Abstract
Background
Epicardial fat may play a role in the pathogenesis of coronary artery disease (CAD). We explored the relationship of epicardial fat volume (EFV) with the presence and severity of CAD or myocardial perfusion abnormalities in a diverse, symptomatic patient population.
Methods and Results
Patients (n=380) with known or suspected CAD who underwent 320-detector row CT angiography, nuclear stress perfusion imaging, and clinically driven invasive coronary angiography for the CORE320 international study were included. EFV was defined as adipose tissue within the pericardial borders as assessed by CT utilizing semi-automatic software. We used linear and logistic regression models to assess the relationship of EFV with coronary calcium score, stenosis severity by quantitative coronary angiography (QCA), and myocardial perfusion abnormalities by SPECT.
Median EFV among patients (median age 62.6 years) was 102 cm3 [interquartile range 53]. Calcium score ≥ 1 was present in 83% of patients with 59% having ≥ 1 coronary artery stenosis of ≥ 50% by QCA, and 49% having abnormal myocardial perfusion results by SPECT. There were no significant associations between EFV and CACS, presence severity of ≥ 50% stenosis by QCA, or abnormal myocardial perfusion by SPECT.
Conclusions
In a diverse population of symptomatic patients referred for invasive coronary angiography, we did not find associations of epicardial fat volume with the presence and severity of coronary artery disease or with myocardial perfusion abnormalities. The clinical significance of quantifying epicardial fat volume remains uncertain but may relate to the pathophysiology of acute coronary events rather than the presence of atherosclerotic disease.
Keywords: epicardial fat, pericardial fat, coronary artery disease, coronary artery calcification, coronary artery stenosis, myocardial ischemia
There is a considerable interest in the reported associations between epicardial fat volume (EFV) and coronary plaque burden, number of coronary arterial stenoses, and presence of provokable myocardial ischemia.1–16 These relationships appear to remain significant even when adjusting for body-mass-index (BMI) or traditional risk factors.17 The mechanisms underlying these associations have not been elucidated but current hypotheses implicate the release of free fatty acids and triglycerides from epicardial fat as a source of inflammatory cytokines.18,19 Specifically, the vasa vasorum in the peripheral arterial wall arising from smaller circular and parallel branches of the epicardial coronary arteries provides a ready source of cytokines which may stimulate inflammation and recruitment of macrophages and B-lymphocytes.19,20,21 These inflammatory processes are connected to the development of atherosclerosis and adverse clinical events.19,22
On the other hand, some clinical studies did not find significant associations between EFV and coronary artery disease (CAD), and other studies revealed results which were no longer significant after adjustment for established risk factors.23, 24 Furthermore, the majority of clinical studies reporting significant associations of EFV with CAD were derived from community-based patient samples with low risk profiles. Thus, there is conflicting evidence whether EFV indeed is an independent risk factor for CAD.25
The purpose of this investigation was to explore the association of EFV with the presence and severity of coronary artery disease as well as myocardial perfusion abnormalities among a diverse, symptomatic population using rigorous methodology.
Methods
Study Population
Three hundred and eighty-one patients who were enrolled for the CORE320 multicenter study were included for this analysis. The details of the CORE320 study design have been previously described. 26–28 Briefly, patients aged 45 to 85 with suspected or known CAD who were referred for clinically indicated invasive coronary angiography were enrolled at 16 centers in Brazil, Canada, Denmark, Germany, Japan, Netherlands, Singapore, and the USA. All patients underwent 320-row CT for coronary artery calcium scanning (CACS), CT coronary angiography, and nuclear stress myocardial perfusion imaging within 60 days of invasive coronary angiography. All enrolled participants provided informed consent approved by institutional and central review boards.
Covariates
Race, gender, age, and smoking status were reported for all study participants. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive medications. Weight (kg) was measured with the use of a standard balance-beam scale. Body mass index was calculated as weight (kg) divided by height squared (in m2). Diabetes was defined as a fasting glucose level ≥ 126 mg/dL or use of medication for diabetes. Dyslipidemia was defined as total cholesterol >200 mg/dL, low-density lipoprotein cholesterol ≥ 130 mg/dL, high-density lipoprotein cholesterol <40 mg/dL for men and high-density lipoprotein cholesterol <50 mg/dL for women, or use of lipid-lowering medications.
CT Imaging and Analysis of Epicardial Fat Volume
Details of CT acquisition and analyses were described elsewhere.26–28
Briefly, after obtaining anteroposterior and lateral scanograms CACS imaging was performed using prospective ECG triggering over a single heartbeat with a gantry rotation of 0.35 seconds, 3-mm slice collimation, tube voltage of 120 kV, and tube current adjusted according to body weight. Coronary calcification was quantified using the Agatston method. A calcium score of ≥ 1 was defined as abnormal (= categorical outcome). The calcium score was also used as a continuous outcome to assess the relationship between EFV and coronary calcification. We defined epicardial fat as all adipose tissue enclosed by the pericardium, including the epicardial fat surrounding the coronary arteries. Epicardial fat quantification was performed using a dedicated software (Virtual Place Advance, Aze Ltd, Tokyo Japan). Image data were processed as follows: First, the upper heart limit - marked by bifurcation of the pulmonary trunk - and lower slice limit - identified as the last slice containing any portion of the heart - were identified from a visual review of the CT images. Next, an experienced reader (18 years in CT interpretation) scrolled through the slices between upper and lower heart limit and traced the pericardium in a transverse view with the aid of the software (Figure 1). Following the pericardial tracing, epicardial fat quantification occurred automatically based on voxel Hounsfield unit (HU) values. Contiguous voxels between the HU limits of (−195, −45) were defined as fat voxels.1, 2, 14 EFV was determined by the sum of cross-sectional areas of fat multiplied by slice thickness (3mm) (Figure 1). The operator of the software was blinded to any clinical information or study results. Inter-observer agreement for our method of EFV quantification was tested among two observers in 14 patients who were enrolled for the run-in phase of the CORE320 study revealing a mean difference of 1.5 cm3 (p=0.61) and no heterogeneity of variance (standard deviation ratio 0.993, p=0.90) by Bland-Altman analysis.
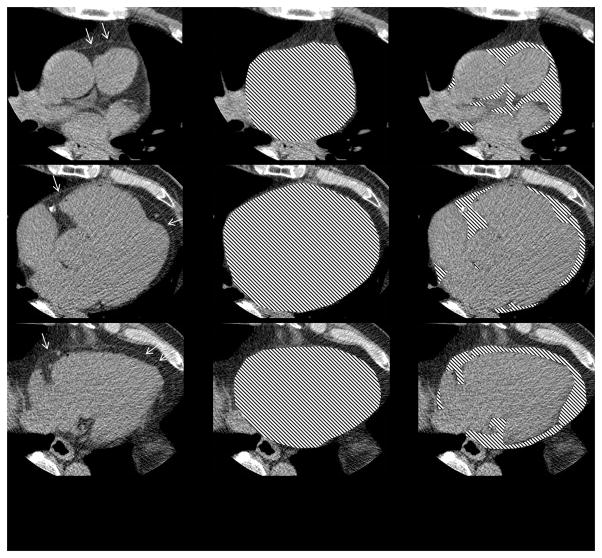
Figure 1. Epicardial Fat Volume Quantification.
The figure illustrates our method of epicardial fat volume quantification using a semi-automated software. After manually tracing the pericardial borders, fat volume is derived based on Hounsfield unit attenuation within the region of interest.
Left: The white arrows point to the pericardial sac as a thin band enveloping the heart. Middle: The pericardial sac is traced by an expert observer.
Right: The overlay represents epicardial fat enclosed by the pericardium.
Invasive Coronary Angiography Data Acquisition and Data Analysis
Invasive coronary angiography was performed using standard angiographic techniques within the 60 days following CT image acquisition and was clinically driven. Coronary angiographic images were saved in digital imaging and communication in medicine (DICOM) format and forwarded to an independent angiographic core laboratory for analysis. The coronary tree segmentation for invasive coronary angiography was previously described.29, 30 Quantitative coronary angiography (QCA) was performed using edge-detection techniques (CAAS II QCA Research version 2.0.1 software, PIE Medical Imaging, Maastricht, the Netherlands). The most severe stenosis within each coronary segment was analyzed, with quantitative assessment performed for all stenoses which were deemed ≥ 30% by visual assessment. Two outcome variables were used: a categorical threshold for significant stenosis defined as ≥ 50% diameter stenosis by QCA and stenosis as a continuous outcome. Since no quantitative measurements were made for stenoses <30%, 15% was imputed for values which were visually assessed as 1–29% narrowed and occluded vessels were marked as 100%.
Nuclear Perfusion Imaging
All SPECT cameras used in the study were required to undergo accreditation for quality assurance before commencement and throughout the enrollment period. The SPECT qualification process was multifocal, involving evaluation of both camera physics and image quality. To account for variability in imaging equipment and image-acquisition techniques, the nuclear core laboratory evaluated images for quality control following guidelines of the American Society of Nuclear Cardiology.31
Myocardial territories were analyzed by SPECT for rest and stress myocardial perfusion abnormalities with a 4-point severity and reversibility-score using a 13-territory model.26 The summed stress score (SSS) was defined as the sum of abnormal myocardial segments at stress phase.32 In the analysis, artifacts did not contribute to the summed stress score (SSS) and therefore a SSS ≥ 1 defined an abnormal SPECT study in accordance with methods used for large multicenter studies and independent core laboratories.33 In addition, SSS was used as a continuous outcome measure to assess the relationship between EFV and myocardial perfusion abnormalities.
Statistical Analysis
Values are presented as median, and the 25th and 75th percentile quartiles (quartiles 1 and 3). Multivariable regression models were generated to evaluate the relationship between EFV with plaque burden, coronary artery stenosis, and ischemia. Because each outcome was positive and highly skewed, we used logistic regression models with clinically meaningful cut points as well as median regression models to assess continuous association. For the logistic regression model outcomes, we defined significant coronary artery stenosis as ≥ 50% stenosis by QCA, coronary calcified plaque as Agatston calcium score > 0, and myocardial hypoperfusion as SPECT SSS ≥ 1. Each model was fit in three ways: (1) a univariate model with only EFV as a predictor; (2) model 1 adjusted for age, sex and race; and (3) model 2 additionally adjusted for BMI, hypertension, diabetes mellitus, dyslipidemia, smoking status, family history of CAD, and (in calcified plaque and myocardial hypoperfusion models only) significant coronary artery stenosis. Group comparisons for differences in EFV were performed using Kruskal-Wallis ANOVA. P-value are two-sided and values less than 0.05 were considered statistically significant. No adjustments were made for multiple testing. The statistical analysis was performed with SAS 9.2 (SAS Institute).
Results
Descriptive Results
Clinical characteristics of study patients are listed in Table 1. Of 381 patients in the final CORE320 cohort we had to exclude one individual because of corrupt imaging data resulting in 380 patients for this analysis. The 380 subjects consisted of 251 men (66%) with a median age of 62.0 years (quartiles 1 and 3 of 55.7, 68.4, respectively). The CORE320 study population consists of predominantly intermediate risk patients (67%) with 31% having known CAD or high pretest probability.32 Median EFV was 102 cm3 [quartiles 1 and 3 of 78,131, respectively]. Distribution of EFV among patients is shown in Figure 2. Table 2 lists the median EFV for subgroups according to risk factors and ethnicity. Notably, obese patients had higher EFV compared to normal weight patients, and African-Americans had lower EFV than Caucasian or Asian patients.
Table 1.
Baseline Characteristics
| Characteristic | Non-obese (BMI< 30) | Obese (BMI=>30) | Overall |
|---|---|---|---|
| Median (Q1, Q3) or N (%) | N=284 | N=96 | N=380 |
| Age, in years | 62.6 (55.7,68.8) | 60.0 (55.3, 66.6) | 62.0 (55.7, 68.4) |
| Male Gender | 195 (69) | 56 (58) | 251 (66) |
| Race | |||
| Asian | 113 (40) | 12 (13) | 125 (33) |
| African American | 27 (10) | 16 (17) | 43 (11) |
| Caucasian | 144 (51) | 68 (71) | 212 (56) |
| Coronary Risk Factor | |||
| Hypertension | 212 (75) | 84 (88) | 296 (78) |
| Diabetes | 95 (33) | 35 (36) | 130 (34) |
| Dyslipidemia | 190 (68) | 63 (68) | 253 (68) |
| Previous myocardial infarction | 80 (28) | 23 (24) | 103 (27) |
| Smoking Status | |||
| Current Smoker | 53 (20) | 11 (12) | 64 (18) |
| Former Smoker | 106 (39) | 26 (28) | 132 (36) |
| Never Smoker | 111 (41) | 56 (60) | 167 (46) |
| Family history of coronary artery disease | 115 (43) | 46 (50) | 161 (45) |
| Epicardial Fat Volume | 96.0 (72.0,126.0) | 122.0 (92.0,160.0) | 102.0 (78.0,131.0) |
| CACS* and CT angiography (CTA) | |||
| Positive Calcium Score (CACS*>0) | 235 (84) | 78 (82) | 313 (83) |
| Coronary artery plaque with stenosis (CTA≥50%) | 195 (69) | 54 (56) | 249 (66) |
| Invasive angiography | |||
| Coronary artery stenosis (QCA†≥50%) | 183 (64) | 45 (47) | 228 (60) |
| Coronary artery severe stenosis (QCA†≥70%) | 138 (49) | 35 (36) | 173 (46) |
| Coronary artery occlusion (QCA†=100%) | 54 (19) | 12 (13) | 66 (17) |
| Single photon emission computed tomography | |||
| Myocardial hypo-perfusion (SSS‡>0) | 142 (50) | 46 (48) | 188 (49) |
| Myocardial ischemia (SDS§ >0) | 217 (76) | 62 (65) | 279 (73) |
CACS: coronary artery calcium score
QCA: quantitative coronary angiography
SSS: summed Stress score
SDS: summed difference score
Figure 2. Distribution of Epicardial Fat Volume in the Study Population.
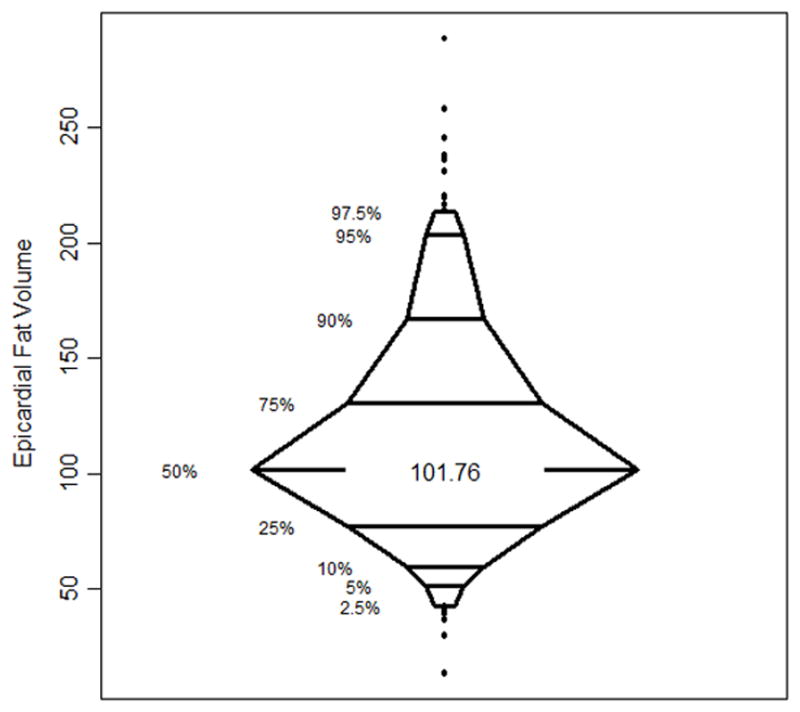
The percentile box plot shows the distribution of epicardial fat volume among the 380 study participants.
Table 2.
Epicardial Fat Volume by Risk Factors and Ethnicity
| Group | N | Epicardial Fat Volume (Median [Q1, Q3]) | p-value** |
|---|---|---|---|
| All patients | 380 | 101.76 [77.58, 131.02] | |
| Diabetes | |||
| Yes | 130 | 100.02 [71.88, 127.89] | 0.34 |
| No | 250 | 103.12 [79.07, 134.53] | |
| Smoking | |||
| Never | 167 | 96.81 [77.61, 123.70] | 0.053 |
| Former | 132 | 108.69 [84.29, 140.13] | |
| Current | 64 | 96.95 [71.03, 132.45] | |
| Hypertension | |||
| Yes | 296 | 103.60 [80.55, 132.05] | 0.01 |
| No | 82 | 90.67 [68.44, 127.60] | |
| Dyslipidemia | |||
| Yes | 253 | 101.80 [78.41, 130.77] | 0.74 |
| No | 119 | 101.58 [71.81, 132.47] | |
| Family History of CAD* | |||
| Yes | 161 | 101.12 [80.40, 130.95] | 0.98 |
| No | 196 | 102.72 [74.94, 131.33] | |
| Previous MI | |||
| Yes | 103 | 104.83 [76.89, 140.09] | 0.41 |
| No | 277 | 100.76 [77.61, 130.95] | |
| BMI | |||
| Normal (<25) | 127 | 94.10 [68.70, 126.15] | <0.0001 |
| Overweight (25–<30) | 157 | 99.28 [78.78, 126.78] | |
| Obese (≥30) | 96 | 122.04 [92.10, 159.52] | |
| Race | |||
| Caucasian | 212 | 103.33 [81.56, 134.76] | 0.01 |
| Asian | 125 | 103.10 [77.55, 138.66] | |
| African-American | 43 | 84.30 [68.44, 111.96] |
p-values calculated by Kruskal-Wallis ANOVA
CAD: Coronary artery disease
Relationship of Epicardial Fat Volume with Coronary Artery Calcification
A total of 313 (83%) patients had a coronary calcium score of greater than 0. Univariate and multivariate analyses suggested no association of EFV with the presence of coronary artery calcium (Table 3). Median regression analysis revealed no significant relationship between calcium score and EFV after adjusting for risk factors. A scatterplot with smoothing demonstrates the relationship between calcium score and EFV in Figure 3. The results for median regression models are shown in Table 4.
Table 3.
Odds Ratios for Calcified Atherosclerosis (Calcium score >0)
| Logistic Regression Models | Odds ratio | Lower CL | Upper CL | p |
|---|---|---|---|---|
| Model 1: Unadjusted | ||||
| Epicardial fat volume (EFV) | 1.005 | 0.999 | 1.012 | 0.1259 |
| Model 2: includes age, gender and race | ||||
| Age | 1.111 | 1.065 | 1.159 | <.0001 |
| Gender (male vs. female) | 3.254 | 1.790 | 5.918 | 0.0001 |
| Race (Caucasian vs. Asian ) | 0.929 | 0.455 | 1.895 | 0.8388 |
| Race (African-American vs. Asian) | 0.572 | 0.225 | 1.455 | 0.2410 |
| Epicardial fat volume (EFV) | 0.999 | 0.992 | 1.006 | 0.8106 |
| Model 3: all predictors | ||||
| Age | 1.117 | 1.058 | 1.179 | <.0001 |
| Gender (male vs. female) | 2.205 | 1.042 | 4.664 | 0.0386 |
| Race (Caucasian vs. Asian ) | 0.875 | 0.346 | 2.214 | 0.7780 |
| Race (African-American vs. Asian) | 0.441 | 0.131 | 1.488 | 0.1871 |
| BMI* (obese [BMI≥30] vs. normal [BMI<25]) | 2.373 | 0.797 | 7.068 | 0.1207 |
| BMI (overweight [BMI 25–29] vs. normal [BMI<25]) | 1.656 | 0.673 | 4.074 | 0.2718 |
| Hypertension | 2.989 | 1.255 | 7.116 | 0.0134 |
| Dyslipidemia | 1.036 | 0.466 | 2.301 | 0.9308 |
| Family History of coronary artery disease | 1.431 | 0.685 | 2.988 | 0.3405 |
| Previous myocardial infarction | 1.657 | 0.566 | 4.857 | 0.3571 |
| Diabetes mellitus | 1.206 | 0.539 | 2.698 | 0.6491 |
| Smoking (current vs. never) | 2.407 | 0.890 | 6.508 | 0.0834 |
| Smoking (former vs. never) | 1.390 | 0.587 | 3.288 | 0.4537 |
| Coronary artery disease (QCA†≥50) | 9.842 | 4.009 | 24.159 | <.0001 |
| Epicardial fat volume (EFV) | 0.997 | 0.987 | 1.007 | 0.5412 |
Odds ratios relate to 1 unit change of EFV.
BMI: body mass index
QCA: quantitative coronary angiography
Figure 3. Relationship between Epicardial Fat Volume and Coronary Calcium Score.
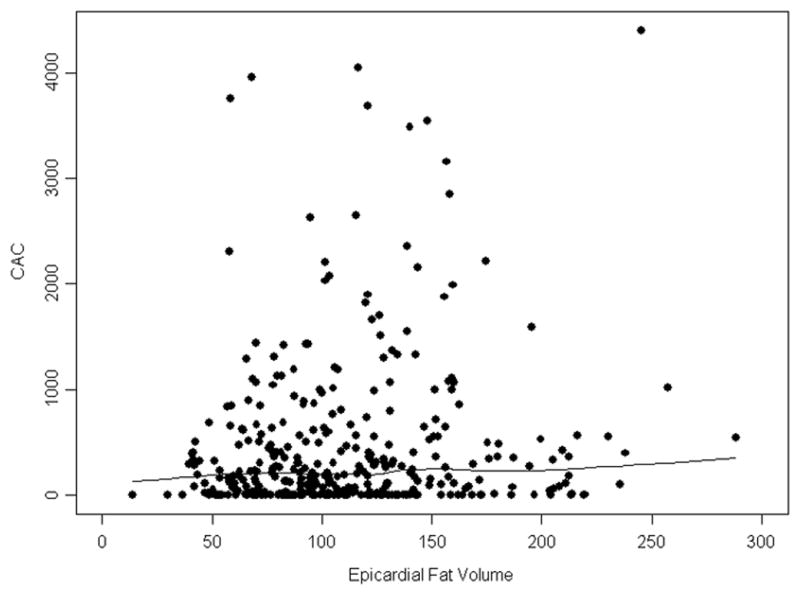
Shown is a scatterplot and Lowess smoothing of epicardial fat volume (EFV) and coronary artery calcium score (CACS) revealing no significant association.
Table 4.
Median Regression for Coronary Calcium Score
| Median Regression Models | Estimate | Lower CL | Upper CL | p |
|---|---|---|---|---|
| Model 1: Unadjusted | ||||
| Epicardial fat volume (EFV) | 1.4991 | 0.1635 | 2.8347 | 0.0279 |
| Model 2: includes age, gender and race | ||||
| Age | 7.4310 | 3.1887 | 11.6733 | 0.0006 |
| Gender (male vs. female) | 177.5607 | 120.7255 | 234.3959 | <.0001 |
| Race (Caucasian vs. Asian ) | 4.8345 | −58.8501 | 68.5191 | 0.8814 |
| Race (African-American vs. Asian) | −40.8245 | −111.636 | 29.9867 | 0.2577 |
| Epicardial fat volume (EFV) | 0.1528 | −0.5923 | 0.8980 | 0.6870 |
| Model 3: all predictors | ||||
| Age | 3.1339 | −0.5691 | 6.8368 | 0.0969 |
| Gender (male vs. female) | 77.0303 | 24.6408 | 129.4197 | 0.0041 |
| Race (Caucasian vs. Asian ) | 4.0177 | −60.9330 | 68.9683 | 0.9032 |
| Race (African-American vs. Asian) | −42.0501 | −118.457 | 34.3563 | 0.2797 |
| BMI (obese [BMI≥30] vs. normal [BMI<25]) | 29.1242 | −38.1791 | 96.4275 | 0.3952 |
| BMI (overweight [BMI 25–29] vs. normal [BMI<25]) | 0.8471 | −58.4900 | 60.1842 | 0.9776 |
| Hypertension | 32.4875 | −29.8496 | 94.8247 | 0.3060 |
| Dyslipidemia | 24.0807 | −24.7714 | 72.9329 | 0.3329 |
| Family History of coronary artery disease | 30.7022 | −18.9112 | 80.3155 | 0.2243 |
| Previous myocardial infarction | −21.7638 | −104.540 | 61.0119 | 0.6053 |
| Diabetes mellitus | −0.5811 | −61.9160 | 60.7539 | 0.9851 |
| Smoking (current vs. never) | 12.1613 | −48.8039 | 73.1266 | 0.6950 |
| Smoking (former vs. never) | −12.4693 | −71.5221 | 46.5835 | 0.6781 |
| Coronary artery disease († QCA≥50) | 255.4947 | 187.1849 | 323.8045 | <.0001 |
| Epicardial fat volume (EFV) | 0.0972 | −0.6022 | 0.7965 | 0.7848 |
Median calcium score for 1 unit change of EFV.
BMI: body mass index;
QCA: quantitative coronary angiography
Relationship of Epicardial Fat Volume with Obstructive CAD
A total of 225 (59%) patients had one or more coronary artery stenosis of at least 50% by QCA. No association between EFV and the presence or absence of obstructive CAD was noted when using QCA as continuous outcome (Figure 4) or on univariate and multivariate analyses using a 50% threshold by QCA for defining a significant stenosis (Table 5). The results for median regression models are shown in Table 6.
Figure 4. Relationship between Epicardial Fat Volume and Coronary Artery Stenoses.
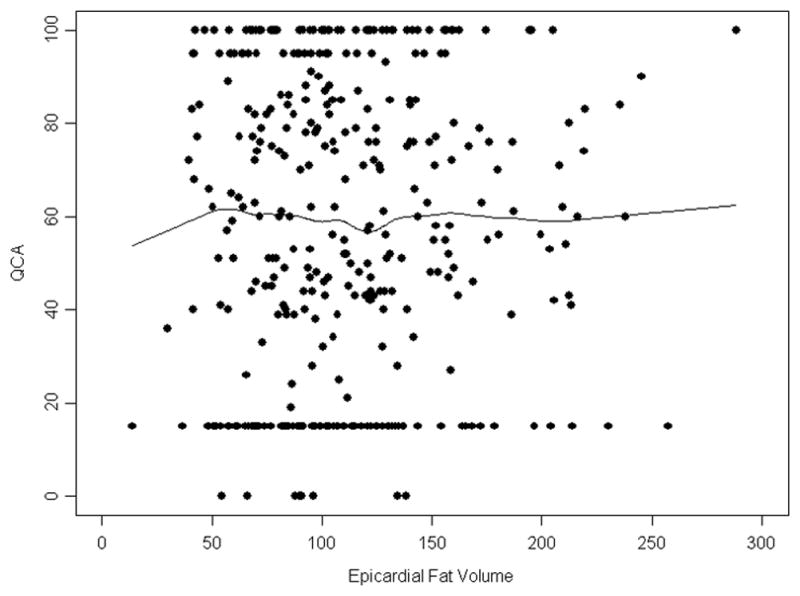
Shown is a scatterplot and Lowess smoothing of epicardial fat volume (EFV) and presence of a ≥50% stenosis by quantitative coronary angiography (QCA) stenosis revealing no significant association. Please note clustering of data points at the extreme ranges due to imputation of values less than 30% and at 100%.
Table 5.
Odds Ratios for Obstructive Coronary Artery Disease (≥1 Stenosis ≥50% by QCA*)
| Logistic Regression Models | Odds ratio | Lower CL | Upper CL | p |
|---|---|---|---|---|
| Model 1: Unadjusted | ||||
| Epicardial fat volume (EFV) | 1.002 | 0.997 | 1.006 | 0.4903 |
| Model 2: includes age, gender and race | ||||
| Age | 1.027 | 1.000 | 1.055 | 0.0533 |
| Gender (male vs. female) | 3.188 | 2.026 | 5.016 | <.0001 |
| Race (Caucasian vs. Asian ) | 0.783 | 0.478 | 1.281 | 0.3299 |
| Race (African-American vs. Asian) | 0.528 | 0.246 | 1.132 | 0.1006 |
| Epicardial fat volume (EFV) | 0.998 | 0.993 | 1.003 | 0.5009 |
| Model 3: all predictors | ||||
| Age | 1.033 | 0.999 | 1.067 | 0.0582 |
| Gender (male vs. female) | 3.219 | 1.885 | 5.498 | <.0001 |
| Race (Caucasian vs. Asian ) | 1.062 | 0.569 | 1.985 | 0.8492 |
| Race (African-American vs. Asian) | 0.582 | 0.234 | 1.450 | 0.2455 |
| BMI* (obese [BMI≥30] vs. normal [BMI<25]) | 0.507 | 0.235 | 1.094 | 0.0834 |
| BMI (overweight [BMI 25–29] vs. normal [BMI<25]) | 1.133 | 0.609 | 2.107 | 0.6939 |
| Hypertension | 1.644 | 0.844 | 3.203 | 0.1437 |
| Dyslipidemia | 1.952 | 1.110 | 3.431 | 0.0201 |
| Family History of coronary artery disease | 1.323 | 0.787 | 2.225 | 0.2908 |
| Previous myocardial infarction | 2.970 | 1.532 | 5.759 | 0.0013 |
| Diabetes mellitus | 1.539 | 0.897 | 2.641 | 0.1173 |
| Smoking (current vs. never) | 0.856 | 0.427 | 1.715 | 0.6615 |
| Smoking (former vs. never) | 0.986 | 0.551 | 1.765 | 0.9616 |
| Epicardial fat volume (EFV) | 1.000 | 0.993 | 1.006 | 0.9740 |
Odds ratios relate to 1 unit change of EFV.
BMI: body mass index
Table 6.
Median regression for Obstructive Coronary Artery Disease
| Median Regression Models | Estimate | Lower CL | Upper CL | p |
|---|---|---|---|---|
| Model 1: Unadjusted | ||||
| Epicardial fat volume (EFV) | −0.0156 | −0.1316 | 0.1004 | 0.7913 |
| Model 2: includes age, gender and race | ||||
| Age | 0.4134 | −0.1582 | 0.9851 | 0.1558 |
| Gender (male vs. female) | 31.8158 | 23.2695 | 40.3621 | <.0001 |
| Race (Caucasian vs. Asian ) | 0.6680 | −9.5785 | 10.9145 | 0.8981 |
| Race (African-American vs. Asian) | −20.6831 | −34.1101 | −7.2560 | 0.0026 |
| Epicardial fat volume (EFV) | −0.0642 | −0.1750 | 0.0467 | 0.2558 |
| Model 3: all predictors | ||||
| Age | 0.2490 | −0.2838 | 0.7817 | 0.3586 |
| Gender (male vs. female) | 30.0674 | 20.3262 | 39.8085 | <.0001 |
| Race (Caucasian vs. Asian ) | 3.0629 | −7.7204 | 13.8463 | 0.5767 |
| Race (African-American vs. Asian) | −8.7321 | −25.1360 | 7.6717 | 0.2957 |
| BMI* (obese [BMI≥30] vs. normal [BMI<25]) | −8.0159 | −21.5971 | 5.5653 | 0.2464 |
| BMI (overweight [BMI 25–29] vs. normal [BMI<25]) | 4.2220 | −6.4752 | 14.9193 | 0.4380 |
| Hypertension | 19.9254 | 8.5336 | 31.3172 | 0.0007 |
| Dyslipidemia | 10.2237 | 0.1095 | 20.3378 | 0.0476 |
| Family History of coronary artery disease | 9.0070 | 0.3296 | 17.6845 | 0.0420 |
| Previous myocardial infarction | 14.0616 | 4.6043 | 23.5189 | 0.0037 |
| Diabetes mellitus | 6.2628 | −3.4387 | 15.9644 | 0.2050 |
| Smoking (current vs. never) | −3.0321 | −14.8693 | 8.8051 | 0.6146 |
| Smoking (former vs. never) | 5.0142 | −4.8388 | 14.8672 | 0.3175 |
| Epicardial fat volume (EFV) | −0.0885 | −0.1876 | 0.0106 | 0.0799 |
Median calcium score for 1 unit change of EFV.
BMI: body mass index.
Relationship of Epicardial Fat Volume with Myocardial Perfusion Defects
A total of 188 (49%) patients had abnormal myocardial perfusion by SPECT. Univariate and multivariate analyses suggested no association of EFV with myocardial perfusion abnormalities (Table 7). A weak trend for a negative relationship between EFV and SSS is visible in the scatterplot with Lowess smoothing (Figure 5), though median regression analysis did not find this effect statistically significant (Table 8).
Table 7.
Odds Ratios for Abnormal Myocardial Perfusion (SPECT SSS*>0)
| Logistic Regression Models | Odds ratio | Lower CL | Upper CL | p |
|---|---|---|---|---|
| Model 1: Unadjusted | ||||
| Epicardial fat volume (EFV) | 0.996 | 0.992 | 1.001 | 0.1341 |
| Model 2: includes age, gender and race | ||||
| Age | 0.976 | 0.951 | 1.001 | 0.0621 |
| Gender (male vs. female) | 2.282 | 1.454 | 3.582 | 0.0003 |
| Race (Caucasian vs. Asian ) | 1.517 | 0.950 | 2.422 | 0.0810 |
| Race (African-American vs. Asian) | 1.172 | 0.561 | 2.451 | 0.6727 |
| Epicardial fat volume (EFV) | 0.996 | 0.991 | 1.001 | 0.0972 |
| Model 3: all predictors | ||||
| Age | 0.967 | 0.936 | 0.999 | 0.0431 |
| Gender (male vs. female) | 1.706 | 0.991 | 2.938 | 0.0541 |
| Race (Caucasian vs. Asian ) | 1.807 | 0.974 | 3.354 | 0.0607 |
| Race (African-American vs. Asian) | 1.532 | 0.621 | 3.783 | 0.3548 |
| BMI†(obese [BMI≥30] vs. normal [BMI<25]) | 0.749 | 0.348 | 1.610 | 0.4591 |
| BMI (overweight [BMI 25–29] vs. normal [BMI<25]) | 0.604 | 0.327 | 1.116 | 0.1071 |
| Hypertension | 1.447 | 0.738 | 2.838 | 0.2817 |
| Dyslipidemia | 0.703 | 0.398 | 1.242 | 0.2251 |
| Family History of coronary artery disease | 1.161 | 0.700 | 1.924 | 0.5629 |
| Previous myocardial infarction | 3.153 | 1.679 | 5.921 | 0.0004 |
| Diabetes mellitus | 1.537 | 0.907 | 2.604 | 0.1100 |
| Smoking (current vs. never) | 0.711 | 0.355 | 1.425 | 0.3361 |
| Smoking (former vs. never) | 1.022 | 0.581 | 1.797 | 0.9411 |
| Coronary artery disease (QCA‡ ≥50) | 2.899 | 1.687 | 4.981 | 0.0001 |
| Epicardial fat volume (EFV) | 0.995 | 0.989 | 1.001 | 0.1197 |
Odds ratios relate to 1 unit change of EFV.
SSS: summed Stress score;
BMI: body mass index;
QCA: quantitative coronary angiography
Figure 5. Relationship between Epicardial Fat Volume and Myocardial Perfusion Abnormalities.
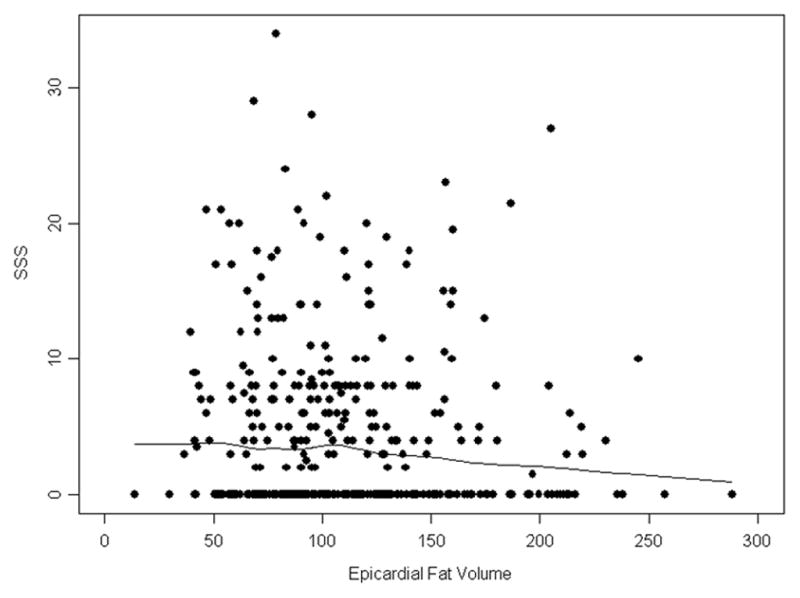
Shown is a scatterplot and Lowess smoothing of epicardial fat volume (EFV) and presence of myocardial perfusion abnormalities using summed stress score (SSS) by SPECT revealing no significant association.
Table 8.
Median Regression for Abnormal Myocardial Perfusion
| Median Regression Models | Estimate | Lower CL | Upper CL | p |
|---|---|---|---|---|
| Model 1: Unadjusted | ||||
| Epicardial fat volume (EFV) | −0.0144 | −0.0324 | 0.0037 | 0.1178 |
| Model 2: includes age, gender and race | ||||
| Age | −0.0810 | −0.1525 | −0.0096 | 0.0263 |
| Gender (male vs. female) | 3.3608 | 2.2005 | 4.5210 | <.0001 |
| Race (Caucasian vs. Asian ) | 0.9842 | −0.4308 | 2.3991 | 0.1722 |
| Race (African-American vs. Asian) | −0.1906 | −2.0793 | 1.6981 | 0.8428 |
| Epicardial fat volume (EFV) | −0.0103 | −0.0238 | 0.0032 | 0.1346 |
| Model 3: all predictors | ||||
| Age | −0.0996 | −0.1601 | −0.0392 | 0.0013 |
| Gender (male vs. female) | 0.9544 | −0.0432 | 1.9520 | 0.0607 |
| Race (Caucasian vs. Asian) | 1.6921 | 0.4589 | 2.9252 | 0.0073 |
| Race (African-American vs. Asian) | 0.5532 | −1.4414 | 2.5478 | 0.5857 |
| BMI* (obese [BMI≥30] vs. normal [BMI<25]) | −0.3259 | −1.9246 | 1.2728 | 0.6886 |
| BMI (overweight [BMI 25–29] vs. normal [BMI<25]) | −0.6627 | −1.9812 | 0.6559 | 0.3235 |
| Hypertension | 0.9793 | −0.3532 | 2.3119 | 0.1492 |
| Dyslipidemia | −0.8929 | −2.0679 | 0.2820 | 0.1358 |
| Family History of coronary artery disease | −0.0794 | −1.1940 | 1.0353 | 0.8887 |
| Previous myocardial infarction | 3.3575 | 1.4069 | 5.3081 | 0.0008 |
| Diabetes mellitus | 1.1218 | −0.1656 | 2.4093 | 0.0874 |
| Smoking (current vs. never) | −1.4779 | −2.8058 | −0.1501 | 0.0293 |
| Smoking (former vs. never) | −0.6715 | −1.8462 | 0.5033 | 0.2616 |
| Coronary artery disease (QCA† ≥50) | 2.4005 | 1.1199 | 3.6811 | 0.0003 |
| Epicardial fat volume (EFV) | −0.0124 | −0.0259 | 0.0010 | 0.0690 |
Median Summed stress score for 1 unit change of EFV.
BMI: body mass index;
QCA: quantitative coronary angiography
Discussion
In contrast to a number of previous studies, we did not find a significant association between EFV and the presence and extent of coronary arterial calcification by CT, presence of obstructive coronary artery disease by cardiac catheterization, or myocardial perfusion abnormalities by SPECT. Accordingly, our results are unexpected and contrary to our hypotheses.
We applied robust methodology and analysis in our study. CT acquisition and assessment for calcification followed standard methods. We used a careful approach to determine EFV with high agreement among observers. Median values as well as distribution of EFV are consistent with those reported in previous studies. We used standard, validated methodology for assessing coronary stenoses and myocardial perfusion. Importantly, all analyses – including statistical evaluations - were performed in independent core laboratories with particular expertise in the respective areas. Strengths of our study also included a solid sample size and the rigorous design structure of a multi-center study.
Possible explanations for the lack of association of EFV with metrics of CAD disease prevalence may be found in our patient population: ethnic diversity, geographical variations, and cardiovascular risk profile. Our patients were referred for cardiac catheterization with clinical suspicion of obstructive coronary artery disease, placing our patients in an intermediate-high risk group in contrast to lower risk populations in community samples.1, 2 Fat distribution in general - and EFV in specific - varies among ethnic groups with African Americans having lower average EFV than Caucasians and Asians – as also shown in our study.34, 35 At the same time, African Americans are at greater risk of adverse events compared to other ethnic groups.36 It is conceivable that by combining patients with different EFV and risk patterns, the associations of EFV with disease markers were diminished. On the other hand, we did not find trends among our data that this indeed was the case.
We noted that increased EFV was associated with BMI≥30 as previously described.1,37 Despite the lack of association between EFV and CAD in our study, we found a trend for greater calcium scores in obese compared to normal weight individuals, consistent with other reports.38, 39 Since the relationship between obesity and coronary artery disease severity is modest, it is possible that such association is weakened for a marker of obesity, i.e., EFV. It is critical to emphasize that we did not investigate the association of EFV with clinical outcome, but rather with coronary artery disease severity. There is strong evidence for an association between obesity and CAD outcome.40, 41 Similarly, recent data provided evidence from large patient cohorts that greater EFV is associated with increased risk of myocardial infarction though it remains uncertain if this association is truly independent from traditional risk factors.42 It is conceivable that the effects of EFV are more important in influencing vascular functions, particularly, in response to plaque alterations,43 rather than directly contributing to atherosclerotic disease burden. As patient outcome is more important than the presence of disease, our results should not lend themselves to disregard the potential significance of EFV for clinical management.
We acknowledge the limitations of our study. The CORE320 patient cohort was enrolled for a separate study design. Accordingly, sample size calculation for our present study was not considered for CORE320 enrollment. While our sample size is larger than many clinical studies of similar design, it is conceivable that some results did not reach statistical significance because of insufficient power. However, the widths of confidence intervals for our main analyses support the strengths of our results. Estimation of EFV is subject to error. We used analytic methods of EFV which were validated in previous studies1, 2, 14 yet variability may occur. Median EFV reported in our study, however, is in agreement with other reports2, 14 from similar populations. In addition, interobserver agreement for EFV was high for our method aided by the semi-automatic nature of EFV analysis. Lastly, we did not differentiate between epicardial and perivascular fat nor did we differentiate between fat types, e.g., brown fat vs. other, which may have different implications for disease associations and patient outcome.18,44
In conclusion, we neither found a significant association of epicardial fat volume with the presence and extent of coronary artery disease as assessed by coronary calcium scannind and invasive coronary angiography, nor with myocardial perfusion abnormalities by SPECT in this diverse, intermediate-high risk population. Our results are in disagreement with some prior reports and they may point to a more complex relationship between epicardial fat volume and coronary artery disease risk. While the current value of assessing epidcardial fat volume for clinical management remains unclear, its evaluation may help predicting future CAD events rather than describing baseline disease burden.
Acknowledgments
Sources of Funding
This work was funded in part by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, USA. The parent study for this research – the CORE320 multicenter study – was sponsored by Toshiba Medical Systems.
Footnotes
Disclosures
Dr. Lima reports receiving grant support from Toshiba Medical Systems. Dr. Dewey reports receiving grants from the Heisenberg Program of the German Research Foundation (DFG) for a Professorship (DE 1361/14-1), European Regional Development Fund, Joint program from the German Science Foundation (DFG) and the German Federal Ministry of Education and Research (BMBF) for meta-analyses, German Heart Foundation/German Foundation of Heart Research, GE Healthcare (Amersham), Bracco, Guerbet, and Toshiba Medical Systems. Dr. Arbab-Zadeh discloses his membership of the steering committee for the CORE320 international study, which is sponsored by Toshiba Medical Systems.
References
- 1.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 2.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The framingham heart study. European Heart Journal. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadi N, Nabavi V, Yang E, Hajsadeghi F, Lakis M, Flores F, Zeb I, Bevinal M, Ebrahimi R, Budoff M. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Academic Radiology. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 4.De Vos AM, Prokop M, Roos CJ, Meijs MF, van der Schouw YT, Rutten A, Gorter PM, Cramer MJ, Doevendans PA, Rensing BJ, Bartelink ML, Velthuis BK, Mosterd A, Bots ML. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. European Heart Journal. 2008;29:777–783. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, Otaki Y, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, Berman DS, Dey D. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: A serial study using non-contrast cardiac ct. Atherosclerosis. 2011;218:363–368. doi: 10.1016/j.atherosclerosis.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 6.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, Ramesh A, Kakadiaris I, Germano G, Slomka PJ, Berman DS. Computer-aided non-contrast ct-based quantification of pericardial and thoracic fat and their associations with coronary calcium and metabolic syndrome. Atherosclerosis. 2010;209:136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Kritchevsky SB, Hsu FC, Harris TB, Burke GL, Detrano RC, Szklo M, Criqui MH, Allison M, Ouyang P, Brown ER, Carr JJ. Association between non-subcutaneous adiposity and calcified coronary plaque: A substudy of the multi-ethnic study of atherosclerosis. The American Journal of Clinical Nutrition. 2008;88:645–650. doi: 10.1093/ajcn/88.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, Jeffrey Carr J Multi-Ethnic Study of A. The association of pericardial fat with calcified coronary plaque. Obesity. 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarin S, Wenger C, Marwaha A, Qureshi A, Go BD, Woomert CA, Clark K, Nassef LA, Shirani J. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. The American Journal of Cardiology. 2008;102:767–771. doi: 10.1016/j.amjcard.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki K, Matsumoto T, Aono H, Furukawa H, Samukawa M. Relationship between epicardial fat measured by 64-multidetector computed tomography and coronary artery disease. Clinical Cardiology. 2011;34:166–171. doi: 10.1002/clc.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, Kawamura A, Yoshikawa T, Tanami Y, Sato K, Kuribayashi S, Ogawa S. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circulation Journal. 2009;73:1927–1933. doi: 10.1253/circj.cj-09-0266. [DOI] [PubMed] [Google Scholar]
- 12.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, Friedman JD, Hayes SW, Thomson LE, Slomka PJ, Rozanski A, Berman DS. Increased pericardial fat volume measured from noncontrast ct predicts myocardial ischemia by spect. JACC. Cardiovascular Imaging. 2010;3:1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janik M, Hartlage G, Alexopoulos N, Mirzoyev Z, mclean DS, Arepalli CD, Chen Z, Stillman AE, Raggi P. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. Journal of Nuclear Cardiology. 2010;17:841–847. doi: 10.1007/s12350-010-9235-1. [DOI] [PubMed] [Google Scholar]
- 14.Bucci M, Joutsiniemi E, Saraste A, Kajander S, Ukkonen H, Saraste M, Pietila M, Sipila HT, Teras M, Maki M, Airaksinen KE, Hartiala J, Knuuti J, Iozzo P. Intrapericardial, but not extrapericardial, fat is an independent predictor of impaired hyperemic coronary perfusion in coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:211–218. doi: 10.1161/ATVBAHA.110.213827. [DOI] [PubMed] [Google Scholar]
- 15.Brinkley TE, Jerosch-Herold M, Folsom AR, Carr JJ, Hundley WG, Allison MA, Bluemke DA, Burke GL, Szklo M, Ding J. Pericardial fat and myocardial perfusion in asymptomatic adults from the multi-ethnic study of atherosclerosis. Plos One. 2011;6:e28410. doi: 10.1371/journal.pone.0028410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HM, Kim KJ, Lee HJ, Yu HT, Moon JH, Kang ES, Cha BS, Lee HC, Lee BW, Kim YJ. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: Its assessment by cardiac magnetic resonance. Cardiovascular Diabetology. 2012;11:83. doi: 10.1186/1475-2840-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marwan M, Achenbach S. Quantification of epicardial fat by computed tomography: Why, when and how? Journal of Cardiovascular Computed Tomography. 2013;7:3–10. doi: 10.1016/j.jcct.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Sacks HS, Fain JN. Human epicardial adipose tissue: A review. American Heart Journal. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Chaldakov GN, Beltowsky J, Ghenev PI, Fiore M, Panayotov P, Rancic G, Aloe L. Adipoparacrinology--vascular periadventitial adipose tissue (tunica adiposa) as an example. Cell Biology International. 2012;36:327–330. doi: 10.1042/CBI20110422. [DOI] [PubMed] [Google Scholar]
- 20.Williams JK, Heistad DD. Structure and function of vasa vasorum. Trends in Cardiovascular Medicine. 1996;6:53–57. doi: 10.1016/1050-1738(96)00008-4. [DOI] [PubMed] [Google Scholar]
- 21.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue F, Sato Y, Matsumoto N, Tani S, Uchiyama T. Evaluation of plaque texture by means of multislice computed tomography in patients with acute coronary syndrome and stable angina. Circulation Journal. 2004;68:840–844. doi: 10.1253/circj.68.840. [DOI] [PubMed] [Google Scholar]
- 23.Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. The American Journal of Cardiology. 2008;102:1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Versteylen MO, Takx RA, Joosen IA, Nelemans PJ, Das M, Crijns HJ, Hofstra L, Leiner T. Epicardial adipose tissue volume as a predictor for coronary artery disease in diabetic, impaired fasting glucose, and non-diabetic patients presenting with chest pain. European Heart Journal Cardiovascular Imaging. 2012;13:517–523. doi: 10.1093/ehjci/jes024. [DOI] [PubMed] [Google Scholar]
- 25.Sacks HS, Fain JN. Human epicardial fat: What is new and what is missing? Clinical and Experimental pharmacology & Physiology. 2011;38:879–887. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 26.Cerci RJ, Arbab-Zadeh A, George RT, Miller JM, Vavere AL, Mehra V, Yoneyama K, Texter J, Foster C, Guo W, Cox C, Brinker J, Di Carli M, Lima JA. Aligning coronary anatomy and myocardial perfusion territories: An algorithm for the core320 multicenter study. Circulation. Cardiovascular Imaging. 2012;5:587–595. doi: 10.1161/CIRCIMAGING.111.970608. [DOI] [PubMed] [Google Scholar]
- 27.George RT, Arbab-Zadeh A, Cerci RJ, Vavere AL, Kitagawa K, Dewey M, Rochitte CE, Arai AE, Paul N, Rybicki FJ, Lardo AC, Clouse ME, Lima JA. Diagnostic performance of combined noninvasive coronary angiography and myocardial perfusion imaging using 320-mdct: The CT angiography and perfusion methods of the CORE320 multicenter multinational diagnostic study. AJR. American Journal of Roentgenology. 2011;197:829–837. doi: 10.2214/AJR.10.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vavere AL, Simon GG, George RT, Rochitte CE, Arai AE, Miller JM, Di Carli M, Arbab-Zadeh A, Dewey M, Niinuma H, Laham R, Rybicki FJ, Schuijf JD, Paul N, Hoe J, Kuribyashi S, Sakuma H, Nomura C, Yaw TS, Kofoed KF, Yoshioka K, Clouse ME, Brinker J, Cox C, Lima JA. Diagnostic performance of combined noninvasive coronary angiography and myocardial perfusion imaging using 320 row detector computed tomography: Design and implementation of the CORE320 multicenter, multinational diagnostic study. Journal of Cardiovascular Computed Tomography. 2011;5:370–381. doi: 10.1016/j.jcct.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row ct. The New England Journal of Medicine. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 30.Miller JM, Dewey M, Vavere AL, Rochitte CE, Niinuma H, Arbab-Zadeh A, Paul N, Hoe J, de Roos A, Yoshioka K, Lemos PA, Bush DE, Lardo AC, Texter J, Brinker J, Cox C, Clouse ME, Lima JA. Coronary ct angiography using 64 detector rows: Methods and design of the multi-centre trial CorE-64. European Radiology. 2009;19:816–828. doi: 10.1007/s00330-008-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA American College of Cardiology Foundation Appropriate Use Criteria Task F, American Society of Nuclear C, American College of R, American Heart A, American Society of E, Society of Cardiovascular Computed T, Society for Cardiovascular Magnetic R, Society of Nuclear M. Accf/asnc/acr/aha/ase/scct/scmr/snm 2009 appropriate use criteria for cardiac radionuclide imaging: A report of the american college of cardiology foundation appropriate use criteria task force, the american society of nuclear cardiology, the american college of radiology, the american heart association, the american society of echocardiography, the society of cardiovascular computed tomography, the society for cardiovascular magnetic resonance, and the society of nuclear medicine. Journal of the American College of Cardiology. 2009;53:2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM, Niinuma H, Yoshioka K, Kitagawa K, Nakamori S, Laham R, Vavere AL, Cerci RJ, Mehra VC, Nomura C, Kofoed KF, Jinzaki M, Kuribayashi S, de Roos A, Laule M, Tan SY, Hoe J, Paul N, Rybicki FJ, Brinker JA, Arai AE, Cox C, Clouse ME, Di Carli MF, Lima JA. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: The CORE320 study. European Heart Journal. 2014;35:1120–30. doi: 10.1093/eurheartj/eht488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachamovitch R, Johnson JR, Hlatky MA, Cantagallo L, Johnson BH, Coughlan M, Hainer J, Gierbolini J, Di Carli MF, Investigators S. The study of myocardial perfusion and coronary anatomy imaging roles in cad (sparc): Design, rationale, and baseline patient characteristics of a prospective, multicenter observational registry comparing pet, spect, and cta for resource utilization and clinical outcomes. Journal of Nuclear Cardiology. 2009;16:935–948. doi: 10.1007/s12350-009-9140-7. [DOI] [PubMed] [Google Scholar]
- 34.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral fat, waist circumference, and bmi: Impact of race/ethnicity. Obesity. 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 35.Willens HJ, Gomez-Marin O, Chirinos JA, Goldberg R, Lowery MH, Iacobellis G. Comparison of epicardial and pericardial fat thickness assessed by echocardiography in african american and non-hispanic white men: A pilot study. Ethnicity & Disease. 2008;18:311–316. [PubMed] [Google Scholar]
- 36.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: The atherosclerosis risk in communities study, the cardiovascular health study, and the multi-ethnic study of atherosclerosis. Circulation. 2012;126:50–59. doi: 10.1161/CIRCULATIONAHA.111.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. The Journal of Clinical Endocrinology and Metabolism. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 38.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, Chung H, Carnethon M. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease. Archives of Internal Medicine. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labounty TM1, Gomez MJ, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann P, Kim YJ, Leipsic J, Lin FY, Maffei E, Raff G, Shaw LJ, Villines TC, Min JK. Body mass index and the prevalence, severity, and risk of coronary artery disease: an international multicentre study of 13,874 patients. European Heart Journal Cardiovascular Imaging. 2013;14:456–63. doi: 10.1093/ehjci/jes179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease-the obesity paradox. Progress in Cardiovascular Diseases. 2014;56:401–408. doi: 10.1016/j.pcad.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, macinnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. The New England Journal of Medicine. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R, Mohlenkamp S. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall study. Journal of the American College of Cardiology. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 43.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzgibbons TP, Czech MP. Epicardial and Perivascular Adipose Tissues and their Influence on Cardiovascular Disease: Basic Mechanisms and Clinical Associations. Journal of the American Heart Association. 2014;3:e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]