Abstract
Microwave-assisted extraction was used for the extraction of alantolactone and isoalantolactone from Inula helenium. Effects of various experimental factors including ethanol concentration, particle size, microwave radiation time, the ratio of material to liquid and extraction temperature on yield of alantolactone and isoalantolactone were evaluated. The optimal extracting process of the alantolactone and isoalantolactone from the root of the Inula helenium was 1 g plant sample (sifted through 140 mesh) mixed with 15 ml of 80% ethanol solution, microwave radiation 120 s at 50°. Under these optimal conditions, the yield of alantolactone and isoalantolactone was 31.83±2.08 mg/g and 21.25±1.37 mg/g, respectively. Compared with heat reflux extraction, ultrasound-assisted extraction, microwave-assisted extraction was more efficient and timesaving for the extraction of alantolactone and isoalantolactone from Inula helenium.
Keywords: Inula helenium, microwave-assisted extraction, alantolactone, isoalantolactone
Inula helenium, which has been used as a traditional medicine in China over 2000 years, is an official plant recorded in Chinese Pharmacopoeia and some European Pharmacopoeias. The root of this plant is used extensively as expectorant, antitussive and diaphoresis in traditional Chinese medicine. Modern scientific research indicates that the main active ingredients of I. helenium are alantolactone and isoalantolactone, which have been demonstrated to have numerous therapeutic activities such as antibacterial, antitumor, antiproliferative, antiinflammatory and antistressor[1,2,3,4].
Conventionally, sesquiterpenes lactone compounds were extracted with different solvents and extraction methods, such as maceration extraction, heat reflux, solid-phase dispersion[5] and ultrasound-assisted extraction[6]. Recently, microwave-assisted extraction (MAE) has drawn more and more attention in various fields, especially in medicinal plant research[7,8] due to its inherent advantages.
Our previous investigation had confirmed that ethanol was an environmentally friendly and high-performance extraction solvent for yielding alantolactone and isoalantolactone from I. helenium. For this reason, ethanol was also used as the extraction solvent in this study. As far as we know, no relevant reports on the MAE of alantolactone and isoalantolactone from I. helenium have been published. The objectives of this study were to optimize MAE parameters such as solvent, particle size, microwave radiation time, the ratio of liquid to material and extraction temperature for the maximum extraction of alantolactone and isoalantolactone.
The roots of I. helenium were obtained from the An-Guo herbal medicine market, China, and identified at the College of Traditional Chinese Medicine, Hebei North University. The specimen (No. 2009-10) was kept in the Department of Pharmacy, Hebei North University. Alantolactone and isoalantolactone were purchased from Nanjing ZeLang Medical Technology Co., Ltd. (purity>98%). Ethanol was of analytical grade and used for the extraction of alantolactone and isoalantolactone from I. helenium. Acetonitrile used was of chromatographic pure grade (Adamas-beta, Shanghai).
Air-dried roots of I. helenium were ground to a powder using a DFY-800B universal high-speed grinder (Wenling Linda machine Co., Ltd. China). The powder sifted through mesh sieve and served for further extraction trails. The process of alantolactone and isoalantolactone extraction from I. helenium was carried out on a MAS-I microwave synthesis system. The powder of I. helenium (5.0 g) was mixed with various solvent, such as water, ethanol in a 300 ml round-bottom flask, and the ratio of liquid to material was set at 5:1, 10:1, 15:1, 20:1, 30:1, respectively. The round-bottom flask was heated at a preset temperature and a stirring speed of 500 rpm was used for all experiments to eliminate the effects of external energy transfer and internal diffusion. After extraction of 0.5-6 min, the extract was allowed to cool to ambient temperature and centrifuged for 15 min at 3000 rpm to deposit suspension particle, the extract was diluted and filtered through 0.45 μm nylon filter before analysis by HPLC.
Ultrasound-assisted extraction (UAE) was carried out by taking 5 g of dried powder and extracted it with 75 ml of 80% ethanol solution in an ultrasonic cleaner JPCQ0628 (Jia-Peng Electronics Co., Ltd, China) for 30 min at 50°. Conventional extraction was carried out in a water-bath. Five grams of I. helenium powder was mixed with 75 ml of 80% ethanol solution and refluxed for 60 min. After centrifugation, the extract was analyzed for the levels of alantolactone and isoalantolactone.
The HPLC equipment included an Agilent 1100 series quaternary pump solvent delivery system, auto degasser, and a variable-wavelength UV detector. The analytical column was an Agilent C18 column (200×4.6 mm, 5 μm). The mobile phase was acetonitrile and 0.1% phosphoric acid solution (50:50, v/v). The flow rate was 1 ml/min and the detection wavelength was set at 220 nm. The column temperature was kept at 30°. The peak area was used to calculate the amount of alantolactone and isoalantolactone using the external standard method.
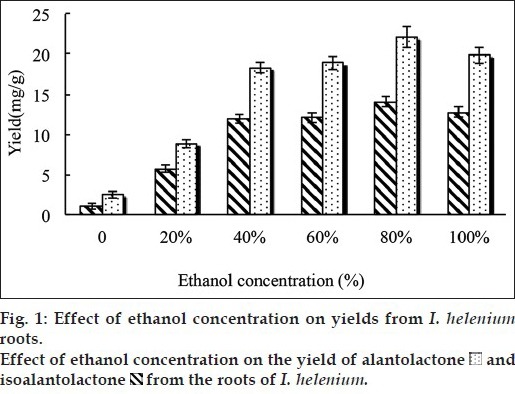
Different solvents produce various amounts and components of extract. The choice of a suitable solvent was the first and the most important step in the extraction, which was beneficial to the extraction of the active component. In this study, water and different concentrations of ethanol were tested as extracting solvents. Specific process is as follows: five grams of powder were extracted with 50 ml of different solvent in a 300 ml round bottom flask microwave heating at 50° for 180 s, respectively. The yield of alantolactone and isoalantolactone, as fig. 1 showed, was reached a maximum when the ethanol concentration was 80%, while either too high or too low degree will suppress the yield. That was because alantolactone and isoalantolactone were nonpolar and ethanol may contribute to the extraction of sesquiterpene lactones. Furthermore, as a polar solvent with a high dielectric constant, water can absorbed more microwave energy and caused the rising temperature in the reaction system. Based on these factors, subsequent experiments were performed with 80% ethanol.
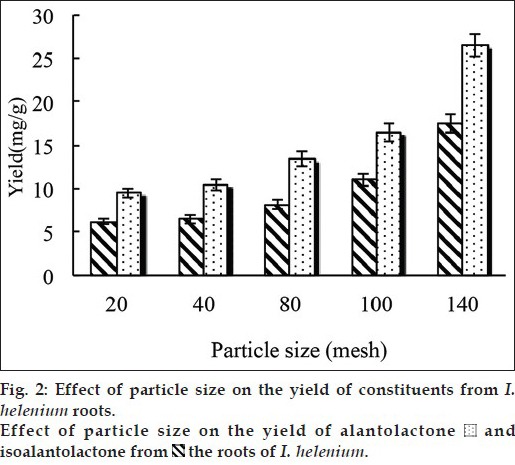
The choice of screen mesh size was another important factor on the extraction. In this experiment, the influence of particle size on the extraction yield of alantolactone and isoalantolactone was investigated. Five grams of powder (sifted through 20, 40, 80, 100, 140 mesh, respectively) were mixed with 50 ml of ethanol. The temperature was set at 50° and the duration of microwave radiation was 180 s. As seen in fig. 2 with other condition remaining unchanged, the yield increased with a decreased in particle size of plant material. The high yield got from the powder sifted through 140 mesh suggested that pulverization steps contributed to the leaching of active substances from plant. An increase in the degree of grinding increased the contact surface area with solvent, which accelerated the diffusion speed of the active ingredients. Furthermore, smaller particle have less penetration thickness that result in uniform microwave exposure. Beyond 140 mesh, adhesion of particles would happen and sieving such tiny particles was difficult and high energy consumption. Consequently, the powder sifted through 140 mesh was used in the experiments.
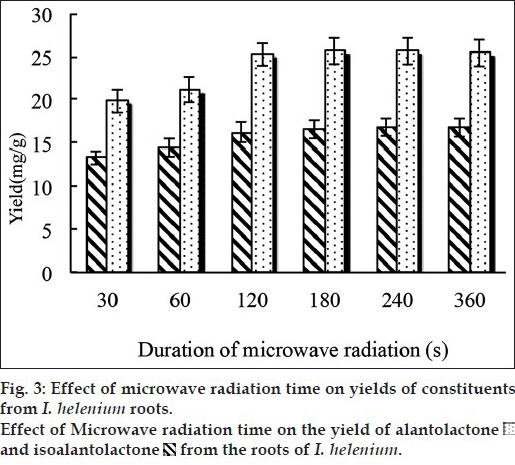
In order to achieve the maximum of alantolactone and isoalantolactone extraction efficiency, different durations of microwave radiation (30, 60, 120, 180, 240, 360 s) was experimented. Other experimental conditions were the ratio of material to solvent (80% ethanol) at 1:10, and the extraction temperature were 50°. The yield of alantolactone and isoalantolactone under different microwave radiation time was shown in fig. 3. The yield of alantolactone and isoalantolactone increased rapidly within 30 s, and come to its maximum on 120 s, then almost unchanged. Longer extraction time may have negative effects because of degeneration or conversion of products. Therefore, in the subsequent experiments, 120 s was chosen as the extraction time.
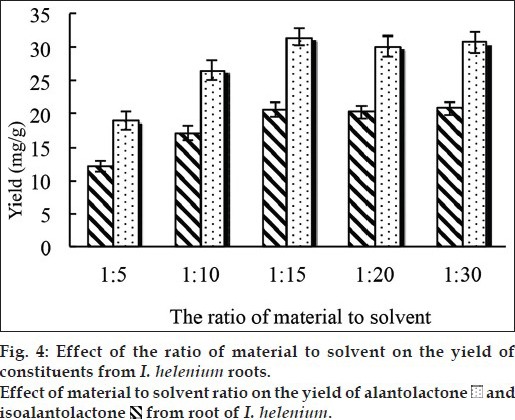
Under the optimal condition as mentioned above, effect of the ratio of material to liquid (1:5, 1:10, 1:15, 1:20, 1:30, g/ml) on yield of alantolactone and isoalantolactone was evaluated with the extraction temperature at 50° in the present study. As it shown in fig. 4, the extraction yield of alantolactone and isoalantolactone was first increased then reached a maximum (31.57 and 20.68 mg/g respectively) when the ratio was 15:1. However, there were nearly unchanged when the ratio increased. This may be caused by reducing the extraction solvent, which would reduce the surface area for solvent to penetrate plant tissue and to solubilize the target compounds that was not beneficial for the extracting process. On the contrary, a larger volume of solvent caused sufficient swelling of material, but inadequate agitation of solvent may occurred during irradiation. For this reason, the solid/liquid ratio of 1:15 was appropriate to reach the high yield of alantolactone and isoalantolactone.
In order to elucidate the influence of MAE temperature on yield of alantolactone and isoalantolactone, five grams powder was mixed with ethanol solution at various temperature (30, 40, 50, 60 and 70°, respectively) for 120 s. fig. 5 showed that the yield of alantolactone and isoalantolactone was increased with the increased of temperature. It meant that the solubility of alantolactone and isoalantolactone increased by elevated temperature. Meanwhile, with the increased of temperature, the diffusion of the active ingredients will accelerate, the solvent viscosity and surface tension decreased, which contribute to the sample wetting and matrix penetration. However, there was no statistical difference on the yield of alantolactone and isoalantolactone among 50, 60 and 70°. Thus, the MAE temperature of 50° was selected as the extraction temperature.
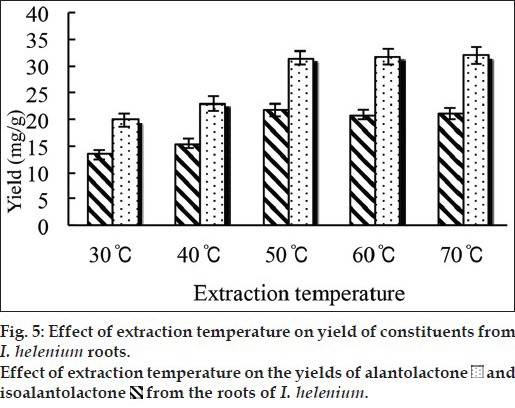
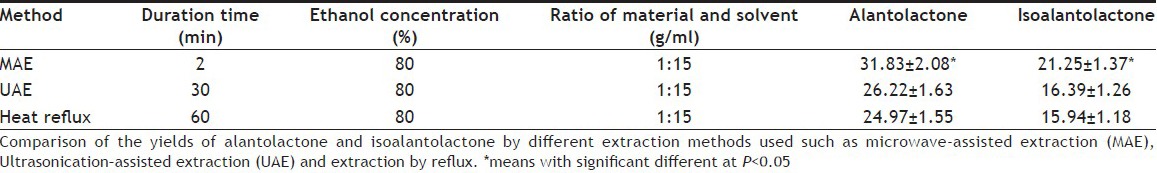
Table 1 showed that MAE for 2 min got a higher extraction yield than UAE for 30 min and heat reflux extraction for 60 min respectively. MAE demonstrated its advantages for improving extraction efficiency and shortening the time required. MAE method has been developed for the extraction of alantolactone and isoalantolactone from the root of I. helenium. The experimental results showed that extraction solvent and particle size played a key role on the extraction yield. Optimal conditions for MAE were evaluated and the optimal extraction conditions were 1 g plant samples (sifted through 140 mesh) mixed with 15 ml of 80% ethanol solution, microwave radiation 120 s at 50°. Under these optimal conditions, the yield of alantolactone and isoalantolactone was 31.83±2.08 mg/g and 21.25±1.37 mg/g, respectively. Compared with the conventional extraction methods, MAE was more efficient and time-saving. The results obtained are useful for the utilization of I. helenium, especially suitable for thermosensitive compounds from plant materials.
TABLE 1.
YIELDS OF ALANTOLACTONE AND ISOALANTOLACTONE BY MAE, UAE AND REFLUX
ACKNOWLEDGEMENTS
This research is supported by, the project of science research, Department of HeBei Education (No.ZD2014075), Key laboratory of Hebei North University and Youth foundation of Hebei North University (Q2013029). We acknowledge the support of Prof. Heng-cheng Zhao, College of Traditional Chinese Medicine, Hebei North University in identifying the plant material.
Footnotes
Zhao, et al.: Alantolactone and Isoalantolactone from Inula helenium
REFERENCES
- 1.Chaadaeva AV, Tenkeeva II, Moiseeva EV, Svirshchevskaia EV, Demushkin VP. Antitumor activity of the plant remedy peptide extract PE-PM in a new mouse T-lymphoma/eukemia model. Biomed Khim. 2008;55:81–8. [PubMed] [Google Scholar]
- 2.Konishi T, Shimada Y, Nagao T, Okabe H, Konoshima T. Antiproliferative sesquiterpene lactones from the roots of Inula helenium. Biol Pharm Bull. 2002;25:1370–2. doi: 10.1248/bpb.25.1370. [DOI] [PubMed] [Google Scholar]
- 3.Nesterova IuV, Zelenskaia KL, Vetoshkina TV, Aksinenko SG, Gorbacheva AV, Gorbatykh NA. Mechanisms of antistressor activity of Inula helenium preparations. Eksp Klin Farmakol. 2002;66:63–5. [PubMed] [Google Scholar]
- 4.Deriu A, Zanetti S, Sechi LA, Marongiu B, Piras A, Porcedda S, et al. Antimicrobial activity of Inula helenium L. essential oil against Gram-positive and Gram-negative bacteria and Candida spp. Int J Antimicrob Agents. 2008;31:588–90. doi: 10.1016/j.ijantimicag.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Cai D, Liu J. Matrix solid-phase dispersion extraction coupled with HPLC-diode array detection method for the analysis of sesquiterpene lactones in root of Saussurea lappa CB Clarke. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2809–14. doi: 10.1016/j.jchromb.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Trendafilova A, Chanev C, Todorova M. Ultrasound-assisted extraction of alantolactone and isoalantolactone from Inula helenium roots. Pharmacogn Mag. 2010;6:234–7. doi: 10.4103/0973-1296.66942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagherian H, Zokaee Ashtiani F, Fouladitajar A, Mohtashamy M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem Eng Process. 2011;50:1237–43. [Google Scholar]
- 8.Zhang G, Hu M, He L, Fu P, Wang L, Zhou J. Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shells and evaluation of its antioxidant and antibacterial activities in vitro. Food Bioproducts Process. 2013;91:158–68. [Google Scholar]


