Abstract
AIM: To explore the biological behavior of gastric carcinoma micrometastasis (MM) with a marker of cytokeratin 18 (CK18) and to evaluate the clinical stage of gastric carcinoma and its prognosis.
METHODS: Reverse transcription-polymerase chain reaction (RT-PCR) was used to examine the expression of CK18 mRNA in 298 lymph nodes from 35 patients with gastric carcinoma and 20 lymph nodes from 10 patients with chronic peptic ulcer and gastric perforation diagnosed by pathological examination and surgery. CK18 mRNA expression of peripheral blood from 54 patients with gastric carcinoma and 10 healthy people were also examined.
RESULTS: Expression of CK18 mRNA was not found in 10 patients with benign pathological changes. CK18 mRNA expression in gastric carcinoma tissues was strongly positive. In gastric carcinoma patients, pathological examination revealed that 99 of 298 (33.2%) lymph nodes were positive, while RT-PCR showed that 133 of 298 (44.6%) lymph nodes had expression of CK18 mRNA. The difference was significant (P < 0.05). Among the 199 negative lymph nodes identified by pathological examinations, 34 (17.1%) displayed positive expression of CK18 mRNA by RT-PCR. The positive expression of CK18 mRNA was associated with lymph node micrometastasis (LMM) of gastric carcinoma. CK18 mRNA was negatively expressed in all 10 healthy cases and positively expressed in 38.9% of 54 blood specimens from gastric carcinoma patients. The positive rate was not correlated with tumor invasion of gastric carcinoma, but was significantly associated with TNM stage, lymph node metastasis (P = 0.0290, P < 0.05) and tumor differentiation (P = 0.2956, P<0.05).
CONCLUSION: RT-PCR with CK18 mRNA as a molecular marker is highly sensitive and specific in detecting LMM of gastric carcinoma. It can benefit the diagnosis of MM and guide studies on biological behavior, clinical phase, and therapy as well as relapse monitoring.
Keywords: Gastric carcinoma, CK18, RT-PCR, Biological behavior
INTRODUCTION
Gastric carcinoma ranks second in alimentary canal diseases and third in all tumors in China. Metastasis and relapse are two major reasons for the death of patients. The two important metastatic pathways of gastric carcinoma are lymph nodes and peripheral blood, which are important factors for the prognosis of patients after curative surgery. This study used reverse transcription-polymerase chain reaction (RT-PCR) to detect the expression of cytokeratin 18 (ck18) mRNA and to explore its correlation with gastric carcinoma micrometastasis (MM).
MATERIALS AND METHODS
Patients and tissue samples
Enrolled in this study were 54 patients (24 females, 30 males) undergone curative gastrectomy for gastric carcinoma at the First and Second Hospitals of Jilin University between October 2001 and August 2002. The patients’ age ranged from 34 to 74 years (mean age of 51.2 years). Control group I included 10 patients with peptic ulcer, control group II consisted of 10 healthy people.
A total of 298 lymph nodes were obtained from 54 patients with gastric carcinoma. Lymph nodes were divided into two parts: one for pathological examination and the other stored at -80 °C for use.
Five milliliters of peripheral blood was collected from 54 patients with gastric carcinoma. Blood specimens were anti-coagulated with heparin. Peripheral blood mononuclear cells (PBMC) were separated from the whole blood by Ficoll-Hypaque density-gradient centrifugation and stored at -70 °C for use. Five milliliters of peripheral blood was collected from negative controls.
Preparation of RNA samples and RT-PCR
RNA was isolated from PBMC and lymph nodes with TRIzol reagent. Lymph nodes were collected from normal controls and gastric carcinoma patients in 1 mL of TRIzol reagent per 100 mg of tissue using power homogenizer. RNA pellet was dried and RNA dissolved in RNase-free water was stored at -70 °C for use. RNA was used as a template for amplification. Oligonucleotides as specific primers and probes for CK18 were synthesized by a company (Biotechnology, Dalian, China). As a PCR control reaction, β-actin was also detected in each run. The sequences of primers are as follows: CK18: 5’-PCR primer AAGAAAACCCGAAGAGG, 3’-PCR primer CTGACTCAAGGTGCAGC; β-actin: 5’-PCR primer GTGGGGCGCCCCAGGCACCA, 3’-PCR primer CTTCCTTAATGTCACGCACGATTTC. The expected sizes of PCR products were 402 bp for CK18 and 540 bp for β-actin. Complementary (c) DNA was synthesized using Rous-associated virus reverse transcriptase (TAKARA Biomedicals).
PCR was performed following the procedure: briefly 130 ng of RNA was used for RT-PCR. For cDNA synthesis, 130 ng in 4 μL of sample RNA solution and 2 μL of oligo dT were heated at 70 °C for 5 min and cooled rapidly. After adding 1 μL of a ribonuclease inhibitor (Takara, Dalian, China), 4 μL of 2.5 mmol/L dNTP (dATP, dCTP, dGTP, dTTP, Takara, Dalian, China) and 4 μL of Rous-associated virus reverse transcriptase (Takara, Dalian, China), 10 μL of 5×PCR buffer, 25 μL of DEPC-water, the mixture was incubated at 65 °C for 60 min, and then at 95 °C for 5 min. The PCR mixture contained 25 μL of cDNA , 7.5 μL of 10×PCR buffer, 6μL of 25 mmol/L MgCl2 ,8 μL of 2.5 mmol/L dNTP, 50 μL of DEPC-water , 2 μL of 5’- and 3’-PCR primer , 0.5 μL of μ-actin and 1.0 μL of thermostable Taq polymerase (Takara, Dalian, China). The amplification was done with a DNA thermal cycler (Geneam PCR System 2400). After denaturation at 94 °C for 4 min, the amplification was conducted for 35 cycles at 94 °C for 45 s, at 55 °C for 45 s, and at 72 °C for 60 s. This was followed by a final extension for 1 min at 72 °C. Ten microliter aliquots of the product was analyzed by electrophoresis on a 2% agarose gel and visualized by UV fluorescence after being stained with ethidium bromide.
Standard of result assessment
The expected sizes of PCR products were 402 bp for CK18 and 540 bp for β-actin. CK18 mRNA expression of two DNA bands in the corresponding location was positive. CK18 mRNA expression of one 540 bp band was negative (Figure 1).
Figure 1.
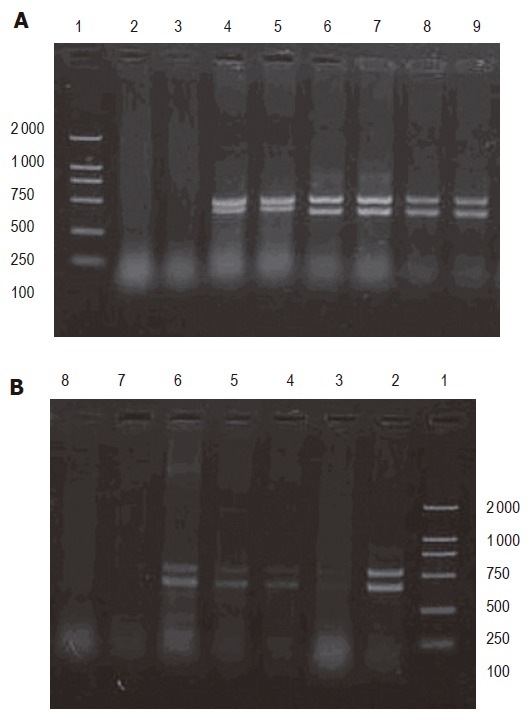
Expression of CK18 mRNA gene in lymph nodes (A) and peripheral blood (B). Lane 1: 2-kb DNA ladder marker; lane 2: positive gastric carcinoma tissue; lanes 3-6: positive peripheral blood specimens; lanes 7 and 8: negative lymph nodes.
Statistical analysis
Data were analyzed by χ2 test using Instat software. P<0.05 was considered statistically significant.
Classification standard for lymph node metastasis
According to the 1997 International Union Contrele Cancer (UICC)/American Joint Committee on Cancer (AJCC) pN classification, PN0: without lymph node metastasis, PN1: 1-6 lymph node metastases, PN2: 7-15 lymph node metastases, PN3: >15 lymph node metastases.
RESULTS
Expressions of CK18 mRNA in lymph nodes and peripheral blood are shown in Figure 1.
Lymph node MM, correlation between CK18 mRNA expression in lymph nodes, peripheral blood and tissue differentiation, tumor stage and invasion are summarized in Table 1, Table 2 and Table 3.
Table 1.
Lymph node MM
| Pathological diagnosis of lymph nodes (carcinoma cells) |
RT-PCR(CK18 mRNA) |
Sum | |
| Positive | Negative | ||
| Positive | 99 | 0 | 99 |
| Negative | 34 | 165 | 199 |
| Sum | 133 | 165 | 298 |
Table 2.
Correlation between expression of CK18 mRNA in lymph nodes, peripheral blood and TNM stage
| Pathologic |
Lymph nodes |
Peripheral blood |
||||||
| parameters | n | + | – | P | n | + | – | P |
| Tissue | >0.05 | <0.05 | ||||||
| differentiation | 15 | 4 | 11 | 29 | 7 | 22 | ||
| Well-moderate | 20 | 10 | 10 | 25 | 14 | 11 | ||
| Poor | <0.05 | >0.05 | ||||||
| Tumor invasion | 11 | 1 | 10 | 23 | 6 | 17 | ||
| Muscularis | 24 | 13 | 11 | 31 | 15 | 16 | ||
| Serosa | <0.05 | <0.05 | ||||||
| Lymph node | 14 | 2 | 12 | 20 | 3 | 17 | ||
| metastasis | 21 | 12 | 9 | 34 | 18 | 16 | ||
| Yes | >0.05 | <0.05 | ||||||
| No | 15 | 4 | 11 | 21 | 4 | 17 | ||
| TNM stage | 20 | 11 | 9 | 33 | 17 | 16 | ||
| I+II | ||||||||
| III+IV | ||||||||
+: positive cases; –: negative cases.
Table 3.
Correlation between expression of CK18 mRNA in lymph nodes, tissue differentiation and tumor invasion
| Pathologic parameters |
CK18 mRNA expression of lymph nodes |
P |
||
| n | Positive | Negative | ||
| Tissue differentiation | >0.05 | |||
| Well-moderate | 31 | 11 | 20 | |
| Poor | 267 | 122 | 145 | |
| Tumor invasion | <0.05 | |||
| Muscularis | 62 | 12 | 50 | |
| (T1+T2) | ||||
| Serosa | 236 | 121 | 115 | |
| (T3+T4) | ||||
DISCUSSION
Micrometastasis
MM spreads from primary tumor to distant secondary tumors in lymph system, blood circulation, bone marrow, liver, kidney, lung, and other organs during the development of malignant neoplasm in non-hematopoietic system. It often has no clinical symptoms[1,2]. Gastric carcinoma is one of the most common malignant neoplasms in China. Many patients showing lymph node metastasis of gastric carcinoma experience relapse and eventually die after curative surgery. Even those with no metastasis of gastric carcinoma ultimately die in the relapse of metastasis. The essential reason is MM, that cannot be detected. MM has become the focus in recent studies on malignant neoplasm. The growth and metastasis of gastric carcinoma are a complicated process with a variety of gene mutations. Although most carcinoma cells entering into circulation are destroyed through mechanisms such as immunization, the residual carcinoma cells can disseminate through a number of mechanisms, such as changes of cell modulation, excretion of protease, growth of blood vessels, and increase of cell dynamics[3]. MM of malignant tumor is controlled by the positive and negative functions of a number of genes at molecule level. Successful clones of ”correlating genes of tumor metastasis” and ”restraining genes of tumor metastasis” are related with tumor metastasis. Genes at molecule level such as c-met, ras, myc and HER-2/neu, and restraining genes of tumor metastasis such as nm23, p53 and CD44 are related to tumor metastasis[4]. Loss, mutation and abnormal expression of the genes are correlated with invasive metastasis of tumor. Lymph node MM is the main pathway of gastric carcinoma MM. Lymph node MM has been considered as cancer metastasis that cannot be detected by traditional pathological methods[4]. MM foci of lymph node could be easily ignored or missed. Amplifying tumor- or tissue-specific mRNA by RT-PCR can detect tumor cells from 106 to 107 normal cells, and further detect the MM foci of lymph nodes. Patients with MM identified by RT-PCR are often in the developing stage of the disease, and symptoms are likely to turn out to be negative after the therapy. Therefore, lymph node metastasis is not only a prognosis indicator but also an indicator for assessing the effectiveness of therapy and monitoring early relapse[5]. Lymph node metastasis was not only an index of dys-outcome, but also has curative effect and monitoring earlier period relapse[3]. The hematogenous dissemination is another pathway of gastric carcinoma MM. Although the presence of carcinoma cells in blood does not necessarily lead to metastasis, entry of carcinoma cells into blood is the first step for the occurrence of tumor transfer in remote areas. Tumor cells shedding from pro-foci in perioperative or postoperative periods have spread to blood, lymph nodes, marrow or other organs even before pro-foci formation. However, it is difficult to detect metastasis in patients without clinical symptoms by traditional methods such as imaging and clinical pathology. Therefore, many researchers are dedicated in searching sensitive and specific methods to detect MM.
CK18 as a marker for micrometastasis
Cytokeratins (CKs) constitute the cytoskeleton of intermediate filament type in most epithelial cells. They consist of at least 20 members, designated as CK1-CK20 according to their molecular weights and isoelectric points[6,7]. Each type of epithelial cells has a rather stable CK composition, termed as CK pattern, which has been used in the identification of different epithelial tissues and their neoplasms[7]. CKs are expressed in the epithelial tissues, but not in the intermediate filament tissues (blood vessel, nerve, lymph tissues). They are continuously expressed in epithelial cells during malignant transformation and tumor formation, but not in normal lymph nodes, which provides the basis for applying CKs in diagnosing gastric carcinoma MM[8]. Molecular markers of malignant tumor MM from epithelial tissues have been explored recently[9-11]. For example, Liu et al[9] from abroad used CK20 mRNA as a marker to examine large intestine carcinoma with RT-PCR. With CK19 mRNA as a marker Ueda et al[10] examined lung carcinoma with RT-PCR. Noguchi et al[11] examined CK19 mRNA in lymph nodes from 10 cases of gastric carcinoma with RT-PCR. Domestic researchers have detected lymph node MM of gastric carcinoma by targeting CK19, CK20 with RT-PCR technique[12,13]. Lymph node MM of gastric carcinoma by choosing CK7, CK8 with immunofluorescence stain and immunocyte chemical technique[1]. Different from other CKs, CK18 has a stricter specific distribution in epithelial cells and a specific expression in cells from glandular epithelium[14]. Therefore, if CK18 mRNA is expressed in lymph nodes from gastric carcinoma patients, it indicates the existence of tumor metastasis in lymph nodes; If CK18 mRNA is expressed in peripheral blood from gastric carcinoma patients, it indicates the possibility that metastasis has been transferred to other distant organs.
Correlation between gastric carcinoma micrometastasis and CK18 expression
RT-PCR was used in this study to detect CK18 mRNA. Results from analyzing 298 lymph nodes of gastric carcinoma and 20 lymph nodes from control group I indicate that: (1) In contrast to the findings of negative expression in all the benign pathological changes of lymph nodes, CK18 mRNA showed positive expression in gastric carcinoma tissues, indicating that RT-PCR technique has a high specificity for detecting CK18 mRNA. Expression of CK18 mRNA was detected in lymph nodes from patients with gastric carcinoma, indicating that the cancer is transferred. CK18 mRNA was positively expressed in 34 of 199 lymph nodes (17.1%), showing that RT-PCR has a high sensitivity for detecting lymph node metastasis of gastric carcinoma. As lymph node metastasis of gastric carcinoma directly affects the prognosis of gastric carcinoma patients, lymph nodes around gastric carcinoma tissues should be removed. According to UICC/AJCC new classification of lymph node metastasis of gastric carcinoma in 1997[15], 199 lymph nodes with PN0 from 35 patients with gastric carcinoma demonstrated by pathological examination in postoperative, contained 17.1% lymph node micrometastases by RT-PCR that should be classified into PN1. The main risk factors for lymph node metastasis include the size of primitive tumor, the degree of differentiation and depth of invasion[16]. Fukagawa et al[17] have found that metastasis occurs more often from tumescent growth tumors than from invasive growth tumors. The current study has revealed that lymph node MM is highly correlated with the depth of invasion (T)[18]. It was reported that patients (PT3N0) without lymph node metastasis detected by routine pathological examination have lymph node metastasis (PT3N1) detected by RT-PCR[19]. Suggesting that MM examination should be conducted for PN0 to improve the accuracy of clinical stage of gastric carcinoma, MM in peripheral blood does not necessarily develop clinical symptoms, but it correlates with biological behavior of gastric carcinoma. Zhang et al[20] demonstrated that CK20 mRNA is a marker of gastric carcinoma MM. Chausovsky et al[21] used CK20 mRNA as a target gene to examine MM of gastric carcinoma in peripheral blood from 116 patients with malignant neoplasm, and found that CK20 mRNA is a target gene for MM of epithelial tumor. Majima et al[22] took CK19 and CK20 mRNA as target genes to examine MM in peripheral blood from 52 patients with gastric carcinoma and found that CK19 mRNA is better than CK20 mRNA as a marker. The current study detected the expression of CK18 mRNA in peripheral blood from 54 patients with gastric carcinoma and found that the positive expression was 38.9% (21/54) compared to control group II, suggesting that patients with gastric carcinoma develop MM in peripheral blood. Suppressed by the organic defense mechanism, carcinoma cells in peripheral blood are in dormant status after they disseminate into the organs. However, carcinoma cells can grow and reproduce again due to multiple factors such as aggravation of the disease which eventually lead to clinical metastasis and relapse, thus influencing prognosis. Therefore, examination of MM in peripheral blood among patients with gastric carcinoma is recommended. It helps to determine relevant assistant therapy and to evaluate prognosis.
In conclusion, RT-PCR has a high specificity and sensitivity in detecting CK18 mRNA in gastric carcinoma patients and can be used to evaluate metastasis of gastric carcinoma at the molecule level.
Footnotes
Supported by the Natural Science Foundation of Jilin Province, No. 20010594
Science Editor Wang XL and Guo SY Language Editor ELsevier HK
References
- 1.Chen XM, Chen GY, Zhang X. The cytological examination of cancer cells in peripheral blood of patients with gastric cancer. Zhonghua Xiaohua Zazhi. 2002;22:481–484. [Google Scholar]
- 2.Zheng JJ, Hu P, F JX. Clinical significance and detection of micrometastasis with gastric carcinoma. Fubu Waike. 2002;15:63. [Google Scholar]
- 3.Liu Y. The evaluation of PCR in diagnosis of genes with gastric carcinoma. Xibei Minzu Zazhi. 2002;23:50–51. [Google Scholar]
- 4.Weng DS, Ding YQ. Studying progression of gene controlling of tumor metastasis. Yixue Zongshu. 2003;9:131–133. [Google Scholar]
- 5.Xiao WD, Peng CH, Wang ML, Zhou YQ, Cheng H, Zhu PQ. Detecting micrometastasis of pathology negative lymph nodes with gastric carcinoma by RT-PCR. Zhongguo Puwai Jichu yu Linchuang Zazhi. 2003;10:487–488. [Google Scholar]
- 6.Gong YL, Zhou QH. Study progression of moleculor diagnosis of lung carcinoma micrometastasis. Zhongguo Feiai Zazhi. 2002;3:75. [Google Scholar]
- 7.Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427–447. [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XW, Liu HW. Studying progression of lymph nodes with gastric carcinoma. Yixue Zongshu. 2001;7:645. [Google Scholar]
- 9.Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Chin Med J. 2002;115:529–531. [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda Y, Fujita J, Bandoh S, Hojo S, Yamaji Y, Ohtsuki Y, Dobashi N, Takahara J. Expression of cytokeratin 19 mRNA in human lung cancer cell lines. Int J Cancer. 1999;81:939–943. doi: 10.1002/(sici)1097-0215(19990611)81:6<939::aid-ijc16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi S, Hiratsuka M, Furukawa H, Aihara T, Kasugai T, Tamura S, Imaoka S, Koyama H, Iwanaga T. Detection of gastric cancer micrometastases in lymph nodes by amplification of keratin 19 mRNA with reverse transcriptase-polymerase chain reaction. Jpn J Cancer Res. 1996;87:650–654. doi: 10.1111/j.1349-7006.1996.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Q, Zhang YCh, Hong HQ, J BC, Xu J, Qian HQ. Detecting lymph nodes micrometastasis with gastric carcinoma by reverse transcriptase-polymerase chain reaction. Zhonghua Waike Zazhi. 2000;38:621. [Google Scholar]
- 13.Lu J, Wu YG, Mu QL, Wu TH. Studying of micrometastasis of peripheral blood preoperative and postoperative with gastric carcinoma. Shandong Yiyao. 2002;42:1–3. [Google Scholar]
- 14.Chen YD, Liu FK, Qi XP, Li JT. The studing of bone marrow micrometastasis and proliferation in gastric carcinoma. Yixue Yanjiusheng Bao. 2001;14:297–298. [Google Scholar]
- 15.Roder JD, Böttcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer. 1998;82:621–631. [PubMed] [Google Scholar]
- 16.Maehara Y, Orita H, Okuyama T, Moriguchi S, Tsujitani S, Korenaga D, Sugimachi K. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–247. doi: 10.1002/bjs.1800790320. [DOI] [PubMed] [Google Scholar]
- 17.Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, Nakanishi Y, Shimoda T. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer. 2001;92:753–760. doi: 10.1002/1097-0142(20010815)92:4<753::aid-cncr1379>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Ishida K, Katsuyama T, Sugiyama A, Kawasaki S. Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer. 1997;79:1069–1076. doi: 10.1002/(sici)1097-0142(19970315)79:6<1069::aid-cncr3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M. Detecting lymph nodes and lymph location drawing with PN0 gastric carcinoma by reverse transcriptase-polymerase chain reaction(British) Guowai Yixue Waikexue Fence. 2003;1:62–63. [Google Scholar]
- 20.Zhang XW, Fan P, Yang HY, Yang L, Chen GY. [Significance of detecting disseminated tumor cells in peripheral blood of gastric and colorectal cancer patients] Zhonghua Zhong Liu Za Zhi. 2003;25:66–69. [PubMed] [Google Scholar]
- 21.Chausovsky G, Luchansky M, Figer A, Shapira J, Gottfried M, Novis B, Bogelman G, Zemer R, Zimlichman S, Klein A. Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer. 1999;86:2398–2405. [PubMed] [Google Scholar]
- 22.Majima T, Ichikura T, Takayama E, Chochi K, Mochizuki H. Detecting circulating cancer cells using reverse transcriptase-polymerase chain reaction for cytokeratin mRNA in peripheral blood from patients with gastric cancer. Jpn J Clin Oncol. 2000;30:499–503. doi: 10.1093/jjco/hyd130. [DOI] [PubMed] [Google Scholar]


