Abstract
In Argentina, Cardiovascular diseases are estimated to cause about 100,000 deaths and more than 250,000 coronary heart disease and stroke events annually, at a cost of more than one billion international dollars. Despite progress in the implementation of several programs to combat non-communicable diseases in Argentina over the last years, most health resources are still dedicated to infectious disease and maternal and child health. The Institute for Clinical Effectiveness and Health Policy, an independent academic institution affiliated to the University of Buenos Aires medical school, runs CESCAS (South American Centre of Excellence in Cardiovascular Health), a center devoted to epidemiological, implementation and policy research. At CESCAS there are three ongoing randomized clinical trials focused on implementation science: 1) A Mobile health intervention to prevent progression of pre-hypertension in poor urban settings in Argentina, Guatemala and Peru; 2) A Comprehensive Approach for Hypertension Prevention and Control in low-resource settings in Argentina; and 3) An Educational Approach to Improve Physician Effectiveness in the Detection, Treatment and Control for patients with Hypercholesterolemia and high Cardiovascular Disease (CVD) risk in low-resource settings in Argentina. All these studies involve the design and implementation of complex interventions to change behaviors of providers and patients. The rationale of each of the three studies, the design of the interventions and the evaluation of processes and outcomes are described in this article together with the barriers and enabling factors associated with implementation research studies. There is a strong need in Argentina and the region at large to build the health research capacity and infrastructure necessary to undertake implementation studies to translate evidence from research findings into improvements in health policy and practice to address CVD and their risk factors.
Cardiovascular disease burden in Argentina
Coronary heart disease (CHD) and stroke are the leading causes of deaths worldwide, killing 12.9 million people in 2010, or one in four deaths, [1]. Eighty percent of these deaths occur in low and middle-income countries and, almost half are in people younger than 70 years old, compared with only 27% amongst corresponding age groups in high-income countries [2]. In Latin America, it is estimated that from 1990 until 2020, death from CVD, including coronary heart disease (CHD) will increase by approximately 145% (for both men and women), compared with an increase of 28% for women and an increase of 50% for men in developed countries during the same period [3]. The Latin American INTERHEART study showed that the majority of cardiovascular risk in the Southern Cone could be explained by tobacco use, abnormal lipids, abdominal obesity and high blood pressure [4]. In Argentina, CVDs are estimated to cause about 100,000 deaths and more than 250,000 events annually, at a cost of more than one billion international dollars [5]. Recent estimates indicate that more than 600,000 Disability Adjusted Life Years (DALY) and almost 400,000 Years of Potential Life Lost (YPLL) are due to CHD and stroke, with modifiable risk factors explaining 75% of fatal and non-fatal acute CHD and stroke events (82% of acute CHD events and 62% of strokes), and 71% of DALYs lost [6]. This is alarming given that the prevalence of all major CVD risks factors in Argentina (hypertension, diabetes, obesity, physical inactivity, and poor nutrition) increased between 2005 and 2009 according to the National Cardiovascular Risk Factors Surveys. The prevalence of diabetes rose 14.1%, from 8.4% to 9.6%; obesity rose 23.3%, from 14.5% to 18.0% and low physical activity rose 19%, from 46.2% to 55% [7]. There has been important progress in the implementation of several programs to combat NCD in Argentina at both national and subnational levels; however, most health resources are still dedicated to infectious disease and maternal and child health. Looking forward, NCD prevention and control is very promising. Argentina recently established strong policies against tobacco and unhealthy diets as well as increased support for universal health coverage for some of the most prevalent NCD’s that are moving up the national health agenda, including CVD, cancer, COPD, diabetes and mental health.
NCD research in Argentina
Insufficient research funding and infrastructure are some of the largest limitations in performing and publishing research in Latin America. For example, Argentina dedicates only 0.65% of the GDP to research, having approximately three researchers per 1,000 economically active adults [8], and only 6% of its biomedical research funds are applied to clinical and public health research [9]. Performance of health research in Latin America has been very poor; from 2001 through 2010, Latin American nations contributed just over 3% of the overall publications worldwide. Almost 80% came from only 3 countries: Argentina, Brazil and Mexico. Most of the research papers were related to basic research and just 22% were publications regarding clinical investigation or public health. Of these, less than 10% (1,338 papers) were focused on CVD. Not surprisingly, only 8 were randomized clinical trials conducted only in Latin American countries and not as part of multinational studies sponsored by the industry [10]. More concerning is the fact that Upper-Middle Income Countries (UMICs) are increasingly being considered ineligible to apply to several funding opportunities from donors and research agencies of developed countries. If this tendency were to spread among funding agencies, the role of research institutions in UMICs will strongly decrease. Since Argentina is an upper-middle income country according to the World Bank classification [11], there may be a further reduction in funding for public health and clinical research in this country.
A Center of Excellence in South America to promote high-quality research to address NCDs
The Institute for Clinical Effectiveness and Health Policy (IECS in its Spanish acronym), an independent academic institution affiliated to the University of Buenos Aires medical school (UBA), runs CESCAS (South American Centre of Excellence in Cardiovascular Health), which was created in 2009 through a competitive award from the National Heart, Lung and Blood Institute (NHLBI) in partnership with the School of Public Health and Tropical Medicine of Tulane University, USA. CESCAS staff is composed of physicians, epidemiologists, nutritionists, nurses, health economists, social scientists and statisticians as well as trainees at a postdoctoral, doctoral and master’s degree level. The center is devoted to epidemiological, implementation and policy research, training in cardiovascular health, and technical cooperation with national, regional and international bodies. The center also advocates for the translation of evidence into policy and practice by promoting a policy dialogue between researchers, policy makers, the media, NGOs, and the public at large to combat CVD at a regional level. Since June 2009, CESCAS has undertaken different observational, implementation, and policy research studies.
Post-translational research studies conducted at CESCAS
Implementation science is about providing the methods to translate research findings from interventions into healthcare policy and practice to help bridge the gap between what is known and what is actually done. It seeks to understand the behavior of healthcare providers, patients, healthcare organizations, and policymakers in a particular context, as key variables to promote the adoption, implementation and uptake of evidence-based interventions. Unlike interventions tested in most randomized clinical trials, which are generally simpler, more straightforward, and targeted directly at patient units, those tested in the implementation research field are more complex since they usually act on patients indirectly, through behavioral changes of providers, organizations or even higher levels such as health services and systems. These complex interventions usually contain multiple interacting components with several dimensions of complexity: the number of elements in the intervention package itself such as the different behaviors required by those delivering or receiving the intervention, the number of groups or organizational levels targeted by the intervention, the number and variability of outcomes, and the degree of flexibility or tailoring of the intervention permitted. All of these imply that lack of effect may reflect implementation failure rather than true ineffectiveness [12]. Moreover, evaluation may be compromised by problems of acceptability, compliance, delivery of the intervention, recruitment and retention, and smaller than expected effect sizes that could have been prevented if a feasibility or pilot study were planned “ex-ante”[13]. For these reasons, it is critical that in the evaluation of the results of these trials, both outcome measures and process measures are planned in order to explore the way in which the interventions have been actually implemented. This approach can disentangle the different components of the planned intervention and provide valuable insight into why an intervention worked, failed, or yielded unexpected results, and how it could be optimized if successful. Process evaluations nested within these trials can be used to assess fidelity and quality of implementation, clarify causal mechanisms, and identify contextual factors associated with variation in outcomes. [14] Notwithstanding, the evaluation of the process is not a substitute for evaluation of the outcomes, however it can be extremely helpful, particularly in negative studies when a complex intervention can be like a black box.
Currently, there are three ongoing implementation research RCTs at our Center. All these studies involve the design and implementation of complex interventions to change behaviors of providers and patients. The rationale of each of the three studies, the design of the interventions and the evaluation of processes and outcomes are described briefly below:
A Mobile health intervention to prevent progression of pre-hypertension in poor urban settings in Argentina, Guatemala and Peru
Pre-hypertensive individuals are at high risk of progressing to hypertension and developing CVD. Early interventions to promote the adoption of healthier lifestyles in these subjects could reduce blood pressure (BP), decrease the rate of progression of BP to hypertensive levels, and even prevent hypertension from occurring [15, 16]. However, primary health care services in most Latin American countries, particularly in poor settings, lack the infrastructure and resources to implement effective health promotion interventions. On the other hand, health promotion is shifting towards new delivery modes (e.g. the internet and cell phones) to reach a larger part of the population. However, evidence regarding the effectiveness of these interventions for lifestyle modification is unclear. Reported overall effects have been small and variable, and reach has been limited to highly educated females from high income countries [17]. Mobile health (mHealth) refers to the use of mobile telecommunication and multimedia technologies for healthcare delivery [18, 19]. This technology is emerging as a useful tool to address several healthcare system constraints such as: limited health care workforce and financial resources, high burden of disease combined with high population growth, and the challenge of extending health care to hard-to-reach and vulnerable populations living in low-resource settings. [20] Mobile phone strategies, using either phone or short message system (SMS) have shown to improve patient-provider communication, encourage behavior change and assist in chronic disease management. [21–24]
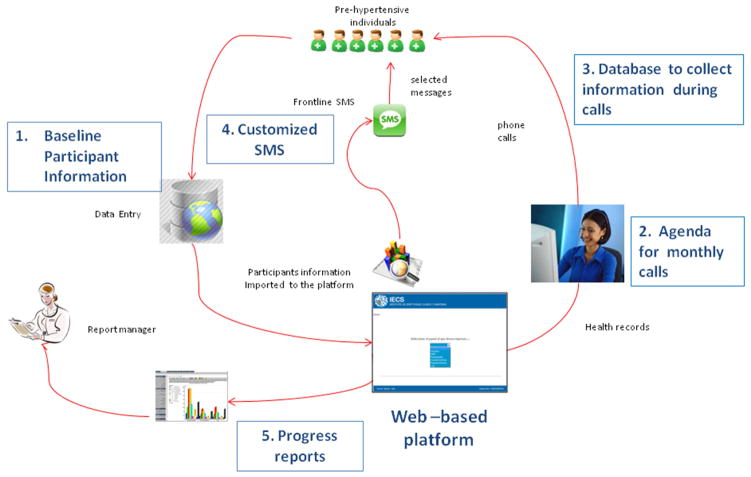
Our study is an individual RCT, sponsored by NHLBI, conducted in collaboration with INCAP in Guatemala and CRONICAS from the Universidad Peruana Cayetano Heredia in Perú. This study aims at promoting lifestyles changes (improvements in diet quality and physical activity) in 660 pre-hypertensive subjects from poor urban settings in Argentina, Guatemala and Perú, through a mobile health intervention (mHealth) along one year of follow-up. This study has just finished and the main process and outcomes results are being evaluated. In short, the intervention, led by nutritionists who completed a three-day training session, had three components: 1) semi-structured counseling interviews through mobile phones to promote lifestyle modification, according to the motivational interviewing (MI) technique [23]. The participants in the intervention arm received counseling on one of the following target behaviors: reduction of dietary sodium intake, reduction of simple sugars and saturated fat intake, increase of fruits and vegetables intake, and promotion of physical activity. Information regarding readiness to change was based on the Transtheoretical Model [25] and was collected during monthly calls to the participants; 2) after the counselor call, participants received a weekly text message (SMS) that was tailored to the state of change regarding a particular target behavior of the subject identified by the counselor. Both the content and wording of SMS had been previously validated in each of the countries (results of the validation process are not yet published); and 3) the use of a web-based application to deliver the interventions. This set-up allowed for the following functions: 1) baseline participant’s information for the nutritionist who made the calls, 2) an agenda for scheduling monthly calls, 3) a database to collect information of the treated behavior and the stage of change, 4) a customized SMS desktop where messages were generated and tailored to the individual’s stage of change and target behaviors, and 5) progress reports (Figure 1). The rationale of the trial was informed by a systematic review previously performed by our group to identify what was already known about mHealth intervention on chronic disease outcomes in developing countries and the methods that have been used to evaluate its effectiveness [24] as well as focus groups conducted in each country with participants with similar characteristics and attributes as the eligible patients (results of the qualitative research are not yet published). The design of the intervention was supported by an appropriate theory [25] that provided a good theoretical understanding that was necessary to know how the intervention causes change, so that weak links in the causal chain can be identified and strengthened [13]. In addition, a feasibility study was performed in 45 pre-hypertensive individuals, prior to the initiation of study enrollment, to test the different components of the intervention program. [26]
Figure 1.
Functions of the web-based platform to provide mHealth interventions
The web-based platform allowed for the following functions: 1) baseline participant’s information for the nutritionist who made the calls, 2) an agenda for scheduling monthly calls, 3) a database to collect information of the treated behavior and the stage of change, 4) a customized SMS desktop where messages were generated and tailored to the individual’s stage of change and target behaviors, and 5) progress reports
We included the following indicators as part of the process evaluation: reach (proportion of the intended target population who received the intervention), dose (components implemented) and attrition (percentage of participants who dropped out of the intervention). The intervention package included an introductory call, monthly mobile phone counseling calls and weekly SMS messages during the year of intervention.
A Comprehensive Approach for Hypertension Prevention and Control in low-resource settings in Argentina
Hypertension is a global public-health challenge because of its high prevalence and concomitant increase in risk of cardiovascular disease [27]. Approximately 80% of the attributable burden of hypertension is in low- and middle-income countries (LMICs) [28]. According to recent estimates from CESCAS I, a large population-based cohort study that our center is conducting in four cities of the Southern Cone [29], the prevalence of hypertension in adult population aged 35–74 years old is 43.3 %. Overall, 62.2% of hypertensives are aware of their diagnosis, 47.7 % are under drug treatment, and only 21.5 % achieve BP control [30]. The hypertension control rate is even lower in underserved populations in Argentina. For example, even though antihypertensive drugs are delivered free of charge at public primary care clinics for uninsured populations, only 57% of uninsured hypertensive patients are actually treated. In those treated, almost 75% of patients received medication for less than 4 months per year, and only 11.8% received it for more than 9 months per year [31]. Barriers to hypertension control have been identified at the health care system, health care provider and patient levels. Lack of access to health care, medication costs and poor insurance coverage are major health system-level barriers [32]. Provider-level barriers include lack of adherence to guidelines, willingness to accept elevated BP, and failure to prioritize BP control among multiple chronic medical issues. Patient-level barriers to BP control are primarily related to therapy adherence, and include low perceived risks of high BP, low health literacy, lack of motivation, out-of-pocket medication costs, and adverse side effects [33–36].
In addition, low-income countries as well as poor settings in middle-income countries are facing a health workforce crisis [37]. Community health workers (CHWs)—also known as lay health workers, non-physician health workers, health educators, patient navigators and promoters, among many other titles—can compensate for this shortage through task shifting and importantly can also provide direct links between health services and communities [38]. CHWs can increase the capacity of an already overburdened health care system by using health care resources more effectively, and by increasing the quality of care [39]. The inclusion of CHWs in the primary care team is an example of an organizational change to address system-level barriers by simplifying the physician’s tasks and transferring some responsibility for patient care to another team member (task shifting). Team change strategies have resulted in median reductions in systolic BP of 9.7 mmHg and in diastolic BP of 4.2 mmHg in a meta-analysis [32]. In addition, CHWs may help remove barriers to blood-pressure (BP) control and medication adherence due to cultural, educational, and language differences between community members and the health care system [40]. A systematic review of RCTs using CHW to implement BP control programs found significant improvement in 7 of 8 studies, primarily in poor, urban, minority communities [41]. Task shifting from physicians to other health team members was an important ingredient of a large-scale hypertension program in an integrated health care delivery system in the US that was able to almost double hypertension control rates in 8 years by reducing appointment times, providing increased scheduling flexibility and decreasing health care costs [42]. Moreover, these interventions have shown to be also effective in LMICs [43].
Our study, a cluster RCT, has been designed to test an intervention strategy for hypertension prevention and control in Argentina, driven by CHW providing education and counseling to patients, and liaison with physicians at the primary care clinic (PCC), training of physicians on hypertension management; and mHealth support tools in 18 primary health care centers of the Argentine public network, enrolling 2,000 uninsured hypertensive individuals and their families. This study, sponsored by NHLBI under the umbrella of the Global Alliance of Chronic Disease (GACD), is implemented in collaboration with the School of Public Health and Tropical Medicine of Tulane University, and the Argentine Ministry of Health [44]. Briefly, we are testing the effectiveness and cost-effectiveness of a comprehensive intervention program, compared to usual care, to decrease systolic blood pressure (SBP) and diastolic blood pressure (DBP) in uncontrolled hypertensive patients and their families and to improve hypertension control in hypertensive patients over an 18-month period. Our strategy integrates individual evidence-based interventions shown to be effective to overcome the barriers for hypertension control, as can be seen in Table 1. The components of the intervention are: 1) task shifting within the primary care team (from physicians and nurses at the PCC to CHWs at patients’ homes). Trained CHWs who completed an initial 2-day workshop reinforced by weekly phone calls by a field supervisor and on-site periodic refreshments, visit participants’ homes monthly for the first six months of the intervention and every other month thereafter. CHWs educate and counsel participants and their families about medication adherence, home blood pressure monitoring, and lifestyle modification strategies. All study participants are given a pill box and a home blood pressure monitor. CHWs also deliver antihypertensive medications to patients’ homes in special cases as needed and help patients to schedule appointments with primary care doctors at the clinic when necessary. During follow-up home visits, CHWs provide tailored counseling to address barriers to hypertension self-management and effective behavior change of targeted lifestyles (i.e.: improving diet quality, training patients to read food labels, increasing physical activity); 2) Physician education through workshops and distance learning modules to reinforce clinical practice guidelines on hypertension prevention and control as well as management of patient’s adherence to prescribed medication; and 3) Individualized SMS sent out weekly to participants to promote lifestyle changes and reminders to reinforce medication adherence. Messages are based on hypertension status and perceived barriers to behavioral change identified during CHW motivational counseling sessions and consist of motivational statements and behavior-change techniques to reinforce in-person education interventions.
Table 1.
Strategies to Overcome Barriers to Hypertension Control
| Barrier | General approach | Specific strategy to overcome the barrier |
|---|---|---|
|
Systems Level
| ||
| Insufficient time | Task shifting |
|
| Lack of time for physician counseling | Task shifting |
|
| Lack of continuity of care | Team change |
|
| Discontinuation of prescribed free medications | Policy change |
|
| Poor access of patients to PHC clinic** | Home visits by CHWs |
|
|
| ||
|
Provider Level
| ||
| Lack of adherence to treatment guidelines, “clinical inertia” | Physician education |
|
| Uncertainty that office BP represents usual BP | Home BP monitoring |
|
|
| ||
|
Patient Level
| ||
| Passive attitude and misperceptions about high BP | Improving self-efficacy |
|
| Poor adherence to medications | Family-support, Patient education, Home BP monitoring |
|
| Hypertension knowledge/ risk perception | Patient education |
|
| Poor memory | Reminding, Family support, Patient education |
|
| Low health literacy | Patient education |
|
| Poor motivation | Reminding, Family-support, Patient education, Home BP monitoring |
|
| Medication costs | Policy change, Physician education, Patient education |
|
| Adverse effects | Physician education, Patient education |
|
CHW = community health worker;
PHC clinic=primary health care clinic
In addition to the outcome measures, such as the lowering of SBP and DBP, some process measures are being taken, including number of CHWs service provided (home visits), percent of CHWs follow-up visits kept, number of SMS sent to each participant, patients’ use of home blood pressure monitors, patients’ weight control and use of pill boxes, as well as improvements in targeted lifestyles such as smoking, diet quality, or physical activity. This data collected in the field, will be analyzed to evaluate whether the desired interventions are adopted by participants, and to what extent they are translated into better outcomes
An Educational Approach to Improve Physician Effectiveness in the Detection, Treatment and Control of Hypercholesterolemia and high Cardiovascular Disease (CVD) risk in low-resource settings in Argentina
Hypercholesterolemia, a major cause of disease burden in both the developed and developing world, is estimated to cause 2.6 million deaths annually (4.5% of all deaths) and one third of ischemic heart diseases, and result in 29.7 million DALYs [45]. In 2008, the global prevalence of elevated total cholesterol among adults was 39% (37% for males and 40% for females) [46]. In Argentina, the National Risk Factor Surveys conducted by the Ministry of Health indicate that between 2005 and 2009 the self-reported prevalence of hypercholesterolemia rose from 27.9% to 29.1%. Of these, only 54.8% received some treatment, 56.3% of which were on lipid-lowering drugs (the rate of those receiving treatment was less than 20% among uninsured subjects, including subjects with more than 3 risk factors) [47]. Recent baseline results from the CESCAS I study [29], found that the prevalence of hypercholesterolemia in Argentina is 23.1% in men and 25.6% in women, and according to the Framingham coronary disease risk measure, the prevalence of non-optimal LDL-C is 28.0%. On the other hand, the percentage of subjects with hypercholesterolemia who are aware of their condition is 37.3% and the percentage of those who are under pharmacological treatment is dismally low: only 11.1%. Furthermore, only one in every four subjects with a self-reported diagnosis of CHD is taking statins and most of those with CHD who are on statins have sub-optimal LDL-C levels (Rubinstein et al. personal communication. Data not yet published). This is especially relevant because hypercholesterolemia accounts for 25% of the burden of CHD in Argentina, as we have shown recently in another study [48]. The Argentine Ministry of Health provides free ambulatory drugs to vulnerable people without health insurance who attend public primary care clinics. Until this year, statins had not been yet included in the list of drugs delivered by the Program.
Despite the availability of evidence-based practice guidelines (CPG), several barriers hinder the appropriate management of hypercholesterolemia in the primary care setting. These can be organizational barriers within primary care clinics; confusing and conflicting guidelines from external sources; errors and omissions by primary care doctors; communication problems at the interface between secondary and primary care [49], multiple competing demands on physicians’ time, and lack of reimbursement for preventive counseling.34 Among the interventions that have been effective in dealing with barriers related to clinical practice are multifaceted educational outreach visits (EOVs) [50], and audit and feed-back [51]. EOVs have the potential to change health professional practice, particularly the prescribing patterns of physicians. The term EOV or “academic detailing” is used to describe an in-person visit by trained individuals to health care professionals in their own workplace. The intervention often includes feedback on existing practices. A recent Cochrane review indicates that patient re-enforcement and reminders seem to be the most promising interventions to increase adherence to lipid-lowering drugs [52]. This study, a cluster RCT recently funded by a competitive independent grant from Pfizer and the International Atherosclerotic Society, will test an educational intervention to improve physician effectiveness in the detection, treatment and control of hypercholesterolemia and high cardiovascular disease risk in 350 patients of 10 primary care clinics from low-resource settings in Argentina. This trial is timely since statins (simvastatin), as mentioned above, are now being incorporated into the package of drugs delivered free-of-charge for patients with high cholesterol, according to CVD risk stratification.
In summary, we will test the effectiveness and cost-effectiveness of a multifaceted educational intervention program, compared to usual care, to lower cholesterol levels and CVD risk in moderate-high cardiovascular risk patients, and to evaluate whether this intervention program improves physician compliance with clinical practice guidelines as well as patient care management and adherence, over one year of follow-up. The components of the intervention are: 1) development of an educational program for physicians. This program will start with an intensive 2-day workshop followed by distance learning modules, to train doctors on global cardiovascular risk assessment and management; epidemiology, diagnosis, treatment and monitoring of patients with dyslipidemia; and management of adherence issues in patients with chronic diseases. This initial training will be reinforced through quarterly EOVs to the PHC clinics of the intervention arm, where respected clinicians-educators (academic detailers) will provide on-site re-training to physicians on CPG, as well as audit and feed-back through medical charts and prescribing patterns review; 2) development of an application for physician’s smartphones to provide evidence-based and guideline-driven decision aids to improve patient management; and 3) design and implementation of a web-based platform to send tailored SMS messages for lifestyle modification, and prompts and reminders for clinic appointments to improve treatment adherence by participating patients in the intervention clinics.
Barriers and enablers of implementation research studies in Argentina
Implementing evidence-based health interventions in low resource settings is a challenge in LMICs [53], particularly when these intervention strategies are tested within a research framework. The main identified barriers to implementation research studies in poor settings include but are not limited to: complexity of interventions with multiple components acting on providers and patients but also on community and clinical settings; limited capacity of local human resources to adopt research methods in a context of a poor evaluative culture; lack of leadership and management skills at the local level; weak health systems, complex social, cultural and political context; and a community usually not engaged or ready for the adoption of the health intervention.
However, to sort-out these bottlenecks, we have also identified some enablers that in our experience can be key for success in implementation research studies, particularly in poor settings. First and foremost, engagement of local authorities, community NGOs, local media and other stakeholders are key to get buy in from the local community. In this regard, continuous involvement of health authorities, participation in community activities, visibility in local media before the beginning of recruitment, and fieldwork for delivery of the intervention facilitates the ownership of and commitment to the study by both health providers and participants. Other factors that have proven to be enablers of success are: 1) Building local capacity to enhance perceived skills and motivation of health personnel participating in the research study as avenue to facilitate scaling-up; 2) Training and periodic re-training of the study personnel, necessary not only to avoid departures from the protocol, which might be a risk in these studies, but also to introduce rigorous methods of assessment derived from clinical research in order to create an evaluative environment; 3). Close monitoring of the field work both on site and through telephone and e-mail follow-up are important for audit and feed-back but perhaps more important to support local research team in dealing with the many hurdles that usually arise during implementation studies of complex interventions; 4). Flexibility to respond to changes in local conditions that can affect the study such as competing activities at the health care centers, lack of personnel, seasonal jobs, etc, and finally; 5) Design of a data management workflow that allows for efficient and timely data and quality control measures.
Conclusions and policy implications
Critical challenges of successful implementation or post-clinical translational research studies include dissemination of results and scaling-up. Scaling-up has been defined as “the ambition or process of expanding the coverage of health interventions” [54]. Interventions that are more likely scaled-up are those that are simple and technically sound with also a widespread consensus about its value. An important aspect of implementation research is therefore to simplify delivery. Also, the chances of success are likely to be increased by strong leadership and governance and the active engagement of a broad range of implementers and key stakeholders, including local community organizations [55]. In this regard, our ongoing studies engaged the National Ministry of Health from the outset on different aspects of the design and the delivery of the intervention. This involvement will probably facilitate the uptake, ownership, dissemination and scaling-up of the intervention strategy across the public primary care clinics countrywide if these studies show positive results.
Despite the increasing burden of CVD in Argentina, which over the last decades has been ranked as the main cause of mortality and morbidity, national health programs and policies are still mostly focused on interventions aimed at tackling communicable diseases or perinatal or childhood conditions. Therefore, there is a strong need in our country and the region at large to build the health research capacity and infrastructure necessary to undertake implementation studies to translate evidence from research findings into improvements in health policy and practice to address CVD and their risk factors. This is even more urgent in poor populations who are disproportionally affected by the epidemic of CVD and hence need effective, cost-effective, acceptable and feasible interventions to help bridge the equity gap and counter CVD, particularly in low-resource settings in developing countries.
Footnotes
COMPETING INTERESTS
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370(9603):1929–38. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Lanas F, Avezum A, Bautista LE, et al. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115(9):1067–74. doi: 10.1161/CIRCULATIONAHA.106.633552. [DOI] [PubMed] [Google Scholar]
- 5.Anuario 2009. Buenos Aires: Statistics and Informatics Department. Ministry of Health; Argentina: 2009. [accessed Aug 30 2014]. http://www.deis.gov.ar. [Google Scholar]
- 6.Rubinstein A, Colantonio L, Bardach A, et al. Estimation of the burden of cardiovascular disease attributable to modifiable risk factors and cost-effectiveness analysis of preventative interventions to reduce this burden in Argentina. BMC public health. 2010;10:627. doi: 10.1186/1471-2458-10-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [accessed Aug-28 2014];Segunda Encuesta Nacional de Factores de Riesgo. para Enfermedades no Transmisibles. 2009 http://www.msal.gov.ar/fesp/descargas_home/seg_encuesta_nac_factores_riesgo_2011.pdf.
- 8. [accessed Sep-15 2014];Bringing science and development together through news and analysis. http://www.scidev.net/global/funding/news/argentina-unveils-plan-to-boost-science-investment-1.html.
- 9.Belizan JM, Rubinstein A, Rubinstein F, Althabe F. Research: increasing value, reducing waste. Lancet. 2014;383(9923):1125–6. doi: 10.1016/S0140-6736(14)60562-6. [DOI] [PubMed] [Google Scholar]
- 10.Jahangir E, Comande D, Rubinstein A. Cardiovascular disease research in Latin America: a comparative bibliometric analysis. World journal of cardiology. 2011;3(12):383–7. doi: 10.4330/wjc.v3.i12.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The World Bank. [accessed Sep-5 2014]; http://data.worldbank.org/income-level/UMC.
- 12. [Accessed on September 6th, 2014];MRC Developing and evaluating complex interventions:new guidance. Available at: http://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/
- 13.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. Bmj. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakley A, Strange V, Bonell C, Allen E, Stephenson J, Team RS. Process evaluation in randomised controlled trials of complex interventions. Bmj. 2006;332(7538):413–6. doi: 10.1136/bmj.332.7538.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA: the journal of the American Medical Association. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahim S, Smith GD. Systematic review of randomised controlled trials of multiple risk factor interventions for preventing coronary heart disease. Bmj. 1997;314(7095):1666–74. doi: 10.1136/bmj.314.7095.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohl LF, Crutzen R, de Vries NK. Online prevention aimed at lifestyle behaviors: a systematic review of reviews. Journal of medical Internet research. 2013;15(7):e146. doi: 10.2196/jmir.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Istepanian RLS, Pattichis CS. Emerging Mobile Health Systems. 2006. [Google Scholar]
- 19.P G. A Mobile Agent. [accessed Sep-15 2014];Approach for Ubiquitous and Personalized eHealth Information Systems. 2005 http://scrat.cs.ucy.ac.cy/pgerman/publications/index.aspx?category=Conferences%20and%20Workshops.
- 20.Mechael PBH, Kaonga N, et al. [accessed Sep 15 2014];Barriers and gaps affecting mHealth in low and middle income countries: Policy white paper. 2010 http://www.globalproblems-globalsolutions-files.org/pdfs/mHealth_Barriers_White_Paper.pdf.
- 21.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2009;15(3):231–40. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 22.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiologic reviews. 2010;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. American journal of preventive medicine. 2009;36(2):165–73. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Beratarrechea A, Lee AG, Willner JM, Jahangir E, Ciapponi A, Rubinstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemedicine journal and e-health: the official journal of the American Telemedicine Association. 2014;20(1):75–82. doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. Journal of consulting and clinical psychology. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 26.Beratarrechea AD-CF, Fernandez A, Kanter R, Letona P, Martinez H, Miranda J, Ramirez-Zea M, Rubinstein A. [accessed Sep 6 2014];A Feasibility and Acceptability Study of the Provision of mHealth Interventions for Behavior Change in Prehypertensive Subjects in Argentina, Guatemala and Peru. http://www.medetel.eu/download/2012/Proceedings_2012_CD_Contents.pdf.
- 27.Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5. 4 million participants. Lancet. 2011;377(9765):568–77. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 28.Lawes CM, Vander Hoorn S, Rodgers A International Society of H. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 29.Rubinstein AL, Irazola VE, Poggio R, et al. Detection and follow-up of cardiovascular disease and risk factors in the Southern Cone of Latin America: the CESCAS I study. BMJ open. 2011;1(1):e000126. doi: 10.1136/bmjopen-2011-000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinstein AIV, Poggio R, Lanas F, Calandrelli M, Ponzo J, Olivera H, Seron P, Bazzano L, Gutierrez L, He J. [accessed Sep 15 2014];Prevalence, Awareness, Treatment, and Control of Hypertension in the Southern Cone of Latin America. 2013 http://circ.ahajournals.org/cgi/content/meeting_abstract/127/12_MeetingAbstracts/AP317.
- 31.Bernztein RDI. Uso de medicamentos en hipertensión arterial en el primer nivel de atención pública argentina: La experiencia del programa remediar. Revista Argentina de Cardiología. 2009;77:187–95. [Google Scholar]
- 32.Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Medical care. 2006;44(7):646–57. doi: 10.1097/01.mlr.0000220260.30768.32. [DOI] [PubMed] [Google Scholar]
- 33.Walsh JM, Sundaram V, McDonald K, Owens DK, Goldstein MK. Implementing effective hypertension quality improvement strategies: barriers and potential solutions. Journal of clinical hypertension. 2008;10(4):311–6. doi: 10.1111/j.1751-7176.2008.07425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. The Journal of family practice. 1994;38(2):166–71. [PubMed] [Google Scholar]
- 35.Lin ND, Martins SB, Chan AS, et al. Identifying barriers to hypertension guideline adherence using clinician feedback at the point of care. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium; 2006. pp. 494–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Ogedegbe G. Barriers to optimal hypertension control. Journal of clinical hypertension. 2008;10(8):644–6. doi: 10.1111/j.1751-7176.2008.08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilbert JJ. The World Health Report 2006: working together for health. Education for health. 2006;19(3):385–7. doi: 10.1080/13576280600937911. [DOI] [PubMed] [Google Scholar]
- 38.Love MB, Gardner K, Legion V. Community health workers: who they are and what they do. Health education & behavior: the official publication of the Society for Public Health Education. 1997;24(4):510–22. doi: 10.1177/109019819702400409. [DOI] [PubMed] [Google Scholar]
- 39.Witmer A, Seifer SD, Finocchio L, Leslie J, O’Neil EH. Community health workers: integral members of the health care work force. American journal of public health. 1995;85(8 Pt 1):1055–8. doi: 10.2105/ajph.85.8_pt_1.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brownstein JN, Bone LR, Dennison CR, Hill MN, Kim MT, Levine DM. Community health workers as interventionists in the prevention and control of heart disease and stroke. American journal of preventive medicine. 2005;29(5 Suppl 1):128–33. doi: 10.1016/j.amepre.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. American journal of preventive medicine. 2007;32(5):435–47. doi: 10.1016/j.amepre.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA: the journal of the American Medical Association. 2013;310(7):699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Annals of internal medicine. 2009;151(9):593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- 44.Mills KT, Rubinstein A, Irazola V, et al. Comprehensive approach for hypertension control in low-income populations: rationale and study design for the hypertension control program in Argentina. The American journal of the medical sciences. 2014;348(2):139–45. doi: 10.1097/MAJ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Organization WH. [accessed Sep 15 2014];Global health risks: mortality and burden of disease attributable to selected major risks. 2009 http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf.
- 46.Farzadfar F, Finucane MM, Danaei G, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3. 0 million participants. Lancet. 2011;377(9765):578–86. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 47.Ferrante DLBKj, King A, Virgolini M, Laspiur S. Encuesta Nacional de Factores de Riesgo 2009: evolución de la epidemia de enfermedades crónicas no transmisibles en Argentina. Revista Argentina de Salud Pública. 2011;2(6):8. [Google Scholar]
- 48.Rubinstein A, Colantonio L, Bardach A, et al. Estimate of the cardiovascular disease burden attributable to modifiable risk factors in Argentina. Revista panamericana de salud publica = Pan American journal of public health. 2010;27(4):237–45. doi: 10.1590/s1020-49892010000400001. [DOI] [PubMed] [Google Scholar]
- 49.Hickling J, Rogers S, Nazareth I. Barriers to detecting and treating hypercholesterolaemia in patients with ischaemic heart disease: primary care perceptions. The British journal of general practice: the journal of the Royal College of General Practitioners. 2005;55(516):534–8. [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. The Cochrane database of systematic reviews. 2007;(4):CD000409. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. The Cochrane database of systematic reviews. 2012;6:CD000259. doi: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schedlbauer A, Davies P, Fahey T. Interventions to improve adherence to lipid lowering medication. The Cochrane database of systematic reviews. 2010;(3):CD004371. doi: 10.1002/14651858.CD004371.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Gwatkin DR. IMCI: what can we learn from an innovation that didn’t reach the poor? Bulletin of the World Health Organization. 2006;84(10):768. doi: 10.2471/blt.06.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangham LJ, Hanson K. Scaling up in international health: what are the key issues? Health policy and planning. 2010;25(2):85–96. doi: 10.1093/heapol/czp066. [DOI] [PubMed] [Google Scholar]
- 55.Yamey G. Scaling up global health interventions: a proposed framework for success. PLoS medicine. 2011;8(6):e1001049. doi: 10.1371/journal.pmed.1001049. [DOI] [PMC free article] [PubMed] [Google Scholar]