Abstract
Objective:
To determine the utility of gingival crevicular fluid (GCF) for the detection of anti-HIV antibodies in human immunodeficiency virus (HIV)-infected individuals using enzyme linked immunosorbent assay (ELISA) with respect to their CD4 counts.
Materials and Methods:
The study was carried out between 37 seropositive (37GCF, 10 saliva) and 37 seronegative (GCF) individuals for a period of 7-8 months in GDC Nagpur. Thirty-seven GCF and 10 whole saliva samples were collected from the same patient. GCF samples were collected from gingival crevice with the help of Kimble disposable microcapillary. Saliva was collected by asking the patient to bend forward. The drooling saliva was collected in a sterile bottle and stored at Minus 20°C (-20°C). After the clinical observations the data were collected and tabulated for statistical analysis.
Results:
When compared with serum, the sensitivity, specificity, and positive and negative predictive values of GCF were 100% respectively.
Conclusion:
All the above findings are suggestive of GCF being a better diagnostic medium than saliva.
Keywords: Antibody, gingival crevicular fluid, immunodeficiency, infection, invasive, microcapillary, pigmentation, replication, reverse transcriptase, transudate
INTRODUCTION
Acquired immunodeficiency syndrome (AIDS) is the most devastating example of secondary immunodeficiency and was discovered in 1981. It is a retroviral disease caused by human immunodeficiency virus (HIV) and is characterized by profound immunosuppression leading to opportunistic infection, secondary neoplasms, and neurologic manifestations.
On an average, it takes 10 years to develop AIDS. The disease has 100% mortality rate and over 359,000 cases were reported to the World Health Organization (WHO) from 162 countries in 2004. By the end of 2004 it was estimated that worldwide 44million people become newly infected, including 800,000 children under 15 yr age.
Over the past two decades, a number of important advances have been made in the arena of retroviral testing. With the evolution of technology, improvements in screening, confirmatory, and HIV monitoring assays have been made and they offer better alternatives to address blood screening, surveillance, diagnosis, and patient management. Many of the newly evolving technologies are essential for use in resource limited countries because they can address the following issues: cost, limited infrastructure, and a lack of formally trained personnel.
During the past decades, technology has evolved to produce newer rapid kits that supplement the classical techniques. The use of oral fluids also has been advocated as a painless and non-invasive alternative to the collection of blood for detection of antibodies to a number of specific bacterial, viral, fungal, and parasitic agents. Oral fluid testing for HIV antibodies was first reported by Archibald et al. and Parry et al. in 1987. Oral fluids are a mixture, with saliva and gingival crevicular fluid (GCF; also called as oral mucosal transudate) being the main components. GCF and saliva are identified as distinct body fluids. Whole saliva and GCF can be distinguished by the fact that the immunoglobulin G (IgG) content of the GCF is several times greater than that of saliva.
Previous studies have been conducted with a limited number of selected samples of whole saliva. The sensitivity of enzyme-linked immunosorbent assay (ELISA) in comparing paired serum and salivary samples ranged from 82 to 98.5%, which was lower than that of GCF as observed in a study carried out by Soto-Ramirez et al. in 1992. However, comparative studies of whole saliva and gingival crevicular transudate GCT or GCF will be necessary to define the true sensitivity of ELISA in these fluids.
Thus the present study was carried out to detect the anti-HIV antibodies in GCF by ELISA with respect to their CD4 counts in seropositive individuals.
MATERIALS AND METHODS
After taking permission from the ethical committee, the study was carried out between 37 diagnosed seropositive (HIV +ve) and 37 seronegative (HIV -ve) individuals for a period of 7-8 months 2006 in GDC Nagpur. HIV positive individuals were selected from antiretroviral therapy (ART) clinic of GMC and H, Nagpur, and HIV negative individuals were Selected from the suspected individuals visiting to the Voluntary Counseling and Testing Centre (VCTC) of Indira Gandhi Government Medical College (IGGMC), Nagpur were included as the control group.
The selected HIV positive patients included in the study group were classified according to the WHO criteria and classified as:
a) stage I- 10 patients b) stage II- 10 patients c) stage III - 10 patients and d) stage IV- 7.
Method of sample collection
After clearance from the ethical committee, informed written consent, case history and their oral manifestations were noted.
The patients who had eatables, drinks, brushed teeth, used mouth wash before 1hour were avoided during sample collection. Figure 1.
Figure 1.
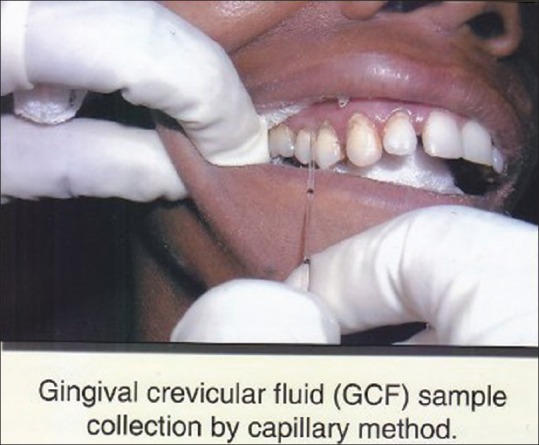
CGF sample collection
Then the GCF sample were collected using Kimble disposable 5 μl microcapillaries by asking the patients were seated comfortably with their head slightly reclined. The sample site was dried and isolated. White color coded 1-5 μl volumetric microcapillary was placed in the crevice. At the same time 10 whole saliva samples were also collected from the same patient and stored at -20°C (minus twenty degree).
By using HIV Chex ELISA kit GCF samples were processed according to the manufacturers instructions. Figures 2 and 3. The samples were brought to room temperatur e. 96 well dilution plate was taken and 250 μl of diluents was pipette into the well. Then 5 μl GCF was added to each well with the help of expirator and mix. Disposable tips were used for each sample. 100 μl of prediluted sample was transferred to the wells and incubated at room temperature for 30 min with cover plate. Wash for five cycles. Add 100 μl of working conjugate to the well, incubate it for 30 min at room temperature with the lid covered. Wash for another 5 cycles. 100 μl of chromogenic substrate was added the well and incubated in dark for 10 min at room temperature. Lastly add 50 μl of stop solution and read under ELISA reader at 450 nm/650 nm within one hour Figures 4 and 5.
Figure 2.

HIV - Kit
Figure 3.
Reagents in HIV Chex kit
Figure 4.
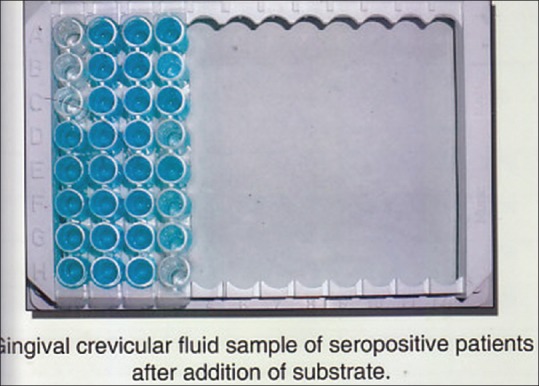
CGF after addition of substrate.
Figure 5.
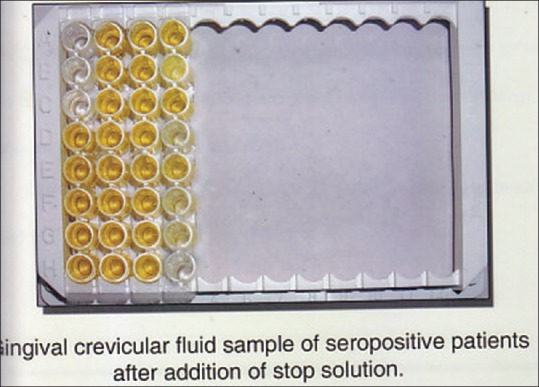
CGF after addition of stop solution.
Precautions
All samples and reagents were handled as potentially infectious agents. Disposable Tips were used. All materials were decontaminated in 5% sodium hypochlorite for 30-60 min before disposal.
Observation and Results
The present study was carried out in the Department of Oral Pathology and Microbiology, GDC and H, Nagpur in association with ART clinic, GMC and H, Nagpur
Study group was having 37 patients and were divided as stage 1, stage II, stage III, stage IV Control group showed 37 patient [Table 1]
In the study and control group, the age of the patients ranged from 20 to 60 years, and 67% (50 males) and 32% (24-females) were noted in both the groups thus it showed male predominance [Table 2]
Only 32% (12) patients showed oral lesions [Table 3]
-
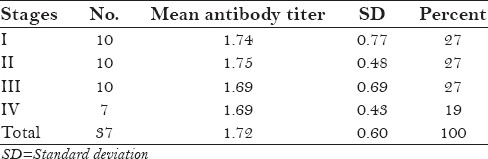
Comparison between different stages on the basis of their mean antibody titer and mean CD4 count showed
- highly significant difference in the CD4 counts of stage I when compared with stages III and IV was noted.
significant difference in the CD4 counts when stages II and IV; stage III and stage IV were compared, respectively
No significant difference was seen when mean antibody titers were compared Table 3 and Table 4.
The mean antibody titer of seropositive group (1.72 ± 0.595) was more than seronegative group (0.026 ± 0.042). Unpaired “t” test was applied and the “P” value was found to be < 0.05, and showed significant difference between the groups [Table 5].
When whole saliva and GCF were compared, the mean antibody titer were 1.737 and 1.780 respectively. Antibody titer of GCF (1.780) was higher than saliva. The mean OD of GCF was more than that of saliva. Paired “t” test was applied and ‘P value’ was less than 0.05 (P = 0.008). This difference was statistically significant [Table 6].
Mean CD4 count of seropositive patients with oral manifestation and without oral manifestation was statistically significant (P = 0.011) but the mean antibody titer was statistically non-significant [Table 7].
When the seropositive patients were examined, out of 37 patients only 12 patients showed oral manifestation, which were linear gingivitis, oral hairy leukoplakia, candidiatis etc. [Table 8].
When antibody titer of GCF were compared with serum the sensitivity, specificity, positive and negative predictive values of GCF were 100%. After the clinical observations, the data collected were tabulated and all the observed results were then subjected to statistical analysis.
Table 1.
Distribution of study and control groups
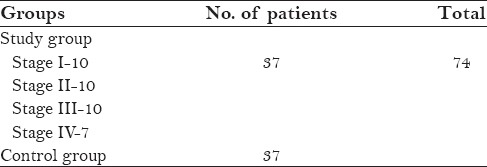
Table 2.
Age and sex distribution of study and control groups
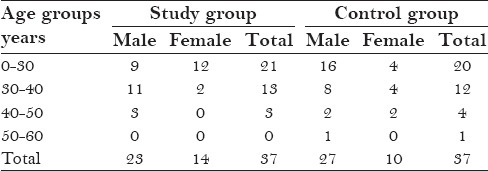
Table 3.
Distribution of oral manifestations
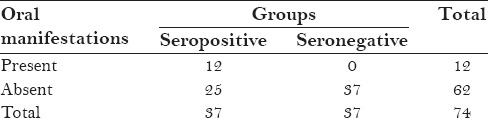
Table 4.
Stage wise distribution of mean CD4 count in serpositive patients
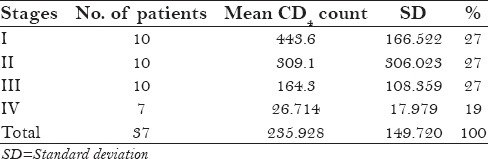
Table 5.
Comparison between seropositive and seronegative groups (on the basis of antibody titer)

Table 6.
Comparision of mean antibody titre of gingival crevicular fluid and saliva in seropositive patients
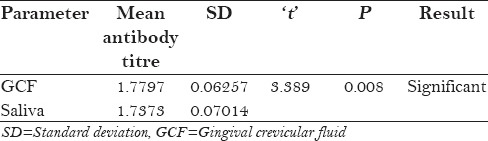
Table 7.
Comparision of mean antibody titre and mean CD4 count in seropositive patients with oral manifestation and without oral manifestation
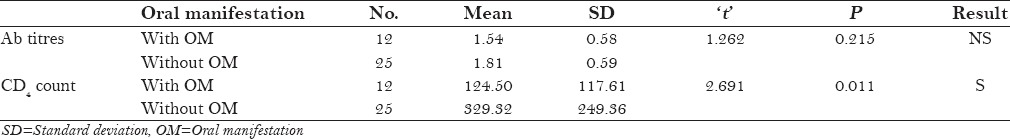
Table 8.
Stage wise distribution of antibody titers in seropositive patients
Chi-square test was applied to determine the statistical difference in sex wise distribution of patients between the study and control groups.
The formulae used to find the following were as follows:
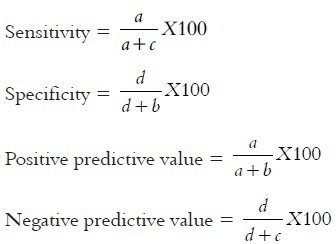
where a denotes true positive, b is false positive, c is false negative, and d is true negative.
Unpaired “t” test was applied to the results of ELISA to find the difference between the means of antibody titers in the following:
Seropositive and seronegative patients
Stage I and Stage II
Stage I and Stage III
Stage I and Stage IV
Stage II and Stage III
Stage II and Stage IV
Stage III and Stage IV
Patients with oral manifestation and without oral manifestation
Continuous clinical parameter is expressed in mean ± standard deviation (SD). The confidence interval for the study was at 95% and the significance level was 5%.
DISCUSSION
Immunodeficiency diseases may be caused by inherited defects affecting immune system development or they may result from secondary effects of other diseases. Clinically patients with immunodeficiency present with increased susceptibility to cancer. Immunodeficiency are of two of two types:
-
a)
Primary immunodeficiency: These are rare and genetically determined and affect either specific immunity or non specific host defense mechanisms mediated by complement proteins and cells such as phagocytes and NK cells
-
b)
Secondary immunodeficiency: May be encountered in patients with malnutrition, infection, cancer, renal disease or sarcoidosis.[1]
Acquired Immunodeficiency syndrome (AIDS) is the most common devastating secondary immunodeficiency.
AIDS is a retroviral disease caused by the human immunodeficiency virus and is characterized by profound immunosuppression leading to opportunistic infections, secondary neoplasms and neurologic manifestations.[2]
The first cases of AIDS were reported by Centre for Disease Control, 1981 in young homosexual men and later in injecting drug users and persons with hemophilia. The virus was first recognized in 1983 (Barre- Sinoussi et al.) and again simultaneously in France by Montagnier et al., 1984 who called the virus human T cell Leukemia/lymphoma virus (HTLV-III). HIV is transmitted by sexual contact with an infected person or through blood or blood products by Grassly et al., 2001. Babies born to HIV infected women may become infected before or during birth or through breast feeding after birth Scarlatti, 2004.[3]
These conditions are prevented by public health measures (screening of donated blood and plasma for antibody to HIV, screening for HIV associated p24 antigen, Heat treatment of clotting factor concentrates and screening for donors on the basis of history), and with all these measures the risk of HIV transmission is reduced. With the invent of serological testing methods the AIDS related death rates continue to progressively decline since 1995. However AIDS still remains the 5th most common cause of death in adults between ages of 25-44 years.[2]
HIV is 120 nm icosahedral, enveloped, single stranded RNA virus. It comprises of an outer envelope consisting of a lipid bilayer with uniformly arranged 72 spikes or knobs of gp120 and gp41. Inside is the protein core surrounding two copies of RNA. Core also contain viral enzymes reverse transcriptase, integrase and protease. Proteins p7 and p9 are believed to involved in regulation of gene expression.[4]
The primary target of HIV virus is CD4 subset of ‘T’ cells. CD4 cells decrease in number as the cells become infected and killed, but the CD8 subset is not affected, resulting in the decrease in the CD4 : CD8 cell ratio. It is quite remarkable that the virus does not replicate inside the CD4 cell until the ‘T’ cell is immunologically activated.[5] Viral neutralizing antibodies are found in AIDS and have been detected by replication of HIV in target cells or by inhibition of syncytium formation. However the diagnosis of HIV infection is routinely carried out by the enzyme-linked immunosorbent assay (ELISA).[6] Though the journey of detection of HIV began in early 1980's, the Enzyme labeled conjugates were first introduced in 1966 for localization of antigens in tissues.[2]
ELISA is the most commonly used type of test to screen HIV infection because of its relatively simple methodology, inherent high sensitivity, and suitability for testing large numbers of samples, particularly blood testing. A common feature of all ELISA is the use of enzyme conjugate that binds to specific HIV antibody, and substrates/chromogens that produce colour in reaction catalyzed by the bound enzyme conjugate.[7]
Body fluids other than serum are now being assayed for various types of antibodies. Urine and oral fluids (Gingival crevicular transudate and saliva) are readily obtained by non- invasive means, not requiring a venipuncture for sample collection.[8] As technology evolves, alternatives to the classic tests and testing strategies arise. Each offers one or more attractive features that may simplify collection, testing or interpretation of results.[9] Although generally referred to as “saliva”, the fluid used for testing is crevicular fluid, which is a transudate of blood and therefore similar to the samples used in serum based tests.[9]
In the Present study HIV antibodies in GCF were detected with the help of new investigational device (the microcapillary), can be promising screening procedure in diagnosis of HIV infection. In the study and control group the age of the patients ranged from 20-60 yrs and male predilection was noted. This finding was comparable with studies carried out by Reichart et al., Soto Ramirez et al.[2,10] So it can be said from the above observation that HIV infections are mostly found in 2nd – 6th decades with male predilection. HIV testing thus becomes necessary to find out the asymptomatic stage of the disease.
The mean antibody titres of patients with and without oral manifestations was statistically non significant whereas mean CD4 count was found to be statistically significant (P= 0.011). The mean antibody titre in 12 patients with oral manifestation was found to be less than the remaining 25 patients without any oral lesion. However no correlation could be established.
Soto Ramirez et al.[10] studied 296 individuals with dental diseases. In this study they found more number of patients with gingivitis. This was in contrast with our study where predominant lesions (3 cases) were oral hairy leukoplakia.
When the antibody titres of seropositive and seronegative individuals were compared, the mean antibody titre of GCF was higher in seropositive patients and was highly significant.
Earlier studies by various authors reported low infection capacity of saliva because it contains approximately 800 – 1000 fold less antibody than serum and plasma and also pointed out that 0.5 mg/L of IgG is required to carry out a very sensitive test for HIV.[6,11] Soto Ramirez et al., related that whole saliva consists mainly of secretory IgA and IgG levels are only 1% of those present in serum. Gingival crevicular transudate however contains mainly IgG in concentrations similar to those in serum and several times greater than saliva. Therefore it could be used for diagnostic procedures.[10,12]
Goodson JM[13] stated that GCF creates flushing action and isolation effect. The second effect dictates that the substance from outside do not penetrate the periodontal pocket i.e., saliva content do not generally enter the periodontal pocket so the concentration of IgG found in GCF from periodontal pocket is approximately 100 times that found in saliva and is now widely appreciated that GCF is formed as a blood ultrafiltrate.
In our study, sensitivity, specificity, positive and negative predictive value GCF were found to be 100% respectively. Soto Ramirez et al.[10] studied Gingival crevicular transudate and obtained sensitivity, specificity, positive negative predictive value were 99.5%, 100% and 99.9% respectively where sensitivity and negative predictive values were higher(100%) in our study. The variation in these values may be due to difference in the collection method/collecting devices used in the studies. “Orasure”, a collecting device, used in their study which consists of a chemically treated cotton fiber pad placed between the lower gum and cheek, rubbed back and forth and then kept in place for 2min. GCT obtained from this device shows four times greater IgG content than saliva,[10] whereas in the present study the collection of GCF was done with a microcapillary method. Microcapillary tubes of known internal diameter were inserted into the entrance of the crevice and the native GCF travels into the microcapillary. So GCF collection by microcapillary appears to be ideal as it provides an undiluted sample of native Gingival crevicular fluid[9] and appears to more accurate.
Gallo et al., 1997[14] reported sensitivity and specificity of GCF is 99.9% respectively but was not comparable with our study where sensitivity & specificity was 100%.
Holm-Hansen et al., 1993[15] proved sensitivity and specificity of 100% which was comparable with our study. On comparing GCF & saliva samples the mean optical density (OD) (1.78 ± 0.06) of GCF was higher than the mean OD of saliva (1.74 ± 0.07). Statistical significant difference was appreciated (p value = 0.008). Above statistical significant difference is indicative that antibody titres in GCF are higher than those found in saliva. Gingival Crevicular Fluid contains more IgG concentration than saliva and because of this it has become the logical focus of diagnosing diseases and hence the Gingival Crevicular Fluid can be used as a diagnostic medium.
GCF can also be of choice instead of serum because of non-invasiveness, painless, low cost, less sample, easy collection, safer disposal and also better for clinicians because of patients better compliance.
All the above findings are suggestive of GCF being a better diagnostic medium than saliva. However a comparative study of these two fluids on a larger sample size should be carried out.
Limitations
Time consuming method
Small sample size.
ACKNOWLEDGEMENTS
I extend my thanks to my guide Dr. Gosavi, and Dr. Ganvir, Dr. Hazare, and Dr. Chaudhari. I would like to thank all the people who directly or indirectly helped me in completing this thesis from which this article is extracted. My special thanks go to Dr. Jalgaonkar (Prof. and HOD) of microbiology department and Dr. Khadse (lecturer) of microbiology department at IGGMC, Nagpur. All the staff of VCTC, ART clinic, Nagpur are acknowledged. This research work could not have been completed without the support and guidance of my seniors Dr. Mehraj Shams (Fatema Saify) and D r. Prajkta Zade. I thank them from the bottom of my heart. Whatever I have achieved so far in my life has been because of the love, care, and support of my parents, my husband, and my two loving kids. Lastly, I thank all my patients who had been the heart and soul of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mindell D. Study theorizes that HIV existed centuries ago in African hamlets. AIDS Policy Law. 1995;10:6. [PubMed] [Google Scholar]
- 2.Riechart PA, Gelderbloom HR, Becker J, Kuntz A. AIDS and Oral Cavity. The HIV infection: Virology, etiology, Origin, immunology, precautions and clinical observation in 110 patients. Int J Oral Maxillofac Surg. 1987;16:129–53. doi: 10.1016/s0901-5027(87)80122-4. [DOI] [PubMed] [Google Scholar]
- 3.Mellanen L. Biomedicum research laboratory, Institute of Dentistry, University of Helsinki; 2006. Jun, Academic dissertation. The influence of HIV infection to periodontium: a clinical, microbiologic, and enzymological study. [Google Scholar]
- 4.Chennai: Publication: Tamil Nadu state AIDS control society, Indian Dental Association; 2004. AVNI health foundation. Oral Health and HIV / AIDS: A resource manual for oral health care practitioners, Mumbai, India. [Google Scholar]
- 5.Balasundaram S. Chennai, India: Ragas Dental College; 2002. HIV antibody screening: Saliva as alternative to serum. [Google Scholar]
- 6.George JR. USA: Proceedings of conference on the laboratory science of HIV; 1998. Alternative Specimen sources: Methods for confirming positives; pp. 47–60. [Google Scholar]
- 7.Mandel ID. Salivary diagnosis: more than a lick and a promise. J Am Dent Assoc. 1993;124:20–1. doi: 10.14219/jada.archive.1993.0007. [DOI] [PubMed] [Google Scholar]
- 8.Constantine NT, Zink H. HIV testing technologies after two decades of evolution. Indian J Med Res. 2005;121:519–38. [PubMed] [Google Scholar]
- 9.Constantine N. United States: University of Maryland School of Medicine, May; 2006. HIV Antibody Assays: HIV Insite Knowledge based Chapter. [Google Scholar]
- 10.Soto-Ramírez LE, Hernández-Gómez L, Sifuentes-Osornio J, Barriga-Angulo G, Duarte de Lima D, López-Portillo M. Detection of specific antibody in gingival crevicular transudate by ELISA for diagnosis of HIV type I infection. J Clin Microbiol. 1992;30:2780–3. doi: 10.1128/jcm.30.11.2780-2783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urquia M, Rodriguez Archilla, Gonzales Moles, Ceballos A. Detection of anti- HIV antibodies in saliva. J Oral Pathol Med. 1993;22:153–6. doi: 10.1111/j.1600-0714.1993.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 12.Roit I, Lehner T. Immunology of oral Disease. 2nd ed. Oxford: Blackwell Scientific Publication; 1983. Acquired Immunodeficiency Syndrome; pp. 139–49. [Google Scholar]
- 13.Goodson JM. Gingival Creviculer fluid flow. Periodontology 2000. 2003;31:43–54. doi: 10.1034/j.1600-0757.2003.03104.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallo D, George JR, Fitchen JH, Goldstein AS, Hindahl MS. Evaluation of a system using oral mucosal transudate for HIV-1 antibody screening and confirmatory testing. OraSure HIV Clinical Trials Group. JAMA. 1997:227, 792. [PubMed] [Google Scholar]
- 15.Holm-Hansen C, Constantine NT, Haukenes G. Detection of antibodies to HIV in homologous sets of plasma, urine and oral mucosal transudate samples using rapid assays in Tanzania. Clin Diagn Virol. 1993;1:207–14. doi: 10.1016/0928-0197(93)90002-m. [DOI] [PubMed] [Google Scholar]


