Abstract
AIM: To investigate the effects of 8-Br-cAMP on differentiation and apoptosis of human esophageal cancer cell line Eca-109, and the related gene expression.
METHODS: The cultured Eca-109 cells were divided into four groups: E1 group (co-cultured with 8-Br-cAMP for 24 h); E2 group (co-cultured with 8-Br-cAMP for 48 h); C1 group (treated without 8-Br-cAMP for 24 h); and C2 group (treated without 8-Br-cAMP for 48 h). The same concentration of cell suspension of each group was dropped separately onto the slides and nitrocellulose membranes (NCM). The biotin-labeled cDNA probes for c-myc, wild-type (wt) p53, bcl-2 and iNOS were prepared for in situ hybridization. The expressions of epidermal growth factor receptor (EGFR), p38 kinase, FAS, FasL and caspase-3 were detected using immunocytochemistry, and the NOS activity and the ratio of differentiated cells/proliferating cells were examined by cytochemistry. Immunocytochemistry, cytochemistry, and in situ hybridization were separately carried out on both slides and NCM specimens for each group. In addition, TUNEL was used to detect the cell apoptosis rate in each group.
RESULTS: The apoptotic rate of E2 group was significantly higher compared to E1 group, while there was no difference in the ratio of differentiated cells/proliferating cells between E1 and E2 groups. The signals of wt p53 and iNOS were markedly stronger, while the signals of c-myc and EGFR were obviously weaker in E1 group than those in C1 group (P<0.05). Moreover, the signals of wt p53, iNOS, p38 kinase, caspase-3 and NOS activity were significantly stronger, whereas, the signals of bcl-2, c-myc and Fas/FasL were markedly weaker in E2 group than those in C2 group (P<0.05).
CONCLUSION: The differentiation and apoptosis of human esophageal cancer cell Eca-109 can be induced after 24- and 48-h treatment with 8-Br-cAMP, respectively. Upregulation of wt p53, iNOS and downregulation of c-myc may be associated with differentiation and apoptosis of Eca-109 cells. Furthermore, upregulation of FasL, p38 kinase and caspase-3 as well as downregulation of bcl-2, and Fas may be involved in the apoptosis of Eca-109 cells.
Keywords: Differentiation, Apoptosis, Gene expression, 8-Br-cAMP, Eca-109 cell line
INTRODUCTION
There are two isoforms of cAMP receptor proteins, type I (PKAI) and type II (PKAII). PKAI can stimulate cell growth, while PKAII can inhibit it. Cho-Chung[1] have reported that the nontoxic 8-Br-cAMP is one of the site-selective cAMP analogs to combine with the PKAII attractively and 8-Br-cAMP could induce cancer cell differentiation via regulatory balance of the intracellular signal transducers of cAMP. It was reported that cAMP analogs or upregulation of cAMP/PKA pathway could suppress tumor malignancy through growth inhibition and differentiation induction[2,3]. In our previous studies, we found that 8-Br-cAMP could inhibit growth-related gene expressions in Eca-109 cells mainly through regulation of c-myc, epidermal growth factor receptor (EGFR), c-fos and wild-type (wt) p53 expressions; and facilitate retinoblastoma Rb44 cell differentiation mainly through iNOS gene expression[4,5]. Some studies demonstrated that the acid-denatured and methyl green-pyronin stain could be used for the identification of differentiated non-proliferating cells[6-8]. It is known that the oncogene/proto-oncogene, EGFR and c-myc can enforce cell proliferation, while the anti-oncogene, wt p53, can inhibit it; iNOS, p38 kinase, Fas/FasL and caspase-3 are mainly associated with apoptosis, while bcl-2 is of anti-apoptotic; besides, different expression levels of the same gene may result in different effects. In this study, we aimed to investigate the effects of 8-Br-cAMP on differentiation and apoptosis of human Eca-109 cells, and to examine the alteration of related gene expressions by using in situ hybridization, immunocytochemistry and cytochemistry.
MATERIALS AND METHODS
Cell culture
The human esophageal cancer cell line Eca-109 was cultured in DMEM (Gibco BRL, USA) supplemented with 100 mL/L fetal bovine serum. The Eca-109 cells were cultured with 2×10-5 mol/L of 8-Bromo-cAMP (Sigma, USA) for 24 h as the experimental 1 (E1) group and for 48 h as the experimental 2 (E2) group. The Eca-109 cells were cultured with the same medium without any drug for 24 h as the control 1 (C1) group and for 48 h as the control 2 (C2) group.
Preparation of specimen
The cultured cell suspension (1×106 cells) of each group was dropped onto the pretreated slides and then the slides were dried, followed by fixation with 20-40 g/L paraformaldehyde and stored at -20 °C.
Furthermore, the cultured cell suspension (1×106 cells) of each group was dropped onto the nitrocellulose membrane (NCM, ProtranTM, USA) pretreated with 20 × SSC for dot blot hybridization or with RNase-free water for immunodot blotting, and then NCM specimens were dried and stored at -20 °C.
Labeling and sensitivity detection of cDNA probes
The denatured cDNAs of wt p53, c-myc, bcl-2, and iNOS were labeled with biotin-11-dUTP (Sigma, USA) by random primer system (Promega, USA), and the sensitivity of each probe was detected using DNA dot blotting.
In situ hybridization
After being pretreated with 5 mg/mL final concentration of proteinase K (Promega, USA) for 10 min, post-fixation with paraformaldehyde and pre-hybridization without the cDNA probes for 3 h, the slide specimens of each group were hybridized with each kind of cDNA probes in final concentration of 0.5 µg/mL at 42 °C overnight. Then the slides were stringently washed four times with 0.1× SSC at 42 °C for 15 min. The streptavidin-alkaline phosphatase (dilution 1:1 000, Promega, USA) was added onto the slides after blocking with 10 g/L acetylated BSA. The nitroblue tetrazolium and 5-bromo 4-chloro 3-indole phosphate (NBT-BCIP, Promega, USA) were used as the substrate to develop the signals in bluish-violet color. For detection of intact cell using dot blot hybridization, the NCM specimens were first rinsed rapidly with chloroform, followed by treating at 80 °C under vacuum for 2 h and subsequently treated basically as aforementioned procedures for the slides.
Immunocytochemistry
The slide specimens of respective groups were treated with 3 g/L Triton X-100/PBS for 10 min, 30 mL/L H2O2 5 min, 0.01 mol/L citrate buffer (pH 6.0) at 95 °C for 10 min, washed with 1× PBS after each step. The slides were treated with 100 mL/L normal goat serum for 20 min, followed by incubation with the primary mAbs against EGFR (Sigma, USA) for E1 and C1 groups, and against p38 kinase and caspase-3 (Invitrogen, USA) for E2 and C2 groups at a dilution of 1:100 at 4 °C overnight. The HRP-labeled or alkaline phosphatase-labeled goat anti-mouse serum was added onto the slides for 1 h as the secondary antibody. The slides were thoroughly washed with PBS after treatment with respective antibodies. The DAB/H2O2 was used as the substrate to develop the positive signals in brownish color, while NBT/BCIP was used as the substrate to develop bluish-violet color. The specific primary antibodies were replaced by PBS for the negative controls. For detection of immunodot blotting, the NCM specimens were treated using the above procedures except for substitution of 0.5 g/L Tween 20 in TBS (Tris-Cl buffer saline) for PBS.
Cytochemistry
The slides of each group were incubated with phosphate buffer (PB) containing 1.0 mg/mL reduced form of NAD 1 (co-enzyme 1) and 0.5 mg/mL NBT at 37 °C for 1.5 h. The negative control was performed simultaneously except for the addition of PB alone.
Ratio of differentiated cells/proliferating cells
The slides of each group were re-fixed with ice-cold Carnoy’ s fixative for 15 min, followed by digestion of intra-nuclear DNA with 1 mol/L HCL in 800 mL/L ethanol for 5 min. The slides were stained with 5 g/L methyl green and pyronin GS (BDH, UK) in an acetate buffer (pH 4.8) for 5-7 min, subsequently washed with D.H2O and acetone.
Detection of apoptosis rate by TUNEL
The slides of each group were treated with 50 mg/mL levamizol in ethanol for 15 min, followed by digestion with proteinase K and post-fixation with paraformaldehyde. Then the slides were incubated with 1 mg/mL terminal deoxyribonucleotidyl transferase (TdT, Promega, USA), biotin-11-UTP (Sigma, USA) and dNTP in TdT buffer at 4 °C overnight. The following procedures were performed similarly as those for in situ hybridization. The negative control was carried out at the same time except for incubation with TdT buffer alone in the total incubation substrate.
Statistical analysis
Each kind of dot blotting was repeated six times. The dot blotting in violet color was scanned at 560 nm by thin-layer chromatography scanner (Shimada, Japan), and that in brownish color was scanned at 420 nm. The signal intensity in slide specimens was calculated as total integration value in more than 100 cells under oil-microscope. The data were analyzed with SSPS 10. A P value less than 0.05 was considered statistically significant.
RESULTS
The sensitivity of each kind of biotin-labeled cDNA probe, including wt p53, c-myc, bcl-2, and iNOS, could approach to 1.0 ng/L as detected by DNA dot blotting. The apoptotic signals in violet color were localized in the nuclei mostly translocated towards cell periphery. The cell apoptosis rate and the ratio of differentiated cells (D)/proliferating cells (P) in each group are shown in Table 1 and Figure 1 A-D.
Table 1.
Apoptosis rate and ratio of differentiated cells (D)/proliferating cells (P) in each group
| E1 group | C1 group | E2 group | C2 group | |
| Apoptosis rate | 16%a | 3.00% | 54%c | 4.00% |
| Ratio of D/P | 1.7 (63/37)e | 0.2 (17/83) | 2.3 (70/30)g | 0.16 (14/86) |
Apoptosis rate:
P<0.05 vs C1 and E2 groups;
P<0.05 vs C2 group. Ratio of D/P:eP<0.05 vs C1,
P<0.05 vs C2.
Figure 1.
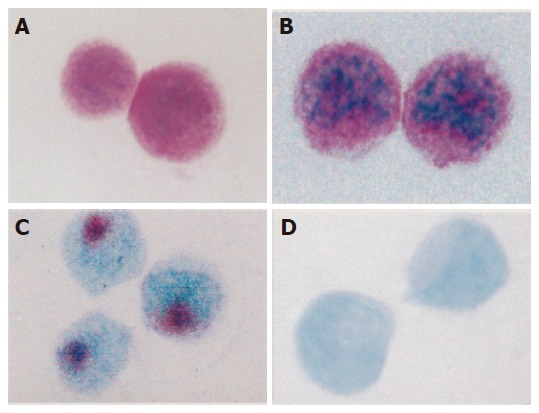
A :HCl denaturation and methyl green-pyronin staining of differentiated Eca-109 cells (×1 000); B: HCl denaturation and methyl green-pyronin staining of proliferating Eca-109 cells (×1 000); C: TUNEL assay showing apoptotic Eca-109 cells (×1 000); D:TUNEL assay showing non-apoptotic Eca-109 cells (×1 000).
The results showed that there was a significant difference in apoptosis whereas no significant difference in differentiation between E1 and E2 groups.The signals of both in situ hybridization (gene transcription of c-myc, wt p53 and iNOS) and EGFR-immunoreactivity (IR) were all localized in the cytoplasm. The hybridization signals appeared as violet color granules; the signal intensity of wt p53 and iNOS in E1 group was markedly higher than that in the C1 group, whereas that of c-myc and EGFR in E1 group was significantly lower than that in the C1 group. The scanning values on NCM specimens for each kind of signals are shown in Table 2.
Table 2.
Comparison of signal scanning values on NCM between E1 group and C1 group (mean±SD)
| Groups | c-myc mRNA | Wt p53 mRNA | iNOS mRNA | EGFR-IR |
| E1 group | 3.38 ± 0.99 | 2.74 ± 0.83 | 4.52 ± 0.74 | 2.37 ± 1.05 |
| C1 group | 5.18 ± 1.39 | 0.38 ± 0.27 | 2.63 ± 0.13 | 4.38 ± 0.48 |
P < 0.05, E1 vs C1 for each signal.
The hybridization signals in violet-colored granules of c-myc, wt p53 and iNOS were localized in the cytoplasm, while that of Fas-IR and FasL-IR were located surrounding the cytomembrane. The signal intensity of bcl-2, c-myc gene expression and Fas/FasL-IR was obviously decreased in E2 group as compared to C2 group, while that of wt p53 and iNOS was markedly increased in E2 group as compared to C2 group. The scanning values of each signal on NCM specimens are shown in Table 3.
Table 3.
Comparison of signal scanning values on NCM between E2 group and C2 group (mean±SD)
| Groups | bcl-2 | c-myc | iNOS | Wt p53 | Fas/FasL-I |
| mRNA | mRNA | mRNA | mRNA | R | |
| E2 group | 1.36 ± 0.54 | 1.72 ± 0.76 | 5.47 ± 0.35 | 4.76 ± 1.28 | 2.69 ± 0.73 |
| C2 group | 3.49 ± 1.53 | 5.22 ± 1.44 | 2.65 ± 1.22 | 0.42 ± 0.28 | 4.17 ± 0.92 |
P < 0.05, E2 vs C2 for each signal.
The brownish-colored granules of caspase-3 IR were scattered in the cytoplasm, the signal intensity of E2 group was obviously higher than that of C2 group. In C2 group, the p38-IR staining appeared as yellow-brownish colored granules in the cytoplasm, while in E2 group, the p38-IR staining with stronger intensity was mostly located in the nuclei (the activated p38 kinase translocated from cytoplasm into nuclei). The violet-colored NOS activity located in the cytoplasm was markedly increased in E2 group as compared to C2 group (Table 4).
Table 4.
Comparison of total integration of signal intensities for p38-IR, caspase-3-IR and NOS activity between E2 and C2 groups
| Group | p38-IR | Caspase-3 IR | NOS activity |
| E2 group | 184 | 348 | 302 |
| C2 group | 122 | 207 | 138 |
P < 0.05, E2 vs C2 for each signal.
DISCUSSION
It is well known that DNA can be stained by methyl green, and RNA by pyronin in cytochemistry. Sen et al[8] demonstrated that the proliferating cells were differentially stained by methyl green in bluish-green color and the differentiated cells were stained mainly by pyronin in red color, since the nuclear DNA of proliferating cells was less sensitive to hydrolysis with hydrochloric acid as compared to the differentiated cells. In this study, the ratio of differentiated cells/proliferating cells had no significant difference between E1 and E2 groups, the effect of cell differentiation or proliferation inhibition could be demonstrated earlier in E1 group induced with 8-Br-cAMP for 24 h. However, there was a significant difference in the apoptosis rate between E1 and E2 groups.
It was reported that the activation of EGFR, c-myc oncogene, and inactivation of wt p53 tumor suppressor gene could be detected in the development of squamous cell carcinoma of the esophagus[9]. The wt p53 encoded by wt p53 tumor suppressor gene antagonized cell cycle progression to inhibit cell growth with contrary effect to EGFR and c-myc through p21waf1, which was elucidated in our previous study[4]. It is known that the EGF receptor in the esophageal cancer, homologous to v-erbB, is a truncated form of EGFR with deletion of extra-cellular ligand domain, and a persistent tyrosine kinase activity displayed to stimulate the cancer cell growth independent of EGF ligand. The c-myc proto-oncogene encodes transcriptional regulator; upregulation of myc enforces cell growth; while abrupt downregulation of myc or withdrawal of any growth cytokine from environmental factors may have an association with cell differentiation and apoptosis[10]. In this experiment, downregulation of myc may be associated with decreased expression of EGFR. The precise mechanism of myc-induced pathways to contribute to apoptotic response is still largely unsolved[11].
It was found that multifunctional gene p53 could be involved in cell differentiation and apoptosis in malignant tumors[12]. Schwartz et al[13] reported that the osteoblasts in an osteosarcoma cell line could be induced by p53 to produce osteocalcin product. Tsumamoto et al[14] indicated that NO could induce cell differentiation in the rat retinal ganglion cells. Our previous study showed that NO and NOS could induce the retinoblastoma HXO-Rb44 cells towards differentiation with enhanced enolase expression[5]. The wt p53 could be upregulated by NO[15]; and apoptosis of human carcinoma cells could be induced by inducible NOS expression[16,17]. The exogenous wt p53 at higher level could inhibit proliferation of K562 cells and induce apoptosis; but could induce the cell differentiation at lower p53 level[18]. Similarly, the NO, product of NOS, in a small amount could involve cell protection, while a great amount of NO could cause cytotoxicity or apoptosis[19]. Interestingly, in this study, we observed that downregulation of EGFR and c-myc and upregulation of wt p53 and iNOS in E1 group (after treatment with 8-Br-cAMP for 24 h) might be associated with differentiation; and further downregulation of c-myc and upregulation of wt p53 and iNOS in E2 group (after treatment with 8-Br-cAMP for 48 h) might be associated with apoptosis in Eca-109 cells.
Our previous study demonstrated that the decreased ratio of bcl-2/bax expression was associated with inhibition of cell apoptosis. The downregulation of anti-apoptotic bcl-2 could cause the cells sensitive to apoptosis, and p53 could inhibit the bcl-2 expression and promote expression of pro-apoptotic Fas; besides, bcl-2 could participate in NO-mediated and p53-mediated apoptosis[14]. The Fas has been shown as a target gene for transcription and activation by p53[20]. The combination of Fas with FasL could participate in apoptosis of tumors, such as ovarian cancer, etc.[21]. In this experiment, the decreased Fas/FasL ratio implicated that the decreased Fas with increased FasL might be induced by 8-Br-cAMP after 48 h of treatment.
Among the mitogen-activated protein kinase (MAPK) family, the p38-MAPK has been reported to be involved in apoptosis, while ERK-1 oppositely stimulated cell growth; and the p53 could be activated by p38 through N-terminal serine in p53 phosphorylated by p38-MAPK[22]. Kim et al[23] indicated that NO-induced p38 kinase activity was an induction signal for apoptosis of chondrocytes in association of p53 accumulation, caspase-3 activation and differentiation status. The caspase-3 is a downstream effector member in the caspase family, playing a role in the final common pathway of apoptosis. It was reported that Fas could induce apoptosis through caspase effector pathway directly, not necessary to be gene-mediated[24]. Our results suggested that 8-Br-cAMP-induced Eca-109 cell apoptosis might mainly be mediated through downregulation of bcl-2 by wt p53 and upregulation of wt p53 by p38 kinase; or wt p53-mediated Fas/NO effector pathway may finally come to the common pathway of activated caspase-3. Hence, upregulation of wt p53 may play an important role, synergistically in combination with regulation of related gene expressions to form a regulatory network involved in Eca-109 cell differentiation and apoptosis. Understanding of the regulatory network may contribute to providing novel strategy and bright insight for cancer therapy.
Footnotes
Supported by the Research Science Foundation of Henan Province, No. 2000180007
Science Editor Kumar M , Li WZ and Guo SY Language Editor Elsevier HK
References
- 1.Cho-Chung YS. Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approaches to therapy. Cancer Res. 1990;50:7093–7100. [PubMed] [Google Scholar]
- 2.Carlson CC, Burnham LL, Shanks RA, Dransfield DT. 8-Cl-adenosine induces differentiation in LS174T cells. Dig Dis Sci. 2001;46:757–764. doi: 10.1023/a:1010740015072. [DOI] [PubMed] [Google Scholar]
- 3.Chen TC, Hinton DR, Zidovetzki R, Hofman FM. Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation, and leads to apoptosis in malignant gliomas. Lab Invest. 1998;78:165–174. [PubMed] [Google Scholar]
- 4.Chen KS, Zheng NG, Wu JL, Ding Y, Wang YL. Effects of 8-Br-cAMP on growth related gene expressions in human esophageal cancer Eca-109 cells. Acta Anatomica Sinica. 1999;30:227–229. [Google Scholar]
- 5.Deng X, Wu J, Guo X, Han X, Ding X. Effect of 8-bromo-cyclic AMP on neuron specific enolase, heat shock protein, nitric oxide, nitric oxide synthase and nitric oxide synthase mRNA in human retinoblastoma HXO-Rb44 cells and cell differentiation. Chin Med J (Engl) 2000;113:198–200. [PubMed] [Google Scholar]
- 6.Iseki S, Mori T. Methyl green-pyronin stain distinguishes proliferating from differentiated nonproliferating cell nuclei after acid denaturation of DNA. J Histochem Cytochem. 1986;34:683–687. doi: 10.1177/34.5.3701031. [DOI] [PubMed] [Google Scholar]
- 7.Chen KS, Zhang LQ, Wang HM, Wang YL, Wu JL. Effects of 8-Br-cAMP on human esophageal cancer Eca-109 cell growth and differentiation. J Henan Med Univ. 1998;33:99–101. [Google Scholar]
- 8.Sen JY, Huang QY, Gao HY, Liu YL, Cheng CF. Modification and application of methyl green-pyronin stain after acid denaturation of DNA. Progress Anat Sci. 1999;5:272–273. [Google Scholar]
- 9.Mandard AM, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res. 2000;462:335–342. doi: 10.1016/s1383-5742(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 10.Mata-Greenwood E, Cuendet M, Sher D, Gustin D, Stock W, Pezzuto JM. Brusatol-mediated induction of leukemic cell differentiation and G(1) arrest is associated with down-regulation of c-myc. Leukemia. 2002;16:2275–2284. doi: 10.1038/sj.leu.2402696. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 12.Mezencev R, Kohút A. [Apoptosis, tumor phenotype and pathogenesis of malignant tumors] Cesk Fysiol. 2004;53:48–65. [PubMed] [Google Scholar]
- 13.Schwartz KA, Lanciloti NJ, Moore MK, Campione AL, Chandar N. p53 transactivity during in vitro osteoblast differentiation in a rat osteosarcoma cell line. Mol Carcinog. 1999;25:132–138. doi: 10.1002/(sici)1098-2744(199906)25:2<132::aid-mc8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Tsumamoto Y, Yamashita K, Takumida M, Okada K, Mukai S, Shinya M, Yamashita H, Mishima HK. In situ localization of nitric oxide synthase and direct evidence of NO production in rat retinal ganglion cells. Brain Res. 2002;933:118–129. doi: 10.1016/s0006-8993(02)02289-8. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura Y, Kamoshima W, Shimohama S, Nomura Y, Taniguchi T. Nitric oxide donor-induced p53-sensitive cell death is enhanced by Bcl-2 reduction in human neuroblastoma cells. Neurochem Int. 1998;32:93–102. doi: 10.1016/s0197-0186(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 16.Hajri A, Metzger E, Vallat F, Coffy S, Flatter E, Evrard S, Marescaux J, Aprahamian M. Role of nitric oxide in pancreatic tumour growth: in vivo and in vitro studies. Br J Cancer. 1998;78:841–849. doi: 10.1038/bjc.1998.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Heldin NE, Grawé J, Ulmsten U, Fu X. Induction of apoptosis or necrosis in human endometrial carcinoma cells by 2-methoxyestradiol. Anticancer Res. 2004;24:3983–3990. [PubMed] [Google Scholar]
- 18.Liang M, Chen YZ, Wu Y, Yang XW, Lu LH. [Effects of Exogenous Wild-Type p53 Gene on K562 Cells] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2001;9:119–123. [PubMed] [Google Scholar]
- 19.Alexander B. The role of nitric oxide in hepatic metabolism. Nutrition. 1998;14:376–390. doi: 10.1016/s0899-9007(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 20.Kannan K, Amariglio N, Rechavi G, Givol D. Profile of gene expression regulated by induced p53: connection to the TGF-beta family. FEBS Lett. 2000;470:77–82. doi: 10.1016/s0014-5793(00)01291-6. [DOI] [PubMed] [Google Scholar]
- 21.Ghahremani M, Foghi A, Dorrington JH. Activation of Fas ligand/receptor system kills ovarian cancer cell lines by an apoptotic mechanism. Gynecol Oncol. 1998;70:275–281. doi: 10.1006/gyno.1998.5091. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 2000;60:2464–2472. [PubMed] [Google Scholar]
- 23.Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002;277:1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- 24.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]


