Abstract
Objectives:
To evaluate psychiatric comorbidity and the cognitive profile in children and adolescents with narcolepsy in western Sweden and the relationship of these problems to H1N1 vaccination.
Patients:
Thirty-eight patients were included in the study.
Design:
We performed a population-based, cross-sectional study to investigate psychiatric comorbidity using a test battery of semistructured interviews generating Diagnostic and Statistical Manual of Mental Disorders, 4th Edition diagnoses, including the Development and Well-Being Assessment and the attention deficit hyperactivity disorder rating scale. The Autism Spectrum Screening Questionnaire and the Positive and Negative Syndrome Scale were used to screen for autistic traits and psychotic symptoms, respectively. The cognitive assessments were made by a clinical psychologist using the Wechsler Preschool and Primary Scale of Intelligence, Third Edition, the Wechsler Intelligence Scale for Children, Fourth Edition, or the Wechsler Adult Intelligence Scale, Fourth Edition.
Measurements and Results:
In the post-H1N1 vaccination (PHV) narcolepsy group (n = 31), 43% of patients had psychiatric comorbidity, 29% had attention deficit hyperactivity disorder (ADHD) inattentive type, 20% had major depression, 10% had general anxiety disorder, 7% had oppositional defiant disorder (ODD), 3% had pervasive developmental disorder not otherwise specified (i.e., atypical autism), and 3% had eating disorder not otherwise specified (anorectic type). In the non–post-H1N1 vaccination (nPHV) narcolepsy group, one of seven patients had ADHD, inattentive type and ODD. The most frequent psychiatric symptom was temper tantrums, which occurred in 94% of the patients in the PHV group and 71% of the patients in the nPHV narcolepsy group. The cognitive assessment profile was similar in both groups and showed normal results for mean full-scale IQ and perceptual speed but decreased verbal comprehension and working memory. Patients with psychiatric comorbidity had a significantly lower full-scale IQ compared to those without.
Conclusion:
Our study indicates increased psychiatric comorbidity in children and adolescents with narcolepsy. The identified cognitive profile with significantly lower verbal comprehension and working memory compared with the normal mean index could have important implications for social relations and schooling. The small numbers of patients with nPHV narcolepsy make it difficult to draw firm conclusions about the possible differences between the two groups of patients.
Citation:
Szakács A, Hallböök T, Tideman P, Darin N, Wentz E. Psychiatric comorbidity and cognitive profile in children with narcolepsy with or without association to the h1n1 influenza vaccination. SLEEP 2015;38(4):615–621.
Keywords: children, cognition, narcolepsy, psychiatric comorbidity
INTRODUCTION
Narcolepsy is a common neurological disorder, affecting approximately 0.06% of the general population.1 It has been hypothesized that a combination of genetic and environmental factors is involved in the pathogenic mechanisms of narcolepsy, where the triggering of an autoimmune process leads to hypothalamic destruction, with the loss of hypocretin-1-containing cells.2 Even though symptoms most frequently begin during childhood or adolescence, the diagnosis is usually not made until adulthood. Narcolepsy is characterized by excessive daytime sleepiness with sudden sleep attacks, and rapid eye movement sleep abnormalities such as cataplexy (sudden reduction or loss of muscular tone not accompanied by loss of consciousness), sleep paralysis (inability to move when falling asleep or waking up), and hypnagogic (upon falling asleep) or hypnopompic (upon awakening) hallucinations and nocturnal dyssomnia with fragmented sleep and awakenings.3
Psychiatric disorders have been shown to be more frequent in adults with narcolepsy compared with the general population. The most common disorders, depressive mood and social anxiety disorders, each have been described in approximately 20–30% of patients.4,5 In contrast, the prevalence of eating disorders or psychotic disorders in adults with narcolepsy does not appear to be increased compared with the general population.6,7 A retrospective study of childhood attention deficit hyperactivity disorder (ADHD) symptomatology among adult patients with narcolepsy identified a more than eightfold to 15-fold greater prevalence than expected, indicating a relationship between narcolepsy and ADHD.8
Adult patients with narcolepsy frequently complain of memory problems. In contrast, the results from standardized neuropsychological investigations of memory performance in these patients have frequently been similar to those of healthy controls.9 Recent studies have revealed somewhat conflicting results. Adult patients with narcolepsy may attain high performance in several neuropsychological domains, but, at the same time, they can have difficulty in areas such as working memory, executive function, reward processing, and decision making.10–13
Although most patients with narcolepsy experience the onset of symptoms in childhood or adolescence, comparatively few studies have focused on aspects of the disease before adulthood and very few studies have addressed the psychiatric and cognitive consequences in childhood. Several retrospective hospital-based studies have described poor school performance in a large proportion of children with narcolepsy.14–16 In addition, behavioral problems were described in approximately half the patients with narcolepsy secondary to the H1N1 vaccination in both Finland and Sweden.16,17 A study of psychosocial and intellectual functioning in 12 children with narcolepsy reported an IQ in the average range tested with the Wechsler Intelligence Scale for Children, Third Edition (WISC-III).18
A 17- to 25-fold increase in the childhood incidence of narcolepsy has recently been described in relation to the 2009 H1N1 pandemic vaccination campaign in Finland and Sweden.16,17 The large number of patients currently has made it possible to study the childhood manifestations of narcolepsy in more detail. We recently performed an epidemiological study of children and adolescents in western Sweden in which we recruited all patients in whom narcolepsy had been diagnosed between January 1, 2000 and December 31, 2010.17 The aims of this cross-sectional study are to describe the prevalence of psychiatric comorbidities, as well as the cognitive profile, in this population-based cohort of children and adolescents with narcolepsy.
MATERIALS AND METHODS
Participants
The study group consisted of 38 children and adolescents, 31 with post-H1N1 vaccination (PHV) narcolepsy and seven with non–post-H1N1 vaccination (nPHV) narcolepsy. PHV narcolepsy was considered in patients with clinical onset within 10 mo from vaccination. Thirty-one patients were recruited from a population-based study in western Sweden. In addition, another seven patients were consecutively recruited from the same population. All patients had onset of disease between January 1, 2000 and December 31, 2010. Patient selection was based on the classification codes of the Swedish version of the International Classification of Diseases, Tenth Revision (ICD-10) and the diagnostic criteria for narcolepsy according to the 2005 International Classification of Sleep Disorders.19
Instruments
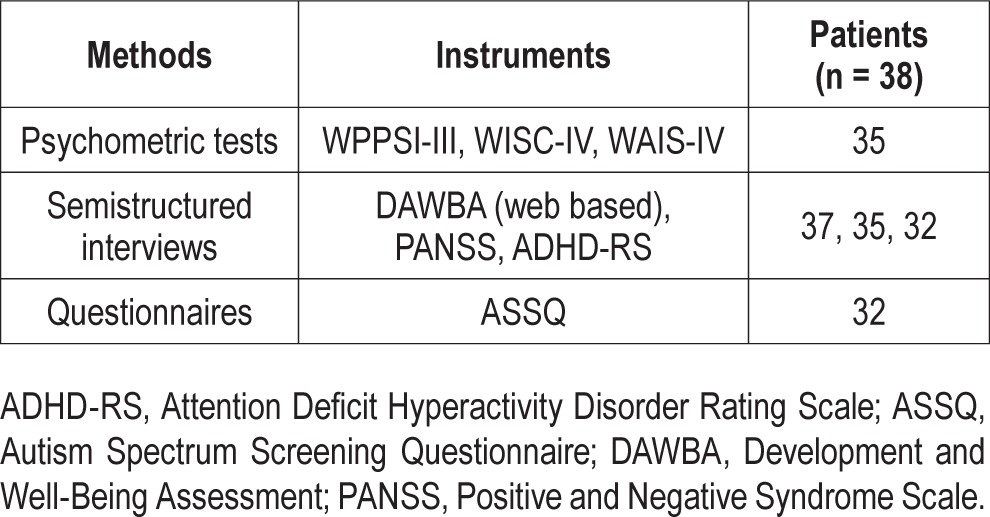
The Development and Well-Being Assessment (DAWBA) parent interview was performed in 37 patients. The DAWBA is a diagnostic tool used for the identification of psychiatric disorders.20 It consists of a package of structured interviews, questionnaires, and rating techniques designed to generate psychiatric diagnoses in children aged 5–17 y according to ICD-1021 and the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV).22 The final rating was made by an experienced child and adolescent psychiatrist, one of the authors (EW). All the subscales, including social behavior, peer problems, hyperactivity, conductive problems, and emotional disturbances, were assessed. Regarding autism spectrum disorders, the DSM-IV diagnoses of autistic disorder (AD), Asperger syndrome, and pervasive developmental disorder not otherwise specified were assessed. In addition, the term “autistic traits”, which is not a DSM-IV diagnosis but indicates social interaction problems causing a significant impairment, was used when three criteria were met for AD.22 The Autism Spectrum Screening Questionnaire (ASSQ) was used in 32 patients to screen for difficulties within the autism spectrum.23 It comprises 27 items rated on a three-point scale, where 11 items refer to social interaction, six to communication problems, and five to restricted and repetitive behavior. The five remaining items embrace motor clumsiness and other associated symptoms, including motor and vocal tics. A cutoff score of 19 or higher has been used in Sweden for the screening of AD in children with normal intelligence or mild mental retardation in a clinical setting.23 The attention deficit hyperactive disorder rating scale (ADHDRS) was used in 32 patients to make the diagnosis of ADHD.24 The ADHDRS consists of 21 questions. Eighteen questions are scored on a two-point scale ranging from 0 to 1 symptom, with a total score ranging from 0 to 18.The cutoff for ADHD was six points of either inattention or hyperactivity/impulsivity corresponding to DSM-IV criteria. Three additional questions specify the onset of symptoms before 7 y of age, before puberty, and if disabilities are caused in at least two areas, such as school and home. ADHD with atypical onset was defined as onset after age 7 y. The Positive and Negative Syndrome Scale (PANSS)25 was used in 35 patients to assess symptoms of psychosis and schizophrenia. The PANSS is only valid for adults, but there is no better instrument for screening psychotic symptoms in children and adolescents. The patient's symptoms are rated from 1 to 7 on 30 different items based on the answers from interviews, as well as reports by family members or primary care hospital workers. Scores are often given separately for the positive items, negative items, and general psychopathology. We only used the positive symptom domain in the PANSS. A pathological deviation was considered when symptom scores within the individual items were 4 or higher.25 A model of study materials and methods is presented in Table 1.
Table 1.
Instruments for evaluation of psychiatric comorbidity and cognition in children and adolescents with narcolepsy.
The cognitive assessments were performed in 35 patients: 19 females and 16 males (three patients were unavailable for testing). The assessments were made by a clinical psychologist, with no previous relationship with the patients. All participants and their parents were given the option to refuse testing if current fitness was not suitable for testing. All participants were also offered the opportunity to pause during testing or stop if needed. No one interrupted the test and only a few needed a break of some minutes.
Wechsler Intelligence Scales, the most widely used of all mental ability tests, were administered. Depending on the age of the patient, the appropriate Wechsler scale (Wechsler Preschool and Primary Scale of Intelligence, Third Edition; Wechsler Intelligence Scale for Children, Fourth Edition; or the Wechsler Adult Intelligence Scale, Fourth Edition) was used. Values for the full-scale IQ (FSIQ), verbal comprehension index (VCI), perceptual reasoning index (PRI), and processing speed index (PSI) were recorded for all the assessed patients. Working memory index (WMI) was obtained for 34 patients (one patient was too young to be able to perform the required tasks). Executive functions such as initiation, cognitive flexibility, and monitoring were examined with two subtests (animal sorting and word generation) from the “A developmental Neuropsychological Assessment” (NEPSY-II) test battery.26 When interpreting the cognitive profiles of the patients, a score of one standard deviation below average was regarded as a cognitive deficit.27–29
This study was approved by the regional ethics committee and written informed consent was obtained from all caregivers and patients participating in the study.
Data processing was performed with the SPSS Statistical Package for the Social Sciences (SPSS) version 21, statistical computer program.30 A significance level of P = 0.05 was used. The statistical calculations of the cognitive profiles were performed with the one-sample t test. The comparison of the cognitive profile of patients with and without psychiatric comorbidity was made using the independent-samples t test and the comparison of VCI and PRI was made using the paired-sample t test.
RESULTS
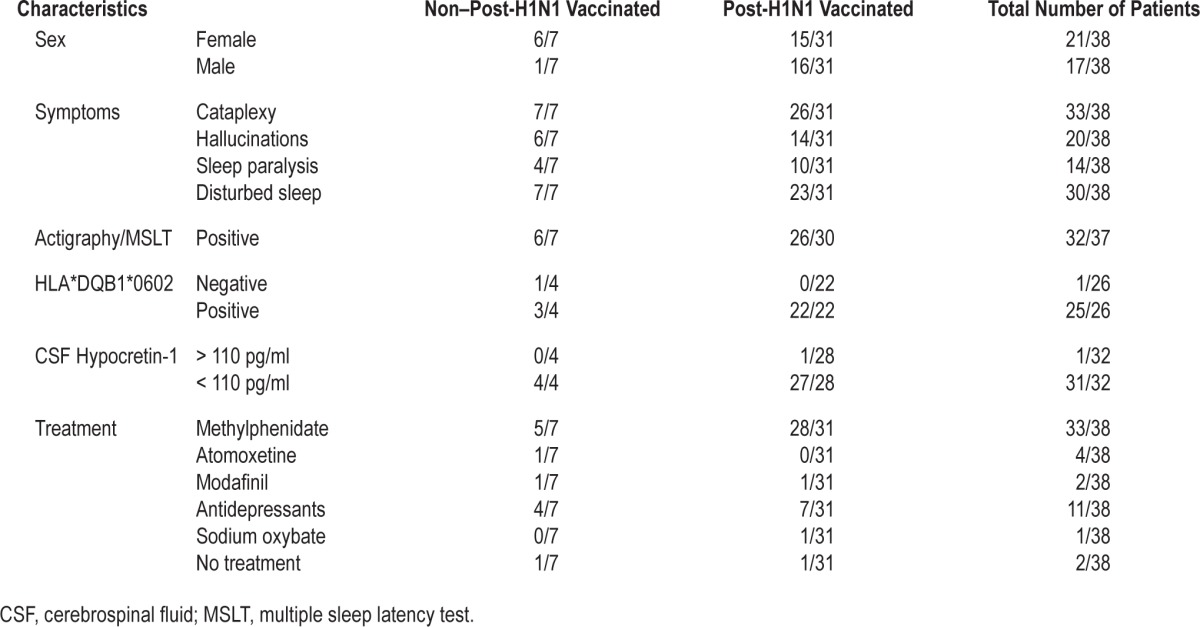
The demographic, clinical, and laboratory characteristics of the 38 children and adolescents with narcolepsy are presented in Table 2. The median age at testing was 15.3 y (range: 5.7–25.0) and the median age at onset was 12.5 y (range: 3.0–17.0). The median narcolepsy duration was 2.8 y (range: 1.9–10.0).
Table 2.
Demographics, clinical and laboratory characteristics in non-post-H1N1 vaccinated and post-H1N1 vaccinated children and adolescents with narcolepsy.
Psychiatric Comorbidity
Psychiatric comorbidity was evaluated in 37 patients. In the PHV narcolepsy group, 13/30 patients (43%) patient had a psychiatric disorder: 8/28 had ADHD, inattentive type, 6/30 had major depression, 3/30 had general anxiety disorder, 2/30 had oppositional defiant disorder (ODD), 1/30 had pervasive developmental disorder not otherwise specified (PDD NOS) (i.e., atypical autism) and 1/30 had eating disorder not otherwise specified (anorectic type). In the nPHV narcolepsy group, 1/7 patients had psychiatric disorders with a combination of ADHD, inattentive type and ODD. Seven of all nine patients with ADHD had atypical onset, i.e., the onset occurred after age 7 y. According to the caregivers onset also started after the debut of narcolepsy. The ADHD criteria were met, even though they received concurrent treatment with stimulants. All three patients with ODD were between age 11–25 y and had a simultaneous diagnosis of ADHD, inattentive type. Three patients, of whom two belonged to the PHV narcolepsy group, screened positive on the ASSQ. One of these three patients fulfilled the criteria for autistic traits and one was the individual who fulfilled the criteria for PDD NOS based on the DAWBA evaluation. According to the PANSS interview, three patients in the PHV narcolepsy group reported daytime hallucinations. The most frequent psychiatric symptom was temper tantrums, which occurred in 94% of the patients in the PHV narcolepsy group and 71% of the patients in the nPHV narcolepsy group. Patients lost their temper often in 35% of cases in the PHV narcolepsy group and in 29% in the nPHV narcolepsy group.
Cognitive Profile
The cognitive profiles of the assessed patients in the PHV and nPHV narcolepsy groups are presented in Figure 1. Both the VCI (mean, 92) and WMI (mean, 89) were significantly lower in the PHV narcolepsy group (VCI: P = 0.000; WMI: P = 0.000) compared with the normal mean index value of 100. The cognitive profile of the nPHV narcolepsy group was similar to the PHV narcolepsy group with decreased VCI (mean, 95) and WMI (mean, 93), but these indices did not differ significantly compared with the normal mean index value of 100. The PRI was significantly higher in the entire study population but not on a group level based on the vaccination status. Seventeen of the patients in the entire study population had a difference of one standard deviation or more, between PRI and VCI, where VCI was the lower one. There was a significant difference (P = 0.000) between PRI (mean 106) and VCI (mean 92). None of the patients had an FSIQ of < 70, i.e., intellectual disability. The test results for executive functions were in the normal range.
Figure 1.
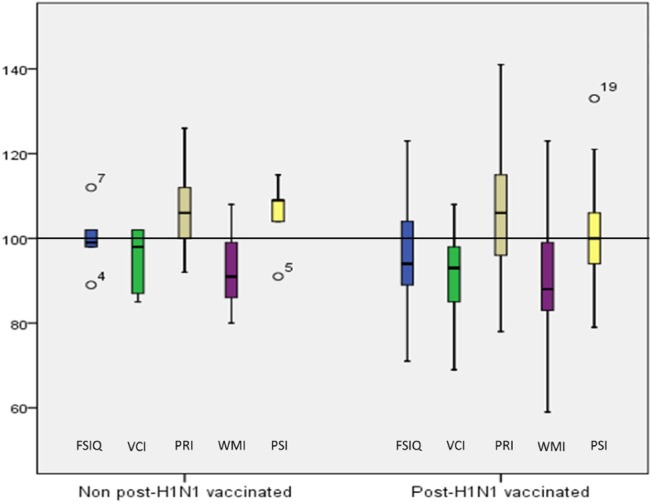
Comparison of the cognitive profile of children and adolescents with non–post-H1N1 vaccinated and post-H1N1 vaccinated narcolepsy. FSIQ, full-scale IQ; PRI, perceptual reasoning index; PSI, processing speed index; VCI, verbal comprehension index; WMI, working memory index.
A cognitive assessment was performed in the 13 of 14 patients with at least one psychiatric diagnosis (Figure 2). The FSIQ in these patients was found to be significantly lower (P = 0.027) compared with the FSIQ in the patients without a psychiatric diagnosis.
Figure 2.
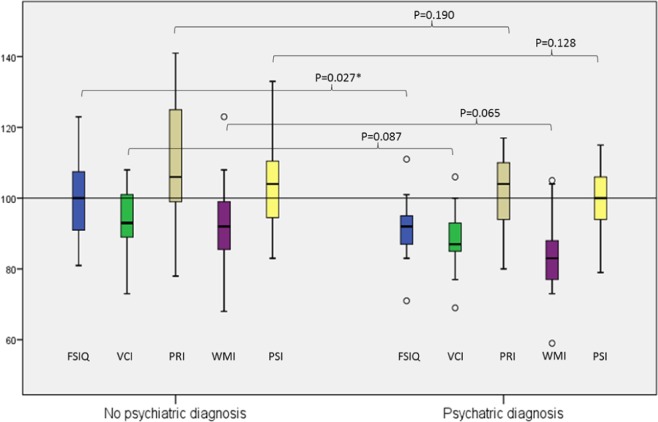
Comparison of the cognitive profile in children and adolescents with narcolepsy with and without psychiatric diagnosis. *Significance level P < 0.05. FSIQ, full-scale IQ; PRI, perceptual reasoning index; PSI, processing speed index; VCI, verbal comprehension index; WMI, working memory index.
DISCUSSION
As far as we know, there are no previous in-depth studies investigating psychiatric comorbidities in children and adolescents with narcolepsy in relationship to the DSM-IV criteria. In this study, 43% of the patients in the PHV narcolepsy group and 14% in the nPHV narcolepsy group fulfilled the DSM-IV criteria for at least one psychiatric disorder—13% and 14% respectively, for two psychiatric diagnoses. Psychiatric comorbidity seemed to be more common in the PHV narcolepsy group but a comparison is difficult to make because of the few patients identified with nPHV narcolepsy in this study. The identified tendency is difficult to explain, but the more sudden and intense onset of narcolepsy after H1N1 vaccination may have been a contributing factor. Another explanation could be a triggering effect of the vaccine itself, although psychiatric comorbidity was not mentioned in a previous Swedish national registry study.31
In the PHV narcolepsy group, major depression was present in 20%, which is almost three times higher than the prevalence in the general Swedish adolescent population, in whom the 1-y prevalence of major depression is 5.8%.32 A high prevalence of depressive mood has also been described in children with narcolepsy using screening forms.14,15 It is not known what causes depression in narcolepsy. One hypothesis is that the increase in daytime sleepiness, itself or in conjunction with disturbed nighttime sleep, leads to depression.33 Another possibility is that the depression is related to hypocretin-1 deficiency, which leads to amygdala dysfunction and results in changes in affective modulation. The amygdala neural system has also been implicated in the pathological emotional processes of patients with major depressive disorder.34 Hypocretin-1 has been described to excite serotonergic neurons,35 which are important in the pathogenesis of depression.
Three patients had problems within the autism spectrum, including one in the PHV narcolepsy group who met the criteria for PDD NOS. It is difficult to draw any conclusions from this finding based on the small number of patients. We have not been able to find any previous studies reporting problems within the autism spectrum in children or adolescents with narcolepsy.
More than one in four of the entire study population had ADHD, inattentive type. The prevalence of ADHD in narcolepsy is clearly higher than in the general population of Swedish children, where it has been estimated to 7% at age 6 to 7 y.36 Our findings are in line with the increased retrospective history of childhood ADHD symptomatology recently reported in adults with narcolepsy.8 The fact that seven of nine of our patients with ADHD developed ADHD after age 7 y and after narcolepsy onset points to a relationship between the onset of narcolepsy and the development of ADHD. Whether this is a direct causal relationship or is dependent on unmasking of a previously unrecognized disease is diffi-cult to tell because of the cross-sectional study approach. The mechanism behind ADHD in narcolepsy is not known. Earlier studies have hypothesized that decreased attention could be reactive because of nocturnal dyssomnia followed by daytime sleepiness.14 Hypocretin-1 has widespread interactions with the brain's neurotransmitter network, including the prefrontal cortex.37 Studies of patients with narcolepsy with functional MRI, as well as with event-related potentials and cognitive evoked potentials, have revealed changes in activation in the executive cortical networks localized in the prefrontal cortex.38 The same centers have been implicated in ADHD and are the main localizations of the attention systems.37 The input to the cortex from the Reticular Activating System (RAS) in the brainstem is another important network facilitated by hypo-cretin-1 that is also involved in ADHD. The RAS system is involved in multiple tasks, such as regulating the sleep-wake cycle and filtering incoming stimuli to discriminate irrelevant background stimuli.39
None of the patients in our study screened positive for psychotic symptoms and the daytime hallucinations reported by three of the patients were regarded as hypnagogic or hypnopompic hallucinations related to narcolepsy. ODD occurred in 8% of the entire study population. For comparison, the pooled prevalence of ODD in children estimated from 25 studies was 3.3%, where children older than 12 y had lower rates than children younger than 12 y.40 The first symptoms of ODD usually appear during the preschool period and they rarely appear after early adolescence.41 Our three patients with ODD were older than 11 y. ODD is frequently secondary to ADHD, the combined type in most cases.42 In the current study, all three patients with ODD had an overlapping diagnosis with ADHD, inattentive type. The most frequent psychiatric symptom in our study was temper tantrums, which often occurred in one-third of the entire study population. Frequent temper tantrums are reported among 6.5–13.5 % of the general population in Sweden depending on age and sex of the children and adolescents.43,44 Our results are in line with previous studies describing significant behavioral problems in 33–66% of children with narcolepsy.15–17
Neuropsychological assessment in this study produced a profile with normal results for mean FSIQ and perceptual speed index in both the PHV narcolepsy group and the nPHV narcolepsy group. Significantly poorer results for VCI and WMI were identified in the PHV narcolepsy group. A similar trend was found in the nPHV narcolepsy group but the change was not statistically significant. The indexes of the cognitive domains did not differ significantly between the PHV narcolepsy and the nPHV narcolepsy groups. A significantly higher PRI was detected on the entire study group level but without correlation to the H1N1 vaccination status. Normal FSIQ has also been demonstrated in a previous study.18 Working memory is closely connected to attention and is a fundamental cognitive function that is required for many mental activities, as well as being essential for many school tasks.45 Working memory is also of great significance for verbal comprehension that reflects abilities that are partially formed by what children learn at school and within social contexts. Many of the children and adolescents participating in this study had missed long periods of their schooling and also reported spending less time with their friends since the onset of the disease. The reduction in working memory may also have led to reduced verbal abilities, because it makes the important encoding process of new information more difficult. WMI in children with narcolepsy has been examined in only one previous study, which showed normal results in 12 patients.18 Executive functions, measured with two brief tests, were normal in the current study. We have not found any previous studies of executive functions in children with narcolepsy.
Studies of memory function including working memory, as well as studies of executive functions in adults with narcolepsy, have been normal in some cases,9,11 whereas other studies have found impairments.10,12,13,46 Patients are able to perform well during short, simple tests, but they perform less well on longer or more demanding tests because a higher proportion of cognitive resources are recruited for the maintenance of alertness.47
The patients with comorbid psychiatric disorders had a significantly lower FSIQ than the group without comorbidity. A low FSIQ, within the normal range, has been associated in young children with a higher rate of psychiatric comorbidity in comparison to typically developing children.48 The subgroup of children and adolescents with narcolepsy and borderline intellectual functioning requires special attention in order to prevent the development of psychiatric symptoms in general and major depression in particular. The way in which narcolepsy influences cognitive functioning is unclear. The assumption is that neural mechanisms secondary to sleepiness could play a causal role.14 However, some studies have identified structural and functional neurocognitive changes in patients with narcolepsy that may point to mechanisms directly related to narcolepsy and its pathology.49,50
There are some limitations to this study that should be considered. First, because of the extensive battery of tests used in each patient, we were not able to include a comparison group. Comparisons are thus made with recent population figures in the literature. Second, applying strict DSM-IV criteria for the diagnosis of psychiatric comorbidities may have led us to underdiagnose milder yet nevertheless significant problems in some individuals. Third, the total number of children with nPHV narcolepsy was too small to establish any significant differences in psychiatric comorbidity and cognitive profile on a subgroup level. The strength of our study is the population-based design. In addition, we used validated diagnostic instruments and diagnoses were made according to the DSM-IV criteria.
Our study highlights the importance of a careful psychiatric and psychological follow-up of children and adolescents with narcolepsy. Potential psychiatric comorbidities need to be examined in detail in each patient and adequate treatment must be started as soon as possible in an effort to reduce disease burden. Cognitive difficulties must be detected and adequate support provided when necessary to promote the patients' everyday functioning, school performance, and mental health. An increased knowledge of psychiatric comorbidities and cognitive consequences in society and health care is important for the care of children and adolescents with narcolepsy. Further studies are needed to explore the consequences of narcolepsy in the daily life of childhood patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Special thanks to Anette Larsson, Veronica Hübinette, Anette Ekberg, and Camilla Mårtensson for their work with the data collection and Ulf Strömberg for the statistical analyses.
REFERENCES
- 1.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25:197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 2.De la Herrán-Arita AK, Kornum BR, Mahlios J, et al. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2014;5:216ra176. doi: 10.1126/scitranslmed.3007762. [DOI] [PubMed] [Google Scholar]
- 3.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14:488–92. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Fortuyn HA, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry. 2010;32:49–56. doi: 10.1016/j.genhosppsych.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Dahmen N, Becht J, Engel A, Thommes M, Tonn P. Prevalence of eating disorders and eating attacks in narcolepsy. Neuropsychiatr Dis Treat. 2008;4:257–61. [PMC free article] [PubMed] [Google Scholar]
- 7.Fortuyn HA, Lappenschaar GA, Nienhuis FJ, et al. Psychotic symptoms in narcolepsy: phenomenology and a comparison with schizophrenia. Gen Hosp Psychiatry. 2009;31:146–54. doi: 10.1016/j.genhosppsych.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Modestino EJ, Winchester J. A retrospective survey of childhood ADHD symptomatology among adult narcoleptics. J Atten Disord. 2013;17:574–82. doi: 10.1177/1087054713480033. [DOI] [PubMed] [Google Scholar]
- 9.Rogers A. Test of memory in narcoleptics. Sleep. 1990;13:42–52. [PubMed] [Google Scholar]
- 10.Naumann A, Bellebaum C, Daum I. Cognitive deficits in narcolepsy. J Sleep Res. 2006;15:329–38. doi: 10.1111/j.1365-2869.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 11.Delazer M, Hogl B, Zamarian L, et al. Executive functions, information sampling, and decision making in narcolepsy with cataplexy. Neuropsychology. 2011;25:477–87. doi: 10.1037/a0022357. [DOI] [PubMed] [Google Scholar]
- 12.Moraes M. Executive attention and working memory in narcoleptic outpatients. Arq Neuropsiquiatr. 2012;70:335–40. doi: 10.1590/s0004-282x2012005000007. [DOI] [PubMed] [Google Scholar]
- 13.Yoon SM, Joo EY, Kim JY, Hwang KJ, Hong SB. Is high IQ protective against cognitive dysfunction in narcoleptic patients? J Clin Neurol. 2013;9:118–24. doi: 10.3988/jcn.2013.9.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stores G, Montgomery P, Wiggs L. The psychosocial problems of children with narcolepsy and those with excessive daytime sleepiness of uncertain origin. Pediatrics. 2006;118:e1116–23. doi: 10.1542/peds.2006-0647. [DOI] [PubMed] [Google Scholar]
- 15.Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PloS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szakacs A, Darin N, Hallbook T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. 2013;80:1315–21. doi: 10.1212/WNL.0b013e31828ab26f. [DOI] [PubMed] [Google Scholar]
- 18.Dorris L, Zuberi SM, Scott N, Moffat C, McArthur I. Psychosocial and intellectual functioning in childhood narcolepsy. Dev Neurorehabil. 2008;11:187–94. doi: 10.1080/17518420802011493. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Sleep Medicine. Diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 20.Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–55. [PubMed] [Google Scholar]
- 21.World Health Organization. International classification of diseases (ICD) Retrieved 23 November 2010. Available from http://www.who.int/classifications/icd/en/
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- 23.Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. 1999;29:129–41. doi: 10.1023/a:1023040610384. [DOI] [PubMed] [Google Scholar]
- 24.DuPaul GJ, Power TJ, Anastopoulus AD, Reid R. ADHD rating scale-IV: checklists, norms, and clinical interpretation. New York, NY: Guilford Press; 1998. [Google Scholar]
- 25.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989:59–67. [PubMed] [Google Scholar]
- 26.Korkman M, Kirk U, Kemp SL. NEPSY II. Clinical and interpretative manual. San Antonio, TX: Psychological Corporation; 2007. [Google Scholar]
- 27.Wechsler D. WPPSI-III. Wechsler Preeschool and Primary Scale of Intelligence-Third Edition Manual. San Antonio, TX: The Psychological Corporation; 2004. (Swedish version 2005) [Google Scholar]
- 28.Wechsler D. WISC-IV. Wechsler Intelligence Scale for Children Manual Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. (Swedish version 2007) [Google Scholar]
- 29.Wechsler D. WAIS-IV. Wechsler Adult Intelligence Scale-Fourth Edition Manual. San Antonio, TX: The Psychological Corporation; 2008. (Swedish version 2010) [Google Scholar]
- 30.Cytel-Inc. Cambridge, MA: StatXact. http://www.cytel.com/software-solutions/statxact. [Google Scholar]
- 31.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population-and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275:172–90. doi: 10.1111/joim.12150. [DOI] [PubMed] [Google Scholar]
- 32.Olsson GI, von Knorring AL. Adolescent depression: prevalence in Swedish high-school students. Acta Psychiatr Scand. 1999;99:324–31. doi: 10.1111/j.1600-0447.1999.tb07237.x. [DOI] [PubMed] [Google Scholar]
- 33.Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: tracking the effects of adolescent sleep loss during the middle school years. Child Dev. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- 34.Kong L, Chen K, Tang Y, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci. 2013;38:417–22. doi: 10.1503/jpn.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–59. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 36.Kadesjo B, Gillberg C. Attention deficits and clumsiness in Swedish 7-year-old children. Dev Med Child Neurol. 1998;40:796–804. doi: 10.1111/j.1469-8749.1998.tb12356.x. [DOI] [PubMed] [Google Scholar]
- 37.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 38.Thomas RJ. Fatigue in the executive cortical network demonstrated in narcoleptics using functional magnetic resonance imaging--a preliminary study. Sleep Med. 2005;6:399–06. doi: 10.1016/j.sleep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–72. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canino G, Polanczyk G, Bauermeister JJ, Rohde LA, Frick PJ. Does the prevalence of CD and ODD vary across cultures? Soc Psychiatry Psychiatric Epidemiol. 2010;45:695–704. doi: 10.1007/s00127-010-0242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 42.Connor DF, SJ, McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J Dev Behav Pediatr. 2010;31:427–40. doi: 10.1097/DBP.0b013e3181e121bd. [DOI] [PubMed] [Google Scholar]
- 43.Smedje H, Broman JE, Hetta J, von Knorring AL. Psychometric properties of a Swedish version of the “Strengths and Difficulties Questionnaire.”. Eur Child Adolesc Psychiatry. 1999;8:63–70. doi: 10.1007/s007870050086. [DOI] [PubMed] [Google Scholar]
- 44.Svedin CG, Priebe G. The Strengths and Difficulties Questionnaire as a screening instrument in a community sample of high school seniors in Sweden. Nord J Psychiatry. 2008;62:225–32. doi: 10.1080/08039480801984032. [DOI] [PubMed] [Google Scholar]
- 45.Alloway TP. How does working memory work in the classroom? Educ Res Rev. 2006;1:134–9. [Google Scholar]
- 46.Smith K. Can we predict cognitive impairments in persons with narcolepsy. Loss Grief Care. 1992;5:103–13. [Google Scholar]
- 47.Naumann A, Daum I. Narcolepsy: pathophysiology and neuropsychological changes. Behav Neurol. 2003;14:89–98. doi: 10.1155/2003/323060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emerson E, Einfeld S, Stancliffe RJ. The mental health of young children with intellectual disabilities or borderline intellectual functioning. Soc Psychiatry Psychiatric Epidemiol. 2010;45:579–87. doi: 10.1007/s00127-009-0100-y. [DOI] [PubMed] [Google Scholar]
- 49.Naumann A, Bierbrauer J, Przuntek H, Daum I. Attentive and preattentive processing in narcolepsy as revealed by event-related potentials (ERPs) Neuroreport. 2001;12:2807–11. doi: 10.1097/00001756-200109170-00011. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz S, Ponz A, Poryazova R, et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131:514–22. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]


