Abstract
In this study, mouth-disintegrating tablets of atenolol and atorvastatin combination were formulated using superdisintegrants to impart fast disintegration. Fifteen formulations were prepared based on different concentrations of two superdisintegrants, croscarmellose sodium and Kyron-T134. Three different techniques such as direct compression, effervescent and sublimation were used to study the effect of manufacturing processes, nature and concentration of superdisintegrants on various features of these tablets. Five formulations were made using each method. Precompression studies like bulk density, tapped density, angle of repose, Carr's compressibility index, Hausner's ratio and compatibility studies such as Fourier transform infrared spectroscopy and differential scanning calorimetry were performed. Various features such as hardness, thickness, diameter, weight variation, friability, disintegration time, dissolution studies, wetting time, wetting volume, water absorption ratio, modified disintegration, uniformity of contents and stability were evaluated. Finally results were statistically analyzed by the application of one way ANOVA test. Formulation F13 containing Kyron-T134 (6%) and croscarmellose sodium (2%) was found to be the best among all fifteen formulations prepared in all aspects evaluated. Sublimation method is found to be the best among three methods of preparation used.
Keywords: Atenolol, atorvastatin, croscarmellose sodium, Kyron-T134, mouth-disintegrating tablets
Different types of dosage forms i.e. tablets, capsules, suspensions, emulsions, syrups, aerosols etc., are available all over the world to treat various pathological and emergency conditions. This era belongs to modern technology and new advances have been made by the scientists in the field of drug delivery systems resulting in development of new dosage forms in order to enhance patient compliance. Solid dosage forms have greater acceptance because of lack of pain, ease of self-administration, accuracy of dose and most importantly patient compliance; all of these make the oral route the most preferred route of administration[1].
Geriatric patients are on the rise and an unmet need exists for the development of convenient drug delivery systems for these patients[2]. Dysphagia is the most common problem associated with frequently used solid dosage forms. This problem occurs not only with geriatric and pediatric patients but also with all other patients. It is effecting 35% of population resulting in noncompliance to the treatment regimen[3].
Problems like dysphasia, bitter taste, nausea, vomiting and diarrhea can effectively be eliminated by the development of mouth-disintegrating tablets (MDTs). Their importance has markedly been increased due to the inclusion of orodispersible tablet in the European Pharmacopoeia as a dosage form that disperses instantaneously in the oral cavity before swallowing[4]. MDTs disperse instantaneously into absorbable form within 15 s to 3 min after placing in the oral cavity with the aid of saliva. MDTs are also called, mouth-dissolving tablets, orodispersible tablets, fast dissolving tablets, melt-in-mouth tablets, rapimelts, porous tablets and quick dissolving tablets[5].
A variety of superdisintegrants such as Crosscarmellose sodium, crosspovidone and sodium starch glycolate are used in formulating mouth-disintegrating tablets. These are used alone or in combination with another in various concentrations[6]. Different absorption sites for drug in saliva would be mouth, pharynx, esophagus and stomach. It depends on how quickly drug comes into solution and how quickly it is absorbed[7].
Different techniques and patent technologies are available for conversion of a raw material into mouth disintegrating tablets. These include direct compression, sublimation and effervescent method. Each method has its own advantages and disadvantages but most commonly direct compression is used in preparing mouth disintegrating tablets[8,9,10].
In the present study MDTs of atenolol and atorvastatin were formulated by using different techniques and superdisintegrants in order to reduce dose frequency and to enhance patient compliance towards therapy. Atenolol, a β-blocker is currently employed in the treatment of hypertension. It is also used in combination therapy of hypertension[11]. Atorvastatin is an antihyperlipidemic drug used to reduce serum cholesterol. It acts by inhibiting the enzyme 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, which is present in liver and plays an important role in cholesterol synthesis[12].
MATERIALS AND METHODS
Atenolol, atorvastatin and Kyron-T134 were obtained as gift sample from Warrick Pharmaceuticals, Islamabad, Pakistan. Saccharine was obtained from Fynk Pharmaceuticals, Islamabad, Pakistan. Croscarmellose sodium and orange flavor were obtained from Saffron Pharmaceuticals Faisalabad, Punjab, Pakistan. All the chemicals used were of high purity.
Preparation of mouth disintegrating tablets:
Fifteen formulations of MDTs were prepared by three different techniques as sublimation, effervescent and direct compression methods. Using each method, five formulations were prepared with different superdisintegrants at different concentrations. Kyron-T134 and croscarmellose sodium were used alone or in combination as superdisintegrants. Required quantity of each ingredient was weighed on analytical weighing balance. Powder blend was sieved through Sieve No. 60 and subjected for grinding in order to bring fineness in the powder[13].
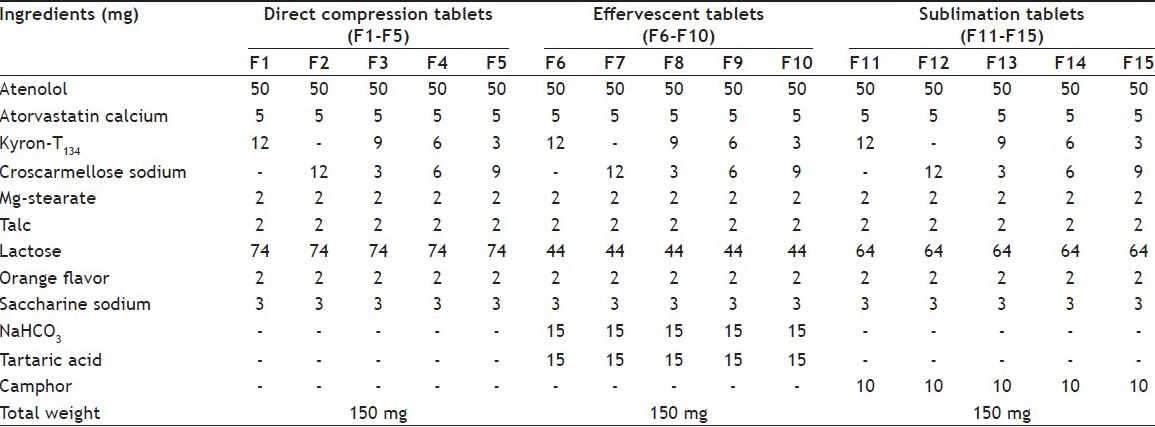
In direct compression method, all ingredients as shown in Table 1 of MDT's were mixed in geometrical order and compressed by using 8 mm round flat punches on a 10 station rotary tableting machine[14]. For the effervescent method, NaHCO3 and tartaric acid were preheated at 80° to remove any traces of moisture before adding to other ingredients of the formulation. All ingredients were sieved, mixed in a tumbling cylindrical blender and were compressed into five formulations by using 8 mm round flat beveled edge punches on a 10 station rotary tableting machine[15]. In the sublimation method, camphor was added as sublimating agent as shown in Table 1. After sieving and mixing, the blend was directly subjected to compression into 150 mg tablets. Then compressed tablets were allowed to sublime by placing them in a hot air oven for 6 h at a temperature of 60±1°[16,17]. A batch of 50 tablets was prepared for each established formulation using each method for further evaluation.
TABLE 1.
COMPOSITION OF MOUTH-DISINTEGRATING TABLETS F1-F15
Evaluation of powder blend, angle of repose:
Funnel method was used for determining angle of repose. Funnel was placed vertically at appropriate height by using support i.e. tripod stand having paper sheet under it. Then powder blend was poured into the funnel[12]. A cone of height (h) was formed having radius (r). TanƟ=h/r, where Ɵ=angle of repose, h=height of cone, r=radius of the cone base.
Bulk density and tapped density:
Cylinder method was used for determining bulk density. Powder after weighing was poured into the cylinder and volume was marked. After that mass of the powder was divided by the volume occupied by the powder in the cylinder[6]. Tapped density was also be determined by using cylindrical method. Weighed amount of powder blend was poured into the graduated cylinder and the cylinder subjected to a given number of tappings. Now the tapped volume is used to calculate tapped density.
Carr's index:
Compressibility is an indication by which a powder or material can be freely induced into free flow. One of the simplest and well known methods for determining the compressibility is Carr's compressibility index.
Hausner's ratio:
It is the ratio of tapped density to the bulk density. It indirectly measures the index of flow of powders. If Hausner's ratio value comes less than 1.25 it shows powder has better flow properties. On the other hand, if its value is greater than 1.25 it represents poor flow properties[20].
Total porosity:
It was determined by measuring the volume occupied by the blend and true volume of the blend. Intramolecular/intraparticle spaces remain smaller as compared to the volume occupied by the whole blend.
Evaluation of MDTs, hardness:
It was measured to ensure integrity and shape maintenance of tablets so that tablets might be able to bear transportation effects[6]. Ten tablets were selected randomly and their average weight was calculated. Monsanto hardness tester was used to find out tablet hardness in kg/cm2. Average of three values was determined.
Tablet thickness and weight variation:
Verneir caliper was used to determine tablet thickness. Tablet was placed in between two arms of the caliper. Average of three values was calculated[6]. Weight variation is determined by taking twenty tablets and weighing them on electronic weighing balance to determine the average weight. At the end, the individual weight was compared with average weight[6].
Friability:
Friability of tablets was determined by using Roche Friabilator (Pharma Test, Germany). Twenty tablets were weighed and placed in the drum of the friabilator and speed was adjusted at 25 rpm. The tablets were allowed to revolve, fall from height of six inches for 4 min. Then tablets were de-dusted using muslin cloth and re-weighed[6].
Tablet disintegration:
Tablet disintegration apparatus was used. Six tablets were taken and placed individually in tubes and properly covered. The temperature of medium was maintained at 37±2° and timely noted by thermometer. The time taken by the tablet to disintegrate completely was noted[18].
Wetting time:
Ten millilitres of the buffer solution of pH 6.80 as of saliva was taken in petri dish. A circular tissue paper having diameter 8 cm folded twice was placed in the petri dish. Single mouth disintegrating tablet was placed on tissue paper and time for complete wetting was noted[18].
Dissolution study:
Dissolution studies of MDTs were performed on USP type-II apparatus. The speed of apparatus was set at 50 rpm. Nine hundred millilitres of phosphate buffer solution of pH 6.80 was taken as dissolution medium in each vessel of the apparatus. A single mouth dissolving tablet was dipped in each of the dissolution vessels and temperature of medium was kept at 37±0.5°. The dissolution sample was taken from each vessel at regular intervals and was replaced by equal quantity of freshly prepared media. Absorbance was measured by using UV/Vis spectrophotometer (UV 1700, Shimadzu, Japan)[19].
In vitro dispersion time:
About 6 ml phosphate buffer of pH 6.80 was taken in a beaker of 10 ml capacity. A tablet was placed in that beaker and time required for the complete dispersion of tablet was noted. The experiment was performed thrice for each formulation.
Water absorption ratio:
It is the amount of water absorbed by the tablet in a unit time. Six millilitres of water was taken in a Petri dish of internal diameter 6.50 cm. A piece of tissue paper folded twice was placed in the petri dish. A tablet was weighed before placing onto the tissue paper in the petri dish and time taken by tablet for complete wetting was noted. Wetted tablet was reweighed and water absorption ratio was calculated by using a previously reported formula[20].
Drug content uniformity:
Twenty tablets were crushed into fine powder. A quantity equivalent to 15 mg was taken and diluted in phosphate buffer of pH 6.80 leading to first dilution. It was filtered and filtrate was diluted again leading to 2nd dilution. Then sample of 2-3 ml was taken and its absorbance was noted on UV/Vis spectrophotometer. The same procedure was repeated thrice and also for the standard. Then amount of the drug present in sample was calculated.
FTIR studies:
FTIR (IR Prestage 21 Shimadzu) spectra were recorded for pure drugs (atenolol and atorvastatin) and superdisintegrants (Kyron-T134 and croscarmellose sodium). The pellets were prepared in KBr press (2 mg sample in 200 mg KBr) under a hydraulic pressure of 150 kg/cm2. The scanning range chosen was 4000-400 cm-1 and the resolution was set at 2 cm-1. Compatibility between pure drugs and superdisintegrants were checked and FTIR-spectra were recorded[21].
DSC studies:
DSC analysis was conducted to analyze any possible drug-drug and drug-superdisintegrants interactions by using thermal analyzer (SDT. Q 600, USA). Thermal curves of pure components alone (drugs and superdisintegrants) and their mixtures were recorded. Superdisintegrants and drugs (atenolol and atorvastatin) were triturated separately to get a finely divided powder and heated in sealed aluminum pans at a rate of 10°/min from 0 to 176° under a nitrogen flow rate of 40 ml/min. Reproducibility was checked by running the sample in triplicate[21].
Stability studies:
The stability studies of MDT's were performed for a period of six months according to ICH (international conference on harmonization) guidelines. All the physical and in vitro tests were performed and any significant changes were observed. Studies were performed under following temperature and humidity conditions 37±1°, 40±1°, 50±1° and RH 75±5%.
Statistical analysis:
All the results were evaluated statistically by using one-way ANOVA after determining mean and standard deviation.
RESULTS AND DISCUSSION
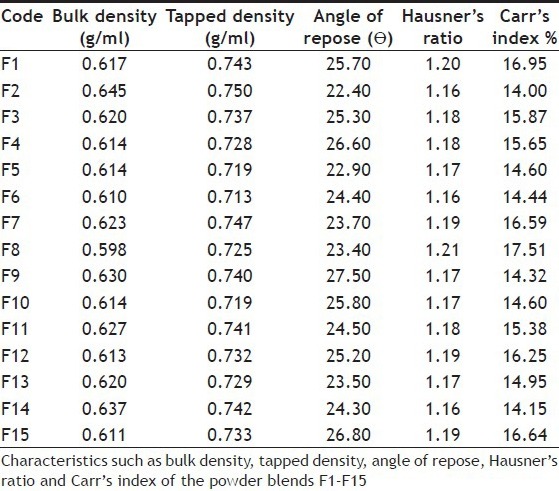
Powder blends for 15 formulations were prepared by using different concentrations of superdisintegrants. These blends were assessed for rheological properties such as bulk density, tapped density, angle of repose, Hausner's ratio and Carr's index. Bulk density of fifteen formulations was in the range of 0.598 to 0.645 g/ml and tapped density 0.713 to 0.750 g/ml. Angle of repose, Hausner's ratio and Carr's index values were found in the range of 22.40 to 27.50, 1.16 to 1.21 and 14.00 to 17.51, respectively. These results of powder blend were well within identified limits as shown in Table 2.
TABLE 2.
CHARECTERISTICS OF POWDER BLENDS
FTIR results showed that there were no interactions between the drugs as well as drugs and superdisintegrants and between the superdisintegrants (fig. 1). DSC study also showed that drugs and excipients had no interactions with each other (fig. 2). Accelerated stability studies had revealed that tablets remained stable for a recommended time period.
Fig. 1.

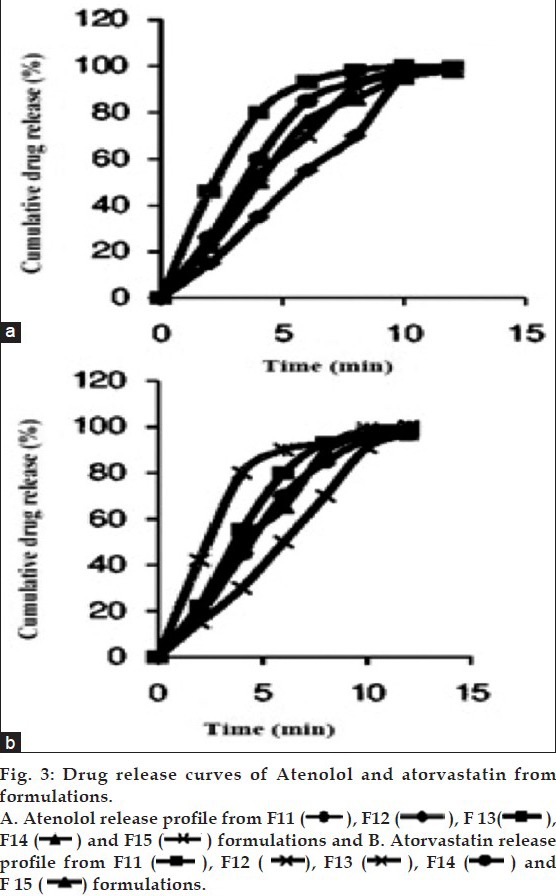
FTIR Spectra of drugs and drug blends with superdisintegrants.
Fourier Transform IR spectra of A. atenolol, B. atorvastatin, C. atenolol+atorvastatin, D. croscarmellose sodium, E. Kyron-T134, F. KyronT134 + croscarmellose sodium, G. atenolol+atorvastatin+ KyronT134+croscarmellose sodium.
Fig. 2.
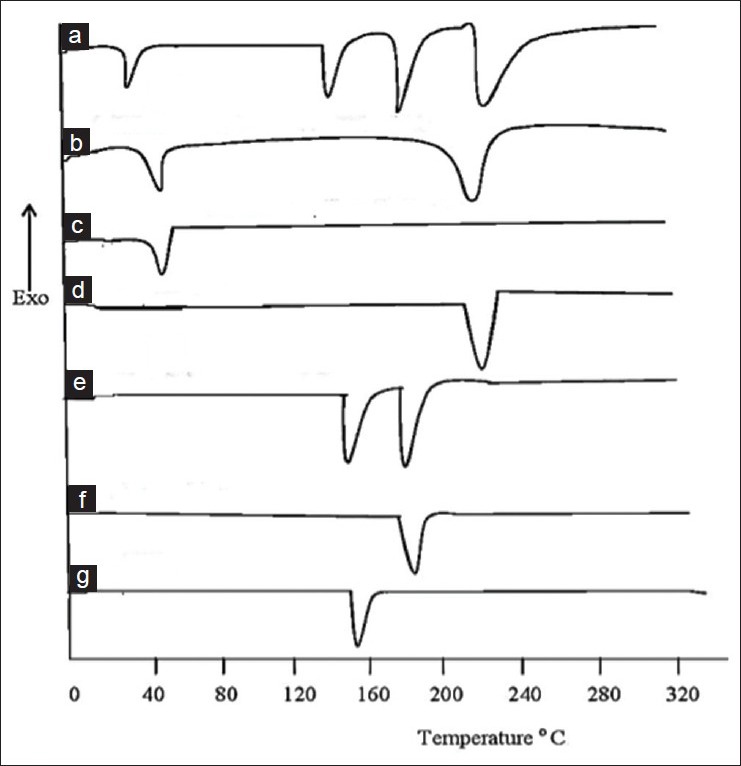
DSC Spectra of individual components and blends.
Differential scanning calorimetric spectra of A. blend of croscarmellose sodium + atenolol + atorvastatin + KyronT134, B. mixture of KyronT134 + croscarmellose sodium, C. croscarmellose sodium alone, D. KyronT134 alone, E. atenolol+atorvastatin mixture, F. atorvastatin and G. atenolol.
Tablets were evaluated for different post-compression parameters. The results of weight variation, hardness, thickness and friability were present within the pharmacopeial limits as shown in Table 3. The weight variation limit with ±7.5% variation for 150 mg tablets was 138.75 to 161.25 mg. All the formulations were in range of 146.67 to 151.58 mg. Friability was found up to 0.714% for all formulations that was less than official value of friability i.e. less than 1%.
TABLE 3.
EVALUATION OF MOUTH-DISINTEGRATING TABLETS
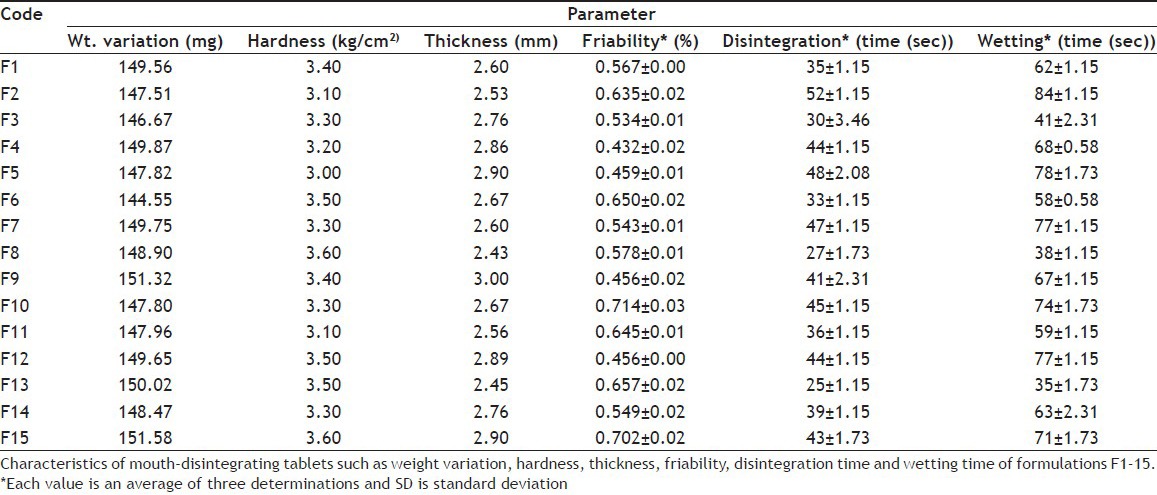
Disintegration time 25 to 52 s, wetting time 35 to 84 s and dispersion time 27 to 53 s were noted for each formulation. Results had clearly revealed that disintegration time was even less than 1 min for all the fifteen formulations. Dispersion time was also less than 1 min. These results of disintegration time and wetting time are shown in Table 3 and dispersion time in Table 4.
TABLE 4.
CHARACTERISTICS OF MOUTH-DISINTEGRATING TABLETS

The release studies of both drugs were measured by using UV/Vis spectrophotometer. The results had shown that the release of atenolol was in the range of 97 to 101% for 15 formulations in 12 min. Atorvastatin release was in the range of 96 to 100.5% (fig. 3). These results had shown that atenolol release is more as compared to atorvastatin. Wetting volume was 10 to 26 ml. Water absorption ratio was up to 1.55 which shown that tablets can absorb water more than 1.5 times of its weight as shown in Table 4.
The values of angle of repose were present between 22.4° to 27.5° that were less than 30° indicating good flow properties. The results of Hausner's ratio and Carr's index have indicated that powder blend fulfilled the required criteria for an excellent flow pattern. The FTIR spectra of atenolol, atorvastatin alone and in combination with superdisintegrants were taken to evaluate any possible interaction between drug molecules as well as with superdisintegrants. The FTIR spectra of pure atorvastatin calcium and superdisintegrants were taken (fig. 1). The FTIR spectra of pure atorvastatin calcium showed characteristic peaks at 2955.15 cm-1 310 (C-N-stretching), 3059.15 cm-1 (C-H-stretching), 1313.56 cm-1 (C-HO-stretching alcoholic group), 1564.97 cm-1 (C=O-stretching amidic group), 3403.27 cm-1 (N-H-stretching), 1656.97 cm-1 (C=C-bending), 751.62 cm-1 696.95 cm-1 (C-F-stretching), 1104.39 cm-1 (O-H-bending). It might be the possibility of intermolecular hydrogen bonding between adjacent atorvastatin calcium molecules. The spectrum of pure atorvastatin calcium was equivalent to the spectra obtained by the addition of superdisintegrants[22].
IR spectrum of atenolol is characterized by the absorption of -COOH group at 1651 cm-1. The spectra of pure drug and superdisintegrants were taken. Same absorption spectra of atenolol were obtained (fig. 1). The results revealed that there were no considerable changes in the IR peaks of atorvastatin calcium, when mixed with atenolol and superdisintegrants Kyron-T134 and croscarmellose sodium. Presence of characteristic peaks of drug molecules and superdisintegrants in the FTIR spectra has established the absence of interaction between drugs and superdisintegrants. These observations indicated that the compatibility of atorvastatin calcium and atenolol with each other as well as compatibility of atorvastatin calcium and atenolol with superdisintegrants[23].
DSC studies of drug molecules and excipients were carried out alone as well as in combination at an increasing temperature rate of 10°/min for specific time period for a particular agent. The typical DSC thermo grams were obtained by using thermal analyzer (SDT. Q 600, USA). The characteristic peaks of alone drugs and with excipients were compared. This study also confirmed that there was no interaction among drugs and excipients (fig. 2). There was no incompatibility among drugs and superdisintegrants used for formulations.
In case of atenolol initially flat or smooth profile was observed but when it entered into its melting range 154°, sharp exothermic peak was observed. Similarly in case of atorvastatin calcium initially flat or smooth profile was observed but when it entered into its melting range 176°, sharp exothermic peak was observed that showed its presence (fig. 2). The characteristic thermograms of drugs alone and with superdisintegrants were compared and no drug-drug, superdisintegrants-drug interaction was found by FTIR and DSC studies[24].
All the outcomes have indicated that powder blend was good for compression into tablets. Consistency of tablet weight and size was calculated by weight variation studies of all the formulations. All the tablets were of appropriate hardness capable of bearing stresses during handling and transport.
The friability of all formulations was less than 0.80% indicating that tablets had good mechanical strength and did not show any unnecessary breakdown of the particles. A few variations in results regarding friability were present but results remained within specified limits. Statistically results of friability were tested by using one way ANOVA. Results of ANOVA between the groups had shown that P value was 0.041, which was less than 0.05 showing significant results.
Uniformity of drug content in all of the formulations was also assessed in order to ensure dose. The analysis proved that drug contents in all of the formulations remained within the range of 96% to 100.5%. These results were within prescribed limits proving a uniform and proper distribution of drug among all the MDTs.
The results of wetting time had shown that all the formulations had wetting time less than 90 s. F13 formulation showed a wetting time of 35 s, which was lowest among all of 15 formulations. The wetting time of the sublimed formulations was less due to the presence of camphor in their composition than other two methods. Wetting time within groups was highly significant, which indicates that wetting of tablets greatly affected by the concentration of superdisintegrants.
Disintegration time for all the formulations was less than 1 min, which is acceptable for MDTs[25]. F13 formulation prepared by sublimation method had lowest disintegration time of all 15 formulations. The results of both wetting time and disintegration time studies were in agreement indicating that all formulations would definitely disintegrate in oral cavity within specified time period[26]. The results of one way ANOVA had shown that P value for disintegration time between the groups was 0.070 that is greater than 0.050. Statistically the results of wetting volume between the groups were significant and within the groups were highly significant. It represents that wetting volume for tablets depends upon the method of preparation and concentration of superdisintegrants.
In vitro dispersion test was performed for all the formulations. Formulation F13 had shown lowest dispersion time 27 s[27]. Results of ANOVA between the groups had shown that P value was 0.024, which was less than 0.05. This proved that results were significant.
Stability studies of three best formulations were conducted for six months period. The tablets were kept under accelerated conditions of temperature and humidity 35±5ξ and 75%±5%, respectively. The sample was taken after 1, 2, 3, 4, 5 and at the end of 6 month. The tablets were evaluated for all of required evaluation parameters. The results had indicated that there were no significant variations occurred in drug content and in vitro dispersion time at the end of 6 months. It means that formulations remained stable under accelerated conditions of temperature and humidity.
Tablets prepared by direct compression, sublimation and effervescent methods were free from fragmentation and sticking. The compatibility of drugs and superdisintegrants was successfully established by DSC and FTIR. The combination of Kyron-T134 and croscarmellose sodium produced faster release than other superdisintegrants. Formulation F13 containing 6% of Kyron-T134 and 2% of croscarmellose sodium had shown the best disintegration time results. Thus it is concluded that kyron-T134 is the best superdisintegrant of two, which were used in present study and sublimation method is the best one of three methods used to prepare mouth disintegrating tablets.
Footnotes
Sarfraz, et al.: Atenolol and Atorvastatin Mouth Disintegrating Tablets
REFERENCES
- 1.Chein YW. New York: Marcel Dekker; 1992. Oral drug delivery and delivery system. [Google Scholar]
- 2.Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20:764–71. doi: 10.1016/s0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuchekar BS, Aruagam V. Design of fast dissolving tablets. Indian J Pharm Educ. 2001;35:150–2. [Google Scholar]
- 4.Shahi SR, Agrawal GR, Shinde NV, Shaikh SA, Shaikh SS, Somani VG, et al. Formulation and in vitro evaluation of orodispersible tablets of etoricoxib with emphasis on comparative functionality evaluation of three classes of superdisintegrants. Rasayan J Chem. 2008;1:292–300. [Google Scholar]
- 5.Sreenivas SA, Dandagi PM, Gadad AP, Godbole AM, Hiremath SP, Mastiholimath VS, et al. Orodispersible Tablets: New-fangled Drug Delivery System - A Review. Indian J Pharm Edu Res. 2005;39:177–81. [Google Scholar]
- 6.Marshal K. Compression and consolidation of powdered solids. In: Lachman L, Lieberman HA, Kanig JL, editors. The Theory and Practice of Industrial Pharmacy. 3rd ed. Mumbai: Varghese Publishing House; 1987. pp. 66–99. [Google Scholar]
- 7.Seager H. Drug-delivery products and the Zydis fast-dissolving dosage form. J Pharm Pharmacol. 1998;50:375–82. doi: 10.1111/j.2042-7158.1998.tb06876.x. [DOI] [PubMed] [Google Scholar]
- 8.Gudas GK, Manasa B, Rajesham VV, Kumar SK, Kumari JP. Formulation and evaluation of fast dissolving tablets of Chlorpromazine HCl. J Pharm Sci Tech. 2010;2:99–102. [Google Scholar]
- 9.Kalia A, Khurrana S, Bedi N. Formulation and evaluation of Mouth Dissolving Tablets of Oxacarbazepine. Int J Pharm Pharma Sci. 2009;1:12–23. [Google Scholar]
- 10.Patel DM, Patel MM. Optimization of fast dissolving etoricoxib tablets prepared by sublimation technique. Indian J Pharm Sci. 2008;70:71–6. doi: 10.4103/0250-474X.40335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baroodo R. Fast dissolving drug delivery systems. JAMA India. 2001;4:27–31. [Google Scholar]
- 12.Kuchekar BS, Atul, Badhan C, Mahajan HS. Mouth dissolving tablets: A novel drug delivery system. Pharma Times. 2003;35:7–9. [Google Scholar]
- 13.Chang RK, Guo X, Burnside BA, Couch RA. Fast dissolving tablets. Pharm Tech. 2000;24:52–8. [Google Scholar]
- 14.Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev Ind Pharm. 1999;25:571–81. doi: 10.1081/ddc-100102211. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik D, Dureja H, Saini T. Formulation and evaluation of olanzapine mouth dissolving tablets by effervescent formulation approach. Indian Drugs. 2004;41:410–12. [Google Scholar]
- 16.Zade PS, Kawtikwar PS, Sakarkar DM. Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res. 2009;1:34–42. [Google Scholar]
- 17.Suresh S, Pandit V, Joshi H. Preparation and evaluation of mouth dissolving tablets of salbutamol sulphate. Indian J Pharm Sci. 2007;69:467–9. [Google Scholar]
- 18.Bi Y, Sunada H, Yonezawa Y, Danjo K, Otsuka A, Iida K. Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity. Chem Pharm Bull (Tokyo) 1996;44:2121–7. doi: 10.1248/cpb.44.2121. [DOI] [PubMed] [Google Scholar]
- 19.Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007;33:1225–32. doi: 10.1080/03639040701377888. [DOI] [PubMed] [Google Scholar]
- 20.Gohel MC, Bansal G, Bhatt N. Formulation and evaluation of orodispersible taste masked tablets of Famotidine. Pharma Boil World. 2005;3:75–80. [Google Scholar]
- 21.Ranjha NM, Khan H, Naseem S. Encapsulation and characterization of controlled release flurbiprofen loaded microspheres using beeswax as an encapsulating agent. J Mater Sci Mater Med. 2010;21:1621–30. doi: 10.1007/s10856-010-4034-4. [DOI] [PubMed] [Google Scholar]
- 22.Laksmi CSR, Akul SP, Totala AV, Chaudhary JJ, Patel NJ. Development and characterization of melt in mouth tablets of atenolol by sublimation technique. Int J Pharm Res Dev. 2011;3:27–36. [Google Scholar]
- 23.Jagdale SC, Sali MS, Barhate AL, Loharkar JN, Chabukswar AR. Design and evaluation of Enteric Press-Coated Tablet for Pulsatile Delivery of Atenolol. Int J Pharm World Res. 2010;1:1–15. [Google Scholar]
- 24.Tayade PT, Kale RD. Encapsulation of water-insoluble drug by a cross-linking technique: Effect of process and formulation variables on encapsulation efficiency, particle size, and in vitro dissolution rate. AAPS J. 2004;6:112–9. doi: 10.1208/ps060112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandari S, Mittapalli RK, Gannu R, Rao YM. Orodispersible tablets: An overview. Asian J Pharm. 2008;2:2–11. [Google Scholar]
- 26.Biswajit B, Joshi V. Formulation and evaluation of orodispersible tablets of Amlodipine Besylate. Int J Pharm Technol. 2011;3:3745–66. [Google Scholar]
- 27.Bhardwaj V, Shukla V, Goyal N, Salim M, Sharma P. Formulation and evaluation of fast disintegrating sublingual tablets of amlodipine besylate using different superdisintegrants. Int J Pharm Pharm Sci. 2010;2:89–92. [Google Scholar]


