Abstract
Objective
The present study tested the independent and interactive contributions of the somatosensory component of pain (pain intensity) and the affective component of pain (pain unpleasantness) on emotional, social, and daily functioning in chronic pain patients.
Subjects
Participants were 472 patients seeking treatment for chronic orofacial pain. Mean age of the sample was 46.0 years (standard deviation [SD] = 14.67, range 18–78), with 82.2% female. Average pain duration at the time of initial appointment was 75.7 months (SD = 106.66).
Methods
Participants completed self-report measures of pain intensity, unpleasantness, and functional outcomes at the time of their first appointment. These data were later extracted from participant’s de-identified medical records. Multivariate linear regression was used to test the interaction of pain intensity and unpleasantness on outcome measures of emotional, social, and daily functioning.
Results
Results revealed that pain intensity contributed to poorer functional outcomes but higher levels of social support even after controlling for pain unpleasantness. After controlling for pain intensity, unpleasantness was associated with higher pain interference and affective distress. There was also pain intensity by unpleasantness interaction on pain interference. Specifically, at lower levels of pain unpleasantness, changes in pain intensity produced greater changes in pain interference than they did at higher levels of pain unpleasantness.
Conclusions
Results suggest that both intensity and unpleasantness contribute unique variance to functional outcomes. The results highlight the importance of interventions that not only try to reduce pain levels but also reduce levels of pain unpleasantness.
Keywords: Pain Intensity, Pain Unpleasantness, Functional Outcomes, Chronic Orofacial Pain
Introduction
Pain has a somatosensory and an affective component [1,2]. The somatosensory component is responsible for determining the location and intensity of pain, whereas the affective component is responsible for producing the unpleasant affective reactions experienced before, during, and after a real or imagined pain experience [1–5]. These two components are thought to be at least partially independent of one another and participants are able to validly distinguish them in laboratory studies [5–7]. In a seminal study, hypnotic suggestion was successfully used to alter the affective component as indexed by pain unpleasantness, but not the somatosensory component as indexed by pain intensity [3]. Further, experiencing social rejection and empathy activated the neural pathways associated with affective but not somatosensory pain networks [8], and induction of negative vs neutral mood increased unpleasantness while leaving somatosensory aspects unaltered [9,10]. Both components work together to produce adaptive outcomes to acute pain. Localized, high-intensity pain serves to protect an injured site from further aggravation and promotes rest and recovery [11]. By having a greater unpleasant affective response to the pain, people are more likely to draw the support of others [12] and are more likely to be careful the next time they encounter a similar situation, for example [11].
However, in chronic pain, unlike in acute pain, intensity and unpleasantness may no longer promote adaptive outcomes like rest and recovery. In fact, higher pain intensity in chronic pain has been associated with greater psychological distress, fatigue, and dysfunction [13,14]. Pain unpleasantness has also been linked cross-sectionally to depression and reductions in everyday activity in chronic pain patients [15]. In the fear-avoidance model of pain, pain unpleasantness produces fear of subsequent pain experiences, which leads to avoidance of activities, generating a downward spiral of pain, fear, and avoidance [15]. Interestingly, however, no study to our knowledge has tested the unique contributions of the somatosensory and affective components to functional outcomes in chronic pain patients.
The current study explored how self-reported pain intensity and unpleasantness interacted to predict social, emotional, and daily functioning in a sample of chronic orofacial pain patients. These are important domains to assess because they reflect how well pain patients are able to live their lives in the face of pain. Based on extant research, it was hypothesized that pain intensity would predict worse functioning on all domains. Additionally, it was hypothesized that pain unpleasantness would predict worse functioning above and beyond pain intensity on domains that assessed avoidance of situations, based on the fear-avoidance model. It is our hope that increasing the information about the somatosensory and affective contributions to functional outcomes sets the groundwork for future studies and interventions aimed at reducing dysfunction and improving quality of life in chronic pain patients.
Methods
Participants
Data were obtained from the records of 472 patients being seen for initial examination at an orofacial pain center between 2008 and 2012. Only participants experiencing pain for longer than 3 months were included. All participants were diagnosed by a licensed dentist based on the guidelines from the American Academy of Orofacial Pain, 5th edition [16]. Primary diagnoses were muscle pain (37.0%), joint pain (24.6%), neuropathic pain (15.1%), primary headache pain (5.2%), and other (i.e., dental pain, and no diagnosis; 18.1%). Seventy-eight percent of patients also had a secondary orofacial pain diagnosis. Average pain duration at the time of initial appointment was 75.7 months (standard deviation [SD] = 106.66). Table 1 presents age and gender information.
Table 1.
Descriptive statistics and bivariate correlations among study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Female gender | 1 | ||||||||||||
| 2. Age | 0.02 | 1 | |||||||||||
| 3. Pain intensity | −0.01 | 0.02 | 1 | ||||||||||
| 4. Pain unpleasantness | 0.01 | −0.04 | 0.75** | 1 | |||||||||
| 5. Pain interference | 0.02 | −0.01 | 0.60** | 0.59** | 1 | ||||||||
| 6. Support | −0.01 | −0.10* | 0.30** | 0.23** | 0.32** | 1 | |||||||
| 7. Pain severity | 0.00 | −0.03 | 0.79** | 0.73** | 0.70** | 0.32** | 1 | ||||||
| 8. Life control | 0.01 | −0.05 | −0.18** | −0.14** | −0.36** | −0.02 | −0.23** | 1 | |||||
| 9. Affective distress | −0.01 | −0.07 | 0.30** | 0.29** | 0.40** | 0.04 | 0.37** | −0.60** | 1 | ||||
| 10. Punishing responses | −0.08 | 0.06 | 0.20** | 0.17** | 0.21** | −0.20** | 0.21** | −0.22** | 0.27** | 1 | |||
| 11. Distracting responses | −0.08 | 0.08 | 0.16** | 0.11* | 0.17** | 0.49** | 0.15** | 0.00 | 0.04 | 0.03 | 1 | ||
| 12. Solicitous responses | 0.11* | −0.08 | 0.23** | 0.15** | 0.23** | 0.61** | 0.18** | −0.04 | 0.10* | −0.04 | 0.63** | 1 | |
| 13. General activity | 0.23** | −0.00 | −0.20** | −0.14** | −0.35** | 0.00 | −0.21** | 0.34** | −0.33** | −0.21** | 0.11* | 0.09 | 1 |
| Mean | — | 46.02 | 50.57 | 60.08 | 46.62 | 62.12 | 52.11 | 60.5 | 47.33 | 28.35 | 51.17 | 54.47 | 56.16 |
| Range | — | 18–78 | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | 0–100 | 0–88 |
| Standard deviation | — | 14.67 | 24.92 | 27.82 | 17.27 | 23.62 | 18.64 | 17.96 | 15.46 | 27.27 | 25.95 | 21.00 | 8.27 |
= P <0.05;
= P <0.00.
Procedures
Patients completed a standardized battery of questionnaires as part of the routine intake protocol at the orofacial pain center where the study was conducted. Prior to filling out the battery, participants consented to their de-identified data being used for retrospective research purposes. The current study used this clinical record of de-identified data. As such, the institutional review board waived requirement for informed consent but gave approval for the study to be conducted.
Measures
Functional Outcomes
The West Haven-Yale Multidimensional Pain Inventory (MPI) is a widely used self-report measure that examines how pain impacts daily functioning across emotional, social, and daily functioning domains [17]. It was specifically designed for use in chronic pain populations and has been validated for use in patients with orofacial pain [13,18]. The MPI is 52 items long and assesses functioning on the following nine domains: Pain Interference, Support, Pain Severity, Life Control, Affective Distress, Punishing Responses (from their partner), Solicitous Responses (from their partner), Distracting Responses (from their partner), and General Activity, which include household chores, outdoor work, activities away from home, and social activities. Participant’s responses were scored using a computerized Rasch-based scoring algorithm. Because the current study examined clinical records that only contained subscale scores and not individual item scores, internal consistency for the subscales could not be computed. However, previous studies have shown the subscales to have high internal consistency in orofacial pain samples (for all scales α= 0.78–0.90) [17]. In the current study, eight people were missing data for the Distracting Responses subscale and one person was missing data for the General Activity subscale. All other subscales had complete data. Those with missing data were excluded from the analyses containing those variables.
Pain Intensity and Unpleasantness
Participants were asked to rate the intensity and unpleasantness of their pain using two visual analog scales (VASs). Participants marked their responses on a 100-mm line and a ruler was then used to quantify the mark, resulting in a possible range from 0 to 100. For pain intensity, participants were asked to rate their “average pain intensity during the past month” with anchors of “no pain at all” and “the most intense pain you can imagine.” For the pain unpleasantness scale, they were asked to rate the unpleasantness of their pain during the past month with anchors of “not at all disagreeable” to “the most disagreeable you can imagine.” The anchors for this unpleasantness rating scale were based on those that were successfully used by orofacial pain patients to rate the intensity and unpleasantness of dry needling procedures [19]. Previous studies have validated VAS scales to assess somatosensory and affective components of pain in chronic pain populations [6]. There were no missing data for the VAS scales.
Data Analysis Plan
All study variables were first checked for normality and outliers using the criteria of 3 SD. Exploratory analyses revealed that there were no outliers. Additionally, all variables were normally distributed. Following data cleaning, bivariate correlations were computed among all study variables.
To determine whether chronic pain patients who were untrained in using the VAS scales were able to validly distinguish pain intensity from unpleasantness, partial correlations were conducted between VAS pain intensity and a numeric rating scale of pain intensity, while controlling for VAS unpleasantness. For the numeric pain rating scale, participants rated the intensity of their pain by circling a number between 1 and 10, with higher numbers representing more intense pain. Likewise, partial correlations between VAS unpleasantness and MPI Pain Severity were conducted while controlling for VAS pain intensity. The Pain Severity subscale of the MPI consists of three questions—two that ask about intensity and one that asks about suffering. Thus, after partialing out intensity, unpleasantness should still significantly correlate with the part of Pain Severity that encompasses suffering.
To test for the effects of pain intensity, unpleasantness, and their interactions on functional outcomes, a pain intensity by unpleasantness interaction term was computed by z-scoring the two VAS variables and multiplying their standardized scores together. Separate regression models were tested for the effects of pain intensity and unpleasantness on eight MPI domains using general linear multivariate regression on SPSS version 20 (SPSS, Inc., Chicago, IL, USA); the ninth domain, Pain Severity, was removed as an outcome because of the high degree of overlapping variance with pain intensity and unpleasantness (r = 0.79 and 0.73, respectively). First, the effects of pain intensity alone were tested for each of the eight outcome variables. Then, the effects of pain unpleasantness alone were tested. Next, models were tested with intensity and unpleasantness entered simultaneously to test for the independent effects of each controlling for the other. Finally, all models were tested with the main effect and interaction terms entered simultaneously, to test whether the interaction of intensity and unpleasantness predicted variance in any of the outcomes above and beyond the effect of intensity or unpleasantness alone. All models were run with and without gender and age entered as covariates. For all models, effect sizes were reported with eta squared, which represents the percentage of variance explained. Significant interactions were probed using the online utility provided by Preacher et al. [20].
Results
Descriptive Statistics and Bivariate Correlations
Table 1 presents the descriptive statistics and bivariate correlations among all study variables. Females (mean [M] = 57.01, SD = 8.01) reported higher general activity than males (M = 51.96, SD = 8.30; t[468] = 5.10, P < 0.001) and also higher solicitous responses (female M = 55.51, SD = 20.05; male M = 49.19, SD = 24.63; t[462] = 2.45, P = 0.02). There were no other gender differences in any domain. Entering gender and age into the models did not change their significance, so the models below do not include the covariates.
Partial Correlations
The bivariate correlation between VAS intensity and unpleasantness was 0.75, revealing a high degree of overlapping variance (approximately 56%). To show that participants were actually using the scales as intended, VAS pain intensity was correlated with a numeric rating scale of pain intensity after removing the variance associated with pain unpleasantness. Results revealed that intensity (without unpleasantness) significantly correlated with a numeric rating scale of pain intensity (r = 0.42, P < 0.001), suggesting that participants were validly using the VAS pain intensity. Likewise, after removing the variance associated with pain intensity, the pain unpleasantness subscale correlated with the MPI Pain Severity subscale (r = 0.34, P < 0.001), again suggesting valid and distinguishable contributions of intensity and unpleasantness in the current study.
Primary Analyses
Hierarchical linear multivariate regression analyses were conducted with each of the eight MPI domains as the outcome variables and VAS pain intensity as the single predictor. As Table 2 shows, higher pain intensity predicted higher support but worse outcomes on all other areas of functioning. The significance of the results remained the same when the models were reran controlling for pain unpleasantness. Likewise, pain unpleasantness alone predicted worse functioning on all domains (except General Activity) but better social support. The pain unpleasantness results only only significantly predicted higher interference and affective distress after controlling for pain intensity.
Table 2.
Effects of pain intensity and unpleasantness on functional outcomes
| Outcome Variable (Total R2) | Predictors | B | t | P | η 2 |
|---|---|---|---|---|---|
| Pain interference (0.41) | Intensity | 10.37 | 16.12 | <0.001 | 0.36 |
| Unpleasantness | 9.94 | 15.50 | <0.001 | 0.34 | |
| Intensity × Unpleasantness | −1.23 | −2.22 | 0.03 | 0.02 | |
| Support (0.08) | Intensity | 7.00 | 6.66 | <0.001 | 0.09 |
| Unpleasantness | 5.50 | 5.18 | <0.001 | 0.06 | |
| Intensity × Unpleasantness | 0.65 | 0.65 | 0.51 | 0.00 | |
| Life control (0.03) | Intensity | −3.38 | −4.15 | <0.001 | 0.04 |
| Unpleasantness | −2.59 | −3.17 | 0.002 | 0.02 | |
| Intensity × Unpleasantness | 0.74 | 0.95 | 0.34 | 0.00 | |
| Affective distress (0.10) | Intensity | 4.84 | 7.13 | <0.001 | 0.10 |
| Unpleasantness | 4.49 | 6.61 | <0.001 | 0.09 | |
| Intensity × Unpleasantness | 0.60 | 0.93 | 0.36 | 0.00 | |
| Punishing responses (0.04) | Intensity | 5.50 | 4.43 | <0.001 | 0.04 |
| Unpleasantness | 4.61 | 3.71 | <0.001 | 0.03 | |
| Intensity × Unpleasantness | −0.09 | −0.08 | 0.94 | 0.00 | |
| Distracting responses (0.02) | Intensity | 4.01 | 3.36 | <0.001 | 0.02 |
| Unpleasantness | 2.76 | 2.31 | 0.021 | 0.02 | |
| Intensity × Unpleasantness | −1.29 | −1.13 | 0.26 | 0.00 | |
| Solicitous responses (0.05) | Intensity | 4.75 | 4.99 | <0.001 | 0.04 |
| Unpleasantness | 3.02 | 3.15 | 0.002 | 0.02 | |
| Intensity × Unpleasantness | −0.22 | −0.24 | 0.81 | 0.00 | |
| General activity (0.04) | Intensity | −1.67 | −4.45 | <0.001 | 0.04 |
| Unpleasantness | −1.14 | −3.01 | 0.93 | 0.02 | |
| Intensity × Unpleasantness | −0.47 | −1.31 | 0.19 | 0.00 |
Multivariate regression analyses were used for all models. The Intensity and Unpleasantness models were tested with z-scored variables of intensity and unpleasantness as single predictors, respectively. The Intensity × Unpleasantness interaction models included the intensity and unpleasantness terms entered simultaneously. The total R2 is for the final model with both main effects and the interaction term. Outcome variables are from the Multidimensional Pain Inventory.
Finally, an intensity by unpleasantness interaction was tested by rerunning the eight models with pain intensity, unpleasantness, and their interaction entered simultaneously as the predictors. Results revealed a significant pain intensity by pain unpleasantness interaction in predicting pain interference. At low levels of pain unpleasantness (−1 SD), the effects of pain intensity on pain interference were greater (B = 8.01, t[468] = 6.38, P < 0.001) than at high levels of pain unpleasantness (+1 SD; B = 5.27, t[468] = 4.45, P < 0.001). In other words, changes in pain intensity produced greater changes in interference at low levels of pain unpleasantness. Figure 1 presents a graphical representation of the interaction.
Figure 1.
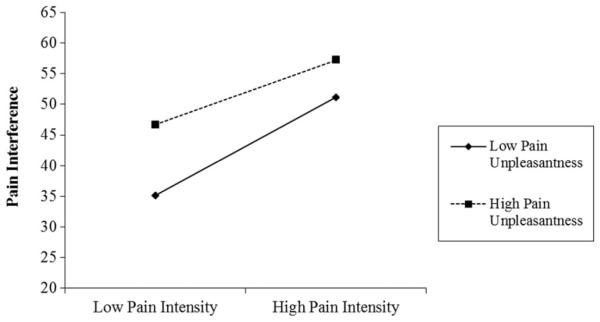
Interaction of pain intensity and pain unpleasantness on pain interference.
Discussion
Somatosensory and affective processes have long been known to contribute to pain processes. This is the first study to our knowledge that tested the unique and interactive effects of somatosensory processes (measured by pain intensity) and affective processes (measured by pain unpleasantness) on emotional, social, and daily functioning in chronic pain patients. Consistent with previous literature (e.g., Osborne et al. [14]) and with the hypotheses, higher pain intensity was significantly associated with poorer functioning across all domains, with the exception of support. These results remained the same after controlling for pain unpleasantness. Similarly, higher pain unpleasantness was also associated with worse functioning, and remained a significant predictor of affective distress and pain interference after controlling for pain intensity. One explanation for these findings is that high intensity and high unpleasantness pain force people to use psychological resources to manage it, leading to fatigue [21]. In support of the role of fatigue as a mediator between pain intensity and poorer functioning, work with orofacial pain patients has found strong associations between fatigue and poor functional outcomes [13]. The contribution of fatigue as a mediator of the relationships between pain intensity and functional outcomes remains speculative and should be tested in longitudinal data.
The fact that higher pain intensity was associated with higher support and solicitous responses may indicate that those experiencing intense pain were also asking for more help from others. Recent evidence finds that those who experience high pain also use more pain behaviors like grimacing and moaning, but only in the presence of another person, suggesting that they may be using pain behaviors as a way to draw social support [12]. Because of the nature of the cross-sectional data in the current study, causal relationships between support and pain intensity could not be established.
The effect sizes from the current study revealed that pain intensity predicted more variance in functional outcomes than did unpleasantness when entered as single predictors, as shown in Table 2. One reason for this is that pain intensity, more than unpleasantness, demands attention and requires cognitive resources, leaving less available for functioning in other domains. Still, unpleasantness is not unimportant. In fact, consistent with our hypothesis and with the fear-avoidance model, pain unpleasantness significantly predicted affective distress and pain interference above and beyond pain intensity. In the fear-avoidance model, aversive pain experiences (i.e., those high in unpleasantness) lead to fear and ultimately avoidance of situations that cause pain [15]. Because in orofacial pain social activities that involve talking, eating, and expressing emotions are the ones that are most likely to be painful and thus feared, and because the pain interference scale includes items like “how much has your pain changed the amount of satisfaction or enjoyment you get from participation in social and recreational activities” (Kerns et al. [17], p. 346), it makes sense that unpleasantness would contribute unique variance to interference.
The Pain Severity subscale consists of three questions—two that ask about intensity and one that asks about how much “suffering” is experienced because of pain. Given the makeup of the subscale, it is perhaps not surprising that it correlated with pain unpleasantness, even after controlling for pain intensity. These results further support the notion that participants were able to validly distinguish the two constructs; if suffering were merely a function of intensity, then controlling for intensity would have nullified the unpleasantness effects. Likewise, the findings that pain unpleasantness predicted affective distress above and beyond pain intensity is also not surprising given that the affective distress subscale consists of three items asking about mood, irritability, and anxiety. In fact, these findings coincide with literature showing that experimental induction of negative mood increases unpleasantness but not intensity [10].
Pain unpleasantness interacted with pain intensity to predict pain interference. At low levels of pain unpleasantness, patients who reported high pain intensity also reported high pain interference, whereas those who reported lower pain intensity reported lower interference. At high levels of unpleasantness, however, even those with low pain intensity reported high interference. As expected, the most interference was reported by those who experienced both high intensity and high unpleasantness pain. The fact that the pain intensity by unpleasantness interaction predicted an additional 2% of variance above and beyond the effects of pain intensity or unpleasantness alone suggests that there may be synergistic effects between them, such that the combination of high intensity and unpleasantness produces the worst outcomes. These interactions suggest that treatment approaches to chronic pain should not focus only on reducing pain intensity but also at reducing unpleasantness. This fits in line with literature showing that cognitive behavioral therapies aimed at making pain less unpleasant, used in combination with medications that reduce pain intensity, produce better functioning [22].
Limitations and Future Directions
The current study is not without limitations. Because data were collected at a single-time point, causality cannot be definitively established. Also, the measures used were all self-report and need to be replicated with objective, experimentally manipulated measures. In the current study, the unpleasantness measure used unconventional anchors of “not at all disagreeable” to “the most disagreeable you can imagine,” and future work should attempt to replicate these findings with more conventional anchors of unpleasantness. Further, more work needs to be done to determine whether chronic pain patients can validly differentiate pain intensity and unpleasantness, although preliminary data from the present study suggest that they can. Finally, because the sample consisted only of orofacial pain patients, the findings may not generalize to other types of chronic pain disorders. Despite these limitations, the current study suggests that pain intensity and unpleasantness are differentially contributing to functional outcomes in chronic pain patients. It is our hope that the study will promote future work investigating how each of these components could be improved—either independently or synergistically—to provide the best help to those experiencing chronic pain.
Acknowledgment
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number F31AG048692. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Melzack R, Wall PD. Gate Control Theory of Pain. Academic Press; New York: 1968. [Google Scholar]
- 2.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 3.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 4.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–43. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez E, Turk DC. Sensory and affective components of pain: Separation and synthesis. Psychol Bull. 1992;112:205–17. doi: 10.1037/0033-2909.112.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Gracely RH. Pain language and ideal pain assessment. In: Melzack R, editor. Pain Measurement and Assessment. Raven Press; New York: 1983. pp. 71–7. [Google Scholar]
- 7.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 9.Singer T, Seymour B, O’Doherty J, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 10.Berna C, Leknes S, Holmes EA, et al. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiat. 2010;67:1083–90. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Loeser JD, Melzack R. Pain: An overview. Lancet. 1999;353:1607–9. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- 12.Cano A. Pain catastrophizing and social support in married individuals with chronic pain: The moderating role of pain duration. Pain. 2004;110:656–64. doi: 10.1016/j.pain.2004.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boggero IA, Kniffin TC, de Leeuw R, Carlson CR. Fatigue mediates the relationship between pain interference and distress in persistent orofacial pain patients. J Orofac Pain Headache. 2014;28:38–45. doi: 10.11607/jop.1204. [DOI] [PubMed] [Google Scholar]
- 14.Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G. Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain. 2007;127:52–62. doi: 10.1016/j.pain.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain. A state of the art. Pain. 2000;85:317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 16.De Leeuw R. Guidelines for Assessment, Diagnosis, and Management. 5th Quintessence Publ. Co.; Chicago: 2011. American Academy of Orofacial Pain. [Google Scholar]
- 17.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–56. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 18.Andreu Y, Galdon MJ, Durá E, et al. An examination of the psychometric structure of the multidimensional pain inventory in temporomandibular disorder patients: A confirmatory factor analysis. Head Face Med. 2006;2:48–57. doi: 10.1186/1746-160X-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan AS, Nolan A, Kelly PJ. The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. J Orofac Pain. 1997;11:307–14. [PubMed] [Google Scholar]
- 20.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–48. [Google Scholar]
- 21.Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain. A review. Ann Behav Med. 2009;37:173–83. doi: 10.1007/s12160-009-9096-5. [DOI] [PubMed] [Google Scholar]
- 22.Stephen M, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]


