Abstract
Hepatocellular carcinoma (HCC) is an epithelial tumor derived from hepatocytes; it accounts for 80% of all primary liver cancers and ranks globally as the fourth leading cause of cancer-related deaths. HCC treatment is a multidisciplinary and a multimodal task, with surgery in the form of liver resection and liver transplantation (LT) representing the only potentially curative modality. However, there are variable opinions and discussions about applying these surgical options and using other supporting treatments. This article is a narrative review that includes articles published from 1984 to 2013 located by searching scientific databases such as PubMed, SCOPUS, and Elsevier, with the main keyword of hepatocellular carcinoma in addition to other keywords such as liver transplantation, liver resection, transarterial chemoembolization, portal vein embolization, bridging therapy, and downstaging. In this review, we focus mainly on the surgical treatment options offered for HCC, in order to illustrate the current relevant data available in the literature to help in applying these surgical options and to use other supporting treatment modalities when appropriate.
Keywords: Bridging therapy, downsizing, hepatocellular carcinoma, liver, liver malignant tumor, liver transplantation, liver resection, transarterial chemoembolization
Hepatocellular carcinoma (HCC), an epithelial tumor derived from hepatocytes, accounts for 80% of all primary liver cancers and ranks globally as the fourth leading cause of cancer-related deaths. Annual mortality rates of HCC remain comparable to its yearly incidence, making it one of the most lethal varieties of solid-organ cancers.[1] HCC treatment is a multidisciplinary and a multimodal task [Table 1], with surgery in the form of liver resection or liver transplantation (LT) representing the only potentially curative modality.[2] This article focuses on the surgical management of HCC.
Table 1.
Different treatment modalities of hepatocellular carcinoma

MATERIALS AND METHODS
This study is a narrative review in which relevant papers, including cross-sectional, cohort, clinical trial, and systematic reviews, were selected using databases and scientific search engines such as PubMed, SCOPUS, and Elsevier, with the main keyword HCC in addition to other keywords such as liver transplantation, liver resection, transarterial chemoembolization, portal vein embolization, bridging therapy, and downsizing. Relevant articles published from the year 1984 up to 2013 were reviewed.
DISCUSSION
Preoperative evaluation and procedures
The preoperative evaluation for the surgical treatment of HCC should focus on the likelihood of the disease being confined to the liver, and whether the anatomical location of the tumor and the underlying liver function will permit resection.
Determining the extent of tumor involvement
Anatomic delineation of tumor extent is best achieved with dynamic multiphase computed tomography (CT) or magnetic resonance imaging (MRI) scanning.[3,4] The typical picture of HCC on a CT scan will appear as an enhanced lesion in the arterial phase [Figure 1a] with early washout of the contrast in the venous phase [Figure 1b]. Lymph node metastases are uncommon overall (between 1% and 8%), but their presence portends a worse outcome. Preoperative detection of nodal metastases is limited by the frequent presence of benign nodal enlargement in patients with cirrhosis.[5] Highly suspicious nodes based on enhancement similar to the intrahepatic HCC lesions indicate the need for biopsy in a patient being considered for resection.[6] Furthermore, chest CT is currently recommended to complete the staging evaluation as well as bone scan if suspicious bone pain or hypercalcemia are present.[7]
Figure 1.
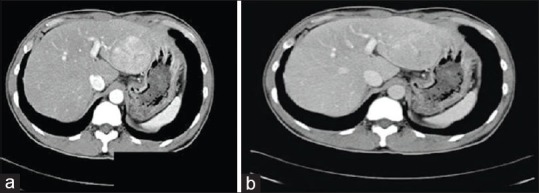
(a) Enhanced HCC lesion in the arterial phase of a CT scan (b) Early washout of contrast in the venous phase
Assessment of hepatic reserve
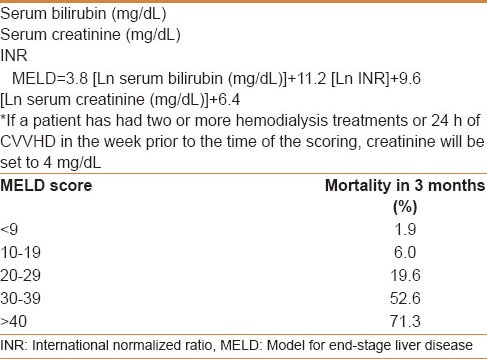
Operative mortality is related to the severity of the underlying liver disease; it is 7%-25% in cirrhotic and less than 3% in noncirrhotic patients.[8] In patients with cirrhosis, surgical resection is most safely performed in those with Child-Pugh class A disease, who has a normal bilirubin and well-preserved liver function [Table 2]. However, even Child-Pugh class A patients may develop rapid hepatic decompensation following surgery due to limited functional hepatic reserve.[9] Although helpful, the Child-Pugh classification and other tools for assessing underlying liver disease, such as the model for end-stage liver disease (MELD) score [Table 3], are not adequate to select patients with sufficient hepatic reserve for major resection.[10] Multiple studies have demonstrated that a normal serum bilirubin level and the absence of clinically significant portal hypertension (i.e. hepatic venous pressure gradient less than 10 mmHg) are the best available indicators of acceptably low risk of postoperative liver failure after liver resection.[9,11,12] In many centers, the Child-Pugh score may be supplemented by specialized investigations such as the indocyaninegreen (ICG) retention test, especially in marginal cases (e.g. Child-Pugh B, possible mild portal hypertension).[13] ICG retention of 14% at 15 min is frequently accepted, mostly in the Asia-Pacific area, as a reflection of adequate functional reserves for major resection (defined as resection of more than two Couinaud segments).[14,15,16] The assessment of the volume and function of residual liver should also be addressed by hepatic volumetry, particularly because portal vein embolization (PVE) can be a valuable tool to increase the liver remnant volume prior to major hepatic resection, particularly for right -sided tumors.
Table 2.
Child-Pugh score
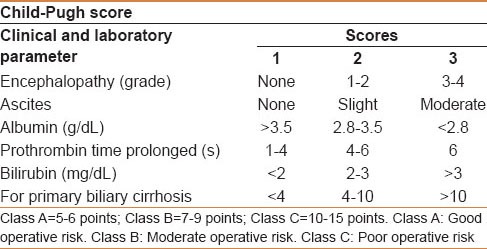
Table 3.
MELD score component, calculation, and mortality prediction
Portal vein embolization
Preoperative PVE is a valuable adjunct to major liver resection. PVE can initiate hypertrophy of the anticipated future liver remnant to enable an extended resection in a patient with normal liver or major resection in a well-compensated cirrhotic patient that would otherwise leave a remnant liver insufficient to support life following liver resection.[17,18,19]
The potential benefits to the use of PVE are as follows:
Conversion of unresectable tumor to insufficient future liver remnant to become resectable for potential cure
Subclinical disease or rapid progression may be detected prior to definitive surgery on postembolization imaging studies, thus sparing the patient an unnecessary operation.[20,21]
The absence of compensatory hypertrophy identifies patient with impaired liver regeneration. Therefore, decreasing the incidence of post–liver resection failure in these patients by avoiding major liver resections.
Transarterial chemoembolization (TACE) has been proposed as a complementary procedure prior to PVE in patients with HCC. TACE not only eliminates the arterial blood supply to the tumor, but it also embolizes potential arterioportal shunts in cirrhotic livers that attenuate the effects of PVE.[22] Most surgeons reserve the “double embolization” procedure for HCC patients with liver disease requiring right hepatectomy.[22,23]
Liver resection versus transplantation
Surgical treatment is the only potentially curable option for patients with HCC. Although surgical resection is the treatment of choice in patients with good hepatic function, it is contraindicated in those with moderate-to-severe cirrhosis (Child class B or C), leaving these patients with liver transplantation as the only option. Moreover, transplantation is the optimal treatment even for small, otherwise resectable disease. This is a reflection of a number of factors. Liver transplantation will most likely result in a microscopically negative resection, which is the most effective oncologic treatment. Most HCCs are multifocal especially in the background of cirrhosis, although preneoplastic lesions may not be visible on perioperative evaluation; they are likely to continue to evolve into new primary HCCs. Furthermore, transplantation eliminates cirrhosis and restores normal hepatic function. However, limited organ availability mandates the restriction of liver transplantation to patients with early-stage tumors, which are not candidates for resection.[24] The debate regarding the best curative option for early HCC in a cirrhotic liver continues.[25] In a study by Adam et al, involving 198 patients with early HCC with liver cirrhosis, who underwent liver resection (97 patients) or liver transplantation (101 patients). The results have shown that liver transplantation has better overall survival (OS) and recurrence-free survival (RFS) than liver resection in 3, 5, and 10 years (OS, P = 0.008; RFS, P < 0.0001) in tumor size 5 cm or less and similar five 5-year OS after resection and liver transplantation (70% vs. 78%, respectively) in tumor size less than 3 cm, although RFS was better with liver transplantation.[26] On the other hand, Rahman et al. have shown in a meta-analysis comparing liver resection with liver transplantation in early HCC patients with liver cirrhosis, 1-year OS was significantly higher in patients undergoing liver resection, 5-year OS was comparable for both groups, and 10-year OS was significantly higher in transplanted patients.[27] The question still remains whether surgical resection and liver transplantation should be considered as alternatives, complimentary, or sequential options in the treatment strategy of HCC. Hence, the decision whether to transplant or resect should be individualized to the patient, institute facility, and waiting list time for transplant.
Liver resection
Liver resection is considered a potentially curative therapy for patients with early-stage HCC (solitary tumor ≤5 cm in size, or three or less tumors each ≤3 cm in size and no evidence of gross vascular invasion) in a Child-Pugh class A score and no evidence of portal hypertension (although a minor resection could be considered in some patients with portal hypertension)[28,29] [Table 4]. However, in highly selected cases, patients with a Child-Pugh class B score may be considered for limited liver resection, particularly if liver function tests were normal and no portal hypertension was evident.[28] There is no general rule regarding tumor size for selection of patients for resection. Certainly, patients with smaller tumors are less likely to harbor occult vascular invasion and have a better outcome after therapy.[30] However, patients with a solitary HCC without vascular invasion have a similar survival probability regardless of the tumor size.[31] Nevertheless, the presence of macro or microscopic vascular invasion is considered to be a strong predictor of HCC recurrence.[32,33] Hepatic resection is controversial in patients with limited and resectable multifocal disease and/or signs of major vascular invasion.[28,33] Multifocality is associated with lower survival, but does not exclude a good outcome in selected patients. In several studies, resection of multifocal HCC was associated with 5-year survival rates of approximately 24%.[34] Patients with multifocal HCC who appear to benefit from resection are those with sufficient liver reserve to tolerate resection, without extrahepatic disease and without major vascular invasion. Liver resection in patients with major vascular invasion should only be performed in highly selected situations by experienced teams. Owing to the high recurrence rate after surgery, it has been proposed that aggressive surgical liver resection with extended lymphadenectomy should be considered for patients with fibrolamellar HCC and the presence of advanced-stage disease. Invasion of tumor to adjacent organs or local lymph node spread, or limited distant metastasis should not be considered as contraindications for curative resection in patients with fibrolamellar HCC.[35,36] Laparoscopy and intraoperative ultrasound (IOUS) may improve the selection of patients for potentially curative resection. IOUS can accurately determine the size of the primary tumor and detect portal or hepatic vein involvement, which precludes curative resection. Another benefit of IOUS is the identification of major intrahepatic vascular structures, which can be used to guide segmental or nonanatomic resections.[37] In noncirrhotic livers, an anatomical resection should be performed. Up to two-thirds of the functional parenchyma can be removed safely depending on the age of the patient and his liver regenerative capacity. For cirrhotic patients, the resection needs to remove the least amount of nonmalignant parenchyma possible, because the capacity for liver regeneration is impaired in these patients, to preserve postoperative liver function. Both anatomic and wedge resection are acceptable, although some studies suggest portal-oriented resections, enabling longer overall and disease-free survival when feasible.[38] The 30-day postoperative mortality for cirrhotic patients ranges from 14% to 24%, compared with 0.8%-7% for noncirrhotic patients.[39,40,41] Intraoperative blood loss of more than 1500 mL and postoperative infection of any type are important factors affecting the development of postoperative liver failure in cirrhotic patients.[6,42] Appropriate selection of patients, meticulous surgical technique, use of low CVP (less than 5 mmHg) anesthesia and inclusion of preoperative volumetry with PVE when appropriate are important factors that have to be considered in order to decrease the rate of morbidity and mortality.
Table 4.
Indications for liver resection in hepatocellular carcinoma

Liver transplantation
HCC is closely associated with chronic liver disease and cirrhotic livers. Although liver resection and local ablation are regarded as potentially curative treatments, the limited functional reserve of the liver restricts their application and there is a high chance of recurrence in the liver remnant.[43] Liver transplantation is the only treatment that offers a chance of cure for the tumor and the underlying cirrhosis by complete extirpation of both.
Organ allocation
In an effort to prioritize liver transplant candidates according to the highest short-term risk of mortality from end-stage cirrhosis, the MELD scoring system was implemented in 2002 [Table 3].[44] To impart more urgent access to liver transplantation for patients with small HCCs, additional points within the scoring system were allotted to these patients. This is done to equilibrate their risk of death in comparison with the mortality of end-stage cirrhosis.[45] Using the American Liver Tumour Study Group Modified TNM staging system, the current UNOS guidelines do not allow upgrading of candidates with Stage I disease, irrespective of biopsy confirmation; only candidates with Stage II HCC disease are upgraded on the waiting list to an MELD score of 22 (equivalent to a 15% probability of candidate death within 3 months) with the intent to shorten their waiting time. From 2002 to 2007 in UNOS database, patients with a “HCC MELD-exception” had similar survival to patients without HCC.[46]
Criteria for transplantation
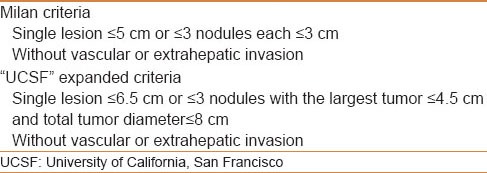
“Milan criteria” has achieved favorable results in patients with cirrhosis [Table 5][46] with a 5-year survival of these early-stage patients exceeding 70%. Several groups reported a 5-year survival of more than 50% in patients transplanted for HCC beyond Milan,[47] in whom there were no macroscopic evidence of vascular invasion or extrahepatic spread, which many investigators have argued that it is the minimum acceptable survival rate. In 2001, Yao and colleagues at the University of California, San Francisco (UCSF), defined an expanded set of HCC criteria [Table 5], for which 1-year and 5-year survival rates after LT were 90% and 75%, respectively.[48] Although, liver transplantation in such candidates is controversial, it is still widely adopted. The short-term outcomes are similar to those who are transplanted within the Milan criteria. The Edmonton group studied the total tumor volume (TTV) in patients with HCC who had liver transplant based on the Milan or UCSF criteria in three centers and they found that TTV < 115 cm3 has lower recurrence rate than TTV > 115 cm3. In the same study, they also found that patients beyond the Milan criteria but within TTV < 115 cm3 had survivals similar to those of patients within the Milan criteria. On the contrary, patients with TTV > 115 cm3 demonstrated lower survival than those within TTV < 115 cm3 when pathology (5 year: 47% vs. 79%, P < 0.001) and radiology staging (5 year: 53% vs. 76%, P < 0.1) were used.[49]
Table 5.
Criteria for liver transplantation
Pretransplant treatment of HCC
The major limitation for liver transplantation as therapy for early-stage HCC is the insufficient number of donor livers. There is always a waiting period between candidate listing and transplantation. If the waiting period extends over a sufficient length of time, the tumor will grow and eventually hinder transplantation. In a study by Yao and colleagues on patients with HCC listed for transplantation, a 6-month waiting period for liver transplantation was associated with a 7.2% cumulative dropout probability, increasing to 37.8% and 55.1% at 12 and 18 months, respectively.[50] In this setting, the treatment of HCC prior to liver transplantation has three potential goals: (1) Controlling tumor growth and vascular invasion during the waiting time and therefore decrease dropouts from the waiting list; (2) carrying out neoadjuvant therapy to improve the post-transplant outcome by reducing the risk of postoperative recurrence, and (3) downsizing the HCC burden to make a patient eligible for transplantation. Follow up for patients on the waiting list is required every 3 months by CT or MRI and serum AFP to ensure continued eligibility for liver transplantation.[51]
Bridging therapy
Bridge therapy is used to decrease tumor progression and the dropout rate from the liver transplantation waiting list.[52] It is considered for patients who meet the transplant criteria. A number of studies have investigated the role of locoregional treatment, surgical resection, and sorafenib as a bridge to liver transplantation in patients on a waiting list.[53,54,55,56,57,58] However, due to the limitations of these studies and the heterogeneous nature of the study populations, as well as the absence of randomized clinical trials evaluating the utility of bridge therapy for reducing the liver transplantation waiting list dropout rate, the conclusions that can be drawn are limited.[10] Therefore, locoregional therapy in patients with T2 (one nodule 2-5 cm or three or fewer nodules each ≤3 cm) HCC (Milan criteria) and a likely waiting time of longer than 6 months, may be appropriate.[51] In fact, patients with anticipated prolonged waiting time are encouraged to have a trial of TACE and/or ablation as bridging therapy to decrease the dropout of transplantation.
Tumor downsizing
Downsizing is done using HCC-directed therapy that aims to reduce and/or number of HCC lesions. Several studies showed successful downsizing with different modalities of treatments and some of them have shown similar survival in patients who initially met the criteria for transplantation. Although such studies have used different selection criteria for downsizing and different transplant criteria, transplantation may be considered after successful downsizing.[51]
Immunosuppression
Immunosuppression is used post-liver transplantation to reduce the risk of graft rejection but some immunosuppressant agents used in HCC, such as cyclosporine and tacrolimus, have been associated with a risk of tumor regrowth and recurrence. Thus, minimizing the effective dose is extremely significant.[59,60,61] On the other hand, the calcineurin-independent immunosuppressive agent sirolimus, a binder of mTOR, inhibits tumor growth in cell lines; it inhibits primary and metastatic tumor growth in vivo. Furthermore, the benefits of sirolimus-based immunosuppression post-LT for HCC have been explored in a meta-analysis. The study showed improved patient outcomes with lower recurrence rates and significantly improved 1-, 3-, and 5-year RFS and OS.[62] Nevertheless, randomized trials would provide further understanding of the effect of sirolimus post-LT for HCC and will help directing future studies in the field.
Long-Term outcomes of HCC
The long-term outcome after liver resection is determined by pathologic tumor factors and the underlying liver disease.
Tumor factors
The most important tumor-related prognostic factors are the presence and the degree of vascular invasion, tumor number and size, and the status of the surgical margins. Other poor prognostic indicators to be considered are the absence of a tumor capsule, preoperative alpha-fetoprotein (AFP) levels >10,000 ng/mL, and poor histologic grade of differentiation.[10]
Underlying liver disease
Preoperative underlying liver disease and cirrhosis are considered important negative prognostic factors. Early recurrence after resection of HCC was found to be related to liver cirrhosis, hepatitis B/C, Child-Pugh score, transaminase level, albumin level, and chronic active hepatitis.[14] A few reports pointed out that chronic active hepatitis and cirrhosis are the most significant risk factors for intrahepatic recurrence through multicentric carcinogenesis, the so-called multicentric occurrence.[20,28]
HCC Surveillance
Although there is no consensus as to the optimal approach for post-transplant surveillance as well, recommendations are based on the consensus that earlier identification of disease may facilitate patient eligibility for investigational studies or other forms of treatment. The Guidelines from the National Comprehensive Cancer Network (NCCN) recommends high-quality cross-sectional imaging every 3-6 months for 2 years, then every 6-12 months. AFP levels, if initially elevated, should be measured every 3 months for 2 years, then every 6-12 months. Re-evaluation according to the initial workup should be considered in the event of disease recurrence.[63]
HCC Survival
Partial hepatectomy (i.e. liver resection) is a potentially curative therapy for patients with early-stage HCC. The 5-year survival rates are over 50% for patients who had liver resection for HCC.[64] Other studies additionally suggested that for selected patients with preserved liver function and early stages of HCC, liver resection can achieve a 5-year survival rate of approximately 70%.[43] However, HCC tumor recurrence rates at 5 years following liver resection have been reported to exceed 70%.[65] Thus, appropriate surveillance should be considered. However, there are clear survival benefits and low recurrence rates after transplantation for HCC. When surgeons adhere to the Milan criteria, 5-year survival rates after transplantation range from 70% to 80%, and tumor recurrence rates are approximately 10%. Furthermore, UCLA Transplant Centre has reported that OS at 1, 3, and 5 years was 82%, 65%, and 52%, respectively. Patients meeting Milan criteria had similar 5-year post-transplant survival to patients meeting UCSF criteria by both preoperative imaging and explant pathology. However, beyond the UCSF criteria the 5-year survival was significantly lower (<50%).[66] Hence, OS is significantly reflected by the proper selection of transplant candidates.
SUMMARY
HCC management is a multidisciplinary and a multimodal task with the surgical treatments representing the only potentially curative modalities available today. Liver resection and liver transplantation are the two offered surgical options for HCC treatment. To apply the best option, careful selection of patients is required. Accurate diagnosis and proper evaluation, including the extent of the disease and the status of the hepatic reserve, are essential elements to determine the treatment strategy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuntz E. Hepatology Textbook and Atlas: History, Part 4. Springer: Heidelberg; 2008. Malignant liver tumor; pp. 795–835. [Google Scholar]
- 3.Oliver JH, 3rd, Baron RL, Federle MP, Rockette HE., Jr Detecting hepatocellular carcinoma: Value of unenhanced or arterial phase CT imaging or both used in conjunction with conventional portal venous phase contrast-enhanced CT imaging. AJR Am J Roentgenol. 1996;167:71–7. doi: 10.2214/ajr.167.1.8659425. [DOI] [PubMed] [Google Scholar]
- 4.Baron RL, Oliver JH, 3rd, Dodd GD, 3rd, Nalesnik M, Holbert BL, Carr B. Hepatocellular carcinoma: Evaluation with biphasic, contrast-enhanced, helical CT. Radiology. 1996;199:505–11. doi: 10.1148/radiology.199.2.8668803. [DOI] [PubMed] [Google Scholar]
- 5.Dodd GD, 3rd, Baron RL, Oliver JH, 3rd, Federle MP, Baumgartel PB. Enlarged abdominal lymph nodes in end-stage cirrhosis: CT-histopathologic correlation in 507 patients. Radiology. 1997;203:127–30. doi: 10.1148/radiology.203.1.9122379. [DOI] [PubMed] [Google Scholar]
- 6.Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D’Angelica M, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006;244:260–4. doi: 10.1097/01.sla.0000217606.59625.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepatobiliary Cancers National comprehensive cancer network 2012. [Last accessed on 2014 Jan 5]. Available from: http://www.nccn.org .
- 8.Molmenti EP, Klintmalm GB. Liver transplantation in association with hepatocellular carcinoma: An update of the International Tumor Registry. Liver Transpl. 2002;8:736–48. doi: 10.1053/jlts.2002.34879. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–22. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–9. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surgical resection for hepatocellular carcinoma, Up to date [Internet] 2012. [Last accessed on 2012]. Available from: http://www.uptodate.com .
- 12.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 13.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 14.Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–9. [PubMed] [Google Scholar]
- 15.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: Decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 16.Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: How I do it. J Hepatobiliary Pancreatic Surg. 2005;12:31–7. doi: 10.1007/s00534-004-0945-0. [DOI] [PubMed] [Google Scholar]
- 17.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: A meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 18.Imamura H, Shimada R, Kubota M, Matsuyama Y, Nakayama A, Miyagawa S, et al. Preoperative portal vein embolization: An audit of 84 patients. Hepatology. 1999;29:1099–105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 19.Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg oncol. 2011;18:1251–7. doi: 10.1245/s10434-010-1423-3. [DOI] [PubMed] [Google Scholar]
- 20.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: Prospective clinical trial. Ann Surg. 2003;237:208–17. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: Rationale, technique and future prospects. Br J Surg. 2001;88:165–75. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 22.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–8. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–74. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 24.Zarrinpar A, Kaldas F, Busuttil RW. Liver transplantation for hepatocellular carcinoma: An update. Hepatobiliary Pancreat Dis Int. 2011;10:234–42. doi: 10.1016/s1499-3872(11)60039-8. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 26.Adam R, Bhangui P, Vibert E, Azoulay D, Pelletier G, Duclos-Vallée JC, et al. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: Does size define the best oncological strategy? Ann Surg. 2012;256:883–91. doi: 10.1097/SLA.0b013e318273bad0. [DOI] [PubMed] [Google Scholar]
- 27.Rahman A, Assifi MM, Pedroso FE, Maley WR, Sola JE, Lavu H, et al. Is resection equivalent to transplantation for early cirrhotic patients with hepatocellular carcinoma. A meta-analysis? J Gastrointest Surg. 2012;16:1897–909. doi: 10.1007/s11605-012-1973-8. [DOI] [PubMed] [Google Scholar]
- 28.Jarnagin WR. Management of small hepatocellular carcinoma: A review of transplantation, resection, and ablation. Ann Surg Oncol. 2010;17:1226–33. doi: 10.1245/s10434-010-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truty MJ, Vauthey JN. Surgical resection of high-risk hepatocellular carcinoma: Patient selection, preoperative considerations, and operative technique. Ann Surg Oncol. 2010;17:1219–25. doi: 10.1245/s10434-010-0976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surg. 2000;127:603–8. doi: 10.1067/msy.2000.105498. [DOI] [PubMed] [Google Scholar]
- 31.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–92. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 32.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–6. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 33.Abdalla EK, Denys A, Hasegawa K, Leung TW, Makuuchi M, Murthy R, et al. Treatment of large and advanced hepatocellular carcinoma. Ann Surg Oncol. 2008;15:979–85. doi: 10.1245/s10434-007-9727-7. [DOI] [PubMed] [Google Scholar]
- 34.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–36. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 35.Ichikawa T, Federle MP, Grazioli L, Marsh W. Fibrolamellar Hepatocellular Carcinoma: Pre-and Posttherapy Evaluation with CT and MR Imaging 1. Radiology. 2000;217:145–51. doi: 10.1148/radiology.217.1.r00se46145. [DOI] [PubMed] [Google Scholar]
- 36.Meriggi F, Forni E. Surgical therapy of hepatic fibrolamellar carcinoma. Ann Ital Chir. 2007;78:53–8. [PubMed] [Google Scholar]
- 37.Lo CM, Lai EC, Liu CL, Fan ST, Wong J. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998;227:527–32. doi: 10.1097/00000658-199804000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surg. 2002;131:311–7. doi: 10.1067/msy.2002.121892. [DOI] [PubMed] [Google Scholar]
- 39.Tsuzuki T, Ogata Y, Iida S, Shimazu M. Hepatic resection in 125 patients. Archives Surg. 1984;119:1025. doi: 10.1001/archsurg.1984.01390210029008. [DOI] [PubMed] [Google Scholar]
- 40.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: Experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–85. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 41.Bozzetti F, Gennari L, Regalia E, Bignami P, Montalto F, Mazzaferro V, et al. Morbidity and mortality after surgical resection of liver tumors. Analysis of 229 cases. Hepatogastroenterology. 1992;39:237–41. [PubMed] [Google Scholar]
- 42.Nagomey DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: Surgical management and determinants of survival. Surgery. 1989;106:740–8. [PubMed] [Google Scholar]
- 43.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 45.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 46.Abrams P, Marsh JW. Current approach to hepatocellular carcinoma. Surg Clin North Am. 2010;90:803–16. doi: 10.1016/j.suc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Bruix J, Fuster J, Llovet JM. Liver transplantation for hepatocellular carcinoma: Foucault pendulum versus evidence-based decision. Liver Transpl. 2003;9:700–2. doi: 10.1053/jlts.2003.50124. [DOI] [PubMed] [Google Scholar]
- 48.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 49.Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–15. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 50.Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: Implications for the current organ allocation policy. Liver Transpl. 2003;9:684–92. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 51.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012;13:e11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujiki M, Aucejo F, Kim R. General overview of neo-adjuvant therapy for hepatocellular carcinoma before liver transplantation: Necessity or option? Liver Int. 2011;31:1081–9. doi: 10.1111/j.1478-3231.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 53.Hwang S, Lee SG, Belghiti J. Liver transplantation for HCC: Its role. J Hepatobiliary Pancreat Sci. 2010;17:443–8. doi: 10.1007/s00534-009-0241-0. [DOI] [PubMed] [Google Scholar]
- 54.Lesurtel M, Müllhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: An evidence-based analysis. Am J Transplant. 2006;6:2644–50. doi: 10.1111/j.1600-6143.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 55.Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–7. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 56.Cheow P, Al-Alwan A, Kachura J, Ho C, Grant D, Catiral M, et al., editors. Hepatology. 111 River St, Hoboken, NJ, USA: John Wiley and Sons Inc; 2005. Ablation of hepatoma as a bridge to liver transplantation reduces drop-out from prolonged waiting time. [Google Scholar]
- 57.Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993–1000. doi: 10.1245/s10434-007-9787-8. [DOI] [PubMed] [Google Scholar]
- 58.Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: A reasonable strategy? Ann Surg. 2003;238:508–18. doi: 10.1097/01.sla.0000090449.87109.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, et al. Low recurrence rate of hepatocellular carcinoma after liver transplantation: Better patient selection or lower immunosuppression? Transplantation. 2002;74:1746–51. doi: 10.1097/00007890-200212270-00017. [DOI] [PubMed] [Google Scholar]
- 60.Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: Key role of immunosuppression. Liver Transpl. 2005;11:497–503. doi: 10.1002/lt.20391. [DOI] [PubMed] [Google Scholar]
- 61.Zhou S, Tan C, Dai Z, Zhu H, Xu M, Zhou Z, et al. Tacrolimus enhances the invasion potential of hepatocellular carcinoma cells and promotes lymphatic metastasis in a rat model of hepatocellular carcinoma: Involvement of vascular endothelial growth factor C. Transplant Proc. 2011;43:2747–54. doi: 10.1016/j.transproceed.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 62.Menon KV, Hakeem AR, Heaton ND. Meta-analysis: Recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:411–9. doi: 10.1111/apt.12185. [DOI] [PubMed] [Google Scholar]
- 63.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: Hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–91. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: Is there a way. A prospective analysis of our approach? Arch Surg. 1999;134:984–92. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 65.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7. [PubMed] [Google Scholar]
- 66.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: A 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–9. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]


