Abstract
Study Objectives:
Evidence is accumulating that electroencephalographic (EEG) sleep slow wave activity (SWA), the key characteristic of deep sleep, is regulated not only globally, but also locally. Several studies have shown local learning- and use-dependent changes in SWA. In vitro and in vivo animal experiments and studies in humans indicate that these local changes in SWA reflect synaptic plasticity. During maturation, when synaptic changes are most prominent, learning is of utmost importance. Thus, in this study, we aimed to examine whether intensive working memory training for 3 w would lead to a local increase of sleep SWA using high-density EEG recordings in children and young adolescents.
Setting:
Sleep laboratory at the University Children's Hospital Zurich.
Participants:
Fourteen healthy subjects between 10 and 16 y.
Interventions:
Three weeks of intensive working memory training.
Measurements and Results:
After intensive working memory training, sleep SWA was increased in a small left frontoparietal cluster (11.06 ± 1.24%, mean ± standard error of the mean). In addition, the local increase correlated positively with increased working memory performance assessed immediately (r = 0.66) and 2 to 5 mo (r = 0.68) after the training.
Conclusions:
The increase in slow wave activity (SWA) correlates with cognitive training-induced plasticity in a region known to be involved in working memory performance. Thus, in future, the mapping of sleep SWA may be used to longitudinally monitor the effects of working memory training in children and adolescents with working memory deficiencies.
Citation:
Pugin F, Metz AJ, Wolf M, Achermann P, Jenni OG, Huber R. Local increase of sleep slow wave activity after three weeks of working memory training in children and adolescents. SLEEP 2015;38(4):607–614.
Keywords: auditory working memory, electroencephalography, plasticity, sleep SWA, training
INTRODUCTION
Sleep slow wave activity (SWA, electroencephalographic [EEG] power between 0.5 to 4.5 Hz), a major characteristic of nonrapid eye movement (NREM) sleep, is globally regulated by a homeostatic process: in parallel to sleep need, SWA increases during wakefulness and decreases during sleep.1 In humans, evidence is accumulating that sleep SWA is associated with cortical plasticity because it is regulated locally in a use- and experience-dependent manner.2–4 For instance, local changes in SWA were associated with learning processes involving specific brain regions.2 As shown by Huber et al.,2 learning a visuomotor adaptation task in the evening induced a local increase in SWA over the right parietal cortex during subsequent sleep.2 In fact, a positron emission tomography study showed that this area is crucial for visuomotor learning during wakefulness.5 Also, other studies indicated that learning induced plastic changes result in corresponding changes of SWA during subsequent sleep.6,7
SWA also reflects plastic changes occurring during development,8 when large modifications of brain networks take place.9–10 Magnetic resonance imaging studies showed a spatiotemporal pattern of how gray matter density changes across development: frontal areas show later maturation than more occipital areas.11 Accordingly, sleep EEG data from childhood to late adolescence reflect brain maturational changes, indicated by an overall decrease of SWA12 and a topographical shift of maximal SWA from more occipital to frontal regions.8
Studies investigating local changes in SWA focused on either immediate effects of before-sleep manipulations in adults,2 or long-term, age-dependent changes during development.8 However, no study thus far investigated longitudinally learning-induced use-dependent changes of SWA in relation to performance that is after several weeks of training or months thereafter. Thus, in our study we were interested to see how a working memory training during 3 w changes SWA locally. Working memory is a fundamental cognitive function that describes the ability to temporarily maintain information and thereby provides an interface between perception, long-term memory, and action.13 We applied working memory training, because it was shown to be beneficial for working memory performance not only in adults,14,15 but also in children and adolescents.16 We demonstrated in a recent study, that visuospatial n-back training serves to assess learning processes in young subjects (10 to 16 y), because we found significant working memory performance changes immediately and even months after the training.16 In the current study, we used high-density (HD) EEG recordings to investigate local changes in SWA induced by the visuospatial n-back training in the same young population as presented in Pugin et al.16 In this age range, cortical and cognitive changes are most prominent.10,17,18 As shown in previous studies,2,8,19 HD EEG recordings allow to detect local changes in SWA with high spatial resolution (128 electrodes20). Moreover, we tested whether local changes in SWA would be predictive for performance changes in auditory n-back (ANB) performance, a working memory task found to be improved after working memory training.16
METHODS
Participants
Fourteen male subjects participated in the study (12.97 ± 0.40 y, mean ± standard error of the mean [SEM]; range 10 y and 4 mo to 16 y and 2 mo). The inclusion criteria were male, right-handed, good sleep (no diagnosed sleep disorder as assessed in a structured telephone interview, nor any reported sleep difficulties) and regular sleep-wake times (similar day-to-day bedtimes and wake-up times, ± 1 h, as assessed by actimetry and sleep-wake diaries), no cognitive or learning disabilities (normal IQ and no reported learning difficulties as assessed in a structured telephone interview16), nonsmokers, moderate caffeine and alcohol consumption (monitored by sleep log, see next paragraphs), no diseases or intake of medication, no travels crossing more than two time zones in the last 6 mo, and no regular daytime sleep. Parents and subjects gave written consent after careful introduction to the study. The study was approved by the local ethics committee (Canton of Zurich, Switzerland).
At least 3 days prior to both overnight sleep sessions at the sleep laboratory, subjects were asked to maintain their habitual sleep-wake schedule, which was checked by wrist motor actigraphs and self-reported sleep-wake diaries. In the sleep diary, in addition to sleep and wake times, subjects were asked to write down daily amounts of caffeine and alcohol intake as well as daytime naps and physical activities. None of the subjects reported any alcohol intake. Caffeine consumption was very low and ranged from 0 to 8 mg/day (mainly from chocolate and ice tea; 100 mL Coca-Cola contains about 10 mg caffeine).
Experimental Design
Sleep was recorded twice at the sleep laboratory of the University Children's Hospital Zurich (PRE and POST training, Figure 1) with 3 w of training in between. In the morning after the first night (PRE), subjects performed an ANB task for 10 min.21 After this ANB session, the subjects started intensive working memory training for 3 w until the next overnight sleep session (POST training). The selection of the training interval was a tradeoff between training as long as possible and compliance of the children/ adolescents to perform the training. A similar training interval was used previously in children.22 Training performance was supervised at the first session and at one home visit within the 3 w. Immediately (POST) and several months after the training (follow-up, FU), ANB performance was reassessed (for more methodological details, see Pugin et al.16).
Figure 1.
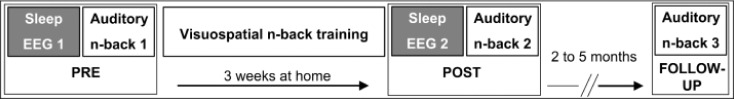
Study design and timeline. In a first session before training (PRE), high-density sleep electroencephalography (EEG) was recorded overnight and in the morning, subjects performed the auditory n-back task (auditory n-back 1). After 3 w of visuospatial n-back training, the procedure at PRE was repeated (POST training). In a follow-up session after 2 to 5 mo, auditory n-back performance was reassessed (auditory n-back 3). To avoid overburdening the young subjects and their families, no EEG recordings took place at the follow-up session.
Intervention: Computerized Visuospatial N-Back Training (BrainTwister Software)
The training consisted of a visuospatial n-back task, developed by the University of Berne.21 In a continuous series of stimuli (i.e., blue squares appearing on a computer screen) subjects had to indicate by pressing a button when the position of the blue square was in the same position as n stimuli before. In other words, for each stimulus in a trial (series of 20 + n stimuli), the position of the current stimulus had to be compared to the stimulus presented n stimuli before. Thus, the larger the n, the more correct positions were memorized. The working memory task was adaptive; thus, the difficulty level (i.e., the n), was adjusted to the individual performance after every trial. For each trial, each subject's performance was saved and retraced during the home visit as well as retrospectively after the training period. Training duration lasted 30 min per day (maximally 20 days).
Working Memory Task: Computerized Auditory N-Back Task (BrainTwister Software)
The adaptive ANB task (University of Berne) was similar to the visuospatial training task, but with a computerized voice speaking letters instead of visual squares appearing on the screen. We used the ANB to test for a “generalization effect” of the visuospatial working memory training.16 ANB was assessed at PRE, POST, and FU and lasted 10 min at each session. The maximally reached n, thus the maximal number of letters that could be memorized, was used for analysis; additional details on the working memory task are discussed by Pugin et al.16
Sleep EEG Recording and Analysis
In all 14 subjects, all-night sleep EEG, electrooculogram, and electromyogram were examined at the sleep laboratory of the University Children's Hospital Zurich. Sleep was recorded with HD EEG (Electrical Geodesics Sensor Net for long-term monitoring, Electrical Geodesics Inc., Eugene, Oregon, USA; 128 channels, referenced to a vertex electrode). Impedances were below 50 kΩ throughout the recording. Preprocessing of sleep data was performed in MATLAB 7.7.0 (R2008b, The Mathworks®, Natick, Massachusetts, USA). Data were sampled at 500 Hz (filtered 0.01–200 Hz). Offline, after high-pass (0.5 Hz) and low-pass (50 Hz) filtering, data were downsampled to 128 Hz. Power spectral density for each derivation and night was estimated with the Welch method (MATLAB function pwelch, average of five 4 sec-epochs, Hanning window). Artifacts were rejected for 20-sec epochs after visual inspection (movement or technical artifacts) and if power exceeded a threshold based on a sliding mean in the 0.75–4.5 Hz (sliding mean of 15 20-sec epochs) and 20–30 Hz bands (sliding mean of 25 20-sec epochs).8 Poor quality EEG channels (noise level exceeded normal time course of power in the aforementioned frequency ranges) were excluded. Each derivation was average referenced to the mean of all unrejected channels. The EEG signal was visually scored according to the standard criteria of the American Academy of Sleep Medicine.23 Artifact detection and visual scoring were done blinded.
For each subject, lights-off time was determined according to their habitual sleep time. Wake-up time had to be adjusted to the subjects' needs (e.g., school start). Because of interindividual differences in sleep duration, we analyzed the minimum common number of NREM-rapid eye movement (REM) sleep cycles found in all subjects and nights (three cycles). Definition of sleep cycles was performed according to Feinberg and Floyd.24 As described by Kurth et al.,8 skipped REM sleep episodes were introduced if necessary.
SWA (EEG power between 1 and 4.5 Hz) was calculated for each subject for both nights (PRE, POST). We used this slightly adjusted frequency range of SWA to reduce the influence of low frequency (< 1 Hz) artifacts like those resulting from sweating. Power values were normalized for each subject and night by dividing SWA in each channel by the mean SWA across all valid channels of a selected time interval. Such a normalization eliminates global changes in SWA because of day-by-day differences in sleep depth/pressure and therefore allows uncovering local topographical changes. For power density spectra, power density values of each frequency bin (0.25 Hz) were normalized in each subject and night by dividing power density in each derivation by the mean power over all derivations.
In order to localize the electrodes' position over respective brain regions, electrodes were digitized and coregistered to the subject's MRI using SofTaxic Optic (E.M.S. s.r.l. Company, Bologna, Italy) and the three dimensional optical digitizer (Polaris Spectra, Northern Digital Inc., Waterloo, Ontario, Canada). With the Talairach Client,25,26 cortical brain regions underlying the single electrodes were estimated as applied previously.8,19
Statistics
Statistics was performed with SPSS (PASW Statistics 18, Quarry Bay, Hong Kong) and MATLAB 7.7.0 (R2008b, The Mathworks®, Natick, Massachusetts, USA). In order to detect local changes in SWA related to the training, we performed paired t test comparing PRE and POST training. To control for multiple comparisons, statistical nonparametric mapping27 was applied. This method is based on permutation testing and was used in previous HD EEG studies.2,19 In brief, EEG power data at each electrode for the PRE and POST session were shuffled according to all possible permutations for all subjects. Based on the distributions obtained from the permutation data, we calculated a t-value for each electrode, and found the maximal t-value over all electrodes for each permutation. The t-value threshold was taken as the 95th percentile of the permutation-derived t-values, and electrodes exceeding that threshold were taken as showing a significant difference between the two sessions. Power spectral density in each bin (0.25 Hz) between the two nights (PRE, POST training) was compared by paired t-tests. Similar to Stadelmann et al.,28 only ≥ 5 consecutive frequency bins were considered for interpretation. A backward regression model performed in SPSS 18 was used to determine the most appropriate model that predicts the dependent variable.29 More specifically, this way we determined how much of the variance in the dependent variable (ANB performance after the training) may be explained by the predictor variables (age, baseline, ANB performance at PRE training and changes in SWA). The model with the highest adjusted R2 was chosen. Beta (β)-values (standard deviation units) describe the relationship between predictors and the dependent variables (i.e., the amount of change in the dependent variable if the predictor changes approximately one standard deviation).
RESULTS
Performance Change In Working Memory
As described in Pugin et al.,16 intense training resulted in significant immediate and long-term improvements in ANB performance. We note that peak training performance was reached before the end of the 3 w training period (i.e., on average after 10.2 ± 1.2 training sessions; see Figure 2 in Pugin et al.16).
Figure 2.
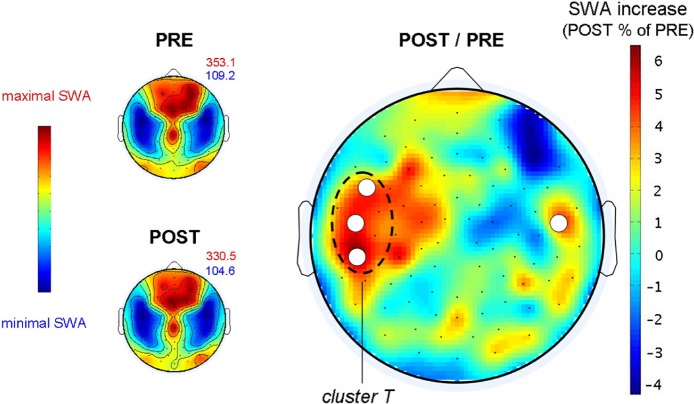
Local changes in slow wave activity (SWA) after working memory training. Left: Spatial distribution of SWA (1–4.5 Hz power) in the night before (PRE) and after (POST) working memory training. Dark blue to brown colors indicate minimal to maximal SWA. The numbers in red and blue indicate minimal and maximal absolute mean power density ([μV2 Hz−1]) values for PRE and POST training recordings. Right: Mean SWA change from PRE to POST in percentage of the PRE recording. The white dots indicate derivations where SWA was significantly increased from PRE to POST (statistical nonparametric mapping, see Methods for details).
Local Increase in SWA after 3 Weeks of Visuospatial N-Back Training
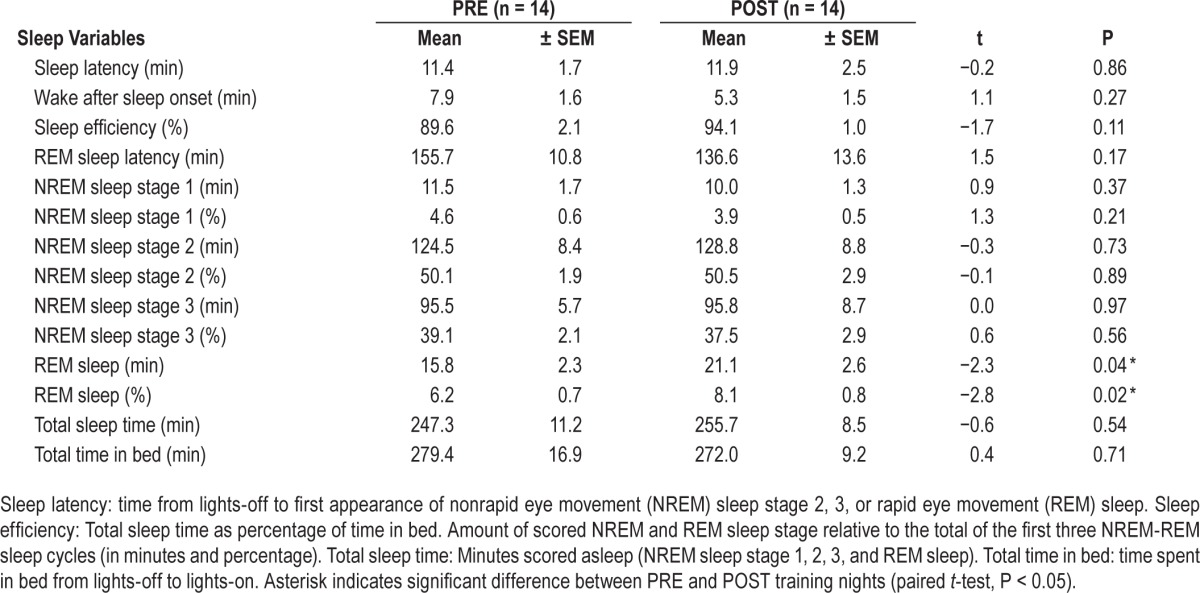
In a first step, we compared sleep quality and sleep stages between the two nights. Neither sleep efficiency nor the amount of NREM sleep stages 1 to 3 differed between PRE and POST training nights (Table 1). Only REM sleep (duration and percentage) was increased in the POST training night compared to the PRE training night.
Table 1.
Sleep variables derived from visual scoring for PRE and POST training nights (see Figure 1).
Next, we analyzed regional training-related changes in SWA by comparing sleep SWA topography before and after the training (PRE, POST). SWA topography (Figure 2, left) was similar for the 2 nights (PRE, POST), with both nights showing an age-typical SWA maximum over frontocentral regions.8 Statistical nonparametric mapping permutation test revealed a significant increase in SWA in three derivations over the left frontal and parietal cortex, including inferior frontal, precentral, and postcentral gyrus, as well as in one derivation over right prefrontal gyrus (Figure 2, right).
We considered the increase in the left three derivations as a cluster (cluster T). Similar to Huber et al.,2 for each individual the derivation with a maximal power increase from PRE to POST within this cluster was determined and used for further analysis. The average increase in cluster T from PRE to POST (ratio POST / PRE) was 11.06 ± 1.24 % (mean ± SEM).
Local Increase in SWA is Related to ANB Performance after the Training
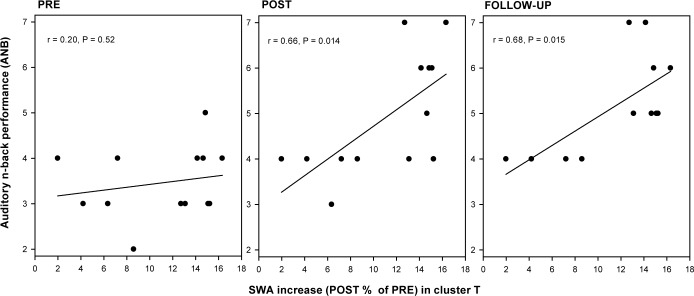
In order to assess whether the improved performance in an ANB task performed at POST and FU was related to the local increase in SWA, we correlated ANB performance with the maximum SWA increase in cluster T. A positive association between the SWA increase and ANB POST and ANB FU was observed (Figure 3). No significant correlation was found in the first session (PRE). Moreover, we found a positive association between the SWA increase and the change in ANB performance from PRE to POST and FU, respectively (PRE- POST, r = 0.57, P = 0.043; PRE- FU, r = 0.55, P = 0.064).
Figure 3.
Local changes in slow wave activity (SWA) correlate with working memory performance. Pearson correlation between SWA increase (%) from PRE to POST in cluster T (three left derivations; see Figure 2) with maximal auditory n-back (ANB) performance at PRE (left) and POST (middle) training and at follow-up (FU; right). The n indicates the maximal performance reached at this session (i.e., 2- to 7-back).
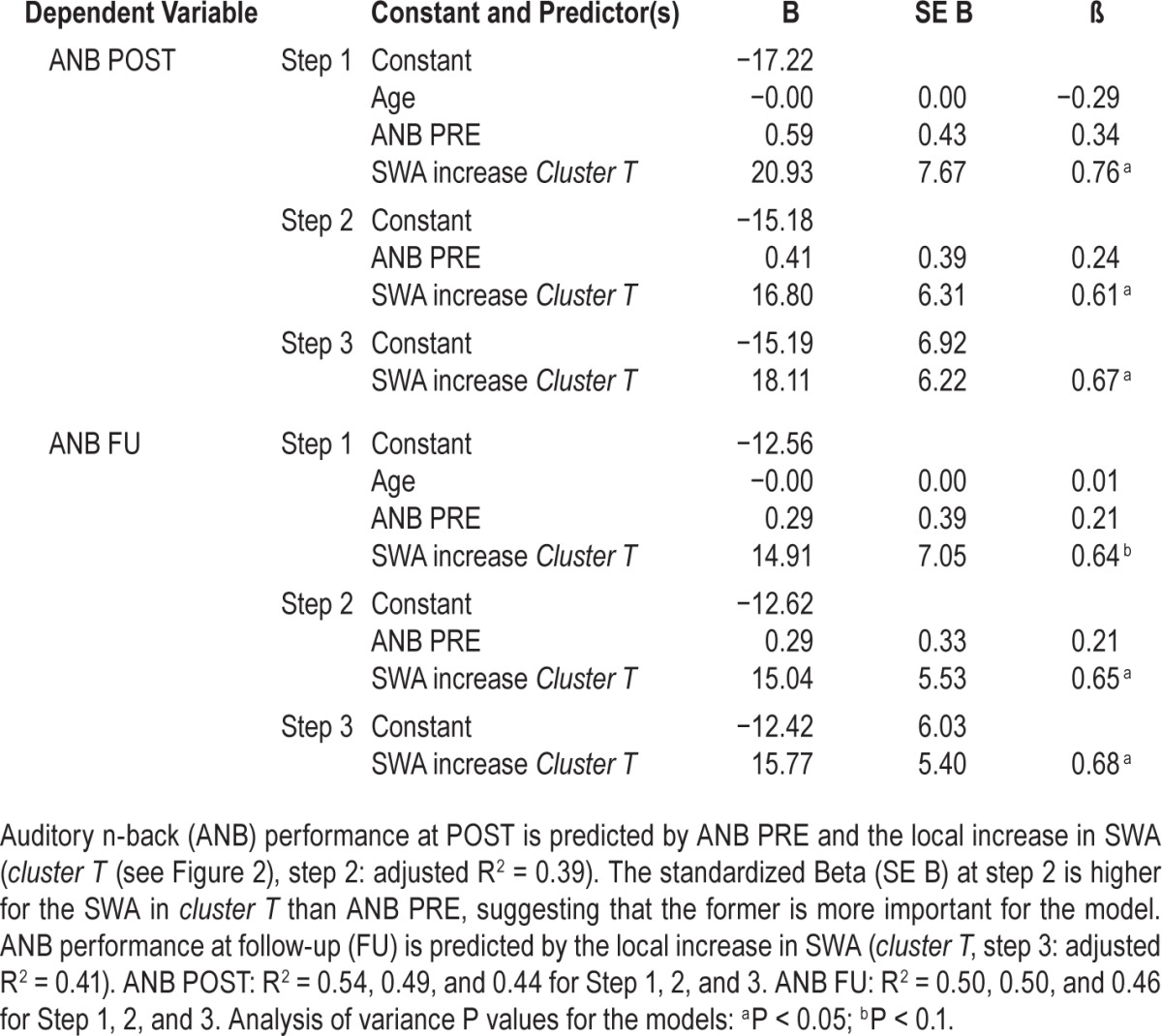
With a backward regression analysis (see Methods for details), we aimed to reveal how predictive the local increase in SWA was for performance in ANB right after the training (POST) and some months later (FU). In addition to the SWA increase in cluster T, the predictors were age and ANB performance at PRE. For ANB POST performance, the model including both ANB PRE performance and the local increase of SWA in cluster T showed the highest adjusted R2 (adjusted R2 = 0.39, F (1, 11) = 4.84, P < 0.05; β (ANB PRE) 24.3 %; β (SWA increase in cluster T) 61.2 %, Table 2). Thus, ANB performance at POST was not predicted by age, but by ANB PRE and, to a larger extent, by the SWA increase in cluster T. ANB FU performance was not predicted by age and baseline performance (ANB PRE), but by the local increase in SWA in cluster T (adjusted R2 = 0.41, F (1,10) = 8.53, Pp < 0.05; β = 67.9 %, Table 2).
Table 2.
Backward regression analysis: predictive variables for auditory n-back performance.
Increase in Spectral Power within Cluster T in Theta and Sigma Range
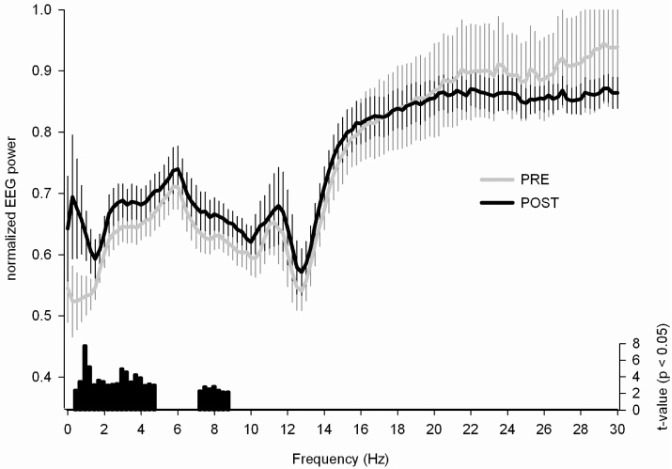
To assess the frequency specificity of the local changes in SWA, we investigated the normalized power spectrum for derivations in cluster T (Figure 4). This analysis revealed a significant increase in the slow wave frequency band (0.5 to 4.75 Hz) and in the theta range (7.25 to 8.75 Hz) from PRE to POST training.
Figure 4.
Power density spectrum for cluster T. Power density spectrum (1 to 30 Hz, bin width = 0.25 Hz) for the derivation showing maximal power in cluster T (see Figure 2) during the PRE (gray) and POST (black) training night (mean ± standard error of the mean). Power density values for the derivations in cluster T were expressed relative to the mean power density across all derivations (see Methods for details about this normalization). Black vertical bars indicate significant t values of paired t tests per bin comparing normalized power density values of PRE and POST training nights.
DISCUSSION
We observed a significant increase in SWA in three left frontoparietal derivations (cluster T) after cognitive training. After 3 w of visuospatial working memory training, this local increase correlated with performance in an ANB task immediately (POST) and some months after the training (FU). Regression analysis revealed that ANB performance variance after the training was best explained by the local increase in SWA.
We highlight three major aspects of our study. First, we investigated not immediate effects of learning on the subsequent sleep EEG, but effects after several weeks of training. Previous studies investigated learning-induced SWA changes in children30 and as well as in adults.2 In these studies, the learning task was performed the day before the sleep EEG recording, with the aim to detect performance-related effects in the subsequent sleep EEG recording. In fact, local changes in SWA as a result of intense use or learning were observed.2,6,7 Our data show that local changes in SWA are also observable after a longer period of learning (3 w). Functionally, the increase in SWA may have resulted from an increase in synaptic efficacy over the 3 w of training, reflected by the induction of learning-related long-term potentiation (LTP) and the synthesis of proteins involved in structural remodeling of synapses.31 Support for a relationship between SWA and plasticity comes from animal studies. For example, in rats, Vyazovskiy et al.32 showed that synaptic potentiation dominates during wake, which was indicated by a net increase in GluR1-containing AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor levels and an increased phosphorylation of receptors and kinases involved in learning and plasticity. Such increased synaptic strength is related to the efficacy of excitatory cortical connections, characterized by increased firing rate and synchrony.33 In other words, the higher the synaptic strength (e.g., after learning during wake and LTP), the higher SWA in the used region in the following sleep episode. In a study in mice, also structural changes such as the number of spines were related to sleep (i.e., increased during wakefulness and decreased during sleep34). These studies support the hypothesis that the SWA increase after intensive working memory training over 3 w may be related to LTP-like or structural plastic changes in a distinct network. As a consequence firing rates and synchrony during deep sleep were altered, resulting in higher SWA, particularly in the three left frontoparietal derivations over cortical areas involved in working memory training. Those functional and structural changes would in turn be reflected in performance changes, building the basis for the observed sustained working memory performance increase over several months. Thus, the local increase of SWA over the left frontoparietal cortex may reflect the “ability to be plastic” also after the intervention, thereby predicting later outcome of cognitive performance. However, our results also show that individual differences in performance can only partially explain the individual differences in the local increase of SWA from PRE to POST training. Other factors such as anatomical or behavioral differences may be contributing factors.
In addition, this is the first study showing changes in sleep SWA after working memory training. So far, studies have reported about local changes in SWA after visuomotor or sensorimotor manipulations,2–4,6–7 but not after manipulations of other cognitive functions. The investigation of local SWA changes in relation to complex cognitive functions such as working memory is challenging because of the high degree of underlying interconnected areas involved in cognitive functions. We found a local increase of SWA over a frontoparietal network that encompassed inferior frontal, precentral and postcentral gyri, including Brodmann areas (BA) 2, 6, 34, 44, and 45. This localization is in agreement with the literature showing cortical activation during n-back tasks in similar regions.35,36 More specifically, activation in the left hemisphere during verbal working memory tasks is enhanced compared to activation during visual tasks.36
Thus, the left increase in SWA and its relation to auditory working memory performance may reflect plastic changes in an area involved in verbal processing. However, working memory processing relies on the activation of both hemispheres.35,36 The increase in SWA in one right derivation (see Figure 2), contralateral to cluster T, may point to such a bilateral activation. Thus, our findings support the notion that SWA can be used to map plasticity-driven changes. For example, in the ongoing debate about the effectiveness of working memory training on cognitive performance, mapping changes of SWA may serve as a promising tool to investigate long-term effects of working memory training, as an addition to the already established purely cognitive assessments or functional neuroimaging measures. Moreover, according to our model of the regression analysis, the increase in SWA in cluster T seems to be the strongest predictor variable for ANB performance at POST and FU. Thus, our results show that local changes in SWA may even have some predictive value for training related changes in working memory performance, even months after the end of the training.
Finally, few studies investigated SWA changes due to learning in young populations. Recently, Wilhelm et al.30 reported learning-related changes in SWA in a broader, but overlapping age range. They found an increase in SWA following a visuomotor learning task in a region similar to the one observed in Huber et al.2 Interestingly, this local increase was more pronounced in children than in adolescent and adult subjects and was correlated with the level of SWA in this particular region.30 In line with this finding, the local increase of SWA after working memory training was larger for those subjects who showed higher SWA in this area prior to training (Pearson correlation between PRE SWA and the increase of SWA in the frontoparietal cluster: r = 0.65, P = 0.013). According to Kurth et al.,8 frontoparietal regions show maximal SWA between 11 and 14 y, which may indicate that these regions undergo major plastic changes during this period. As hypothesized by Wilhelm et al.,30 these regions may be more prone to intervention. In our study, the positive correlation of local SWA (cluster T) with ANB performance after training (see Figure 3) supports these views, because increased susceptibility for plastic changes, indicated by higher initial SWA and a larger increase in SWA, seems to be advantageous for later working memory performance (Figure 3, Table 2). Consequently, as working memory performance reaches adult-level performance around 20 y of age,17 older subjects may show less prominent SWA increases because a plasticity plateau is reached. These observations indicate that developmental aspects may be relevant when investigating learning-related local changes in SWA.
However, some limitations have to be considered when interpreting the findings of our study. One issue is the small number of subjects within a rather broad age range. As a result, we cannot exclude that with more statistical power, for example, the single derivation showing increased SWA on the contra-lateral site of cluster T might extend into a cluster. Moreover, correlational analyses (Figure 3) with a low number of subjects are limited in their validity. However, it is needless to say that such a demanding study protocol is challenging for a young subject population. This limitation is also the reason why we did not perform a third HD EEG recording during the FU session. We also would like to point out that the power spectral changes from night 1 to night 2 in cluster T were not restricted to the slow oscillation (< 1 Hz) and slow waves range (1 to 4.75 Hz), but were also observed at 7.25 to 8.75 Hz. However, other studies reported changes in theta range. For example, during recovery sleep after 40 h of sleep deprivation both slow wave and theta activity were increased in frontal derivations.37 Also Huber et al.2 reported a parallel increase in theta activity after the visuo-motor learning task. Finally, whether SWA changes can be observed after a certain time interval may also depend on the task and the subject's age.30 Moreover, how the nonlinear time course of performance improvement during training is related to the observed local increase of SWA is unclear. More frequent EEG recordings in the course of the training would have provided interesting insights. However, this approach was not feasible.
In conclusion, we show that SWA can be used to map long-term effects of an intensive learning period in young subjects. Hereby, the local changes in SWA seem to be an indicator for learning-induced plasticity related to complex cognitive functions such as working memory. In the future, mapping of SWA may be used to longitudinally monitor the effects of working memory training in children and adolescents with working memory deficiencies.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the University Research Priority Program “Integrative Human Physiology” of the University of Zurich (Switzerland), the Anna Müller Grocholsky foundation (Switzerland), and the Swiss National Science Foundation (grant P00P3-135438). The funding institutions were not involved in study design, data collection and analysis, decision to publish, nor preparation of the manuscript. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Madlaina Stauffer and Urs Bachofner for their support with data collection.
REFERENCES
- 1.Achermann P, Borbély AA. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th edition. Philadelphia: Elsevier Saunders; 2011. pp. 405–17. [Google Scholar]
- 2.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 3.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 4.Kattler H, Dijk DJ, Borbély AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 5.Ghilardi M, Ghez C, Dhawan V, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–45. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 6.Landsness EC, Crupi D, Hulse BK, et al. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–84. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maatta S, Landsness E, Sarasso S, et al. The effects of morning training on night sleep: a behavioral and EEG study. Brain Res Bull. 2010;82:118–23. doi: 10.1016/j.brainresbull.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–9. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huttenlocher PR. Synaptic density in human frontal cortex--developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 10.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–39. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 14.Buschkuehl M, Jaeggi SM, Hutchison S, et al. Impact of working memory training on memory performance in old-old adults. Psychol Aging. 2008;23:743–53. doi: 10.1037/a0014342. [DOI] [PubMed] [Google Scholar]
- 15.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–33. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugin F, Metz AJ, Stauffer M, Wolf M, Jenni OG, Huber R. Working memory training shows immediate and long-term effects on cognitive performance in children [v3; ref status: indexed, http://f1000r.es/4y9] F1000Research. 2015;3:82. doi: 10.12688/f1000research.3665.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Devel. 2004;75:1357–72. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 19.Ringli M, Souissi S, Kurth S, Brandeis D, Jenni OG, Huber R. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex. 2013;49:340–7. doi: 10.1016/j.cortex.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Lustenberger C, Huber R. High density electroencephalography in sleep research: potential, problems, future perspective. Front Neurol. 2012;3:77. doi: 10.3389/fneur.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschkuehl M, Jaeggi SM, Kobel A, Perrig WJ. Department of Psychology, Division of Experimental Psychology and Neuropsychology, Universität Bern; 2007. BrainTwister: A Collection of Cognitive Training Tasks. Version 1.0.2. [Google Scholar]
- 22.Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A. 2011;108:10081–6. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1 ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 24.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 25.Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stadelmann K, Latshang TD, Lo Cascio CM, et al. Quantitative changes in the sleep EEG at moderate altitude (1630 m and 2590 m) PLoS One. 2013;8:e76945. doi: 10.1371/journal.pone.0076945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agresti A, Finlay B. Statistical methods for the social sciences. 4th edition. Pearson Prentice Hall; 2008. [Google Scholar]
- 30.Wilhelm I, Kurth S, Ringli M, et al. Sleep slow-wave activity reveals developmental changes in experience-dependent plasticity. J Neurosci. 2014;34:12568–75. doi: 10.1523/JNEUROSCI.0962-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 32.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 33.Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–20. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeggi SM, Seewer R, Nirkko AC, et al. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage. 2003;19:210–25. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 36.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]