Abstract
Study Objectives:
People with insomnia complain of cognitive deficits in daily life. Results from empirical studies examining associations between insomnia and cognitive impairment, however, are mixed. Research is needed that compares treatment-seeking and community-based insomnia study samples, measures subjective as well as objective cognitive functioning, and considers participants' pre-insomnia cognitive function.
Design and Participants:
We used data from the Dunedin Study, a representative birth cohort of 1,037 individuals, to examine whether insomnia in early midlife was associated with subjective and objective cognitive functioning. We also tested whether individuals with insomnia who reported seeking treatment for their sleep problems (treatment-seekers) showed greater impairment than other individuals with insomnia (non-treatment-seekers). The role of key confounders, including childhood cognitive ability and comorbid health conditions, was evaluated.
Measurements:
Insomnia was diagnosed at age 38 according to DSM-IV criteria. Objective neuropsychological assessments at age 38 included the WAIS-IV IQ test, the Wechsler Memory Scale, and the Trail-Making Test. Childhood cognitive functioning was assessed using the Wechsler Intelligence Scale for Children-Revised (WISC-R).
Results:
A total of 949 cohort members were assessed for insomnia symptoms and other study measures at age 38. Although cohort members with insomnia (n = 186, 19.6%) had greater subjective cognitive impairment than their peers at age 38, they did not exhibit greater objective impairment on formal testing. Treatment-seekers, however, exhibited significant objective impairment compared to non-treatment-seekers. Controlling for comorbidity, daytime impairment, and medications slightly decreased this association. Childhood cognitive deficits antedated the adult cognitive deficits of treatment-seekers.
Conclusions:
Links between insomnia and cognitive impairment may be strongest among individuals who seek clinical treatment. Clinicians should take into account the presence of complex health problems and lower premorbid cognitive function when planning treatment for insomnia patients.
Citation:
Goldman-Mellor S, Caspi A, Gregory AM, Harrington H, Poulton R, Moffitt TE. Is insomnia associated with deficits in neuropsychological functioning? Evidence from a population-based study. SLEEP 2015;38(4):623–631.
Keywords: insomnia, neuropsychological, cognitive
INTRODUCTION
Cognitive complaints among people suffering from insomnia are common. Individuals with insomnia frequently self-report experiencing memory and concentration problems in their daily lives.1,2 Because acute sleep deprivation is strongly associated with acute deficits in memory and attention, it is plausible that sleep loss due to chronic insomnia could lead to longer-term cognitive impairment.3–5 Furthermore, the hyperarousal theory of insomnia6 suggests that the disorder is accompanied by increased physiological activation, which has been linked to poorer cognitive functioning.7–9 Insomnia is the most common sleep disorder, affecting 10% to 20% of adults.10–13 The disorder's high prevalence means that any cognitive impairment associated with it could pose major public health, economic, and/or safety burdens.10–12 Indeed, individuals with insomnia experience significantly higher rates of motor-vehicle and other unintentional fatalities, non-motor-vehicle accidents, and poor work productivity.10,14,15 These findings, if causal, suggest that successfully treating sleep difficulties such as insomnia might be one strategy to reduce the rising population burden of cognition-related health risks.16,17
Empirical studies, however, have provided mixed evidence about the magnitude and functional significance of any differences in objective cognitive ability between individuals with versus those without insomnia. For example, a meta-analysis of studies using mostly small clinical or volunteer-based samples concluded that individuals with insomnia exhibited mild to moderate impairment on objective measures of memory, problem solving, and attentional processes, although they did not exhibit impaired functioning on other objective cognitive tasks.18 To our knowledge, only one study has used a population-based sample (a subset of adults aged > 20 years from the Penn State cohort) to examine this question.19 In contrast to findings from the meta-analysis, the Penn State cohort found that, overall, the presence of chronic insomnia was not associated with cognitive deficits—only insomniac individuals with objectively measured short sleep duration had any signs of impaired cognition.
Inconsistencies in this literature may be resolved, in part, by examining whether individuals with insomnia drawn from community-based samples and those who self-refer for clinical attention differ with respect to their cognitive impairment. These two groups may be different on a range of factors, including presence of psychiatric comorbidity, medical comorbidity, and severity of daytime impairment due to insomnia.13,20,21 All of these factors are known to influence health treatment-seeking decisions and to impair objectively measured cognitive functioning.13,22,23 Furthermore, objective neuropsychological test scores are imperfectly correlated with patients' subjective complaints about cognitive difficulties, making it useful to examine both outcomes. Objective testing is designed to measure an individual's maximum cognitive capacities under ideal performance conditions, whereas patients' complaints may reflect difficulties they encounter in everyday life, under less-than-ideal conditions.
Moreover, individual differences in objective cognitive functioning are known to emerge in childhood and be relatively stable over the lifecourse.24 It is therefore important to also consider individuals' childhood cognitive ability when considering whether a health problem such as insomnia has affected their adulthood ability, to rule out the possibility that premorbid cognitive differences may better explain any observed associations between the health problem and objective cognitive functioning in adulthood.25
In this study, we made use of a large, population-based, representative cohort of adults to test whether individuals diagnosed with insomnia manifest impaired cognitive functioning, measured using both subjective cognitive assessments as well as a battery of standardized neuropsychological tests. We then subdivided the insomnia cases into those who (a) reported seeking attention from a doctor or other professional for their sleep problems (henceforth labeled “treatment-seekers”) and those who (b) did not seek medical attention for their sleep problems (“non-treatment-seekers”). We compared cognitive functioning between these two subgroups to examine whether treatment-seeking status may account for the heterogeneity of results in the earlier literature. To assess whether key confounding factors explain any observed association between insomnia and cognitive functioning, we also sequentially controlled for concurrent psychiatric comorbidity, medical comorbidity, and daytime impairment due to insomnia. Lastly, we controlled for study members' childhood IQ to assess the role of premorbid differences in cognitive functioning.
METHODS
Sample
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (N = 1,037; 91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible for the longitudinal study based on residence in the province at age 3 and who participated in the first follow-up assessment at age 3. The cohort represents the full range of socioeconomic status in the general population of New Zealand's South Island and is primarily white. Assessments were carried out at birth and at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 95% of the 1,007 study members still alive took part. At each assessment wave, study members are brought to the research unit for a full day of interviews and examinations. The Otago Ethics Committee approved each phase of the study and informed consent was obtained.
Measures
Insomnia Diagnosis
At the age-38 assessment in 2010–2012, study members were asked how often they had problems sleeping in the past month because they could not get to sleep within 30 minutes, woke up in the middle of the night, woke up in the early morning, or felt that their sleep was unrefreshing.26 Individuals were diagnosed with insomnia if they reported having at least 1 of the 4 sleep difficulties ≥ 3 times per week, and also reported that their sleep problems affected their lives ≥ 3 times per week in at least one of the following domains: (a) work, (b) ability to concentrate, (c) memory, (d) daytime sleepiness, (e) levels of energy or fatigue, or (f) levels of irritability,26 or (g) staying awake while driving, eating, or engaging in social activity.27,28 This definition meets the primary diagnostic criteria for insomnia according to the then-current Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV); however, we did not impose the DSM-IV exclusionary criteria (e.g., presence of comorbid conditions).
Treatment-Seeking for Sleep Problems
At the age-38 assessment, study members were also asked whether there was any time during the past year when they wanted to talk to a doctor or other professional about their sleep difficulties, and whether or not they did so. Study members who met the diagnostic criteria for insomnia and who also reported talking to a doctor or other professional about their sleep problems were categorized as treatment-seeking. All other study members with insomnia were categorized as non-treatment-seeking. (One study member who met criteria for insomnia was missing information on treatment-seeking and was excluded from the analyses.)
Cognitive Functioning in Adulthood
We assessed study members' adulthood cognitive functioning in two ways: Subjective assessments, comprising self-reported cognitive difficulties and informant-reported cognitive difficulties, and objective assessments, comprising tests of study members' neuropsychological functioning. All measures were assessed at age 38 years.
For self-reported cognitive functioning, study members were queried about experiencing problems related to memory and attention. Study members reported how often in the past year (never, sometimes, or often) they experienced problems with, e.g., keeping track of appointments, remembering why they went to a store, and repeating the same story to someone, among other items. Scores on each of the 19 questions were summed to create an overall measure of cognitive difficulties (score range = 0 to 31; mean [standard deviation (SD)] = 9.1 [5.3]; internal consistency reliability = 0.83).
For informant reports, study members nominated people who knew them well. These informants were mailed questionnaires and asked to complete a checklist, including whether the study members had problems with their attention and memory over the past year (possible responses for each problem included “doesn't apply,” “applies somewhat,” or “certainly applies” to the study member).29 The informant-reported attention problems scale consisted of 4 items: The study member “is easily distracted, gets sidetracked easily,” “can't concentrate, mind wanders,” “tunes out instead of focusing,” and “has difficulty organizing tasks that have many steps” (range = 0 to 8; mean [SD] = 0.93 [1.21]; internal consistency reliability = 0.79). The informant-reported memory problems scale consisted of three items: “has problems with memory,” “misplaces wallet, keys, eyeglasses, paperwork,” and “forgets to do errands, return calls, pay bills” (range = 0 to 6; mean [SD] = 0.72 [0.91]; internal consistency reliability = 0.63).
For objective assessments of adulthood neuropsychological functioning, we used tests of IQ, executive function, learning and memory, and processing speed. Full-scale IQ and its component index scores for working memory, processing speed, perceptual reasoning, and verbal comprehension were measured using the WAIS-IV.30 These scores have standardized mean = 100 and SD = 15. Additional tests of learning and memory, executive function, and processing speed included the Rey Auditory Verbal Learning Test,31 subtests of the Wechsler Memory Scale-III,32 the Trail-Making Test,33 and subtests of the Cambridge Neuropsychological Test Automated Battery (CANTAB).34 For these tests, the sample is a representative birth cohort and therefore formed its own norms. Table S1 (supplemental material) provides further details about each test. All testing occurred in the morning in two 50-min counterbalanced sessions.
Full-scale IQ score was negatively correlated with self-reported cognitive difficulties (r = −0.152, P < 0.0001), informant-rated attention problems (r = −0.261, P < 0.0001), and informant-rated memory problems (r = −0.139, P < 0.0001).
Confounding Factors
Social class at age 38 was assigned based on the study member's current or most recent occupation.35 The scale places each occupation into one of six categories based upon the educational levels and income associated with that occupation in data from the New Zealand census. The scale ranges from 1 = unskilled laborer to 6 = professional. For study members not currently in the labor force, age 32 occupation was used (n = 37).
Psychiatric conditions were assessed at age 38 in private standardized interviews using the Diagnostic Interview Schedule,36 with diagnoses made following the then-current DSM-IV criteria.37 Psychiatric comorbidity was defined using an ordinal covariate that indexed how many of the following psychiatric diagnoses the study member received at age 38 (0, 1, 2, 3, or 4+): depression, generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), any fear or phobia (including panic disorder, social phobia, agoraphobia, and simple phobia), alcohol dependence, cannabis dependence, hard drug dependence, or research diagnoses of lifetime schizophrenia.38
Medical comorbidity was assessed using a variable that indexed how many physical health conditions the study member reported at age 38 (observed range: 0 to 4; mean [SD]: 0.62 [0.80]). Health conditions were chosen to include those that can predict both insomnia and cognitive impairment: asthma, obesity, hypertension, type 1 or 2 diabetes, arthritis, cancer, Crohn disease, hepatitis C, lupus, and multiple sclerosis. Study members' asthma was defined by a positive response to “Has a doctor ever told you that you have asthma?” as well as self-reported presence of asthma symptoms in the past year (including recurrent wheezing, asthma attack, or treatment for asthma). Obesity was defined as a body mass index (BMI; kg/m2) ≥ 30; study members' heights were measured using a portable Harpenden Stadiometer (Holtain, Crymych, UK) and their weights were recorded to the nearest 0.1 kg using calibrated scales. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg.8,39 Diabetes was defined by HbA1c values ≥ 6.5%.40 Presence of other conditions was self-reported.
Medication use was measured with 2 variables. Psycho-tropic medication use indexed whether the study member was currently taking any antianxiety, antipsychotic, antidepressant, antihistamine, or attention deficit hyperactivity disorder medication (queried on the day of the study assessment at age 38). These medications are known to impair cognitive functioning and may also interfere with sleep. Sleep medication use was assessed separately as part of the age-38 sleep interview. Study members were asked “In the past month, how often have you taken medicine (prescribed or over-the-counter) to help you sleep?” Those who reported taking such medicine at least once a week were classified as currently using sleep medication.
Daytime impairment due to insomnia was indexed using a score of how many domains of persistent non-cognitive impairment study members experienced as a result of their sleep problems (i.e., their ability to work, daytime sleepiness, levels of energy or fatigue, level of irritability, or ability to stay awake while driving, eating, or engaging in social activity; range = 0 to 5; mean [SD] = 0.40[0.93]).41
Study members' cognitive functioning in childhood was measured using their full-scale IQ scores, assessed at ages 7, 9, and 11 years using the Wechsler Intelligence Scale for Children-Revised (WISC-R).42 Scores were averaged across the 3 assessments, with a mean of 100 and a standard deviation of 15.
Statistical Analysis
We used linear regression analyses to model the relationship between insomnia and subjective and objective measures of study members' cognitive functioning, all at age 38. Subjective cognitive functioning outcome variables included scores on the self-reported cognitive difficulties scale and the 2 informant-reported cognitive (memory and attention) difficulties scales. Objective cognitive functioning outcome variables included scores on the WAIS-IV IQ and the other tests of executive function, memory, and processing speed. This first analysis controlled for study members' sex and age-38 socioeconomic status (SES).
We then excluded all those without insomnia from the analyses, and examined whether treatment-seeking (yes vs. no) predicted cognitive performance scores among study members with insomnia. Model 1 for this subgroup analysis controlled for sex and SES. Model 2 additionally controlled for psychiatric comorbidity, medical comorbidity, and medication use at age 38. Model 3 additionally controlled for daytime impairment due to insomnia. Lastly, Model 4 included all covariates from Models 1–3 and added a control for study members' childhood cognitive functioning. All statistical analyses were conducted using Stata 12.0 (StataCorp LP).
RESULTS
Of the 949 Dunedin cohort members assessed at age 38, nearly one-fifth (19.6%, n = 186) met diagnostic criteria for insomnia. Of the n = 185 study members with insomnia and information on treatment-seeking, 79.5% (n = 147; 15.5% of the cohort) did not report seeking any treatment for their sleep problems in the past year; the remaining 20.5% (n = 38; 4.0% of the cohort) reported seeking treatment from a doctor or other professional for their sleep problems.
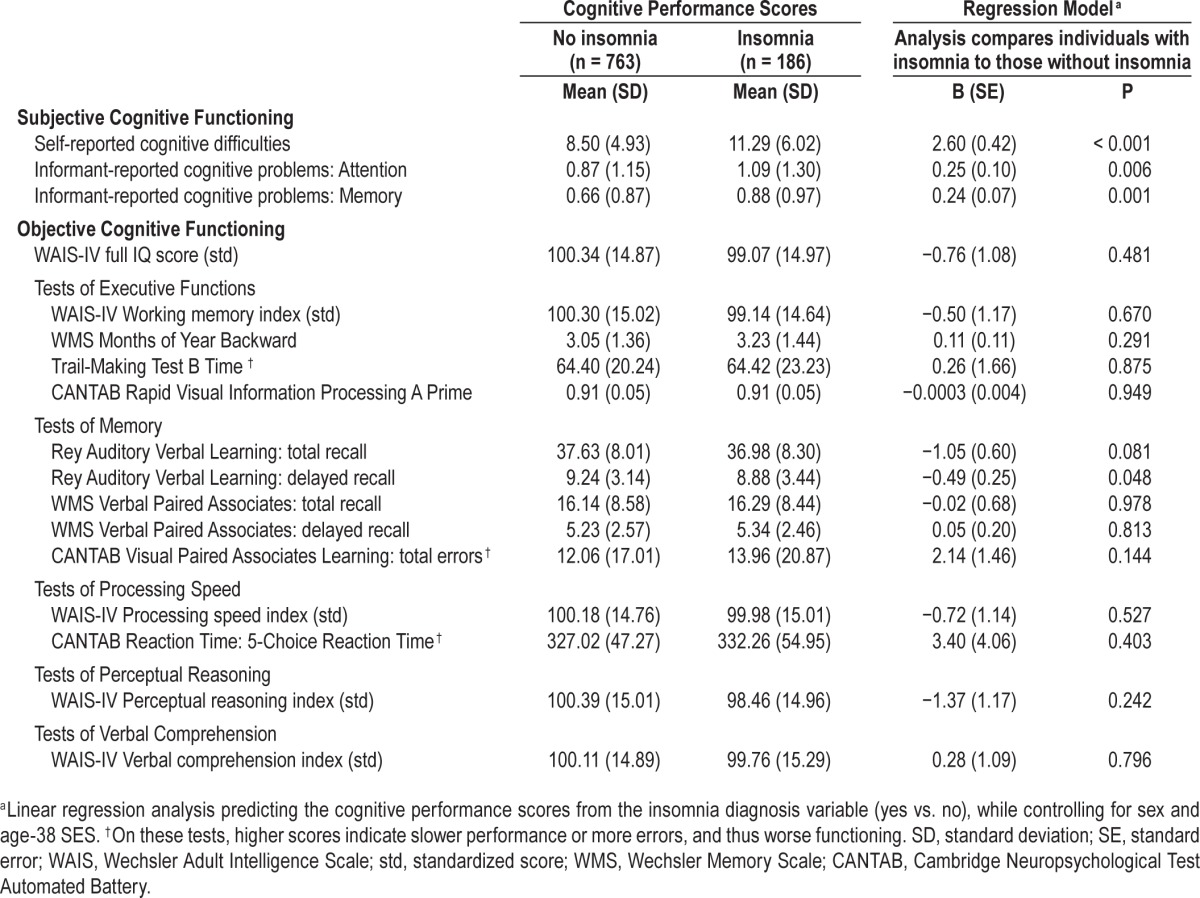
We first examined whether study members diagnosed with insomnia evidenced subjective and objective cognitive impairment compared to good sleepers, controlling for sex and SES (Table 1). Study members with insomnia scored higher on all 3 measures of subjective cognitive impairment (all P < 0.01), indicating that both the insomniac study members themselves, as well as their informants, believed that their memories and attention were worse than those of good sleepers. However, these results were not corroborated by their performance on objective measures of cognitive function. Scores on the full-scale IQ test, IQ subtests, Wechsler Memory Scale, Trail-Making Test, and the CANTAB were statistically and substantively indistinguishable between study members with and without insomnia (all P > 0.10). The only exception was the Rey Auditory Verbal Learning delayed recall memory task, on which the insomnia group performed slightly but significantly worse compared to good sleepers (P < 0.05).
Table 1.
Subjective and objective cognitive functioning at age 38 in the Dunedin Study: Descriptive statistics and results of regression analyses comparing good sleepers to individuals with insomnia.
We then examined whether cognitive functioning among treatment-seeking study members with insomnia differed from that of non-treatment-seeking study members with insomnia. Columns 2 and 3 in Table 2 show mean scores on all cognitive functioning measures in these 2 insomnia subgroups. Results (Table 2, Model 1) show that after controlling for sex and SES, treatment-seekers scored higher than non-treatment-seekers on the 3 subjective measures of cognitive problems: Self-reported cognitive difficulties (B = 2.12, P = 0.055), informant-reported attention problems (B = 0.74, P = 0.002), and informant-reported memory problems (B = 0.37, P = 0.036).
Table 2.
Subjective and objective cognitive functioning at age 38 among the subset of cohort members with insomnia: Descriptive statistics and results of regression analyses comparing non-treatment-seeking individuals to treatment-seeking individuals.
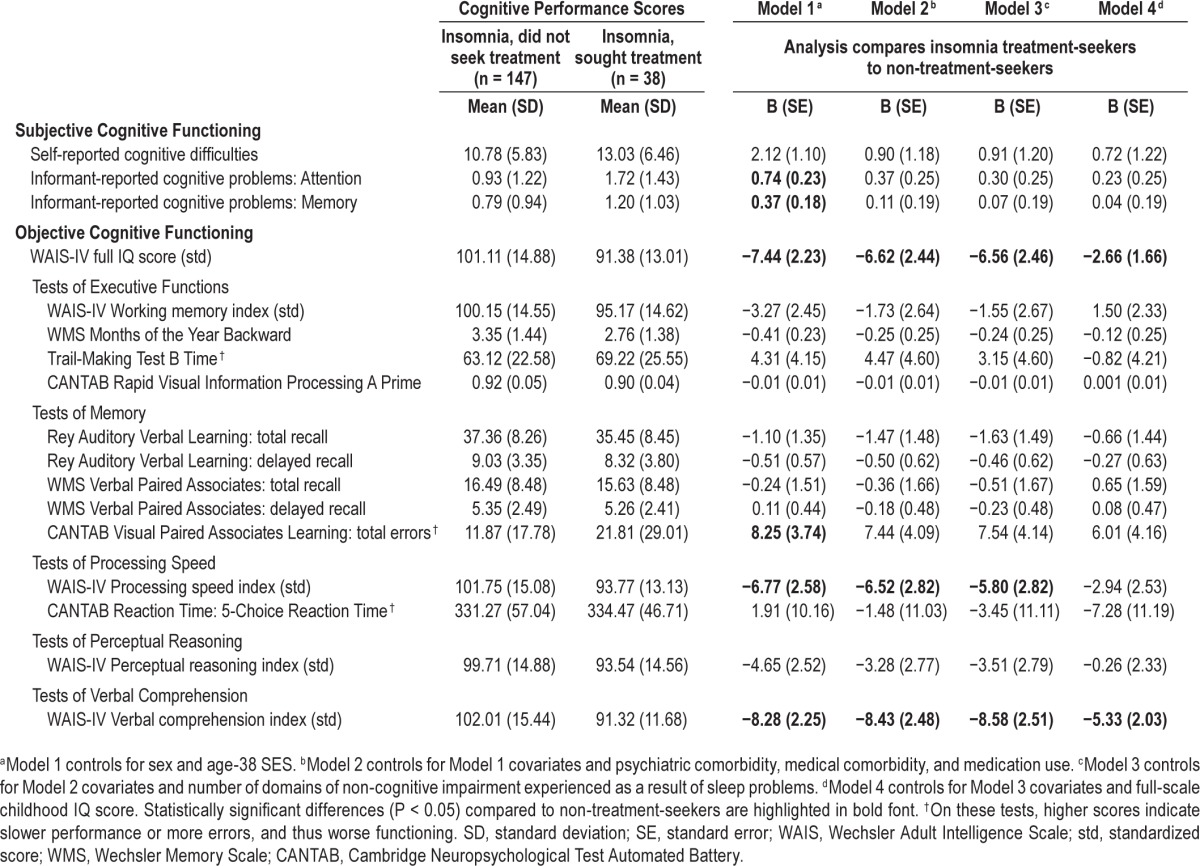
The treatment-seeking insomnia group also evidenced statistically greater cognitive impairment on several objective measures of functioning. Their average unadjusted full-scale IQ score was 10 points lower than non-treatment-seekers (mean = 91.4 vs. mean = 101.1; Model 1 B = −7.44, P = 0.001). Treatment-seekers did not exhibit impaired function on tasks related to executive functioning and memory, with the exception of the CANTAB Visual Paired Associates Learning task. On this test of episodic memory and new learning, treatment-seekers made nearly twice as many errors compared to non-treatment-seekers (unadjusted means = 21.8 vs. 11.9; Model 1 B = 8.25, P = 0.029). Treatment-seekers scored more poorly on the WAIS-IV processing speed index (unadjusted means = 93.8 vs. 101.8, Model 1 B = −6.77, P = 0.000) but not on the CANTAB reaction time test (another measure of processing speed). On the tasks related to perceptual reasoning, treatment-seekers scored 6 IQ points lower than non-treatment-seekers, but this analysis did not reach conventional levels of statistical significance (Model 1 B = −4.74, P = 0.07). Lastly, treatment-seekers' scores on the verbal comprehension index were nearly 11 points lower than non-treatment-seekers' (un-adjusted means = 92.3 vs. 102.0; Model 1 B = −8.28, P < 0.001).
As noted previously, treatment-seeking individuals with insomnia may differ from those who do not seek treatment on a range of factors, including psychiatric and medical comorbidity, medication use, and daytime impairment. Table 3 shows descriptive statistics for these variables among both treatment-seeking and non-treatment-seeking Dunedin study members with insomnia (as well as in good sleepers, for purposes of comparison). When compared to non-treatment-seekers, treatment-seekers had significantly higher levels of psychiatric comorbidity (z = 4.43, P < 0.001), increased rates of psychotropic and sleep medication use (both P < 0.001), and more domains of non-cognitive impairment arising from their insomnia (z = 2.54, P = 0.011). Treatment-seekers also reported experiencing marginally more medical comorbidity (z = 1.80, P = 0.071).
Table 3.
Demographic characteristics, comorbidity, daytime impairment, and childhood cognitive functioning among the full Dunedin cohort as well as three cohort subgroups: individuals without insomnia, individuals with insomnia who did not seek treatment for their sleep problems, and individuals with insomnia who sought treatment for their sleep problems.
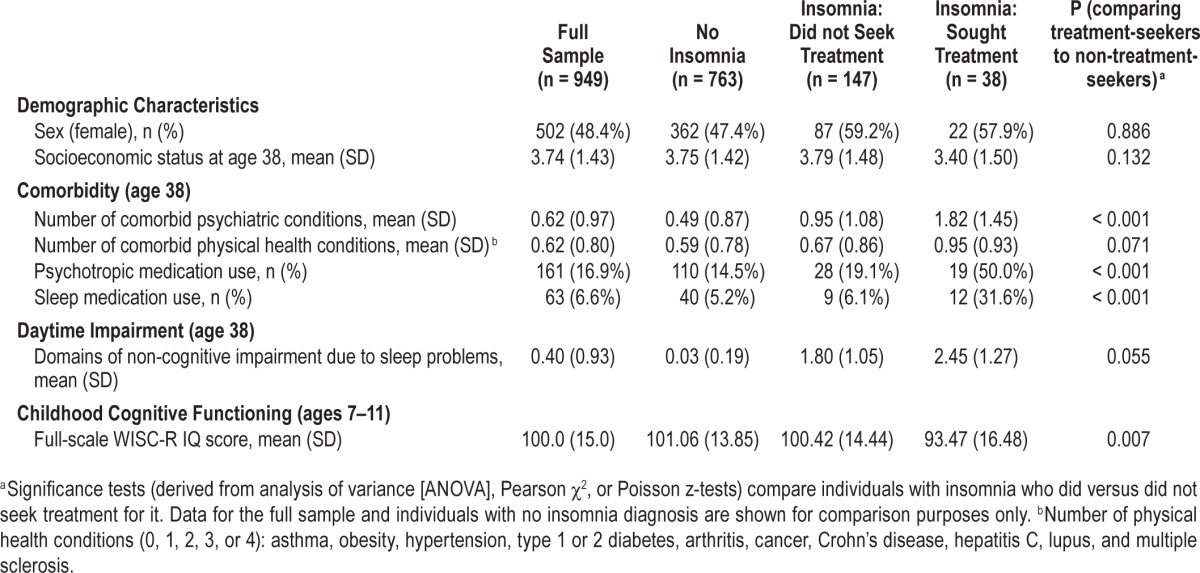
Table 2 results for Models 2 and 3 show that controlling for comorbidity, medication use, and daytime impairment completely explained group differences in subjective cognitive functioning. These factors also partially accounted for differences in objective measures of cognitive functioning, although the effect estimates for treatment-seeking remained large and statistically significant in most cases. We calculated the percent reduction in effect estimate magnitudes, averaging across all objectively measured cognitive performance tests from Model 1 to Model 3; the mean reduction in magnitude was 44%. Thus, while higher levels of comorbidity, medication use, and daytime impairment account for a proportion of the objective cognitive impairment observed from testing treatment-seekers, these factors do not explain the entire association.
Table 3 also indicated that treatment-seekers had lower IQ scores in childhood compared to non-treatment-seekers (mean = 93.47 vs. mean = 100.42, P = 0.007). In analyses additionally controlling for this childhood IQ variable (Table 2, Model 4), effect estimates of treatment-seeking status on adulthood cognitive functioning were substantially reduced and became nonsignificant, except in the case of verbal comprehension. Figure 1 depicts childhood and adulthood full-scale IQ scores for treatment-seekers, non-treatment-seekers, and cohort members without insomnia. The figure shows that study members who sought treatment for insomnia exhibited impaired cognitive functioning in adulthood largely because they started out life already suffering from cognitive deficits. Lastly, we wanted to ensure that our results were not confounded by study members' sleep problems earlier in life, because children who sleep poorly may not do well on cognitive testing. As children aged 5 to 9 years, a total of 106 study members (who took part in the age-38 assessment) had been reported by their mothers as having persistent sleep problems (16.2% of treatment-seekers [n = 6], 9.4% of non-treatment-seekers [n = 13], and 12.3% of cohort members without insomnia [n = 87]).43 We re-estimated each model in Tables 1 and 2 with an additional covariate indexing the presence of persistent childhood sleep problems; results were largely unchanged (see Tables S2 and S3, supplemental material).
Figure 1.
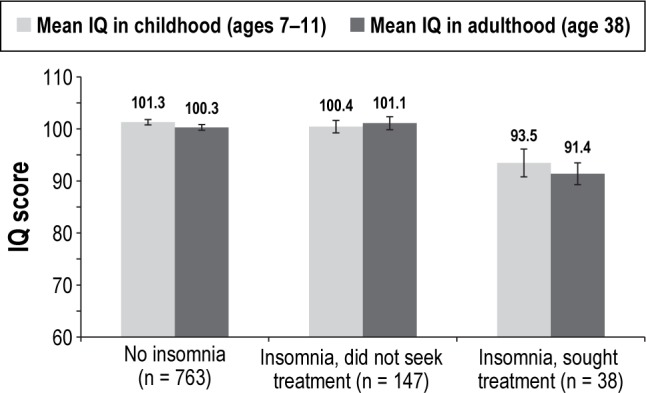
Mean full-scale IQ scores in childhood and adulthood, by cohort members' insomnia diagnosis and treatment-seeking status. Error bars represent standard error of the mean.
DISCUSSION
Findings from cross-sectional study designs have suggested, albeit inconsistently, that individuals with insomnia suffer from impairments in cognitive functioning.18 Some data from the United States and elsewhere show that the population incidence of sleep problems, including insomnia symptoms, is rising with societal changes such as increasing rates of shift work and nighttime use of television and Internet-enabled devices.44–46 If insomnia does lead to cognitive deficits, these reports raise the possibility that insomnia-related cognitive impairment—along with its associated threats to public health, such as unintentional injuries—will increase in the coming decades.
However, data from our population-based cohort of 1,000 adults suggest that at the community level insomnia is not associated with deficits in cognitive functioning. Although individuals with insomnia (and people who knew those study members well) believed they suffered from significant memory and attention problems, this belief was not confirmed by impairment on objective measures of overall IQ, executive functioning, memory, processing speed, perceptual reasoning, or verbal comprehension. We see two possible explanations for this inconsistency. On the one hand, it is already well-established that discrepancies exist between objective and subjective functioning among certain people with insomnia—for example, it has been repeatedly demonstrated that some individuals with insomnia perceive their average nightly sleep duration to be significantly shorter than it is when measured objectively using polysomnography.47,48 Discrepancies between objective and subjective functioning in insomnia may therefore extend to cognitive capacities, at least among the general population. On the other hand, the inconsistency between objective and subjective performances may emerge from fundamental differences in measurement context. While our neuropsychological testing sessions are designed to ensure that an individual can perform to his or her maximum ability (i.e., in a quiet room with no distractions), the study members' self- and informant reports reflect everyday circumstances in which the study member may be fatigued, upset, or overwhelmed by multitasking. Our results provide reassuring evidence that insomnia does not lead to gross neuropsychological damage. However, insomnia sufferers may notice that their attention and memory capacities deteriorate more acutely in situations where they are already cognitively vulnerable.
Our finding that individuals with insomnia experienced worse subjective, but not objective cognitive functioning, is also in accordance with Harvey's cognitive model of insomnia.49 This model suggests that insomnia sufferers may be excessively concerned about the consequences of their disorder, mistakenly believing that it will result in multiple problems in functioning. Insomnia sufferers may also adopt behavioral techniques like imagery control to ameliorate their perceived functional problems; however, these techniques can result in anxiety which in turn causes objective difficulties in daytime functioning.49
Despite the absence of objective neuropsychological deficits among all study members with insomnia, we did find that a key subgroup—those who sought clinical treatment for their sleep problems—exhibited impaired cognition when compared to non-treatment-seeking study members with insomnia. Our finding that only a certain subset of those with insomnia showed poorer neuropsychological functioning is consistent with other literature on this topic. For example, in another population-based study, Fernandez-Mendoza et al. found that those with insomnia and objectively measured short sleep duration showed neuropsychological deficits, whereas those with insomnia and normal length sleep did not.19 Similarly, Edinger et al. found that only insomnia-diagnosed individuals with long sleep latency (a measure of physiological hyperarousal) experienced deficits in neuropsychological functioning.50 Harvey's cognitive model could also be implicated: The treatment-seeking subgroup of our study members may have been particularly concerned about the impact of their symptoms, culminating in worse neuropsychological impairment.
In our study, a remarkable 80% of adults reporting insomnia with significant life impairment had not consulted a doctor (even in New Zealand, which has a national health-care system). The 20% who were treatment-seekers tended to have other psychiatric and medical conditions, indicating that they were likely already engaged with medical specialists; this engagement may have facilitated their asking for help with insomnia. These figures reflect a high level of potential service need among community members suffering from insomnia. Additional efforts to raise awareness about sleep-problem treatment options and opportunities for reducing the public health burden of insomnia may be warranted.
Treatment-seeking study members' objective cognitive impairments were observed in their full-scale IQ scores as well as their scores on specific aspects of verbal comprehension, memory, and processing speed. These specific domains of impairment, particularly memory and processing speed, are consistent with those found in previous studies.18,19 Mental abilities of working memory and processing speed are often termed “fluid” mental abilities,51 because they are known to be easily disrupted by acute factors such as illness and infection, intoxication, or severe fatigue. Here insomnia is also implicated. In contrast, verbal comprehension is termed a “crystallized” ability, which is usually established in childhood-adolescence and is difficult to disrupt thereafter, remaining stable into late life. Treatment-seekers' deficit in verbal comprehension may reflect, in part, a pre-insomnia level of cognitive vulnerability.
Our study adds to the literature by showing that individuals who seek treatment for insomnia in adulthood already evinced diminished cognitive capacities in childhood. Premorbid differences in childhood cognitive functioning explained the vast majority of these treatment-seeking study members' deficits in adulthood cognitive functioning. These results suggest that treating insomnia patients for their sleep problems is not likely to alleviate their longstanding cognitive problems. However, if clinicians can take insomnia patients' cognitive problems into account when planning treatment, therapeutic outcomes may improve. We observed that individuals in the treatment-seeking insomnia group had a mean IQ of 91, indicating that the individual's ability to process information is weaker than that of 73% of the population.52 This degree of cognitive deficit may warrant some adaptation of clinical treatment.53–57 Harvey and colleagues noted in a recent review that specific cognitive support strategies may enhance patients' memory for the contents of therapy and their adherence to a treatment plan.53 Although cognitive behavioral therapy for insomnia (CBT-I) has already been shown to be effective,58,59 implementing such cognitive support strategies may improve adoption and success rates for CBT-I.
Although treatment-seekers also exhibited higher levels of psychiatric and medical comorbidity, medication use, and daytime impairment, accounting for these three factors only partially explained treatment seekers' relative cognitive deficits. It is widely acknowledged that cases of psychological disorder observed in clinical patient samples tend to be of longer duration and greater severity than cases observed in general population samples.21 Our finding that objective cognitive impairment is observed only among the treatment-seeking subgroup is in accordance with this principle. Our study also underscores, however, that clinicians need to be aware that their insomnia patients may be struggling with neuropsychological impairment (as well as complex health conditions), and that they should factor that possibility into their clinical evaluations. Patients may feel validated if clinicians acknowledge that patients in treatment for insomnia often struggle with problems of attention and memory.2,18 Certain cognitive abilities—e.g., working memory and problem solving—are necessary for carrying out complex tasks, and even mild impairment of these abilities may compromise individuals' safety and work performance.
Methodological advantages of the current study include its use of a population-representative birth cohort, a rigorous operational definition of insomnia, a comprehensive battery of standard neuropsychological tests, and the inclusion of multiple raters as well as important confounding factors (such as comorbidity and medication use). The study was also strengthened by our measure of childhood IQ scores, which allowed us to examine the role of study members' premorbid cognitive functioning when assessing the link between insomnia and cognitive functioning in adulthood.
There were also several limitations to this study. First, objective measures of our study members' sleep habits (such as overnight sleep studies), as well as information on the presence of sleep disorders such as narcolepsy or sleep apnea, were not available and thus could not be incorporated into our analyses. Additionally, our insomnia assessment did not include queries about the frequency of insomnia episodes (e.g., number of episodes in the past year), duration of current insomnia symptoms, or the age of onset of study members' insomnia symptoms. We therefore could not assess whether study members with longer-standing insomnia symptoms manifested greater cognitive impairment.19 Furthermore, our categorization of study members with insomnia into treatment-seekers vs. non-treatment-seekers is based on self-report, and thus differs from previous studies using insomnia patients who sought treatment in a clinic. Finally, our data were right-censored at age 38, and the relation between insomnia and neuropsychological function may differ in older groups.60
Many adults reporting diagnosable insomnia likewise report cognitive complaints, but these cognitive complaints are confirmed by formal neuropsychological testing only in the smaller subset who are seeking a doctor's help for insomnia. This insight may help to resolve inconsistent findings in the literature on insomnia and cognition. It is doubtful that insomnia causes cognitive decline in midlife adults; in our cohort of 38-year-olds, the cognitive status of treatment-seeking individuals had remained below the population average since their childhood. Regardless of causality, clinicians treating individuals with insomnia have the opportunity to acknowledge their patients' cognitive complaints and address them. Clinicians also have the opportunity to enhance the effectiveness of insomnia treatment for cognitively impaired patients if therapeutic communications are tailored to compensate for patients' compromised verbal comprehension, slower mental processing speed, and impaired memory capacity. Doing so may help to improve patients' understanding and implementation of insomnia treatment, relieve their sleep problems, and reduce insomnia-related functional impairment.
DISCLOSURE STATEMENT
This was not an industry supported study. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research received support from U.S. National Institute of Aging grant AG032282 and U.K. Medical Research Council grant MR/K00381X. Additional support was provided by the Jacobs Foundation. Goldman-Mellor is supported by a postdoctoral fellowship provided by the National Institute of Child Health and Human Development (T32-HD07376) through the Center for Developmental Science, University of North Carolina at Chapel Hill. The study protocol was approved by the institutional ethical review boards of the participating universities. Study members gave informed consent before participating. The authors have indicated no financial conflicts of interest. The work was performed at Duke University, Durham, NC. All authors contributed to the analysis of data and drafting of this manuscript.
ACKNOWLEDGMENTS
The authors thank the Dunedin Study members, their families, Unit research staff, and Study founder Phil Silva.
SUPPLEMENTAL MATERIAL
Neuropsychological tests administered in adulthood (age 38) in the Dunedin Study.
Subjective and objective cognitive functioning at age 38 in the Dunedin Study: Descriptive statistics and results of regression analyses comparing good sleepers to individuals with insomnia, with additional control for persistent childhood sleep problems.
Subjective and objective cognitive functioning at age 38 among the subset of cohort members with insomnia: Descriptive statistics and results of regression analyses comparing non-treatment-seeking individuals to treatment-seeking individuals.
REFERENCES
- 1.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey II. Sleep. 1999;22(Suppl 2):S354–8. [PubMed] [Google Scholar]
- 2.Fortier-Brochu É, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep. 2014;37:1787–98. doi: 10.5665/sleep.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo S-S, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 5.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–29. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–53. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Coulouvrat C, et al. Insomnia and the performance of U.S. workers: results from the America Insomnia Survey. Sleep. 2011;34:1161–71. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS) Sleep. 2011;34:997–1011. doi: 10.5665/SLEEP.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the National Comorbidity Survey Replication. Biol Psychiatry. 2006;60:1364–71. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10:427–38. doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Laugsand LE, Strand LB, Vatten LJ, Janszky I, Bjorngaard JH. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37:1777–86. doi: 10.5665/sleep.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandi-Perumal SR, Verster JC, Kayumov L, et al. Sleep disorders, sleepiness and traffic safety: a public health menace. Brazilian J Med Biol Res. 2006;39:863–71. doi: 10.1590/s0100-879x2006000700003. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Global status report on road safety 2013: supporting a decade of action. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 18.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16:83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22(Suppl 2):S359–65. [PubMed] [Google Scholar]
- 21.Cohen P, Cohen J. The clinician's illusion. Arch Gen Psychiatry. 1984;41:1178–82. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- 22.Raimo EB, Daeppen J, Smith TL, Danko GP, Schuckit MA. Clinical characteristics of alcoholism in alcohol-dependent subjects with and without a history of alcohol treatment. Alcohol Clin Exp Res. 1999;23:1605–13. [PubMed] [Google Scholar]
- 23.Goldberg D, Huxley P. Mental illness in the community: the pathway to psychiatric care. New York: Tavistock Press; 1981. [Google Scholar]
- 24.Deary IJ. Intelligence: a very short introduction. Oxford: Oxford University Press; 2001. [Google Scholar]
- 25.Belsky DW, Caspi A, Goldman-Mellor S, et al. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Am J Epidemiol. 2013;178:1461–8. doi: 10.1093/aje/kwt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, Hall M. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5:41–51. [PMC free article] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Goldman-Mellor S, Gregory AM, Caspi A, et al. Mental health antecedents of early midlife insomnia: evidence from a four-decade longitudinal study. Sleep. 2014;37:1767–75. doi: 10.5665/sleep.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci. 2012;109:E2657–64. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler adult intelligence scale–fourth edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 31.Lezak MD. Neuropsychological assessment. Oxford University Press; 2004. [Google Scholar]
- 32.Wechsler D. Wechsler memory scale. 3rd edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 33.Army Individual Test Battery. Manual of directions and scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 34.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399. [PMC free article] [PubMed] [Google Scholar]
- 35.Milne BJ, Byun U, Lee A. New Zealand socio-economic index 2006. Wellington, NZ: Statistics New Zealand; 2013. [Google Scholar]
- 36.Robins LN, Cottler L, Bucholz KK, Compton W. Diagnostic Interview Schedule for DSM–IV. St. Louis, MO: Washington University School of Medicine; 1995. [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 38.Meier MH, Caspi A, Reichenberg A, et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171:91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James PA, Oparil S, Carter B, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee. JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 40.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Academy of Sleep Medicine. International classification of sleep disorders, revised. Chicago, IL: American Academy of Sleep Medicine; 1997. [Google Scholar]
- 42.Wechsler D. Manual for the Wechsler intelligence scale for children, revised. Psychological Corporation; 1974. [Google Scholar]
- 43.Gregory AM, Caspi A, Eley TC, Moffitt TE, O'Connor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33:157–63. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- 44.Ferrie JE, Kumari M, Salo P, Singh-Manoux A, Kivimaki M. Sleep epidemiology--a rapidly growing field. Int J Epidemiol. 2011;40:1431–7. doi: 10.1093/ije/dyr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975-2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kronholm E, Partonen T, Laatikainen T, et al. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: a comparative review and re-analysis of Finnish population samples. J Sleep Res. 2008;17:54–62. doi: 10.1111/j.1365-2869.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manconi M, Ferri R, Sagrada C, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010;19:478–86. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 49.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 50.Edinger JD, Means MK, Krystal AD. Does physiological hyperarousal enhance error rates among insomnia sufferers? Sleep. 2013;36:1179–86. doi: 10.5665/sleep.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 52.Sattler JM, Ryan JJ. Assessment with the WAIS-IV. Jerome M. Sattler Publisher; 2009. [Google Scholar]
- 53.Harvey AG, Lee J, Williams J, et al. Improving outcome of psychosocial treatments by enhancing memory and learning. Perspect Psychol Sci. 2014;9:161–79. doi: 10.1177/1745691614521781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottfredson LS. Why g matters: the complexity of everyday life. Intelligence. 1997;24:79–132. [Google Scholar]
- 55.Wild J, Gur RC. Verbal memory and treatment response in post-traumatic stress disorder. Br J Psychiatry. 2008;193:254–5. doi: 10.1192/bjp.bp.107.045922. [DOI] [PubMed] [Google Scholar]
- 56.Aharonovich E. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–11. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers M. Patient recall of recommendations in the behavioural treatment of insomnia. Sleep Res. 1991;20:222. [Google Scholar]
- 58.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 60.Jelicic M, Bosma H, Ponds RWHM, Van Boxtel MPJ, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuropsychological tests administered in adulthood (age 38) in the Dunedin Study.
Subjective and objective cognitive functioning at age 38 in the Dunedin Study: Descriptive statistics and results of regression analyses comparing good sleepers to individuals with insomnia, with additional control for persistent childhood sleep problems.
Subjective and objective cognitive functioning at age 38 among the subset of cohort members with insomnia: Descriptive statistics and results of regression analyses comparing non-treatment-seeking individuals to treatment-seeking individuals.


