Abstract
Study Objectives:
This study aimed to determine whether tongue stiffness (shear modulus) in patients with obstructive sleep apnea (OSA) is different for controls matched for age, sex, and body mass index (BMI), and to investigate the effect of continuous positive airway pressure (CPAP) on stiffness.
Design:
Controlled experimental study.
Setting:
Medical research institute.
Participants:
Patients with OSA and age-, sex-, and BMI-matched healthy controls.
Measurements:
Magnetic resonance elastography was performed in nine patients with OSA (apnea-hypopnea index (AHI) > 15 events/h) and seven controls (AHI < 10 events/h) matched for age, sex, and BMI. Six of these OSA subjects were also scanned while 10 cmH2O CPAP was applied. Mean isotropic shear modulus and anisotropic shear moduli parallel and perpendicular to the muscle fascicles in the tongue were calculated.
Results:
Tongue shear modulus in patients with OSA was lower than that in matched controls (2.68 ± 0.35 (mean ± standard deviation) kPa versus 2.98 ± 0.44 kPa, P < 0.001). Shear modulus decreased with increasing AHI (R = −0.496, P = 0.043), but not age, BMI, or percentage tongue fat. Anisotropic analysis revealed that reduction in stiffness was greatest parallel to the muscle fibers. CPAP had no significant effect on tongue shear modulus.
Conclusions:
In awake subjects with obstructive sleep apnea, the tongue is less stiff than in similar healthy subjects and this difference occurs in the muscle fiber direction. CPAP did not significantly reduce tongue stiffness. Thus, any change in neural drive to genioglossus during wakefulness is insufficient to restore normal tongue stiffness.
Citation:
Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC, Bilston LE. Tongue stiffness is lower in patients with obstructive sleep apnea during wakefulness compared with matched control subjects. SLEEP 2015;38(4):537–544.
Keywords: collapsibility, elastography, obstructive sleep apnea
INTRODUCTION
It has been estimated that one third of the variability in severity of obstructive sleep apnea (OSA) is caused by variation in mechanical load to the pharyngeal dilator muscles and two thirds due to their neuromuscular responses.1 Passive mechanical loads (the forces the muscle contracts against) are uniformly elevated in severe OSA. Normal subjects with elevated passive mechanical loads increase neuromuscular activity and maintain airway patency, which suggests that abnormal loading and blunted neuromuscular responses are both required for the development of OSA.2 The stiffness of upper airway tissues is composed of two components: the passive behavior of the tissues (the passive muscle and the connective tissue) and the variable contribution of muscle activation influenced by chemical and central drives which may change with sleep/wake state.3 The passive component is likely to be altered in patients with OSA because there is increased fat in the posterior tongue,4 inflammation and fibrosis,5 an increased proportion of the muscle fibers are either atrophied or hyper-trophied,6 and there are changes in muscle fiber type.7 The active component of the tissue stiffness may also vary in patients with OSA because of changes in neural drive to the airway dilator muscles. During wakefulness, multiunit electromyography (EMG) recordings suggest increased drive to the muscle,8 whereas single motor unit EMG data indicate neuropathic change.9,10 Dynamic imaging of the upper airway during quiet respiration in patients with severe OSA showed minimal movement of the posterior tongue throughout the respiratory cycle compared with normal subjects,11 but it could be that, in severe OSA, the muscle responds to altered neural drive by isometric contraction rather than movement, in which case the stiffness of the muscle would be expected to increase.
Magnetic resonance elastography (MRE) is a recently developed imaging technique for noninvasive investigation of tissue mechanical properties.12–14 The stiffness and viscosity of the tissue can be measured from magnetic resonance images because the propagation speed and attenuation of a mechanical vibration wave through the tissue are directly related to its physical properties. The shear storage modulus (G') is the resistance of the tissue to propagation of shear waves and reflects the elastic (or recoverable) resistance of the tissue to shearing motion and is related to the stiffness of the tissue. The shear loss modulus (G'') represents the viscous component or energy dissipation part of the tissue's response. MRE has previously been used to measure the shear modulus of upper airway tissues in healthy volunteers.15 Isotropic analysis assumes that the tissue properties are equal in all physical directions,16 but anisotropic analysis measures the shear moduli perpendicular and parallel to a nominated direction, assuming that the properties in that direction differ from the perpendicular directions.
The aim of this study was to compare the stiffness (defined as the viscoelastic shear storage modulus, G') and viscous properties (defined as the shear loss modulus, G”) of the tongue and soft palate in patients with OSA with age and body mass index (BMI)-matched normal subjects during wakefulness, in order to quantify the changes in mechanical properties of the airway due to OSA. It has previously been suggested that patients with OSA have increased fat deposition4 and other tissue compositional changes5–7 in the tongue that might reduce tongue stiffness, but it is not known whether these changes outweigh any stiffening effect of increased neural drive to genioglossus during wakefulness in patients with OSA. The hypothesis for this study was that the tongue would be less stiff during wakefulness in patients with OSA. To validate our technique we performed genioglossus EMG to confirm that the vibration stimulus used in MRE did not alter muscle activation. Because continuous positive airway pressure (CPAP) is known to suppress EMG in the genioglossus during wakefulness,17–19 a secondary aim of the study was to determine whether the lowered drive induced by CPAP affected tongue stiffness in patients with OSA. It was hypothesized that the tongue would be less stiff during CPAP.
METHODS
This study was designed as a case-control study of subjects older than 18 y. Nine patients with OSA were individually matched for age, BMI, and sex to seven controls. Ethics approval was obtained from the Human Research Ethics Committees of both the University of New South Wales and South Eastern Sydney and Illawarra Area Health Service. Informed written consent and MRI safety clearance was obtained in all subjects. The study conformed to the Declaration of Helsinki.
Participants
Subjects were recruited through the sleep clinics at the Prince of Wales Hospital and had no contraindications for MRI. Patients with OSA had moderate or severe OSA (apneahypopnea index [AHI] > 15) and had not started treatment. Controls (AHI < 10) were recruited either through the sleep clinic (n = 2), or from the community (n = 7). Exclusion criteria included severe chronic illness, in particular respiratory or neuromuscular disorders or medication that could affect the muscles of the upper airway. Previous surgery to the upper airway was also a contraindication, apart from minor orthodontic work or nasal surgery.
Polysomnography
Subjects with OSA underwent polysomnography (PSG) using the standard technique in a clinical sleep laboratory, scored by an experienced technician and reported by a sleep physician. Electroencephalography (EEG), electrooculography, electrocardiogram, submental EMG, and airflow via nasal pressure transducer, oximetry, and abdominal and thoracic respiratory effort were recorded. Control subjects recruited through the clinic underwent clinical PSG, and community recruits were studied at home using an Embletta X50 (Medcare, Iceland) with one EEG lead, airflow, oximetry, and thoracic and abdominal bands. Home studies were set up, scored, and reported by a sleep physician. Sleep stages were scored in 30-sec epochs. All studies were scored according to American Academy of Sleep Medicine criteria,20 with hypopneas defined as a 50% reduction in airflow accompanied by a desaturation of 3% or respiratory arousal. The Epworth Sleepiness Scale (ESS) score was recorded prior to magnetic resonance imaging (MRI).
Magnetic Resonance Elastography
Sagittal MRE scans were performed on a 3T MRI (Achieva TX, Philips, Best, The Netherlands) using a spin-echo MRE sequence.13–15,21 Prior to the experiment, a sports mouth guard was molded for each subject (Double Shield, Dingzhou Peng Ao Sports Goods Factory, China). During MRI, the subject was instructed to rest the tongue on the mouth guard and to breathe through the nose. The subject was positioned in the neurovascular coil with the Frankfort plane aligned vertically and head stabilized with padding to minimize head motion. The technique has been described previously.15 A three-dimensional (3D) anatomical scan of the pharyngeal region (T1TFE, 3D mode, 1 mm isotropic voxels, 256 × 256 matrix, 130 slices) was also acquired.
Vibration was propagated through the tongue with a mechanical transducer consisting of two coaxial coils attached to a bite bar and the custom-molded mouth guard.15 The vibration stimulus was set to 80 Hz, which had been determined in previous studies to deliver the greatest wave penetration in the upper airway tissues.15 Imaging parameters were repetition time/ echo time (TR/TE) 550/60 msec, scan resolution 64 × 64 pixels, field-of-view (FOV) 200 mm, and slice thickness 2 mm. Measurements consisted of seven slice images per direction and four time offsets, with each MRE dataset taking approximately 10 min to acquire. Diffusion tensor imaging (DTI) was used to determine the direction of the muscle fibers at each pixel to allow anisotropic analysis of the MRE data.16 A single-shot echo planar imaging sequence with 32 gradient directions, b-factor 800 sec/ mm2 and TR/TE = 1038/72 msec was used with matching slice geometry to MRE data. This anisotropic MRE method has been validated previously.16 Figure 1 depicts a typical set of DTI data on a midsagittal slice, indicating the primary direction of the muscle fascicles within the tongue (color coded) that is used for the anisotropic analysis (see next paragraphs).
Figure 1.
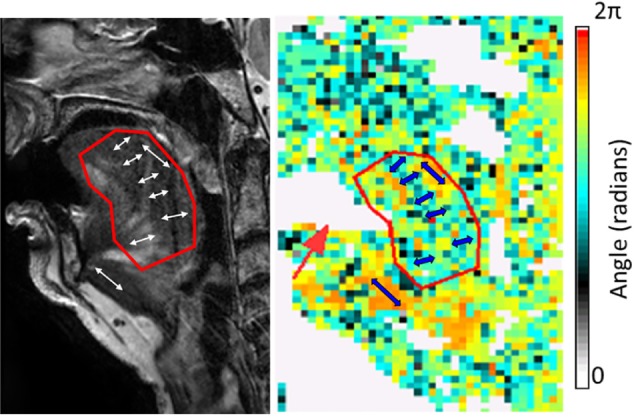
Sample diffusion tensor imaging (DTI) results. The DTI angle image (right panel) shows variation in fiber angle, where each pixel is color-coded with the mean fiber orientation at that location. The angle scale is in radians (2π = 360°, zero is horizontal). Indicative angles for the genioglossus and geniohyoid are shown as blue arrows. Note that fibers in a given direction may be represented by either an angle between 0 and π, or an angle between π and 2π. The left panel shows the anatomy from a high-resolution anatomical scan in the same subject. There is signal dropout from the mouth guard (white region) on the DTI angle image (red arrow).
Continuous Positive Airway Pressure
For the patients with OSA, MRE was performed twice in the same imaging session: without CPAP or mask in situ and with CPAP pressure of 10 cmH20 via nasal mask (Swift FX, ResMed, Bella Vista, New South Wales, Australia). CPAP was delivered using a Bilevel Positive Airway Pressure (BiPAP) machine (S/T model 332108, Philips Respironics, Botany, New South Wales, Australia) with manual dials set to deliver 10 cmH2O in inspiration and expiration. CPAP was applied for 5 min prior to imaging. Because of the magnetic field, the BiPAP machine was outside the MRI and pressure was delivered via 6-m tubing. A manometer confirmed that the pressure delivered at the mask was 10 cmH2O. Note that three of the nine patients with OSA declined to undergo the second MRE measurement, citing discomfort from the combined CPAP mask and MRE setup.
Analysis
MRE images were analyzed off-line using custom software16,22 with a gaussian filter to reduce noise (ς gauss = 3 mm × 2 pixels in each direction). Both isotropic and anisotropic analysis were performed, and the average shear moduli calculated over manually selected regions of interest for the tongue, excluding the geniohyoid, and the soft palate outlined on the matching anatomical image and verified against the magnitude image from MRE to ensure no tissue movement had occurred. For the anisotropic analysis, the mean mechanical anisotropic ratio (G'‖ : G'⊥) was also calculated for these regions of interest. Shear moduli were averaged over the central three slices (6-mm thickness) in the mid-sagittal plane. The percentage of tongue fat was estimated from the matching midsagittal image by measuring the area of the central tongue fat band and total tongue area.
Electromyography
The effect of the vibration stimulus on EMG of genioglossus was assessed prior to starting recruitment for MRI, in separate subjects. Six healthy volunteers were recruited (age range 28–53 y, BMI 19–23, three females, three males). EMG was performed in the supine position with a 27-gauge needle containing two Teflon-coated 51-μm hooked stainless steel fine-wire electrodes (A & M Systems, Carlsborg, WA, USA) inserted to a depth determined to be in the genioglossus by prior ultrasonography (model 128XP/4; Acuson, Mountain View, CA, USA). A mouth guard was vibrated with a transducer which produced a sinusoidal wave at 90 Hz (the vibration frequency used in MRE pilot studies). Bipolar EMG was recorded with and without the stimulus for three stable breaths. The EMG signal was amplified and band-pass filtered (53 Hz–3 kHz) and sampled at 10 kHz using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Multiunit EMG was analyzed off-line. The raw EMG data were passed through a leaky integrator (decay time constant 100 msec). Change in EMG activity from baseline, time of inspiratory activation where there was a phasic component, and tidal volume were recorded.
Statistics
The data were analyzed using SPSS (IBM, Version 20, Chicago, IL). Power calculations based on data collected from normal individuals15 suggested a sample of nine would have a power of 0.80 to find a difference of 15% in G' between patients with OSA and controls. All datasets for comparison between subjects and controls and the CPAP data were approximately normally distributed, so Student paired t-tests were performed. All subjects (n = 16) were included in the correlation analysis of isotropic shear modulus with AHI, BMI, age, and tongue fat. With all subjects included, the data were non-parametric so Spearman correlations were performed. Data are presented as mean ± standard deviation.
RESULTS
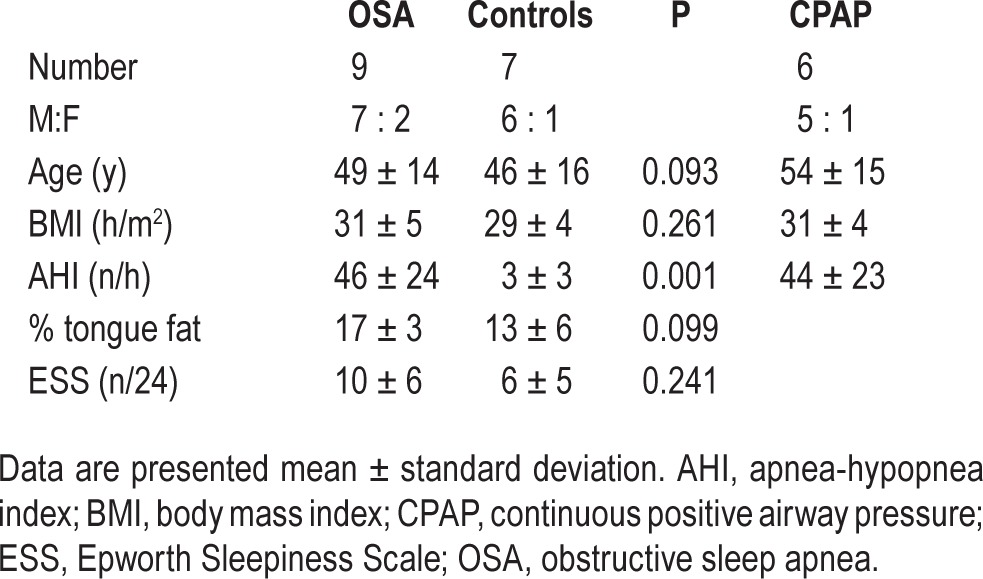
Subject characteristics for the MRE and CPAP experiment are shown in Table 1. There were two double matches in the control group. There were no significant differences in age, BMI, or ESS between the subjects and controls. Intraobserver repeatability of the analysis of genioglossus shear modulus was excellent, with an intraclass correlation coefficient between repeat analyses of 0.984.
Table 1.
Subject characteristics for the magnetic resonance elastography study and for the continuous positive airway pressure experiment.
Genioglossus EMG With Vibration
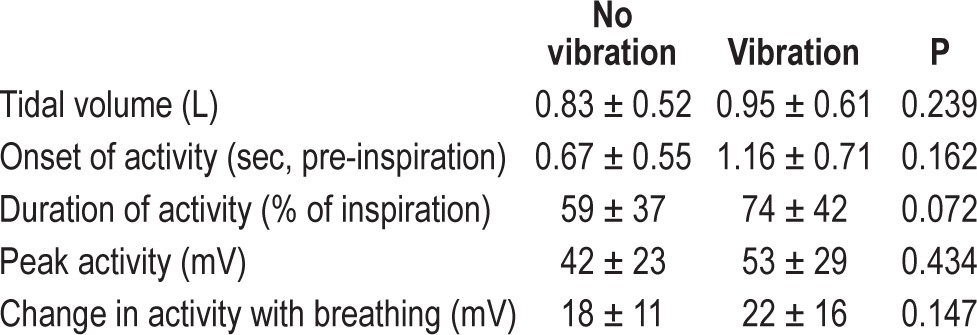
In the six healthy subjects studied, no significant changes were observed in any EMG variables during vibration (Table 2). From this we concluded that the vibration had no significant reflex or other effect on muscle activity.
Table 2.
Electromyography activity, start of inspiratory-related activity, and tidal volume with and without vibration.
Isotropic MRE Analysis
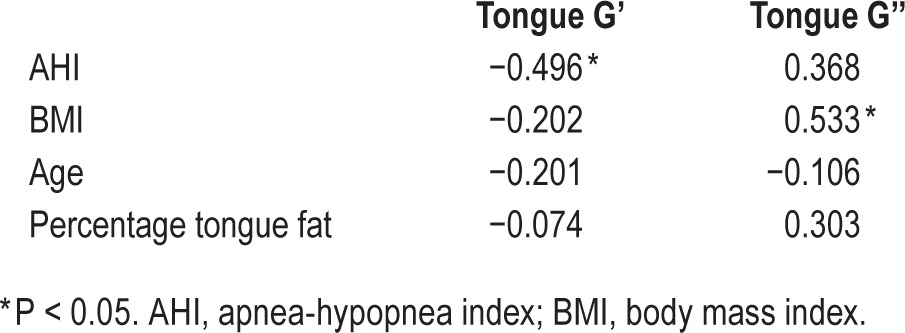
The tongue was less stiff in subjects with OSA compared to controls (P < 0.001). MRE results for a typical subject with OSA are shown in Figure 2. The mean isotropic shear storage modulus (G') and loss modulus (G'') are shown quantitatively in Table 3 and depicted graphically in Figure 3. AHI was significantly negatively associated with isotropic tongue shear modulus (R = −0.496, P = 0.04) and BMI was significantly associated with loss modulus (R = 0.533, P = 0.027). No other significant correlations were found (Table 4).
Figure 2.
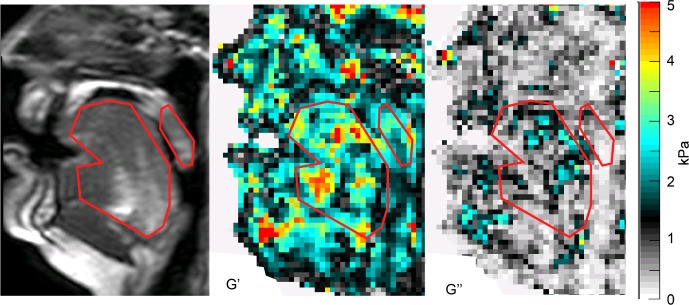
Sample isotropic elastography data in a patient with OSA. Anatomy (left panel), shear modulus representing stiffness (G' – center panel), and loss modulus representing viscosity (G'' – right panel) showing the outline for analysis of the tongue and soft palate in red. The most anterior portion of the tongue was not included in the area of interest because of artifact from the mouth guard in the raw data. The scale (far right) is in kPa.
Table 3.
Mean isotropic shear moduli and anisotropic ratio from magnetic resonance elastography for the tongue and soft palate.

Figure 3.
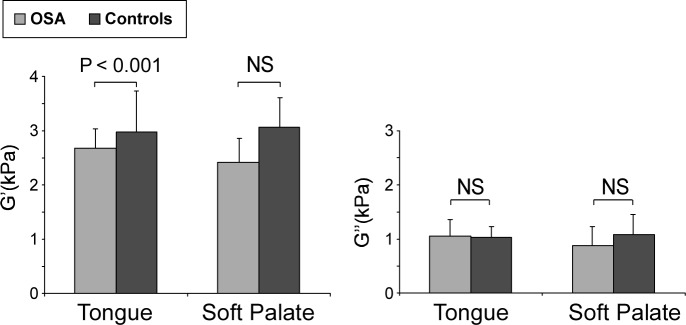
Isotropic shear storage moduli of patients with obstructive sleep apnea (OSA) compared to matched controls. Mean isotropic shear moduli storage modulus (G') and loss modulus (G''). Error bars represent the standard deviation.
Table 4.
Spearman correlation R values for correlations between isotropic shear moduli for the tongue and other measured variables.
Anisotropic Analysis
Shear modulus parallel to the muscle fiber direction (G'‖) was significantly lower in both the tongue and soft palate of subjects with OSA (P = 0.028 and 0.041 respectively, see Figure 4, Table 4). The mechanical anisotropic ratio (G'‖ : G'⊥) of the tongue was significantly lower in the patients than controls (P = 0.021), indicating that the shear modulus of subjects with OSA is lower in the direction of the muscle fibers compared to the controls. Figure 1 shows the direction of the fibers in the tongue of a typical subject as calculated from the DTI data.16
Figure 4.
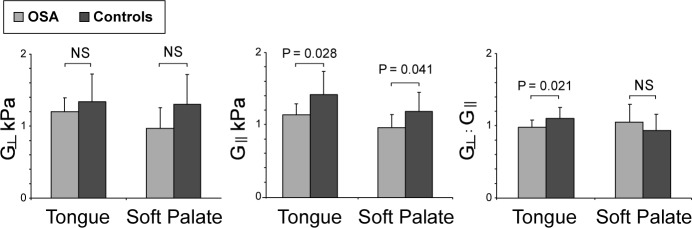
Anisotropic shear modulus and mechanical anisotropic ratio of the tongue and soft palate in patients with obstructive sleep apnea (OSA) and matched controls.
Effect of CPAP
CPAP had no significant difference on tongue storage modulus (G') or the viscous component (G'') of the tongue or soft palate, but the soft palate shear modulus was significantly higher with CPAP than without (P = 0.018). Quantitative results are shown in Table 5, and Figure 5 shows MRE results for a typical subject with and without CPAP. The anisotropic analysis showed no significant differences in the anisotropic ratio with CPAP (1.08 ± 0.10 with CPAP compared to 0.94 ± 0.19 without CPAP for the tongue, P = 0.08).
Table 5.
Mean isotropic shear storage (G′) and loss moduli (G″) of the tongue and soft palate with and without continuous positive airway pressure from magnetic resonance elastography.
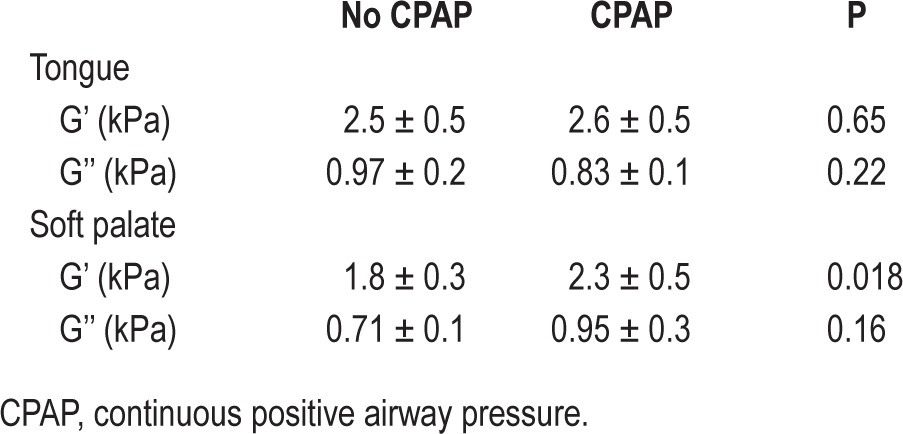
Figure 5.
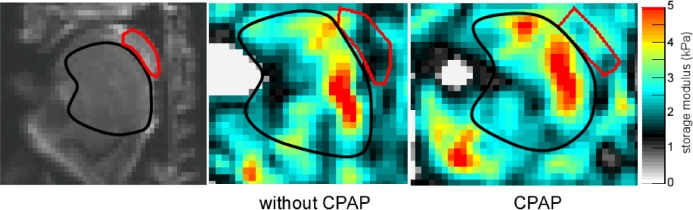
Shear storage modulus with and without continuous positive airway pressure (CPAP). Left panel is the raw anatomical image with the tongue and uvula marked. Shear storage modulus of the tongue (black outline) and soft palate (red) without (center panel) and with (right panel) CPAP in one subject. Mean stiffness of the tongue did not change with CPAP but the stiffness of the soft palate increased.
DISCUSSION
This is the first study to measure the mechanical properties of the upper airway tissues in patients with OSA and controls. Using MRE, this study showed that the stiffness (quantified as the shear modulus) of the tongue was lower in patients with OSA compared to age- and BMI-matched controls. The anisotropic data suggest that the decrease in tongue shear modulus in OSA occurred in the direction of the muscle fibers. The soft palate shear modulus was higher during CPAP, but there was no significant change in tongue shear modulus during CPAP. This suggests that previously observed reductions in genioglossus EMG during CPAP8,17,23 during wakefulness are not resulting in a significantly less stiff tongue.
The results of this study suggest there are fundamental differences in the mechanical properties of the tongue tissue in patients with OSA, and these changes are not the result of only age or obesity. Changes in both tissue composition and neural drive may contribute to the observed changes in tongue tissue stiffness. The observed reduction in shear modulus of approximately 10% is similar in magnitude to the increase in stiffness measured during a contraction of approximately 5–10% MVC in the biceps muscle.24 These data suggest that any increase in neural drive associated with OSA during wakefulness8,25 is not sufficient to return the stiffness of the tissue to “normal” values. The precise changes in tissue composition underpinning these observations require further study, although previous studies have shown OSA is associated with an inflammatory infiltrate and fibrosis5 and alterations in the extracellular matrix.26,27
The precise effect of a 10% reduction in stiffness of the tongue is not yet known, but it probably contributes to increased collapsibility of the airway, both awake and asleep. The reduction in tongue stiffness observed here is consistent with previous suggestions that the pharynxes of patients with OSA are more collapsible than those of healthy controls even without reduced neuromuscular activation associated with sleep.2 A reduced tongue stiffness may also contribute to the lower active Pcrit (i.e., Pcrit with neuromuscular drive intact) observed in asleep patients with OSA patients2 (although stiffness does not directly correspond to Pcrit).
Muscle fibers, including those in the tongue, can respond to neural drive by contracting concentrically (shortening), isometrically (no length change), or eccentrically (lengthening).28 During wakefulness in patients with severe OSA, it was recently shown that the posterior tongue did not contract ante-riorly at the beginning of inspiration,29 despite this being a time of increased inspiratory phasic neural drive.9,10 This study shows that any possible isometric contraction was not enough to restore the shear modulus to control values.
The isotropic shear modulus of the tongue and soft palate in matched controls in this study was similar to previously published data in young normal subjects.15 Our findings are in contrast with those of Series et al.,31 who found that by stretching soft-palate surgical specimens ex vivo that elastance (defined as the ratio of passive tension to percentage change in length) was increased in the uvula of patients with OSA compared to snoring controls. Their results may be different from ours because they compared ex vivo surgical specimens so they do not reflect in vivo conditions, particularly the effect of muscle activation. Also, tissue mechanical properties are well known to be altered post-mortem. Moreover, they did not account for differences in sample dimensions, which would confound their results, as there were systematic differences in cross-sectional area of their specimens between patients and controls.
The anisotropic data showed that the decreased stiffness in the tongues of patients with OSA compared to controls was directional, occurring parallel to the muscle fibers. This suggests that changes in the properties of the muscle fibers or their related connective tissue are contributing to the altered physical properties of the tongue. The mechanism for such changes is not known, but patients with OSA have been reported to have changes in length-tension characteristics, muscle fiber atrophy,6,32 and hypertrophy,6,7 changes from type I to type IIA muscle fibers,7,33 and heterogeneity of fiber size6 or decreased muscle fiber diameter.26 How these might contribute to the mechanical behavior requires further study.
Although this study matched subjects for age and BMI, and no differences in the percentage of gross tongue fat were found in the midsagittal region of the tongue where the MRE data was collected, we cannot rule out effects caused by possible differences in the distribution of tongue fat between patients with OSA and matched controls, as some studies suggest that patients with OSA have more fat in the posterior tongue than BMI-matched controls.4,34,35 This requires further study because conventional MRI is poor at distinguishing between fat and water, although there are a number of experimental techniques in development to estimate tongue fat.36,37 Although it is possible that our technique is not sensitive enough to detect small changes in tongue stiffness associated with small changes in muscle activation, muscle shear modulus measured using MRE is strongly correlated with EMG activity in limb muscles (R2 ≥ 0.82 in calf muscles),38 albeit at higher levels of activation (10–44% maximum voluntary contraction) than are typical during quiet breathing. The lower shear modulus seen in patients with OSA therefore suggests that any changes in net neural drive in patients with OSA39,17 are not causing sufficiently strong muscle contractions to be detected as changes in stiffness by MRE. This is consistent with the observation that CPAP did not significantly change the shear modulus of the tongue during wakefulness, even though CPAP decreases dilator muscle EMG during wakefulness and CPAP reduces the total number of active units and the firing frequency of genioglossus inspiratory phasic units,17 so application of CPAP acutely lowers neural drive. If muscle activity were primarily responsible for stiffness, then application of CPAP should have decreased tongue stiffness in patients with OSA, but this was not observed.
In contrast to the tongue, the shear modulus of the soft palate was significantly higher with CPAP. The precise reason for this is not known definitively, but it may be that CPAP alters the length-tension relationship within the muscle which in turn affected shear modulus, or that the soft palate, which was directly exposed to the CPAP pressure, was compressed, thus increasing its apparent stiffness because of the nonlinear behavior of soft tissues. It is unlikely that the increased stiffness in the soft palate during CPAP occurred due to increased EMG activity in the soft palate, because CPAP is not known to increase EMG in the soft palate.
Higher AHI was associated with lower stiffness, but other demographic variables did not explain the difference in stiffness between patients with OSA and controls. However, it must be noted that the study was not powered to find differences in these variables. BMI, but not AHI, was significantly related to the viscous properties of the tongue. Physically, a more viscous material attenuates the propagating vibration more, either because of a more ‘liquid-like’ behavior, or because the tissue microstructure scatters the waves more. In the liver, increased fat deposition (in the absence of fibrosis) increases tissue viscosity measured with MRE,40 which may explain the observed correlation between BMI and the viscous modulus.
Some key limitations of MRE of the upper airway are: that measures must be averaged over relatively large regions due to the analytical technique used to estimate the moduli from the wave images14,41; that good data quality relies on the subject not moving or swallowing during acquisition; that it is difficult to perform during sleep due to scanner noise; and that data are averaged over a number of respiratory cycles. Ongoing improvements in design of the MRE transducer and reduced scan times (newer techniques may reduce scan time from 10 min to approximately 3 min42 may improve the practicality and patient acceptability of the technique. EMG measurements during vibration confirmed that the vibration did not alter muscle activity, ruling this out as a significant confounding effect. Cheng et al.15 validated tongue MRE at this institution using normal subjects and showed that results were comparable to other published methods of measuring stiffness and also that position of the tongue did not affect the results. Anisotropic MRE has been validated against mechanical testing in phantoms and ex vivo muscle tissue.16 Although the researcher performing analysis was not blinded to the OSA status of the subject, the analysis is fully automated, apart from the region of interest selection, which is based on the tongue and soft-palate anatomy, so it is unlikely that the lack of blinding influenced the results. The power of the CPAP data was limited (post hoc power was 20% to find a large effect) because CPAP was poorly tolerated by subjects in the MRI and not all subjects recruited were able to complete the whole protocol. A larger sample is required to rule out a smaller effect, and replication of these studies is highly desirable to confirm the findings. It is unlikely that the result in the subset of subjects with CPAP was due to inadequate time for changes to occur in the reflex control of genioglossus, as there was 5 min of CPAP prior to imaging. The negative pressure reflex acts on a breath-by-breath basis43 so CPAP should be effective almost immediately. A follow-up study of MRE after long term CPAP would be informative because long term CPAP reduces tissue edema44 and improves airway caliber,44,45 which could lead to changes in tissue shear modulus.
In summary, this study showed that the stiffness of the tongue in patients with OSA was approximately 10% lower than age- and BMI-matched control subjects during wakefulness. The stiffness differences appeared to occur in the direction of the muscle fibers, as assessed by diffusion tensor imaging. CPAP did not significantly further reduce the stiffness of the tongue, despite its known action in reducing neural drive to the genioglossus.8,17,23 The lower stiffness observed in patients with OSA may contribute to the increased propensity of the upper airway to collapse during sleep.
DISCLOSURE STATEMENT
This research was supported by an NHMRC Project Grant 455228. Drs. Bilston, Butler and Gandevia are supported by NHMRC fellowships. Dr. Bilston has received consulting income from Linguaflex LLC not related to this study. The other authors have indicated no financial conflicts of interest. All research was conducted at Neuroscience Research Australia.
ACKNOWLEDGMENTS
The authors would like to thank the staff of the NeuRA Imaging Centre, Diagnostic MRI Services and the Sleep Laboratory at Prince of Wales Hospital for their assistance.
REFERENCES
- 1.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 2.Patil S, Schneider H, Marx J, Gladmon E, Schwartz A, Smith P. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 3.Longobardo GS, Evangelisti CJ, Cherniack NS. Analysis of the interplay between neurochemical control of respiration and upper airway mechanics producing upper airway obstruction during sleep in humans. Exp Physiol. 2007;93:271–87. doi: 10.1113/expphysiol.2007.039917. [DOI] [PubMed] [Google Scholar]
- 4.Zohar Y, Sabo R, Strauss M, Schwartz A, Gal R, Oksenberg A. Oropharyngeal fatty infiltration in obstructive sleep apnea patients: a histologic study. Ann Otol Rhinol Laryngol. 1998;107:170–4. doi: 10.1177/000348949810700214. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–6. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 6.Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Resp and Crit Care Med. 1998;157:586–93. doi: 10.1164/ajrccm.157.2.96-06049. [DOI] [PubMed] [Google Scholar]
- 7.Sériès F, Côté C, Simoneau J, et al. Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J Clin Invest. 1995;95:20–5. doi: 10.1172/JCI117640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saboisky JP, Stashuk DW, Hamilton-Wright A, et al. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:322–9. doi: 10.1164/rccm.201106-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saboisky J, Butler J, McKenzie D, et al. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol. 2007;585:135–46. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC, Bilston LE. Respiratory movement of upper airway tissue in obstructive sleep apnoea. Sleep. 2013;36:1069–76. doi: 10.5665/sleep.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–7. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 13.Sinkus R, Lorenzen J, Schrader D, Lorenzen M, Dargatz M, D Holz D. High-resolution tensor MR elastography for breast tumor detection. Phys Med Biol. 2001;45:1649–64. doi: 10.1088/0031-9155/45/6/317. [DOI] [PubMed] [Google Scholar]
- 14.Sinkus R, Tanter M, Catheline S, et al. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn Reson Med. 2005;53:372–87. doi: 10.1002/mrm.20355. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Gandevia SC, Green M, Sinkus R, Bilston LE. Viscoelastic properties of the tongue and soft palate using MR elastography. J Biomechanics. 2011;44:450–4. doi: 10.1016/j.jbiomech.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Qin EC, Sinkus R, Geng G, et al. Combining MR elastography and diffusion tensor imaging for the assessment of anisotropic mechanical properties: a phantom study. J MRI. 2013;37:217–26. doi: 10.1002/jmri.23797. [DOI] [PubMed] [Google Scholar]
- 17.Saboisky JP, Jordan AS, Eckert DJ, et al. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol. 2010;109:1939–49. doi: 10.1152/japplphysiol.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Y, Jordan A, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–7. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mezzanotte W, Tangel D, White D. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21:755–64. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 22.Sinkus R, Tanter M, Catheline S, et al. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn Reson Med. 2005;53:372–87. doi: 10.1002/mrm.20355. [DOI] [PubMed] [Google Scholar]
- 23.Lo Y, Jordan AS, Malhotra A, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–7. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brauck K, Galban CJ, Maderwald S, Herrmann BL, Ladd ME. Changes in calf muscle elasticity in hypogonadal males before and after testosterone substitution as monitored by magnetic resonance elastography. Eur J Endocrin. 2007;156:673–8. doi: 10.1530/EJE-06-0694. [DOI] [PubMed] [Google Scholar]
- 25.Saboisky JP, Butler JE, McKenzie DK, et al. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol. 2007;585:135–46. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina FD, Santos FC, Falleiros LR, et al. Microscopical evaluation of extracellular matrix and its relation to the palatopharyngeal muscle in obstructive sleep apnea. Microscopy Res Tech. 2011;74:430–9. doi: 10.1002/jemt.20927. [DOI] [PubMed] [Google Scholar]
- 27.Dantas DA, Mauad T, Silva LF, Lorenzi-Filho G, Formigoni GG, Cahali MB. The extracellular matrix of the lateral pharyngeal wall in obstructive sleep apnea. Sleep. 2012;35:483–90. doi: 10.5665/sleep.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winmaier PW, Raff H, Strang TS, editors. Vander's human physiology, the mechanisms of body function. 12th edition. New York: McGraw-Hill; 2010. [Google Scholar]
- 29.Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC, Bilston LE. Respiratory movement of upper airway tissue in obstructive sleep apnea. Sleep. 2013;36:1069–76. doi: 10.5665/sleep.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98:3284–91. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 31.Series F, Cote C, St. Pierre S. Dysfunctional Mechanical Coupling of Upper Airway Tissues in Sleep Apnea Syndrome. Am J Respir Crit Care Med. 1999;159:1551–5. doi: 10.1164/ajrccm.159.5.9804124. [DOI] [PubMed] [Google Scholar]
- 32.Edström L, Larsson H, Larsson L. Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnea: a muscle biopsy study. J Neurol Neurosurg Psych. 1992;55:916–20. doi: 10.1136/jnnp.55.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1960–6. doi: 10.1164/ajrccm.159.6.9809052. [DOI] [PubMed] [Google Scholar]
- 34.Horner R, Mahiaddin R, Lowell D, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnea and weight matched controls. Eur Respir J. 1989;2:613–22. [PubMed] [Google Scholar]
- 35.Mortimore I, Marshall I, Wraith P, Sellar R, Douglas N. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–3. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 36.Humbert IA, Reeder SB, Porcaro EJ, Kays SA, Brittain JH, Robbins J. Simultaneous estimation of tongue volume and fat fraction using IDEAL-FSE. J Magn Reson Imaging. 2008;28:504–8. doi: 10.1002/jmri.21431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi L, Kim C, Jackson N, Maislin G, Schwab RJ. Quantification of tongue fat by novel MRI techniques and its relationship to BMI. Sleep. 2011;34:B72. [Google Scholar]
- 38.Heers G, Jenkyn T, Dresner MA, et al. Measurement of muscle activity with magnetic resonance elastography. Clin Biomech. 2003;18:537–42. doi: 10.1016/s0268-0033(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 39.Fogel R, Malhotra A, Pillar G, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects: mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–30. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- 40.Salameh N, Larrat B, Abarca-Quinones J, et al. Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology. 2009;253:90–7. doi: 10.1148/radiol.2523081817. [DOI] [PubMed] [Google Scholar]
- 41.Van Ee CA, Chasse AL, Myers BS. Quantifying skeletal muscle properties in cadaveric test specimens: effects of mechanical loading, postmortem time, and freezer storage. J Biomech Eng. 2000;122:9–14. doi: 10.1115/1.429621. [DOI] [PubMed] [Google Scholar]
- 42.Garteiser P, Sahebjavaher RS, Ter Beek LC, et al. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR Biomed. 2013;26:1326–35. doi: 10.1002/nbm.2958. [DOI] [PubMed] [Google Scholar]
- 43.Fogel RB, Trinder J, Malhotra A, et al. Within-breath control of genioglossus muscle activation in humans: effect of sleep-wake state. J Physiol. 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan CF, Lowe AA, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144:939–44. doi: 10.1164/ajrccm/144.4.939. [DOI] [PubMed] [Google Scholar]
- 45.Corda L, Redolfi S, Montemurro LT, La Piana GE, Bertella E, Tantucci C. Short- and long-term effects of CPAP on upper airway anatomy and collapsibility in OSAH. Sleep Breath. 2009;13:187–93. doi: 10.1007/s11325-008-0219-1. [DOI] [PubMed] [Google Scholar]


