the pleiotropic effects of AngII on a variety of second messenger systems, such as calcium, protein kinase C, mitogen-activated protein kinase, and other tyrosine kinases, there are many potential mechanisms by which AngII may stimulate a cell survival signal[27,28]. It has been reported that AngII inhibits apoptosis mainly via the stimulation of the AT1[26]. Other anti-apoptotic mechanism might be associated with down-regulation of iNOS expression and requires an intact phosphatidylinositol 3-kinase-Akt survival signal pathway[29]. Whether the interaction between Losartan and AT1 could activate downstream molecular signals inducing apoptosis is worthy of further investigation. Losartan treatment also lowers the content of collagen I in culture media and hPSCs compared to control group determined by Western blot and immunofluorescence. This contention coincides with other reports that the specific blockade of AT1 causes a reduction in collagen I.
It is demonstrated that PSCs possessing AT1, have a possible impact on the pathogenesis of acute and chronic pancreatitis. In the injured pancreas, AngII production by the local RAS may contribute to an impaired microcirculation, resulting in increased intrapancreatic vascular resistance, reduced pancreatic juice efflux, and fibrotic reorganization[30]. Kuno et al.[12] recently demonstrated in an in vivo model of chronic pancreatitis that inhibition of the ACE attenuates pancreatic inflammation and fibrosis, further corroborating the view that AngII and AT1 may play an important role in pancreatic pathophysiology. Taken together, our data provide evidence to support that Losartan has direct anti-migration, antifibrotic and pro-apoptotic effects on hPSCs in vitro, these processes might be mediated in part, by interaction with AT1. These findings have important implications for understanding the role of AT1 and its antagonist in pancreatic fibrosis. If this is confirmed, the inhibition of AT1 by Losartan could represent a novel approach to inhibit fibrogenesis in patients with chronic pancreatic disease.
Footnotes
Supported by Shanghai Sanitary Bureau Foundation, No. 40306
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Valderrama R, Navarro S, López JM, Caballería J, Giménez A, Parés A, Adrián MJ, Fernández-Cruz L, Terés J. Synthesis and degradation of collagen in pancreatic fibrogenesis. Pancreas. 1999;18:34–38. doi: 10.1097/00006676-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Wang XP, Zhang R, Wu K, Wu L, Dong Y. Angiotensin II mediates acinar cell apoptosis during the development of rat pancreatic fibrosis by AT1R. Pancreas. 2004;29:264–270. doi: 10.1097/00006676-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J Gastroenterol. 2003;9:2751–2758. doi: 10.3748/wjg.v9.i12.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi N, Miyata T, Ohnishi H, Yasuda H, Tamada K, Ueda N, Mashima H, Sugano K. Activin A is an autocrine activator of rat pancreatic stellate cells: potential therapeutic role of follistatin for pancreatic fibrosis. Gut. 2003;52:1487–1493. doi: 10.1136/gut.52.10.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 7.Chan WP, Fung ML, Nobiling R, Leung PS. Activation of local renin-angiotensin system by chronic hypoxia in rat pancreas. Mol Cell Endocrinol. 2000;160:107–114. doi: 10.1016/s0303-7207(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 8.Regoli M, Bendayan M, Fonzi L, Sernia C, Bertelli E. Angiotensinogen localization and secretion in the rat pancreas. J Endocrinol. 2003;179:81–89. doi: 10.1677/joe.0.1790081. [DOI] [PubMed] [Google Scholar]
- 9.Ohta T, Amaya K, Yi S, Kitagawa H, Kayahara M, Ninomiya I, Fushida S, Fujimura T, Nishimura G, Shimizu K, et al. Angiotensin converting enzyme-independent, local angiotensin II-generation in human pancreatic ductal cancer tissues. Int J Oncol. 2003;23:593–598. [PubMed] [Google Scholar]
- 10.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 11.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, Nakazawa T, Ohara H, Nomura T, Joh T, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–1019. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- 13.Tsang SW, Cheng CH, Leung PS. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul Pept. 2004;119:213–219. doi: 10.1016/j.regpep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam KY, Leung PS. Regulation and expression of a renin-angiotensin system in human pancreas and pancreatic endocrine tumours. Eur J Endocrinol. 2002;146:567–572. doi: 10.1530/eje.0.1460567. [DOI] [PubMed] [Google Scholar]
- 16.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745–750. doi: 10.1053/jhep.2001.28231. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology. 2001;121:148–155. doi: 10.1053/gast.2001.25480. [DOI] [PubMed] [Google Scholar]
- 18.Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodés J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–1156. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 19.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161:1999–2004. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- 20.Nagashio Y, Asaumi H, Watanabe S, Nomiyama Y, Taguchi M, Tashiro M, Sugaya T, Otsuki M. Angiotensin II type 1 receptor interaction is an important regulator for the development of pancreatic fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G170–G177. doi: 10.1152/ajpgi.00005.2004. [DOI] [PubMed] [Google Scholar]
- 21.Ishida T, Ishida M, Suero J, Takahashi M, Berk BC. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest. 1999;103:789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaya K, Ohta T, Kitagawa H, Kayahara M, Takamura H, Fujimura T, Nishimura G, Shimizu K, Miwa K. Angiotensin II activates MAP kinase and NF-kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int J Oncol. 2004;25:849–856. [PubMed] [Google Scholar]
- 23.Klonowski-Stumpe H, Reinehr R, Fischer R, Warskulat U, Lüthen R, Häussinger D. Production and effects of endothelin-1 in rat pancreatic stellate cells. Pancreas. 2003;27:67–74. doi: 10.1097/00006676-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Phillips PA, Wu MJ, Kumar RK, Doherty E, McCarroll JA, Park S, Pirola RC, Wilson JS, Apte MV. Cell migration: a novel aspect of pancreatic stellate cell biology. Gut. 2003;52:677–682. doi: 10.1136/gut.52.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González A, López B, Ravassa S, Querejeta R, Larman M, Díez J, Fortuño MA. Stimulation of cardiac apoptosis in essential hypertension: potential role of angiotensin II. Hypertension. 2002;39:75–80. doi: 10.1161/hy0102.100788. [DOI] [PubMed] [Google Scholar]
- 26.Pollman MJ, Yamada T, Horiuchi M, Gibbons GH. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK, Alexander RW. The angiotensin (AT1) receptor. Semin Nephrol. 1993;13:558–566. [PubMed] [Google Scholar]
- 28.Ip SP, Tsang SW, Wong TP, Che CT, Leung PS. Saralasin, a nonspecific angiotensin II receptor antagonist, attenuates oxidative stress and tissue injury in cerulein-induced acute pancreatitis. Pancreas. 2003;26:224–229. doi: 10.1097/00006676-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Tian B, Liu J, Bitterman P, Bache RJ. Angiotensin II modulates nitric oxide-induced cardiac fibroblast apoptosis by activation of AKT/PKB. Am J Physiol Heart Circ Physiol. 2003;285:H1105–H1112. doi: 10.1152/ajpheart.01139.2002. [DOI] [PubMed] [Google Scholar]
- 30.Reinehr R, Zoller S, Klonowski-Stumpe H, Kordes C, Häussinger D. Effects of angiotensin II on rat pancreatic stellate cells. Pancreas. 2004;28:129–137. doi: 10.1097/00006676-200403000-00003. [DOI] [PubMed] [Google Scholar]


