Abstract
Study Objectives:
To compare a positive airway pressure (PAP) device's detection of respiratory events and airway status during device-detected apneas with events scored on simultaneous polysomnography (PSG).
Design:
Prospective PSGs of patients with sleep apnea using a new-generation PAP device.
Settings:
Four clinical and academic sleep centers.
Patients:
Forty-five patients with obstructive sleep apnea (OSA) and complex sleep apnea (Comp SA) performed a PSG on PAP levels adjusted to induce respiratory events.
Interventions:
None.
Measurements and Results:
PAP device data identifying the type of respiratory event and whether the airway during a device-detected apnea was open or obstructed were compared to time-synced, manually scored respiratory events on simultaneous PSG recording. Intraclass correlation coefficients between device-detected and PSG scored events were 0.854 for apnea-hypopnea index (AHI), 0.783 for apnea index, 0.252 for hypopnea index, and 0.098 for respiratory event-related arousals index. At a device AHI (AHIFlow) of 10 events/h, area under the receiver operating characteristic curve was 0.98, with sensitivity 0.92 and specificity 0.84. AHIFlow tended to overestimate AHI on PSG at values less than 10 events/h. The device detected that the airway was obstructed in 87.4% of manually scored obstructive apneas. Of the device-detected apneas with clear airway, a minority (15.8%) were manually scored as obstructive apneas.
Conclusions:
A device-detected apnea-hypopnea index (AHIFlow) < 10 events/h on a positive airway pressure device is strong evidence of good treatment efficacy. Device-detected airway status agrees closely with the presumed airway status during polysomnography scored events, but should not be equated with a specific type of respiratory event.
Citation:
Li QY, Berry RB, Goetting MG, Staley B, Soto-Calderon H, Tsai SC, Jasko JG, Pack AI, Kuna ST. Detection of upper airway status and respiratory events by a current generation positive airway pressure device. SLEEP 2015;38(4):597–605.
Keywords: central sleep apnea, obstructive sleep apnea, positive airway pressure, respiratory event related arousal, upper airway
INTRODUCTION
Most patients with obstructive sleep apnea (OSA) are treated with positive airway pressure (PAP). Sensors in the airway circuit of PAP devices measure airflow, vibration, and flattening of the airflow profile. Auto-adjusting PAP devices use this feedback to make online adjustments in pressure to maintain upper airway patency. The device identifies and stores mask-on time and the presence of snoring, apneas, hypopneas, and total air leak. Some newer-generation PAP devices detect respiratory event-related arousals (RERAs). Clinicians use this information to objectively evaluate patient adherence to PAP treatment and its efficacy.
The newest generation of PAP devices also offer the innovative feature of detecting whether the airway is obstructed or clear (i.e., open) during device-detected apneas. When an apnea is identified, the device generates a transient pressure pulse in the breathing circuit. A resulting increase in airflow indicates a clear airway, whereas the absence of such a response indicates an obstructed airway. This information is incorporated into the software algorithm of auto-adjusting devices to control the delivered pressure. For example, if the auto-adjusting device detects that the airway is obstructed, it is programmed to increase pressure delivery to open the airway; but if the airway is identified as being clear, pressure delivery does not change.
Several previous studies,1–6 including a recent event-by-event comparison of an older- generation PAP device (REMstar Auto M-Series, Philips-Respironics, Murrysville, PA) by our group,1 compared simultaneous recordings of a PAP device and PSG data to document the accuracy of PAP event detection. The results consistently show that PAP devices tend to overestimate the measures at relatively lower values and underestimate them at higher levels. A device-detected apnea-hypopnea index (AHIFlow) < 10 events/h is highly predictive of a manually scored polysomnography (PSG) AHI < 10 events/h. However, no previous studies have evaluated the ability of the PAP devices to determine airway status, i.e., whether the airway is clear or obstructed during device-detected apneas, or to detect RERAs.
Recognizing the important differences between indices derived by the PAP device and manually scored PSG, a recent task force of the American Thoracic Society recommended that PAP indices be designated with the subscript “flow”, i.e., AHIFlow.7 Given the differences between how PAP units and PSG detect respiratory events and generate respiratory event indices, the task force report emphasized the need for further studies to better understand how the automatically adjusting PAP devices operate and how to interpret breathing event information reported by PAP devices. The purpose of this industry-supported study was to compare device-detected respiratory events and upper airway status to manually scored respiratory events on a simultaneous PSG using a newer generation of PAP device that, unlike models used previously, detects RERAs and airway status during device-detected apneas.
METHODS
We enrolled 45 subjects at three clinical sites: University of Florida, Gainesville, FL; Sleep Health, Portage, MI; and National Jewish Health, Denver, CO. A convenience sample of individuals who had been on any type of PAP treatment for at least 1 y and had previous PSGs showing the presence of central apneas were recruited in order to increase the likelihood that these events would be present for our comparison. Inclusion criteria were: age 21–80 y and a diagnosis of OSA or complex sleep apnea (Comp SA). Individuals with OSA had an AHI ≥ 10 events/h on diagnostic PSG; Comp SA was diagnosed when the diagnostic PSG showed AHI ≥ 10 events/h and central apnea index (CAI) ≥ 5 events/h, or the PSG during PAP treatment had a central apnea index ≥ 5 events/h after obstructive apneas resolved. Exclusion criteria included: premenopausal women known to be pregnant or sexually active and not using a reliable method of birth control; head and neck surgery within the previous 90 days; previous upper airway surgery for OSA treatment; chronic respiratory failure caused by neuromuscular disease, moderate to severe chronic obstructive pulmonary disease or other pulmonary disorders, or any condition with elevated arterial carbon dioxide levels (> 45 mm Hg) while awake; use of supplemental oxygen; ventilator-induced barotrauma in the past 6 mo; untreated insomnia; or other major medical conditions that, in the judgment of the clinical site investigator, precluded participation. The Institutional Review Board at each clinical site approved the protocol, and all participants provided signed informed consent. The Institutional Review Board at the University of Pennsylvania did not require protocol approval because participants were not enrolled at this site and all of the PSG files sent to that site for centralized score were deidentified.
Participants performed an overnight PSG while using PAP (System One REMstar Auto A-Flex, Philips-Respironics). Although different PSG equipment was used across the three sites, the recordings were standardized by using the same montage of specified signals, filters, and digitization rates. The polysomnographic technologists used a standardized protocol to perform the recordings. Because participants on their prescribed PAP setting would not have a sufficient number of respiratory events to make a reasonable comparison, the settings of the PAP device were adjusted during the PSG to increase the number of respiratory events. Participants went to sleep initially at their prescribed pressure until sleep onset. For participants with OSA, pressure was manually decreased by 2 cm H2O every 5 min until breathing events were observed. Participants remained at this pressure for 45 min, then pressure was returned to the starting level for 30 min. This process was repeated throughout the night. For participants with Comp SA, the same process was used except that pressure was increased by the same amount at the same intervals. Participants used their usual mask interface during the PSG. Mask fit was verified prior to initiating the PSG and the technologist checked the mask fit if excessive mask leak developed during the recording. Interventions were rarely needed except for bathroom breaks. A DC analog output signal from the PAP device was recorded on PSG to indicate instances of device-detected apneas, hypopneas, and RERAs.
Device-Detected Events
The System One devices (Philips-Respironics) measure changes in air flow by an internal pneumotachograph to identify respiratory events. A moving window of 3 or 4 min is established and, if flow decreases by 40–80% for at least 10 sec, the event is labeled a hypopnea; a decrease in flow by more than 80% for at least 10 sec is labeled an apnea. A RERA is defined as a sequence of breaths that exhibit both a subtle reduction in airflow and progressive flow limitation that is terminated by a sudden increase in airflow without flow limitation, and the event does not meet the conditions for an apnea or hypopnea.
Status of Upper Airway
A device-detected apnea triggers the device to administer a square-wave pressure pulse of 2 cm H2O. The device measures the resulting airflow to detect whether the airway is clear or obstructed. If no airflow occurs in response to the pressure pulse, the device flags the airway as being obstructed. If airflow occurs in association with the pressure pulse, the airway is flagged as being clear (i.e., no obstruction). A DC analog output signal from the PAP device was recorded on PSG to indicate instances when a pressure pulse was delivered.
Manual PSG Scoring
Deidentified PSGs were converted to European Data Format and transmitted to the University of Pennsylvania Clinical Research Center for Sleep, where they were scored manually with computer assistance (Sandman, Natus Medical Inc, San Carlos, CA) by one scorer who also performed quality control. The scorer participated in the ongoing quality assurance program for PSG scoring at the sleep center during the project and had an intrascorer intraclass correlation coefficient of 0.999 for AHI, 0.964 for apnea index, and 0.999 for hypopnea index. The scorer initially scored the studies using 2007 American Academy of Sleep Medicine (AASM) recommended criteria while blinded to all of the signals from the PAP device.8 All epochs were scored manually for sleep stage and respiratory events. Oxygen desaturation events were determined by automatic scoring with manual assistance. On a second scoring pass, the technologist took into account the pressure pulses generated by the device to avoid mistaking airflow and chest wall movement that were caused by the device pressure pulse. When the airflow and chest wall movement during an event initially scored as a hypopnea were caused by pressure pulses, the event was rescored as a central apnea. When airflow during an event initially scored as a hypopnea was caused by pressure pulses and respiratory-related chest wall movements were present that were not temporally associated with the pressure pulses, the event was rescored as an obstructive apnea. The latter situation was rarely encountered.
Event-by-Event Analysis
To facilitate the comparison of the device-detected events to manually scored events, a customized computer program was developed to identify whether a device-detected event was a true positive (identified on both the manually scored PSG and by the device), false positive (identified by the device but not on the manually scored PSG), or false negative (identified on the manually scored PSG but not the device). True negative events were estimated by determining the number of 30-sec epochs during the PSG when neither method identified an event. The computer program for event-by-event analysis was validated as previously described.1 The customized computer program generated a time-ordered list of manually scored events and events identified by the device (event marks in the event detection channel). After each manually scored event, a 30-sec window was analyzed for the occurrence of a device-detected event. This accounted for any device delay in reporting the event (e.g., the device does not report a hypopnea until at least two breaths after the end of the actual event). Specifically, the detected event was considered a true positive if at least one device-detected event (apnea or hypopnea) occurred at some point between the start of a manually scored event (apnea or hypopnea) and 30 sec after the end of the same manually scored event. If the automatic event marker preceded the manually scored event, it was considered a false positive. The absence of an event marker during or within 30 sec after a manually scored event was considered a false negative.
Statistical Analysis
The following respiratory event indexes were calculated by manual scoring and the PAP device software: AHI, apnea index (AI), hypopnea index (HI), and RERA index (RERAI). The indices for manually scored events were computed as the number of events per hour of sleep. The indices for device-detected events were the number of events per hour of therapy time. Agreement of respective indices between the two detection methods was assessed using the nonparametric Wilcoxon signed-rank test due to the asymmetric distribution of the data. Intraclass correlation was performed using a two-way random model measuring absolute agreement for single measures. In addition, Bland-Altman plots of the AHI, AI, HI, and RERAI data were generated for visualization of the bias and limits of agreement. The sensitivity, specificity, and positive and negative predictive values for given device-detected event cutoff values were calculated, and receiver operating characteristic (ROC) curves were constructed. The accuracy of device-detected airway status during device-detected apneas was evaluated by comparing these results to the manually scored events on PSG. The data were analyzed using computer programs: SPSS (IBM, version 20; Chicago, IL) and Analyse-it (Analyseit Software, Ltd. version 2.26; UK). Descriptive statistics include mean, standard deviation, and median values. Statistical trends were considered significant at P < 0.05.
RESULTS
Demographics
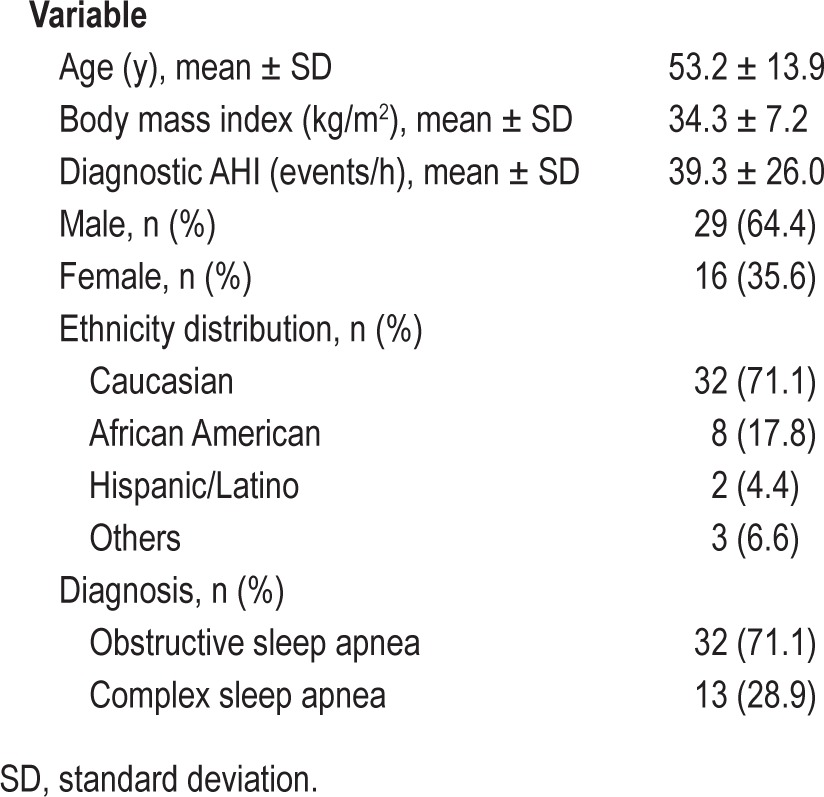
Forty-five adults (29 males) were enrolled: mean age 53.2 y, body mass index 34.3 kg/m2, and diagnostic AHI 39.3 events/h (Table 1). On the manually scored PSGs performed for the current study, sleep efficiency was 78.2 ± 17.2%, AHI 18.1 ± 18.6 events/h, AI 11.5 ± 16.0 events/h, and HI 6.6 ± 7.5 events/h. Central apneas were present in 32 of the subjects (5 with CAI > 5 events/h).
Table 1.
Participant characteristics (n = 45).
Correlation and Bland-Altman Analysis
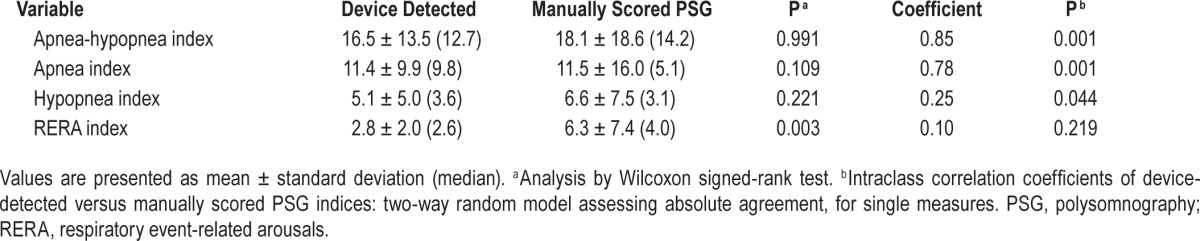
No significant differences were observed on Wilcoxon signed-rank tests between the device-detected AHIFlow, AIFlow, and HIFlow and manually scored AHI, AI, and HI, respectively (Table 2). The device-detected RERAIFlow was less than the manually scored RERAI: 2.8 ± 2.0 (2.6) vs. 6.3 ± 7.4 (4.0) [mean ± standard deviation (median)], P = 0.003.
Table 2.
Comparison of device-detected and manually scored polysomnography respiratory event indices.
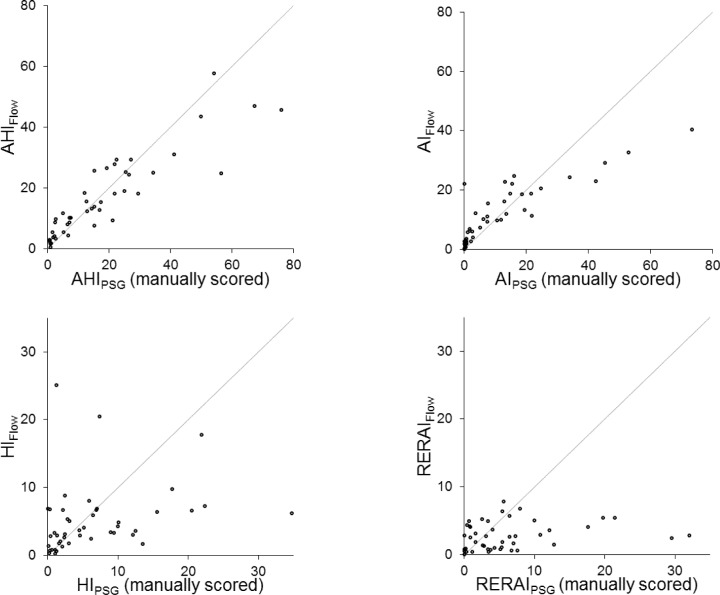
AHI and AI on manually scored PSG and device-detected AHIFlow and AIFlow were highly correlated with intraclass correlation coefficients of 0.854 and 0.783, respectively (Table 2, Figure 1). PSG versus device-detected hypopneas and RERAs showed considerably more scatter with respective intra-class correlation coefficients of 0.252 and 0.098.
Figure 1.
Plots of apnea-hypopnea index (AHI), apnea index (AI), hypopnea index (HI), and respiratory event related arousal index (RERAI) detected by the device versus the corresponding indices determined by manually scored polysomnography (PSG). Each point represents the results of one subject. The solid lines are the lines of identity.
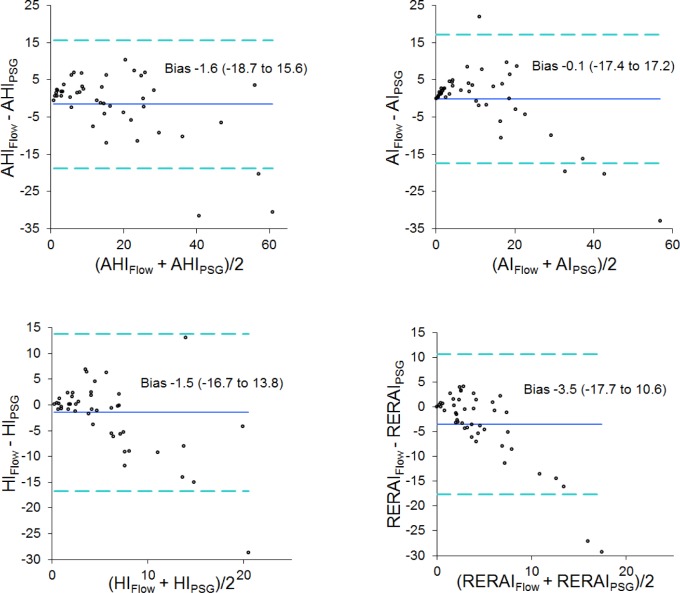
The Bland-Altman graphs in Figure 2 show the difference between AHI, AI, HI, and RERAI values (device-detected events minus manually scored PSG events) plotted against their respective averages. The device tended to overestimate the measures at relatively lower values and underestimate them at higher levels. Scatter tended to increase as all of the indices increased.
Figure 2.
Bland-Altman plots of apnea-hypopnea index (AHI), apnea index (AI), hypopnea index (HI), and respiratory event related arousal index (RERAI) with the difference between device detected and manually scored polysomnography (PSG) measures plotted against the average of the two values. The solid horizontal line indicates the mean difference. The dashed lines show the 95% limits of agreement.
ROC Analysis
AHI values (manually scored and device-detected) for each subject were analyzed using the manually scored PSG results as the gold standard. True-positive and true-negative pairs as well as false-positive and false-negative pairs were determined at a PSG AHI cutoff of 10 events/h. Area under the resultant ROC plot was 0.98 (Figure 3). The sensitivity was 0.92 and specificity was 0.84 at an AHIFlow of 10 events/h. The positive and negative predictive values were both 0.89.
Figure 3.
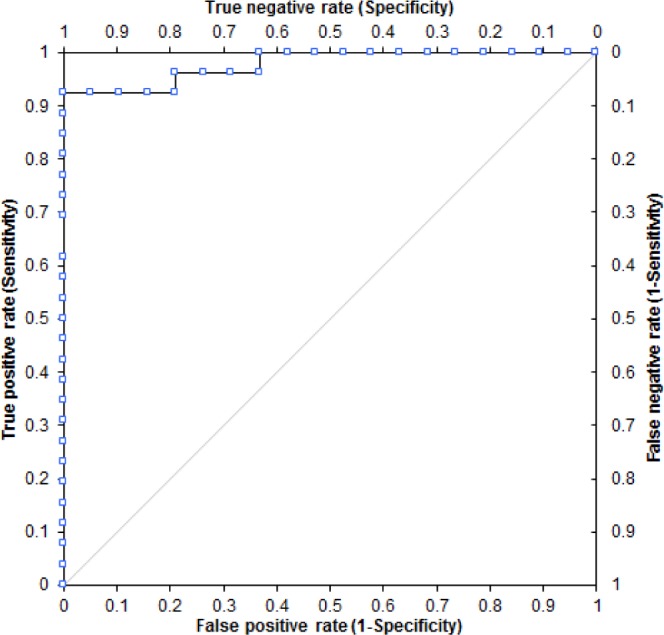
Receiver operating characteristic plot using an apneahypopnea index (AHI) polysomnography (PSG) cutoff of 10 events/h to determine sensitivity and specificity for various device-detected AHIFlow values.
Airway Status Detection During Device-Detected Apneas
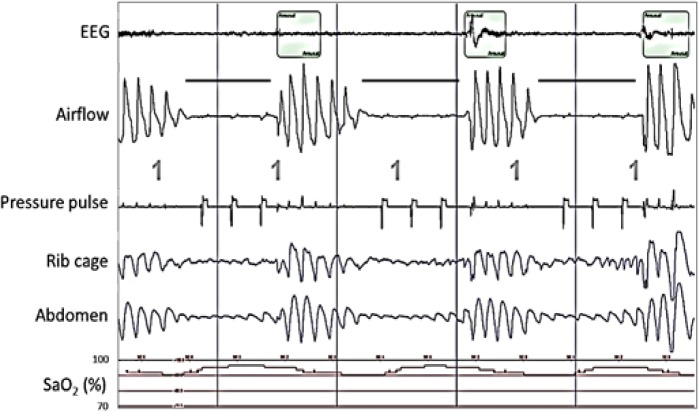
Figure 4 shows two consecutive central apneas. The pressure pulse generated by the device during the first apnea did not result in significant airflow and therefore the device designated this event as being associated with an obstructed airway. Without the device's pressure pulse signal in view, the second event would be manually scored as a hypopnea. However, when the pressure pulses are taken into account, the airflow and chest wall movement during the event are clearly associated with the pressure pulses and therefore, the event was manually scored as a central apnea. Because the first two pressure pulses generated during the second event were associated with biphasic airflow, this event was designated as being associated with a clear airway. None of the pressure pulses in Figure 5 resulted in airflow, and the device designated these events as being associated with an obstructed airway.
Figure 4.
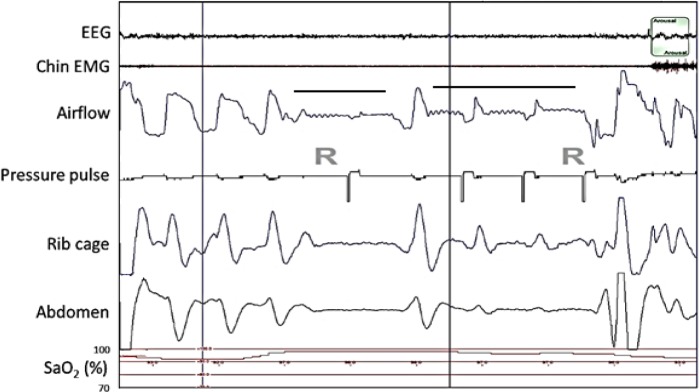
Polysomnography (PSG) recording showing airflow (positive airway pressure device's flow signal) and device-generated pressure pulses during two central apneas (horizontal bars) during rapid eye movement sleep (R) in a patient on subtherapeutic positive airway pressure. Downward deflection of the airflow waveform denotes inhalation. The sharp, transient, downward deflection at the beginning of each pressure pulse is an artifact generated by the device to identify pulse delivery. The near-absence of an airflow response to the first pressure pulse indicates an obstructed airway, and the presence of a biphasic airflow response with the second and third pressure pulses indicates a clear airway. Note the small, relatively rapid fluctuations in the airflow signal that were in-phase with the electrocardiogram (not shown). The presence of these cardiac-generated flow oscillations in the first half of the first apnea and throughout the second apnea demonstrate that the airway was clear during those periods. The absence of the cardiac flow oscillations in the latter half of the first apnea, the period during which the first pressure pulse occurred, confirms that the airway was obstructed during this time. The fluctuations in chest wall movement during the second apnea are secondary to the pressure pulses, another indication that the airway is clear. Taking this into consideration, both apneas were manually scored as central apneas. The balloon on the top trace marks an arousal. The vertical lines denote 30-sec intervals.
Figure 5.
Polysomnogram showing airflow (positive airway pressure device's flow signal) and device generated pressure pulses during three obstructive apneas (solid bars) during stage 1 sleep in a patient on subtherapeutic positive airway pressure. Downward deflection of the airflow waveform denotes inhalation. The lack of an airflow response to the pressure pulse indicates an obstructed airway. The balloons on the top trace mark arousals. The vertical lines indicate 30-sec intervals.
Comparison of Device-Detected Airway Status and Manually Scored PSG Respiratory Events
An event-by-event analysis was performed to compare the device-detected events to the manually scored events using the methodology of our previous study.1 This analysis found that the device-detected event agreed with manual scoring regarding the presence of a respiratory disordered event (i.e., apnea, hypopnea, or RERA) with a sensitivity of 0.63 and a specificity of 0.93.
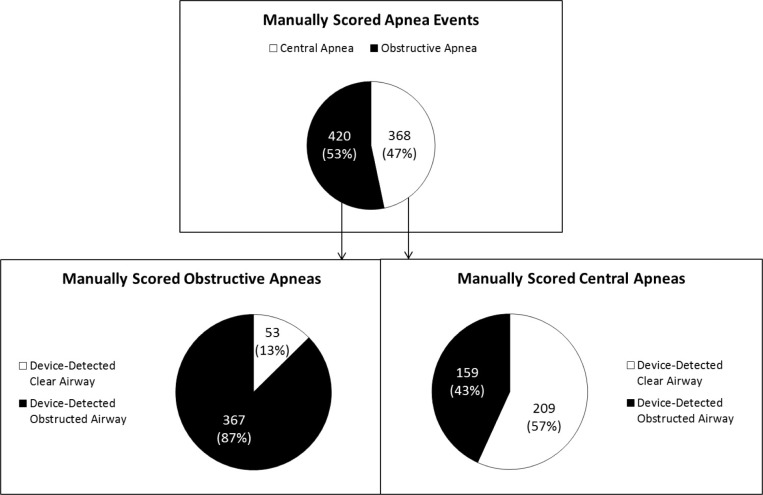
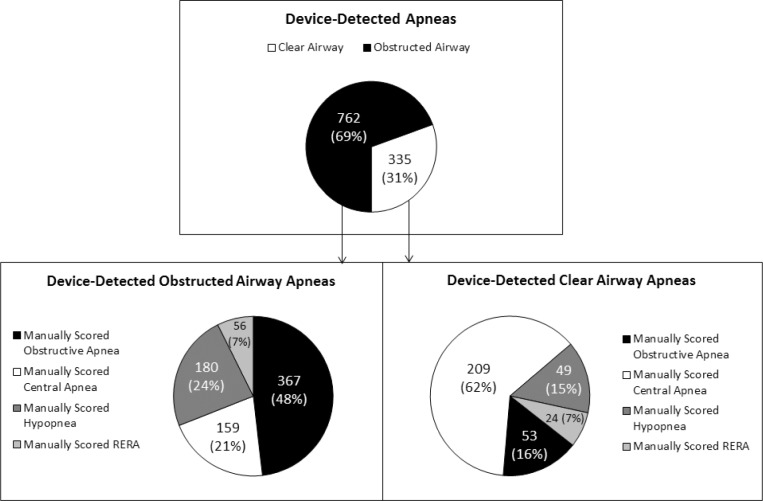
Table 3 shows how 1,543 device-detected events were manually scored on PSG. Of the 1,097 device-detected apneas, 71.8% were manually scored as apneas. Of the apneas identified by both the device and manual PSG scoring, 53.3% were manually scored as obstructive apneas and 46.7% were manually scored as central apneas. The device scored 66.8% of the 788 apneas as obstructed airway and 33.2% as clear airway (Table 3, Figure 6).
Table 3.
Comparison of device-detected and manually scored polysomnography respiratory events (n = 1,543).
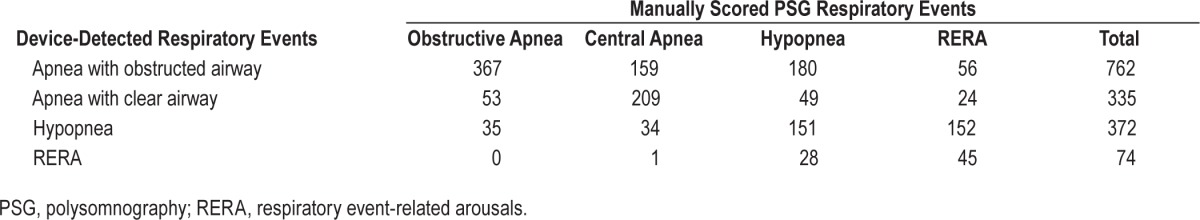
Figure 6.
Comparison of manually scored apnea (central or obstructive) and device classification of airway status (clear or obstructed).
Of the 420 device-detected apneas that were manually scored as obstructive, the device detected 87.4% as obstructed airway apneas and 12.6% as clear airway apneas. Of the 368 device-detected apneas that were manually scored as central, the device detected 43.2% as obstructed airway apneas and 56.8% as clear airway apneas (Figure 6).
Of the 335 device-detected apneas with clear airway (Figure 7), 15.8% were manually scored as obstructive apneas, 62.4% as central apneas, 14.6% as hypopneas, and 7.2% as RERAs. Of the 762 device-detected apneas with obstructed airway, 48.2% were manually scored as obstructive apneas and 51.8% were manually scored as either central apneas (20.9%), hypopneas (23.6%), or RERAs (7.3%). Visual inspection of device-detected apneas that were manually scored as RERAs revealed breaths with a particularly prolonged expiratory time, during which the absence of airflow was identified by the device as an apnea, but could not be manually scored as apnea because the duration of the absence of airflow was less than 10 sec (Table 3).
Figure 7.
Comparison of device determined airway status (clear or obstructed airway apnea) to manually scored event (obstructive apnea, central apnea, hypopnea, or respiratory event-related arousals).
DISCUSSION
Addressing the need identified by the recent American Thoracic Society taskforce on PAP adherence,7 this study provides the first evaluation of airway status detected by a PAP device. The results demonstrate that device-detected airway status does not identify the particular type of apnea on PSG. This is because of a combination of factors: the different criteria for respiratory events used by the PAP device and on PSG; the inability of the PAP device to detect respiratory effort; and the inability of PSG to determine airway status during central apneas. However, comparison of device-detected airway status to that presumed during respiratory events scored on PSG revealed close agreement. Of the device-detected apneas with clear airway, 84.2% were manually scored as events other than obstructive apneas (Figure 7).
Consistent with previous studies evaluating other PAP models, the results demonstrate that the AHIFlow and AIFlow obtained from a current-generation PAP device are in close agreement to the indices generated on PSG with intraclass correlation coefficients of 0.854 and 0.783, respectively. The device was particularly accurate in detecting apneas across a broad range of values, but tended to overestimate AHI at values less than 10 events/h and underestimate higher AHI values. The skew in the AHI relationship was due to the device's detection of hypopneas. The device tended to overestimate hypopneas when the number of hypopneas was relatively low and underestimate hypopneas as their number increased. Further, the PAP model tested in the current study offers the new feature of identifying RERAs. We found that RERAIFlow detected by the PAP device was poorly correlated with RERAI on PSG (intraclass coefficient, 0.098) (Table 2). This is not surprising because the PSG criteria require an arousal on event termination to score a RERA, but the device only uses airflow.7
The results show that airway status determined by the PAP device does not distinguish obstructive from central apneas scored by PSG. For example, an obstructed airway was detected in about a third of device-detected apneas that were scored as central apneas on PSG (Table 3). Although it is not possible to use PSG signals to determine if the pharyngeal airway is clear or obstructed during a central apnea, it is well known that the airway may be closed during this event. Badr et al.9 used a transnasal fiberoptic scope to view the pharyngeal airway in subjects with sleep apnea during nonrapid eye movement sleep and observed pharyngeal airway collapse during central apneas that were either spontaneous or induced by mechanical hypocapnic hyperventilation. The device uses its airway status determination to decide whether to change pressure delivery. Determination of an obstructed airway, even if it might be a central apnea, warrants an increase in pressure by the device in order to open the airway and allow resumption of airflow.
The device classified 13% of the obstructive apneas on PSG as having a clear airway (Figure 6). This may be explained by the different criteria used by the PAP device and manually scored PSG to identify an apnea. The device defines an apnea as at least an 80% reduction from baseline in pneumotach airflow, whereas 2007 AASM criteria for an apnea on PSG require at least a 90% reduction in airflow on oronasal thermistor.8 However, it can be hypothesized that the pharyngeal airway during an obstructive apnea may open during active expiratory efforts and collapse again on inspiration. Under this circumstance, it is possible that a pressure pulse that occurred during expiration may have resulted in airflow. The device also detected an obstructed airway in about 44.1% of manually scored hypopneas (Table 3). It is possible that when the narrowed pharyngeal airway has a very high airway resistance, the relatively small pressure pulse generated by the PAP device results in very blunted airflow (such as that in the first event in Figure 5); a response that the device would identify as an obstructed airway.
PAP downloads report the same variables obtained on PSG, including AHI, AI, HI, and RERAI. However, the methods used in PAP devices and PSG to generate these measures differs markedly. Respiratory events detected by the PAP devices are based solely on measurement of airflow.7 In contrast, multiple signals are used to score respiratory events on PSG, including airflow, arterial oxygen saturation, chest wall movement, and, depending on the scoring criteria, electroencephalographic (EEG)/electromyographic (EMG) arousals.8 Furthermore, the respiratory event indices reported by the PAP device are based on hours of device use whereas the indices on PSG are based on hours of sleep.
Several previous studies have documented the accuracy of the respiratory event detection by a PAP device.1–6 About 18 y ago, two studies evaluated PAP units employing automatic event detection algorithms; the devices have since undergone extensive modification.2,3 Three more recent studies compared the AHI manually scored on PSG with the AHI exported from an auto-adjusting device used during the PSG. All three studies reported that the AHI obtained from the devices was reasonably accurate.4–6 One study did not distinguish apneas from hypopneas, and no studies performed an event-by-event comparison of the two methods of measurement. Most recently, our group compared individual events by manual PSG scoring and PAP device and found an AHI sensitivity of 0.58 and a specificity of 0.98.1 The AHI, AI, and HI by the two methods were highly correlated. Bland-Altman analysis showed better agreement for AI than HI. Using a manually scored AHI ≥ 10 events/h to denote inadequate treatment, an AHIFlow ≥ 10 events/h had a sensitivity of 0.58 and a specificity of 0.94.1 In this study, we not only confirmed the accuracy of the respiratory event detection by a PAP device, but also determined whether the airway is obstructed or clear during device-detected apneas.
The availability of accurate, objective information about residual respiratory events and airway status during respiratory events on PAP treatment is essential to adequately manage patients with OSA. Studies have found that a surprisingly large percentage of patients thought to be well treated on PAP still have a considerable number of residual respiratory events.10,11 Some of these patients may have Comp SA, i.e., the emergence of central apneas in patients with OSA during PAP therapy.12–14 In reviewing PAP download reports, clinicians must take into account that the device is using different criteria to detect respiratory events than those used on PSG scoring. However, if the AHIFlow is less than 10 events/h, clinicians can be confident that the patient is on efficacious treatment because the device tends to overestimate AHI at values below this cutoff. Patients with an AHIFlow greater than 10 events/h may or may not be on adequate treatment and, depending on clinical correlation, may require sleep testing to further evaluate the finding.
Clinicians also need to be aware that a device-detected apnea identified by the device as having a clear airway is not always a central apnea and a device-detected apnea identified by the device as having an obstructed airway is not necessarily an obstructive apnea. However, a preponderance of events with clear airway on PAP download strongly suggests the presence of central apneas. An in-laboratory PSG should be performed to confirm the finding and possibly evaluate more efficacious modes of PAP delivery such as adaptive servoventilation. In-laboratory PSG should also be considered when the device reports a preponderance of events with an obstructed airway. However, when this finding is confined to lower levels of PAP in patients on an auto-adjusting device, increasing the lower pressure of the operating pressure range and obtaining a new download on the narrowed pressure range prior to further sleep testing should be considered. In addition, there needs to be better education of sleep physicians and sleep technologists regarding how to interpret the download data and understand the differences between PAP download data and PSG data.
This study has some possible limitations. Participants of the current study included patients with OSA and Comp SA. Unfortunately, given the small sample size, it was not possible to determine if the proportion of clear versus obstructed airway events differed between these two groups. We selected a convenience sample of individuals who were more likely to have central apneas in order to increase the number of these particular events in our analysis. Because this was not an intervention study and the outcomes were objectively measured, we do not believe that subject selection biased our results. Enrollment of participants at multiple sites increased the generalizability of the findings. The standard protocol for PSG recordings used across the three sites and the centralized PSG scoring helped to reduce site variability. We used PAP device-generated data based on recording time rather than selecting events that only occurred during sleep. It is likely that even better correlations would have been found using device-detected events during sleep time. However, we chose to use PAP device-generated event data during recording time to be consistent with previous publications.1–6 Furthermore, because wakefulness is not excluded in the daily breathing event reports generated by the device and used by clinicians, this analysis better shows how the two methods compare from a clinical perspective. The manufacturer of the device used in this study uses the same detection algorithm in all of its current PAP models. However, the results of the current study may not generalize to devices made by other PAP manufacturers or to patients with conditions mentioned in the exclusion criteria.
In summary, using a current generation PAP device, we found that device-detected airway status agrees closely with PSG respiratory event scoring but should not be equated with a specific type of respiratory event. Device-detected RERAs are weakly correlated with manually scored RERAs. In agreement with previous studies, AHIFlow < 10 events/h on the PAP device is strong evidence of good treatment efficacy. When interpreted appropriately, PAP device reports provide important information needed for adequate management of patients on PAP treatment.
DISCLOSURE STATEMENT
This study was supported by NIH 1P01-1HL094307 and Philips Respironics, Inc. Mr. Jasko is employed by Philips Respironics. The other authors have indicated no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Donna O'Malley, Bill Hardy, Ray Vasko, Benjamin Shelly, and Susan Purdy, RPSGT for their support on this project.
REFERENCES
- 1.Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST. Respiratory event detection by a positive airway pressure device. Sleep. 2012;35:361–7. doi: 10.5665/sleep.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley P, Mortimore IL, Douglas NJ. Comparison of polysomnography with ResCare Autoset in the diagnosis of the sleep apnoea/hypopnea syndrome. Thorax. 1995;50:1201–3. doi: 10.1136/thx.50.11.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gugger M, Mathis J, Bassetti C. Accuracy of an intelligent CPAP machine with in-built diagnostic abilities in detecting apnoeas: a comparison with polysomnography. Thorax. 1995;50:1199–1201. doi: 10.1136/thx.50.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueno K, Kasai T, Brewer G, et al. Evaluation of the apnea-hypopnea index determined by the S8 auto-CPAP, a continuous positive airway pressure device, in patients with obstructive sleep apnea-hypopnea syndrome. J Clin Sleep Med. 2010;6:146–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Desai H, Patel A, Patel P, et al. Accuracy of auto-titration CPAP to estimate the residual apnea-hypopnea index in patients with obstructive sleep apnea on treatment with auto-titrating CPAP. Sleep Breath. 2009;13:383–90. doi: 10.1007/s11325-009-0258-2. [DOI] [PubMed] [Google Scholar]
- 6.Cilli A, Uzun R, Bilge U. The accuracy of auto-titrating CPAP-determined residual apnea-hypopnea index. Sleep Breath. 2013;17:189–93. doi: 10.1007/s11325-012-0670-x. [DOI] [PubMed] [Google Scholar]
- 7.Schwab RJ, Badr SM, Epstein LJ, et al. ATS Subcommittee on CPAP Adherence Tracking Systems. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188:613–20. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Isreal S, Chesson AS, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 9.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/ occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 10.Pittman SD, Pillar G, Berry RB, Malhotra A, MacDonald MM, White DP. Follow-up assessment of CPAP efficacy in patients with obstructive sleep apnea using an ambulatory device based on peripheral arterial tonometry. Sleep Breath. 2006;10:123–31. doi: 10.1007/s11325-006-0058-x. [DOI] [PubMed] [Google Scholar]
- 11.Baltzan MA, Kassissia I, Elkholi O, Palayew M, Dabrusin R, Wolkove N. Prevalence of persistent sleep apnea in patients treated with continuous positive airway pressure. Sleep. 2006;29:557–63. [PubMed] [Google Scholar]
- 12.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35:17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005;11:485–93. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 14.Lehman S, Antic NA, Thompson C, Catcheside PG, Mercer J, McEvoy RD. Central sleep apnea on commencement of continuous positive airway pressure in patients with a primary diagnosis of obstructive sleep apnea hypopnea. J Clin Sleep Med. 2007;3:462–6. [PMC free article] [PubMed] [Google Scholar]