Abstract
The present investigation aimed to evaluate the protective effects of sitagliptin, glimepiride, rosuvastatin and their combinations on oxidative stress and endothelial dysfunction in the aortic tissues in fructose-fed type-2 diabetic rats. Sitagliptin (20 mg/kg, p.o.), glimepiride (2 mg/kg, p.o.), rosuvastatin (5 mg/kg, p.o.) and their combinations were administered for 6 w after induction of diabetes by fructose (66%, w/v solution, p.o. for 8 w) in wistar rats. The effects were examined on body weight, serum glucose, triglyceride, cholesterol, blood pressure, heart rate, nitric oxide and antioxidant defensive enzymes. After completion of treatment schedule, the blood pressure was determined by invasive method and vascular reactivity was tested with adrenaline, noradrenaline and phenylephrine. Endothelial dysfunction was determined by acetylcholine and sodium nitroprusside-induced vasorelaxation studies on isolated rat aortas. Long term treatments significantly decreased body weight gain, serum glucose, triglyceride and cholesterol levels; normalize the heart rate, and blood pressure in fructose fed rats. The treatments significantly improved vascular reactivity to catecholamines with reduction in elevated blood pressure in type-2 diabetic rats. The significant improvement in the relaxant response to acetylcholine and sodium nitroprusside was obtained on isolated aortas. All the treatments were effective in restoring defensive antioxidant enzymes. Sitagliptin and rosuvastatin were able to reverse endothelial dysfunction in type-2 diabetes, but better ameliorating potential was found when used in combination.
Keywords: Endothelial dysfunction, blood pressure, catecholamines, vascular reactivity
Recent estimates indicate that more than 171 million people in the world suffer with diabetes and this is projected to increase 366 million by 2030. It is believed to develop as a result of genetic and lifestyle factors producing a state of high insulin resistance[1]. There is a strong association between insulin resistance, hypertension, glucose intolerance, low HDL-cholesterol and raised VLDL-triglycerides[2]. Diabetes mellitus (DM) is associated with, significant morbidity due to specific diabetes related microvascular and macrovascular complications (i.e. ischemic heart disease, stroke and peripheral vascular diseases), and diminished quality of life. Insulin resistance is associated with many risk factors that commonly precede the development of hyperglycemia and these typically include obesity, dyslipidaemia characterized by high triglyceride, elevated blood pressure, oxidative stress, endothelial dysfunction[3]. It has been suggested that hyperglycemia and hypertriglyceridemia induce endothelial dysfunction and inflammation through the production of an oxidative stress. Endothelial dysfunction refers to a condition in which the endothelium loses its physiological properties: the tendency to promote vasodilation, fibrinolysis and antiaggregation[4]. Oxidative stress is an increase in the steady state levels of reactive oxygen species and may occur as a result of increased free radical generation and/or decreased antioxidant defence mechanism[5]. It has been shown that, when the activities of superoxide dismutase (SOD, which capture O2•) and catalase (CAT, which capture H2O2) were maintained, the endothelial function was not altered even in cases of hyperglycemia[6]. Insulin resistance leads to abnormalities in regulatory mechanism of blood pressure resulting into cardiovascular complications in type-2 DM[7]. The dyslipidaemia is a central player in the development of atherosclerosis and other cardiovascular complications in the setting of insulin resistance in type-2 DM[8].
The increase in blood pressure (BP) and triglyceride level in type-2 diabetes is secondary to the hyperinsulinemia, and then a drug intervention that reverses these effects should also attenuate the hypertension and cardiovascular complications[9,10]. A drug that modulates endothelial functions can significantly alter morbidity and mortality associated with endothelial dysfunction[11]. Antidiabetic drugs like sitagliptin (DPP-4 inhibitor) and glimepiride (sulfonylureas) are known to be effective in increasing the insulin sensitivity and thereby reduce hyperinsulinemia[12]. While rosuvastatin [3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor] has been shown to exert antiinflammatory effects on the microvascular endothelial independent by its lipid lowering action as well as due induction of nitric oxide from the vasculature[13,14].
Therefore, we initiated long-term treatments with sitagliptin, glimepiride, rosuvastatin and their combinations in fructose-fed rats. In this study, an attempt has been made to investigate the efficacy of sitagliptin, glimepiride, rosuvastatin and their combinations on endothelial dysfunction in type-2 diabetic rats.
MATERIALS AND METHODS
Rosuvastatin and glimepiride (Glenmark Pharmaceuticals Ltd., Nashik, India) and sitagliptin (Merck Ltd., Mumbai, India) were obtained as gift samples. The drug solution of sitagliptin was freshly prepared in distilled water and suspension of rosuvastatin and glimepiride were made in distilled water using 0.5% carboxy methylcellulose. Biochemical kits for glucose, triglyceride and cholesterol (Auto Span, Surat, India) were used. All the chemicals used were of analytical grade and purchased from standard manufacturers.
Experimental animals:
Male wistar rats (150-170 g) were used. Animals were housed in polypropylene cages and maintained under the standard laboratory environmental conditions; temperature 25±2°, 12:12 h light:dark cycle and 50±5% Relative humidity with free access to food and water ad libitum. Animals were acclimatized to laboratory conditions before the test. All the experiments were carried out during the light period (08:00–16:00 h). The studies were carried out in accordance with the guidelines given by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India). The Institutional Animal Ethical Committee (IAEC) of M.V.P.S College of Pharmacy, Nashik approved the protocol of the study (IAEC/2011/01).
Induction of type–2 DM in rats:
Rats were administered with fructose (66%, w/v solution, 5 ml/kg/day, p.o., for 8 w) to induce type-2 DM[15]. The animals with fasting blood glucose level more than 280 mg/dl and BP over 145 mmHg were selected for the study.
Experimental design:
The diabetic animals were randomly assigned to six groups (n=6). Group I (Control) - vehicle (distilled water; 5 ml/kg, p.o.), group II (diabetic) - fructose (66% w/v solution, 5 ml/kg/day, p.o., for 8 w), group III -sitagliptin+fructose (20 mg/kg, p.o+66% w/v p.o., for 8 w), group IV - glimepiride+fructose (2 mg/kg, p.o+66% w/v p.o., for 8 w), group V -rosuvastatin+fructose (5 mg/kg, p.o+66% w/v p.o., for 8 w), group VI - sitagliptin+glimepiride+fructose (20 mg/kg, p.o and 2 mg/kg, p.o+66% w/v p.o., for 8 w, respectively), group VII - sitagliptin+rosuvastatin+fructose (20 mg/kg, p.o and 5 mg/kg, p.o+66% w/v p.o., for 8 w, respectively). The drugs were administered daily for a period of 6 w after induction of diabetes.
In vivo experimental method:
After induction of diabetes, the treatments were initiated. Body weight of each animal was measured before the start of treatments and thereafter weekly of drug treatments. The systolic blood pressure by tail-cuff method and pulse rate were recorded weekly during the treatments on Power-lab data acquisition system (AD Instruments, Australia). Blood samples were collected through retro-orbital plexus under ether anaesthesia weekly for determination of serum glucose, triglyceride and cholesterol. After completion of treatments, BP was determined by invasive method and vascular reactivity was tested with adrenaline (ADR), noradrenaline (NA) and phenylephrine (PE). Acetylcholine (ACh) and sodium nitroprusside-induced vasorelaxation were studied on isolated rat aortas. Oxidative stress indices such as SOD, CAT, LPO and NO levels were determined in rat aortas at end of the treatments[16,17].
Estimation of serum glucose, triglyceride and cholesterol levels:
The rats were anaesthetized under light ether; blood was removed from the retro orbital plexus using a capillary in micro sample tubes, serum was separated and used for biochemical investigations. Serum glucose, triglyceride and cholesterol levels were determined by using standard biochemical kits.
Vascular reactivity to catecholamines:
After the completion of treatment schedule, rats from each group were anesthetized by ketamine and xylazine (75 and 15 mg/kg i.p., respectively). Right jugular vein was cannulated with fine polyethylene catheter for the administration of drugs. BP was recorded from left common carotid artery using pressure transducer (MLT-125) by direct method on Power-lab data acquisition system. Heparinised saline (100 IU/ml) was filled in the transducer and in the fine catheter cannulated to the carotid artery to prevent clotting. After 30 min of stabilization, mean change in BP to ADR (1 μg/kg), NA (1 μg/kg) and PE (1 μg/kg) were recorded[18].
Endothelial-dependent and independent vasorelaxation on isolated rat aortas:
Immediately after completion of vascular reactivity studies, rats were sacrificed by cervical dislocation. Thoracic aorta from the arch down to diaphragm was isolated and was placed in Krebs solution of composition (mM), NaCl: 118.4; KCl: 4.7; CaCl2: 2.5; KH2PO4: 1.2; MgSO4: 1.2; NaHCO3: 25.0, Glucose: 11.0, at 37° and aerated. The rings of 3 mm length were prepared and mounted in organ bath containing 15 ml of Krebs solution. The contractions were recorded by a force transducer (Power-Lab, AD Instruments). The resting tension of 1 g was applied to the preparation and equilibrated for 90 min before the experiment with exchange of bathing solution every 15 min. After the equilibration, the rings were exposed to 1×10-6 M PE for precontraction. When the contractile response of PE was plateaued, acetylcholine or sodium nitroprusside were added in a cumulative fashion for endothelium dependent vasorelaxation studies respectively. The concentrations of acetylcholine or sodium nitroprusside were made in range of 1×10-9 to 1×10-4 M. To verify the integrity of smooth muscle in thoracic aortas, sodium nitroprusside-induced vasorelaxation (a NO donor) was investigated which causes aortas to relax due to endothelial nitric oxide[17].
Estimation of antioxidants activity:
The 10% w/v tissue homogenate of aorta was prepared in ice cold 0.1 M phosphate buffer (pH 7.4). The post nuclear fraction for catalase assay was obtained by centrifugation (Remi–C-30, Remi Industries Ltd. Mumbai, India) of the homogenate at 1000 g for 20 min at 4°; for other enzyme assays, centrifugation was at 12 000 g for 60 min at 4°. Bio-spectrophotometer (BL-200, Elico, India) was used for further assay procedures.
Estimation of SOD and CAT activity:
Superoxide dismutase activity was assayed according to the method of Kono[19], where in the reduction of nitrobluetetrazolium chloride (NBT) was inhibited by the superoxide dismutase and measured at 560 nm spectrophotometrically. Briefly the reaction was initiated by the addition of hydroxylamine hydrochloride to the reaction mixture containing NBT and post nuclear fraction of brain homogenate. Results were expressed as percentage inhibition of reduction of NBT[19].
Catalase activity was assessed by the method of Luck[20], where the breakdown of H2O2 was measured. The assay mixture consisted of 3 ml of H2O2 phosphate buffer (0.0125 M H2O2) and 0.05 ml of supernatant of aortic homogenate (10%) and the change in the absorbance were measured at 240 nm. The enzyme activity was calculated using the millimolar extension coefficient of H2O2 (0.07). The results were expressed as micromoles of H2O2 decomposed per minute per milligram of protein[20].
Estimation of LPO level:
The quantitative measurement of lipid peroxidation was done by the method of Wills[21]. The amount of malondialdehyde (MDA) formed was measured by reaction with thiobarbituric acid at 532 nm. The results were expressed as nanomloes of MDA per mg protein, using the molar extension coefficient of chromophore (1.56×105 M-1 cm-1)[21].
Estimation of NO level:
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO), was determined with a colorimetric assay using Greiss reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide and 2.5% phosphoric acid). Equal volumes of supernatant and Greiss reagent were mixed, the mixture was incubated for 10 min at room temperature in the dark and the absorbance at 543 nm was determined. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and expressed as μmol of nitrite per ml of homogenate[22].
Statistical analysis:
Results are expressed as mean±SEM, and the statistical analysis of data was done using one-way analysis of variance (ANOVA) followed by Dunnett's test. Probability level less than 0.05 was considered statistically significant.
RESULTS
Effect of sitagliptin, glimepiride, rosuvastatin and their combinations on body weight:
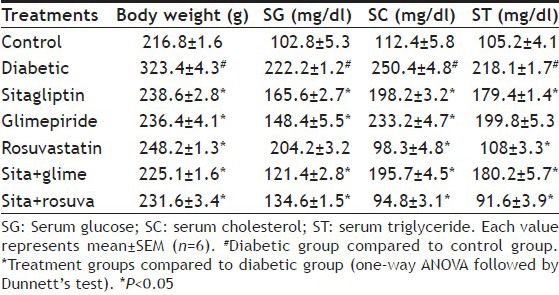
Before induction of diabetes the rats had mean body weight of 162.7±8.8 g. After induction of diabetes the rats showed increase in body weight to 272.4±9.2 g. Treatment with sitagliptin, glimepiride, rosuvastatin and their combinations were able to significantly (P<0.05) reduce the diabetes induced body weight gain (Table 1).
TABLE 1.
EFFECT ON BODY WEIGHT AND BIOCHEMICAL PARAMETERS
Effect of sitagliptin, glimepiride, rosuvastatin and their combinations on biochemical parameters:
Fructose treated group showed significant (P<0.05) rise in serum glucose, cholesterol and triglyceride levels as compared to control group. Long term treatment of the combination of sitagliptin with glimepiride and rosuvastatin significantly (P<0.05) reduced elevated serum glucose, cholesterol and triglyceride levels as compared to fructose treated group. There was no significant difference in serum glucose, but cholesterol and triglyceride levels were significantly (P<0.05) reduced in group treated with rosuvastatin alone (Table 1).
Effect of sitagliptin, glimepiride, rosuvastatin and their combinations on heart rate and blood pressure (by noninvasive and invasive method):
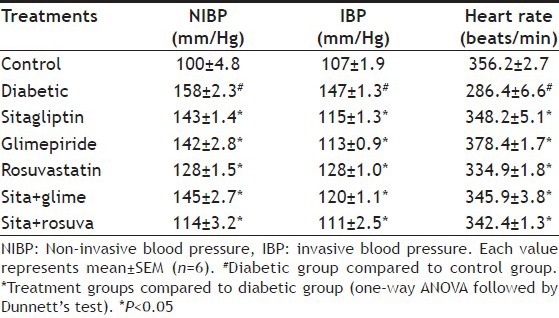
The control group animals showed the normal heart rate and BP, whereas fructose treated group showed significant decrease (P<0.05) in heart rate and rise in (P<0.05) BP as compared to control group. Administration of sitagliptin, glimepiride and rosuvastatin alone for a period of 6 w significantly (P<0.05) restored the heart rate and reduced BP compared to fructose treated group. But the reduction was most significant with the combination of sitagliptin-rosuvastatin (Table 2).
TABLE 2.
EFFECT ON BLOOD PRESSURE AND HEART RATE
Effect on defensive antioxidant enzyme levels:
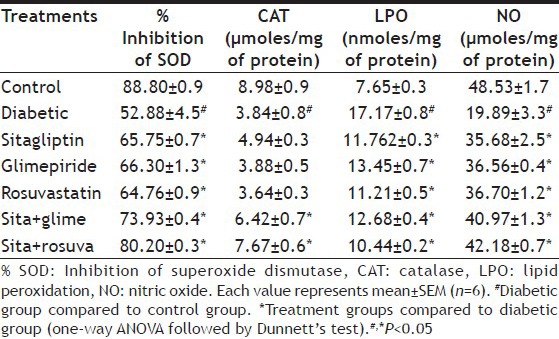
The SOD and CAT levels were significantly (P<0.05) decreased, whereas the significant (P<0.05) rise in LPO levels found in fructose fed diabetic rats compared to control group. Treatment with sitagliptin, glimepiride, rosuvastatin and their combinations significantly (P<0.05) increase the levels of SOD and CAT with concomitant decrease in LPO (P<0.05) levels as compared to fructose treated group (Table 3).
TABLE 3.
EFFECT ON OXIDATIVE STRESS PARAMETERS
Effect on NO Levels:
The NO levels were significantly (P<0.05) decreased in fructose fed diabetic rats compared to control group. Treatment with sitagliptin, glimepiride, rosuvastatin and their combinations significantly (P<0.05) increase NO levels compared to fructose treated group. The combination of sitagliptin with rosuvastatin was found to be more effective (P<0.05) than single drug treatment (Table 3).
Effect of sitagliptin, glimepiride, rosuvastatin and their combinations on vascular reactivity to catecholamines:
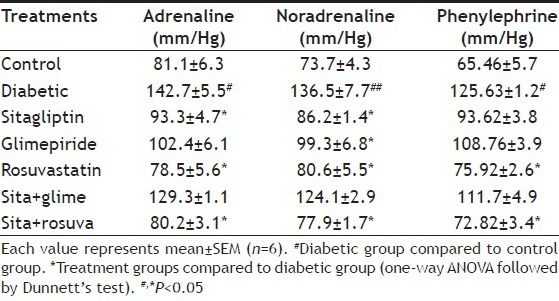
The control group showed normal vascular response to ADR, NA and PE (1 μg/kg, iv.), whereas fructose treated group showed a significant (P<0.05) elevation in mean change in BP to ADR, NA and PE. Single drug treatment with sitagliptin, glimepiride and rosuvastatin significantly (P<0.05) reduced the mean change in BP to ADR, NA and PE compared to fructose treated group. Sitagliptin-rosuvastatin combination showed the most significant reduction in mean change in BP to ADR, NA and PE (P<0.05, Table 4).
TABLE 4.
EFFECT ON VASCULAR REACTIVITY TO VARIOUS CATECHOLAMINES
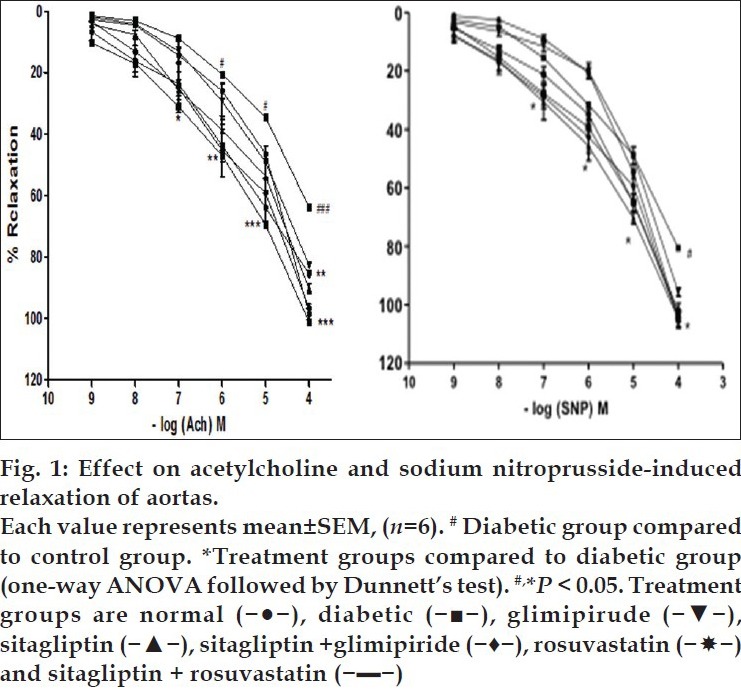
Effect on acetylcholine- or sodium nitroprusside-induced relaxation of rat aorta:
The aortas of control group animals showed normal relaxant response to cumulative doses of acetylcholine or sodium nitroprusside precontracted with PE. The aortas isolated from fructose treated group showed significant (P<0.05) impairment of relaxation with cumulative doses of acetylcholine or sodium nitroprusside, indicating diabetes induced vascular damage. The aortas of sitagliptin, glimepiride and rosuvastatin treatments showed significant improvement in relaxation, which indicates improvement in endothelial function in diabetic rats. Improvement in relaxation was most significant (P<0.05) with sitagliptin and rosuvastatin combination compared to fructose treated group (fig. 1).
DISCUSSION
In this study, we demonstrated that inhibition of HMG-CoA reductase and DPP-4 in experimental animals improve endothelial function in diabetes. Insulin resistance induced by high fructose diet in rats was associated with an oxidative stress. Insulin resistance was also associated with an early development of moderate hypertension and vascular remodelling of arteries. Insulin resistance, endothelial dysfunction and low-grade chronic inflammation are frequently present and closely associated in subjects with impaired glucose tolerance or impaired fasting glucose[23]. Increased release of free fatty acids from adipocytes and their delivery to the liver provide additional substrate for hepatic production of cholesterol and triglycerides rich VLDL, which contributes to the susceptibility to atherosclerotic disease[24,25].
The results of the present study indicated that the oral administration of 66% fructose induce type-2 diabetes in experimental animals. Administration of fructose leads to increase in body weight of rats. This might be due to increase in the adiposity of the body. All the treatments significantly reduced body weight. In our study, the serum glucose, triglyceride and cholesterol levels were significantly increased in fructose fed rats, suggesting the presence of hyperglycaemia and hyperlipidaemia. Sitagliptin, glimepiride and combination of sitagliptin with rosuvastatin were significantly able to reduce the hyperglycaemia. The heart rate was found to be lower in fructose fed diabetic rats; suggesting early autonomic dysfunction due to diabetes[26]. Chronic treatment with the combinations of sitagliptin-rosuvastatin and sitagliptin-glimepiride restored the heart rate to normal. This indicates that the autonomic dysfunction was brought to normal. The fructose fed rats showed significant increase in systolic BP when measured by both invasive and noninvasive method. Long term administration of rosuvastatin alone and its combination with sitagliptin significantly reduced the elevated BP in fructose fed hypertensive rats. Rosuvastatin and sitagliptin found to be effective in reduction of BP. Again this activity of sitagliptin may attributes to its property to suppress hyperinsulinemia by increasing insulin sensitivity and anticholestremic activity of rosuvastatin, respectively. The vascular reactivity to ADR, NA and PE was tested on rat aortas. There was significant increase in pressor response to ADR, NA and PE in diabetic rats. This increase in pressor response might be due to presence of endothelial dysfunction and hypertension in diabetic rats. All the treatments significantly reduced the increase in pressor response to ADR, NA and PE in fructose treated group. The most significant reduction in pressor response was observed with rosuvastatin and sitagliptin combination.
Endothelial dysfunction is a clinical feature of metabolic syndrome; there is decreased availability of endothelium derived NO for proper vasodilatation to occur[27,28]. Sodium nitroprusside mediates its vasorelaxant effects by production of NO in the endothelial cells by the activation of nitric oxide synthase (NOS)[17]. In present investigation, the normal relaxant response was observed to cumulative doses of acetylcholine and sodium nitroprusside on isolated aortas from control group. The relaxant response to cumulative doses of acetylcholine and sodium nitroprusside were significantly reduced on aortas isolated from fructose fed rats, suggesting endothelial damage in the diabetic rats. Treatment with sitagliptin, glimepiride, rosuvastatin and their combinations significantly improved the relaxant response to acetylcholine and sodium nitroprusside on aortas isolated from fructose treated hyperinsulinemic group. The combination of sitagliptin with rosuvastatin was found to be most effective in restoring the relaxant response to acetylcholine and sodium nitroprusside, indicating improvement in endothelial function.
Oxidative stress is one of the major reasons of endothelial dysfunction in metabolic syndrome[29,30]. The effect of sitagliptin, glimepiride, rosuvastatin and their combinations were evaluated on various oxidative stress indices such SOD, CAT, LPO and NO in isolated rat aortas. There was significant reduction in the levels of defensive antioxidant enzymes such as SOD, CAT and NO were observed. The level of LPO was increased in rats treated with fructose, suggesting increase in oxidative stress in type-2 diabetes. Chronic treatment of sitagliptin and its combination with rosuvastatin significantly increased the levels of SOD, CAT and NO in rat aortas. Treatments also significantly reduced LPO level. Thus, the combination of sitagliptin with rosuvastatin was found to be most significant in restoring the antioxidant defensive enzymes.
This work clearly demonstrated that oxidative stress participates in the progression of cardiovascular complications and endothelial dysfunction in insulin resistance. Inhibition of both HMG-CoA reductase and DPP-4 results in the improvement in endothelial function and oxidative stress in fructose-fed diabetic rats. Most impressive results were seen with simultaneous inhibition of DPP-4 and HMG-CoA reductase by sitagliptin and rosuvastatin combination as compared to their individual effect.
Footnotes
Nade, et al.: Sitagliptin and Rosuvastatin Reverse Endothelial Dysfunction in Diabetes
REFERENCES
- 1.Shannon A, Erin L. Sitagliptin: A dipeptidyl peptidase IV inhibitor for the treatment of type. 2 diabetes. Ann Pharmacother. 2006;40:1336–43. doi: 10.1345/aph.1G665. [DOI] [PubMed] [Google Scholar]
- 2.Wild SH, Byrne CD. The Global burden of the metabolic syndrome and its consequences for diabetes and cardiovascular disease. In: Wild SH, Byrne CD, editors. The metabolic syndrome. 2nd ed. Chichester, UK: John Wiley and Sons Ltd; 2005. pp. 2–5. [Google Scholar]
- 3.Sattar N, McInnes IB. Vascular co-morbidity in rheumatoid arthritis; potential mechanisms and solutions. Curr Opin Rheumatol. 2005;17:286–92. doi: 10.1097/01.bor.0000158150.57154.f9. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type-2 diabetic patients. Circulation. 2005;111:2518–24. doi: 10.1161/01.CIR.0000165070.46111.9F. [DOI] [PubMed] [Google Scholar]
- 5.Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Tramontano A, et al. Rosuvastatin (3-Hydroxy-3-Methylglutaryl coenzyme-A reductase inhibitor), decreases cardiac oxidative stress and remodeling in Ren-2 transgenic rats. Endocrinology. 2007;148:2181–8. doi: 10.1210/en.2006-1355. [DOI] [PubMed] [Google Scholar]
- 6.Guerci B, Bohme P, Kearney S, Zannad F, Drouin P. Altered endothelial function and the effects of treatments in type-2 diabetes mellitus. Diabetes Metab. 2001;27:436–47. [PubMed] [Google Scholar]
- 7.O’Rahilly S. Bristol, UK: BioScientifica; 1999. Insulin resistance and cardiovascular disease; pp. 54–70. [Google Scholar]
- 8.Kumar S, O’Rahilly S. West Sussex: John Wiley and Sons; 2004. Insulin resistance, insulin action and its disturbances in disease; pp. 467–78. [Google Scholar]
- 9.Krentz AJ, Wong ND. London, UK: Informa Healthcare; 2007. Metabolic syndrome and cardiovascular disease; Epidemiology, assessment, and Management; pp. 212–7. [Google Scholar]
- 10.Vasdev S, Longerich L, Gill V. Prevention of fructose-induced hypertension by dietary vitamins. Clin Biochem. 2004;37:1–9. doi: 10.1016/j.clinbiochem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh K, Shenoy K. Endothelial dysfunction; many ways to correct that promise. Indian J Pharmacol. 2003;35:73–82. [Google Scholar]
- 12.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type-2 diabetes. Diabetes. 2006;55:1695–704. doi: 10.2337/db05-1602. [DOI] [PubMed] [Google Scholar]
- 13.Schäfer A1, Fraccarollo D, Vogt C, Flierl U, Hemberger M, Tas P, et al. Improved endothelial function and reduced platelet activation by chronic HMG-CoA reductase inhibition with rosuvastatin in rats with streptozotocin-induced diabetes mellitus. Biochem Pharmacol. 2007;73:1367–75. doi: 10.1016/j.bcp.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Nangle MR, Cotter MA, Cameron NE. Effects of rosuvastatin on nitric oxide dependent function in aorta and corpus caverno-sum of diabetic mice. Diabetes. 2003;52:2396–402. doi: 10.2337/diabetes.52.9.2396. [DOI] [PubMed] [Google Scholar]
- 15.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–6. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 16.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin, a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 17.Zeydanli EN, Kandilci HB, Turan B. Doxycycline ameliorates vascular endothelial and contractile dysfunction in the thoracic aorta of diabetic rats. Cardiovas Toxicol. 2011;11:134–47. doi: 10.1007/s12012-011-9107-1. [DOI] [PubMed] [Google Scholar]
- 18.Bunag RD. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J Appl Physiol. 1973;34:279–82. doi: 10.1152/jappl.1973.34.2.279. [DOI] [PubMed] [Google Scholar]
- 19.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 20.Luck H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1971. pp. 885–93. [Google Scholar]
- 21.Wills E. Mechanism of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannebaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez MA, Selwyn AP. Endothelial function, inflammation and prognosis in cardiovascular disease. Am J Med. 2003;115(Suppl 8A):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Calles-Escandon J, Garcia-Rubi E, Mirza S, Mortensen A. Type-2 diabetes: One disease, multiple cardiovascular risk factors. Coron Artery Dis. 1999;10:23–30. [PubMed] [Google Scholar]
- 25.Cosentino F, Luscher T. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl 3):54–61. [PubMed] [Google Scholar]
- 26.Schaan BD, Maeda CY, Timm HB, Medeiros S, Moraes RS, Ferlin E, et al. Time course of changes in heart rate and blood pressure variability in streptozotocin-induced diabetic rats treated with insulin. Braz J Med Biol Res. 1997;30:1081–6. doi: 10.1590/s0100-879x1997000900006. [DOI] [PubMed] [Google Scholar]
- 27.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–74. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 29.Nakazono K, Watanabe N, Matsono K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991;88:10045–8. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]


