Abstract
Synapses are the functional connection between neurons which are necessary for the transfer of electric activity or chemical activity from one cell to another. Synapses are formed by the pre- and postsynaptic membrane which communicates between pre- and postneurons while a neurochemical modulator is operated in this process. H2S has been known as a toxic gas with rotten eggs smell. However, increasing number of researches show that it regulate a variety of physiological and pathological processes in mammals. Hence, H2S is a physiologically important molecule and has been referred to as the third gaseous molecule alongside carbon monoxide and nitric oxide. The previous era has made an exponential development in the physiological and pathological significance of H2S. Specifically, in the central nervous system, H2S facilitates long-term potentiation and regulates intracellular calcium concentration in brain cells. We as well as others have also shown that H2S has antioxidant, antiapoptotic, and anti-inflammatory properties against various neurodegenerative disorders such as stroke, Alzheimer's disease, and vascular dementia. In this chapter, we highlight the current knowledge of H2S and its neuroprotective effects with a special emphasis on synaptic remodeling.
1. INTRODUCTION
Hydrogen sulfide (H2S) was found to be produced endogenously in various parts of the body such as the heart (Geng et al., 2004), blood (Zhao, Chen, Shen, Kahn, & Lipke, 2001), and central nervous system (CNS) (Warenycia et al., 1989). H2S is synthesized endogenously by a variety of mammalian tissues by two pyridoxal-5′ -phosphate-dependent enzymes responsible for metabolism of l-cysteine which is a by-product of l-methionine, homocysteine, and cystathione. Cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and a newly identified enzyme, 3-mercaptopyruvate sulfurtransferase (3MST) (Sen et al., 2012) are involved in generation of H2S. The substrate of CBS and CSE can be derived from alimentary sources or can be liberated from endogenous proteins (Rezessy-Szabo et al., 2007; Zhu, Song, Li, & Dao, 2008). In the CNS, CBS was found highly expressed in the hippocampus and cerebellum (Abe & Kimura, 1996). CBS is mainly confined to astrocytes (Enokido et al., 2005; Ichinohe et al., 2005) and microglial cells. CSE is mainly expressed in the cardiovascular system, but was also found in microglial cells (Oh et al., 2006), spinal cord (Distrutti et al., 2006), and cerebellar granule neurons (Garcia-Bereguiain, Samhan-Arias, Martin-Romero, & Gutierrez-Merino, 2008). However, 3MST is also an important enzyme for the synthesis of H2S in the brain which is localized within neurons and astrocytes (Shibuya et al., 2009; Zhao, Chan, Ng, & Wong, 2013). 3-Mercaptopyruvate is converted from cysteine by the action of cysteine aminotransferase (Tanizawa, 2011) (Fig. 1). By comparing the production of H2S in different brain cells, Lee, Kim, Kim, and Ahn (2009) found that H2S production in astrocytes was 7.9-fold higher than in cultured microglial cells, 9.7-fold higher than in neuron-committed teratocarcinoma NT2 cell line (NT-2 cells), and 11.5-fold higher than in neuroblastoma cell line (SH-SY5Y cells) (Lee et al., 2009). These data clearly indicate that astrocytes may be the main cells that produce H2S in the brain. The estimated physiological concentration of H2S was recently measured to be around 14–30 µM, based on measurements of the brains of mice (Furne, Saeed, & Levitt, 2008) and consistent with values reported by another group (Ishigami et al., 2009). Above information regarding H2S in the brain indicates the impact of H2S sulfide on neuronal function. Neural circuits are composed of mainly glutamatergic and GABAergic neurons, which communicate through synaptic connections. GABAergic synapse maturation occurs in many brain regions. In addition, changes in GABAergic output are cell wide and not target-cell specific. Chang et al. (2014) found that glutamatergic neuronal activity also determined the AMPA receptor (a non-NMDA-type ionotropic transmembrane receptor) for glutamate that mediates fast synaptic transmission in the CNS. AMPA receptor also determines the properties of synapses on the partner with GABAergic neuron. The N-methyl-d-aspartate (NMDA) receptor is a major type of ionotropic glutamate receptor that plays a pivotal role in the CNS under both physiological and pathological conditions. The functional diversity of NMDA receptors can be mainly attributed to their different subunit compositions that perform multiple functions in various situations. Recent reports have indicated that synaptic NMDA receptors have a distinct role in neuronal cell survival. NMDA receptors modulate the LTP and synaptic plasticity which is responsible for learning and memory function and therefore maintains synapse function. Although H2S enhances the induction of hippocampal LTP, the mechanism by which H2S modulates synaptic activity is still in debate. H2S enhances the responses of neurons to glutamate in hippocampal slices, and H2S alone induces the increase in intracellular Ca2+ in astrocytes (Nagai, Tsugane, Oka, & Kimura, 2004). In the presence of H2S, the induction of LTP is enhanced at the synapse and Ca2+ waves are induced in the surrounding astrocytes. Ca2+ waves propagate and reach another synapse and may modulate it. H2S may therefore modulate synaptic activity by enhancing the responses to glutamate in neurons and inducing Ca2+ waves in astrocytes that propagate and modulate the neighboring synapse. Some of the reports showed that H2S controls the neuronal signaling. As signaling passes through neuronal synapse, it is also modulated by H2S. Thus, understanding the role of H2S in the brain will help in gaining a mechanism for controlling synapse function. This review presents an overview of the current evidence that H2S probably acts as a neuromodulator and/or as an intracellular messenger and plays an important role in synaptic remodeling.
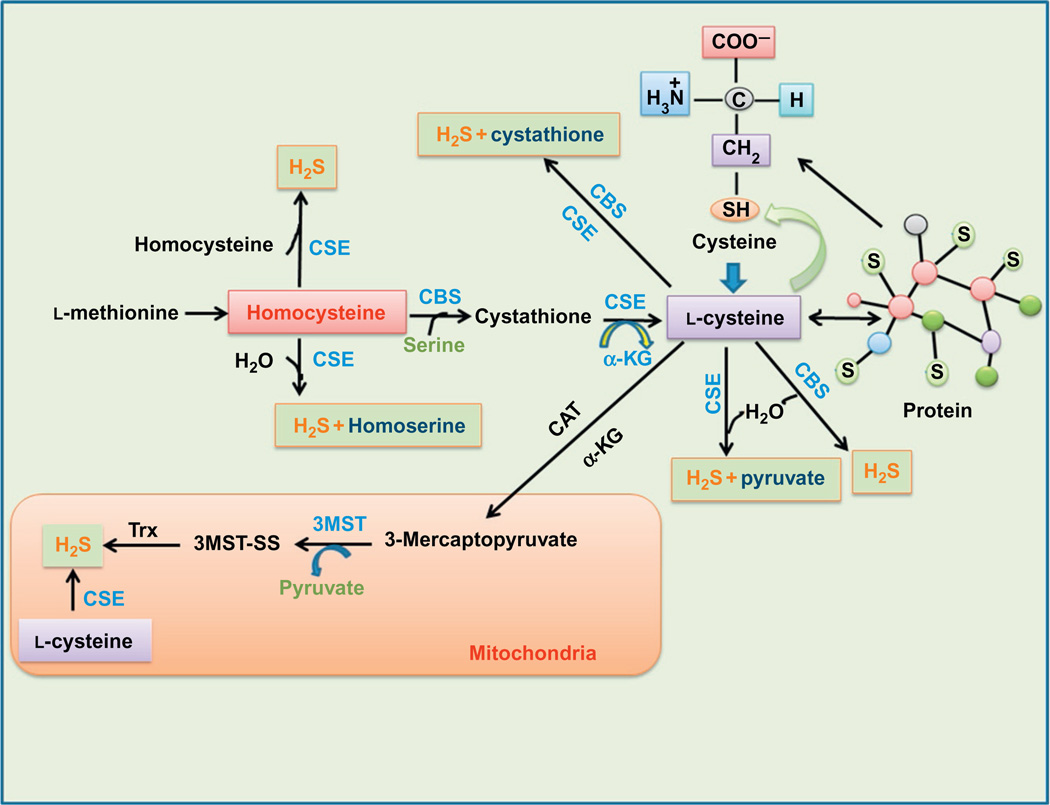
Figure 1.
H2S formation in cytosol as well as in mitochondria. The three enzymes CBS, CSE, and 3MST are responsible for H2S generation in cells. CBS and CSE are usually present in cytosol while 3MST is present in mitochondria. The sources of cytosolic H2S generation are l-methionine, homocysteine, cystathione, and l-cysteine. l-Cysteine also originates from a protein and cysteine and moreover also produces pyruvate and cystathione. In mitochondria, the source of H2S synthesis are mercaptopyruvate and l-cysteine. CBS, cystathionine β-synthase; 3MST, 3-mercaptopyruvate sulfurtransferase; CSE, cystathionine γ-lyase; α-KG, α-ketoglutarate; CAT, cysteine aminotransferase; H2S, hydrogen sulfide; TRX, thioredoxin.
2. PHARMACOLOGICAL AND PHYSIOLOGICAL EFFECT OF H2S
Dietary garlic has long been known for its beneficial effects on the cardiovascular system. Their components are the main source of H2S donor naturally. The paste of garlic cloves allows the enzyme alliinase to metabolize the amino acid alliin, producing allicin (diallyl thiosulfinate), which decomposes to polysulfides, including diallyl disulfide and diallyl trisulfide (Banerjee & Maulik, 2002). When these compounds react with reduced thiol groups, including reduced glutathione, in cells, they produce H2S. In experimental set of condition, H2S or H2S donors (most commonly NaHS) provide protection in many physiological systems including the cardiovascular system and central nervous system (Wallace, 2007). Most notably, H2S mainly attenuate vasoconstriction and reduce damage via dilating blood vessels (e.g., myocardial infarct size) in several animal models of cardiovascular disease. However, in the brain, H2S acts as anti-inflammatory, antioxidant, and finally as a neuroprotective agent which can also be designated as neuromodulator. Most researchers prefer to use pharmacological inhibitors that inhibit H2S synthesis such as dl-propargylglycine (PAG); a CSE inhibitor and β-cyano-l-alanine (BCA), l-amino ethoxyvinylglycine, aminooxyacetic acid, trifluoroalanine, and hydroxylamine. Though BCA is a selective inhibitor for CSE, it also inhibits CBS at high concentrations. In contrast, none of the compounds tested exhibited significant selectivity toward CBS. In addition, the above-mentioned compounds did not inhibit 3MST. The effects of H2S on cells studied using H2S donors are variable in nature. Lower (micromolar) (lower micromolar concentrations) of H2S are generally cytoprotective, with protection often ascribed to a reduction of free radicals generation such as neutralization of reactive oxygen and nitrogen species. These effects have been reported in neurons, myoblasts, neutrophils, and macrophages. At higher concentration such as millimolar levels, H2S is often proapoptotic or cytotoxic, via free radical and oxidant generation, glutathione depletion, and thus promotes apoptosis. In addition to that, when sulfide donors or precursors administered systemically can convert into H2S gas, this gas can be absorbed via the lung into the circulation and after that all over the body. Rezessy-Szabo et al. (2007) have reported the capacity and potential of intravenously (i.v.) administered H2S donors to induce exhalation of H2S. Previously, we and others have used H2S donors such as IK-1001 (sodium sulfide for injection), a parenteral injectable GMP formulation of H2S, and H2S donor (NaHS, GYY-4137) which acts as a neuroprotector and vasoprotector. This compound has recently been proven to be protective against various physiological and pathological conditions. Moreover, the effect of H2S donors were used in many experimental studies to characterize the pharmacological effects of H2S in vitro and in vivo (Esechie et al., 2008; Kamat et al., 2013; Mishra, Tyagi, Sen, Givvimani, & Tyagi, 2010; Qipshidze, Metreveli, Mishra, Lominadze, & Tyagi, 2012; Sen et al., 2012; Simon et al., 2008; Sodha et al., 2008; Tyagi, Vacek, Givvimani, Sen, & Tyagi, 2010).
3. EFFECT OF H2S ON THE CNS
Several findings suggested that H2S exists in the CNS at a nanomolar (nM) or very low micromolar (µM) concentrations. In contrasts to previous literature, CNS concentrations of H2S 50–160 µM/l have been reported (Lopez, Prior, Reiffenstein, & Goodwin, 1989; Warenycia et al., 1989). This has significant impact on most previously published papers which used H2S concentrations in the range of µM. Previous findings may yet be valid based on recent evidence showing that µM concentrations of H2S rapidly decays in vitro to undetectable levels within 30 min suggesting single µM doses of H2S might have already exerted their effects before they decay to undetectable levels and imply that the action of H2S is likely a molecular “switch” that activates downstream pathways that persist long after H2S decay. H2S is usually stored as bound sulfane sulfur in neurons and astrocytes (Ishigami et al., 2009). Upon neuron excitation or other stimulation, the bound sulfane sulfur then releases free H2S. Free H2S is mainly oxidized to thiosulfate, sulfite, and finally sulfate by thiosulfate:cyanide sulfurtransferase in mitochondria (Lowicka & Beltowski, 2007). H2S can also be methylated by the enzyme thiol-S-methyltransferase to methanethiol and dimethylsulfide or be bound to methemoglobin, an oxidized form of hemoglobin (Lowicka & Beltowski, 2007). The clearance of H2S via transport from the brain to the clearance organs such as kidneys, lungs, or liver is less likely as concentrations of H2S in the blood are lower than 14 nM (Whitfield, Kreimier, Verdial, Skovgaard, & Olson, 2008) and H2S has a short half-life in vitro (Hu et al., 2009). It was proposed that the neurological and cardiovascular actions of H2S were continuously modulated primarily by circulating sulfide rather than by endogenous production (Olson et al., 2008). This was disproved based on recent studies reporting undetectable H2S levels in mouse and rat blood samples using sensors that can detect 14 nM of H2S (Whitfield et al., 2008), implying that H2S found in the CNS is more likely to be derived directly from the CNS than from the blood. This also supports the hypotheses put forth by recent reviews (Li et al., 2005; Rezessy-Szabo et al., 2007) that H2S does not circulate in the plasma at measurable conditions (Whitfield et al., 2008) which is consistent with speculations that H2S has neuromodulatory functions in vivo (Abe & Kimura, 1996; Kim, Lee, Jang, Han, & Kim, 2011). In the CNS, H2S acts as a messenger in response to specific stimuli (usually noxious) such as febrile seizures (Han et al., 2005), stimuli leading to pain (Kubo, Kajiwara, & Kawabata, 2007), and cerebral ischemia (Qu, Chen, Halliwell, Moore, & Wong, 2006), which do not occur frequently. Therefore, it is clear that H2S is present in the brain and comes from different sources and is involved in the regulation of intracellular signaling molecules, ion channel function, and the release and function of amino acid neurotransmitters (Fig. 2).
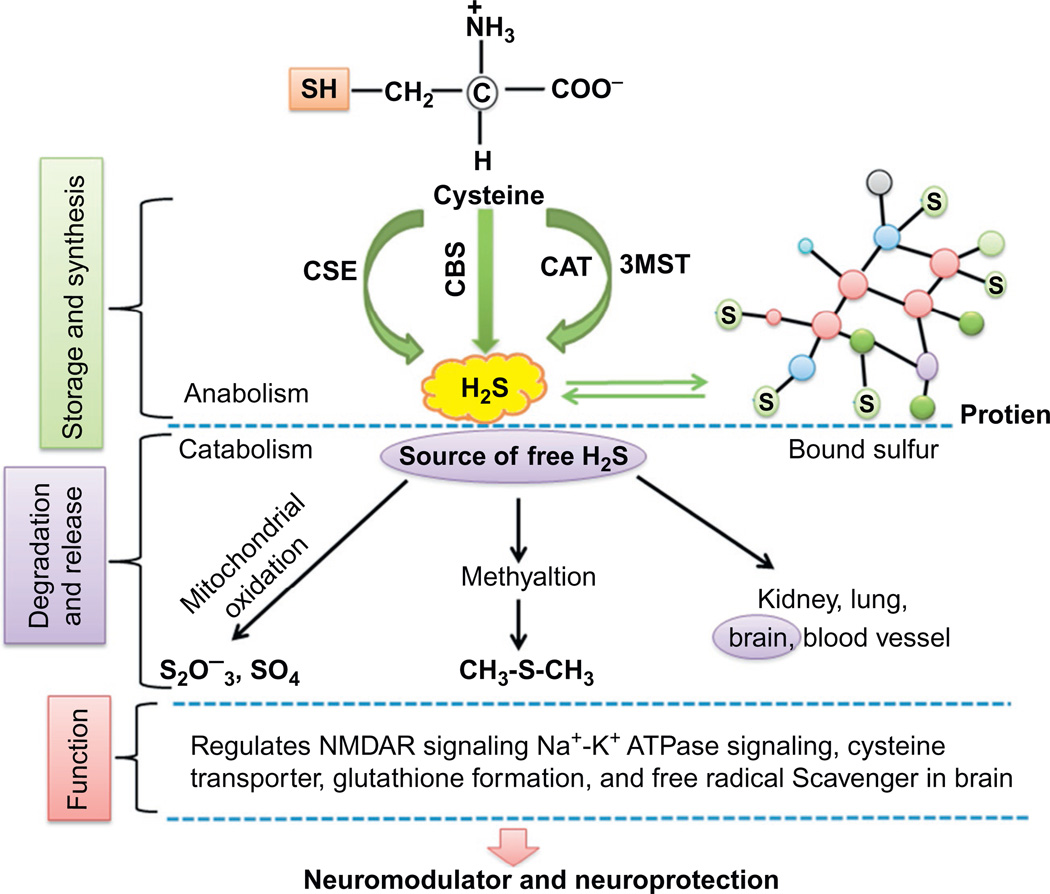
Figure 2.
Diagrammatic representation shows the sources of H2S and moreover anabolism and catabolism of H2S. The usual sources of H2S are blood lung, kidney, heart, and brain. Free H2S gets converted into sulfate and thiosulfate through mitochondrial oxidation and sulfomethane via methylation which becomes a source of H2S. Diagram also depicts the synthesis, storage, and function of H2S in neuronal synapse. These free H2S involved in NMDA receptor signaling, Na+ K+ ATPase signaling, and glutathione formation maintains and regulates neuronal function.
As reports suggesting that H2S might play a role in synaptic transmission, it also maintains excitatory postsynaptic potentials (EPSPs). EPSPs are necessary for electrical and chemical stimulation in synapse. In the hippocampus field and population spikes evoked by the electrical stimulation of the Schafer collaterals in the CA1 region, there is a concentration-dependent sensitivity toward H2S (Abe & Kimura, 1996). H2S concentrations greater than 130 µM were found to suppress both field EPSPs and population spikes. The suppression by H2S was specific to EPSPs and the population spikes as the action potentials generated by direct stimulation of presynaptic fibers were not affected by H2S. This indicates that the physiological concentration of H2S is a determining factor for synapse to work properly or improperly.
4. EFFECT OF H2S ON BRAIN CELLS (ASTROCYTE, MICROGLIA, AND OLIGODENDROCYTE)
Glial cells (astrocytes, oligodendrocytes, and microglia) are significantly abundant in the brain and are pathologically linked to neurodegenerative disorders. Large numbers of available data imply that neurodegeneration in different brain pathology is associated with the alterations in glial cells (Klegeris & McGeer, 2002; McGeer, Yasojima, & McGeer, 2002). Microglia play important roles in responses of the brain to injury and activated microglia congregate around degenerating neurons, and may produce toxins and inflammatory cytokines that contribute to the neurodegenerative process (Li et al., 2005; Rai, Kamat, Nath, & Shukla, 2013, 2014). The severe changes in glial cells and microglial cells in brain disease may promote neuronal degeneration (Jantzen et al., 2002). In addition, although synapses degenerate in vulnerable neuronal circuits, the remaining synapses may increase in size to compensate and astrocytes may play a role in this process (Murai, Nguyen, Irie, Yamaguchi, & Pasquale, 2003). Studies show that glial activation modifies long-term depression (LTD) and potentiation of synaptic transmission in the hippocampus (Albensi & Mattson, 2000). Finally, changes in the mitochondrial membrane permeability in synaptic terminals have been associated with impaired synaptic plasticity in the hippocampus (Albensi, Alasti, & Mueller, 2000). Progressively, accumulating evidence suggested that astrocytes play roles in synaptic transmission through the regulated release of synaptically active molecules including glutamate, purines (ATP and adenosine), GABA, and d-serine (Perea & Araque, 2010; Perea, Navarrete, & Araque, 2009; Shigetomi, Bowser, Sofroniew, & Khakh, 2008). Synaptic stimulation through NMDA receptors is important for learning and memory functions, but excess glutamate can over stimulate these receptors resulting in excitotoxicity and neurodegeneration (Kamat, Rai, Swarnkar, Shukla, & Nath, 2014; Michaels & Rothman, 1990). The release of such gliotransmitters occurs in response to changes in neuronal synaptic activity, which involves astrocyte excitability as reflected by increases in astrocyte Ca2+ and can alter neuronal excitability (Halassa, Fellin, & Haydon, 2007; Halassa, Fellin, Takano, Dong, & Haydon, 2007; Nedergaard, Ransom, & Goldman, 2003). Such evidence has given rise to the “tripartite synapse” hypothesis (Perea et al., 2009). Synaptically associated astrocytes are considered as an integral modulatory element of tripartite synapses consisting of the presynapse, the postsynapse, and the glial element (Fig. 3). Astrocytes may secrete glial binding proteins into the synaptic cleft, thus binding free neurotransmitters and thereby reducing the levels of neurotransmitters available for stimulating the postsynapse through receptors. Astrocytes also have membrane-bound receptors for neurotransmitters, and when these bind to neurotransmitters, the astrocytes upregulate the amount of binding protein secreted into the synapse. Thus, astrocytes play an important role in the formation, maintenance, and proper functioning of synapses (Christopherson et al., 2005; Ransom, Behar, & Nedergaard, 2003). Astrocytes exert a powerful influence on the synapse remodeling and pruning of the healthy adult CNS or in response to CNS disorders (Barker & Ullian, 2008). It has been reported that H2S enhances the induction of hippocampal long-term potentiation (LTP) and induces calcium waves in astrocytes. Based on these observations, it could be strongly suggest that H2S acts as a synaptic modulator in the brain mediated through astrocytes. Tsugane, Nagai, Kimura, Oka, and Kimura (2007) showed that differentiated astrocytes acquire sensitivity to H2S that is diminished by their transformation into reactive astrocytes.
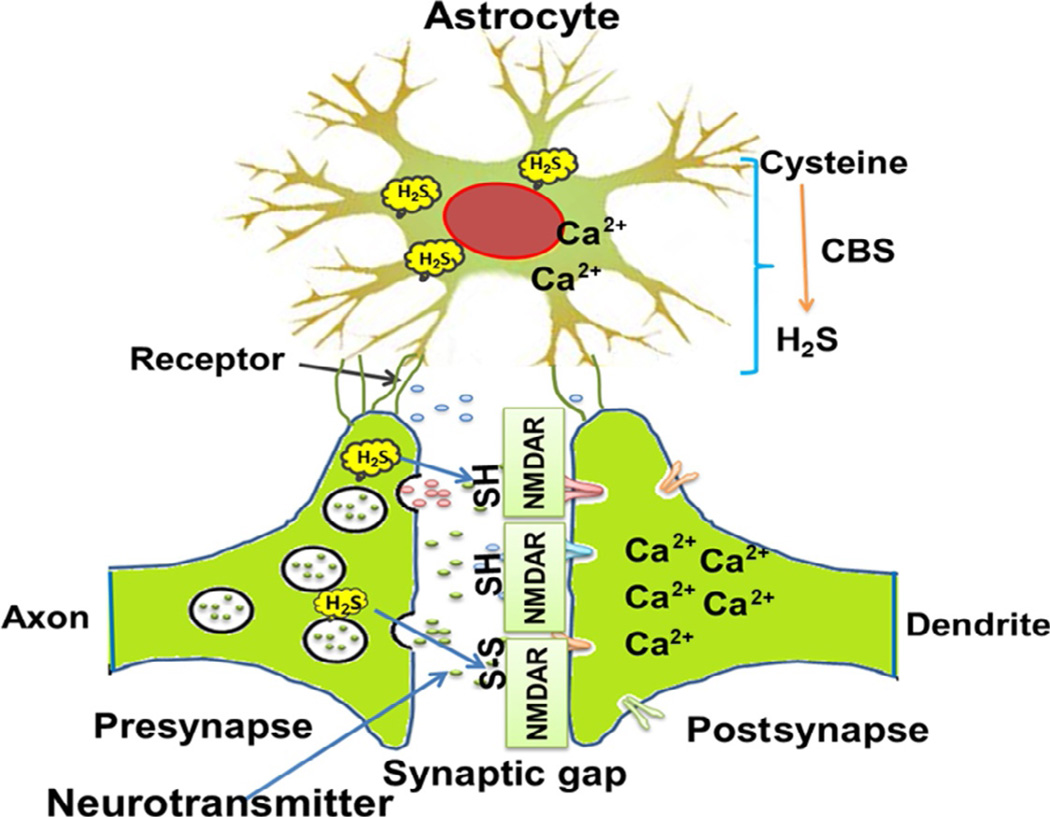
Figure 3.
Cartoon represents the structure of tripartite synapse (presynapse, postsynapse, and astrocyte). In astrocyte, H2S interacts with H2S and slow down the excessive glutamate and calcium ion release from astrocyte. On the other hand, in pathological condition H2S moderates the calcium ion influx through NMDA receptor in postsynapse and prevents from excitotoxic cell death. Neuronal synapses showing the presence of H2S in presynapse and postsynapse. Moreover, it also represents how H2S interacts with NMDA receptors; actually, H2S modulates the NMDA receptor and controls synapse function properly. Apart from that H2S also associated with glutathione production in cells which acts as cellular antioxidant.
5. SYNAPSE
Synapses are the structures where neurons exchange the neurotransmitter which is essential for neuronal functions and mediate signals to individual target cells. At a synapse, the plasma membrane of the signal-passing neuron (the presynaptic neuron) comes into close proximity with the membrane of the target (postsynaptic) cell. Both the presynaptic and postsynaptic sites of neurons contain extensive groups of molecular machinery that link the two membranes together and carry out the signaling process. Astrocytes also exchange information with the synaptic neurons, responding to synaptic activity and, in turn, regulating neurotransmission (Fig. 3). It is well known that the synapse plays an important role in the formation of learning and memory through molecular machinery. Memory formation process occurs by way of synaptic strengthening which is known as LTP. Memory formation is related to altered release of neurotransmitters and plasticity of synapses. The postsynaptic cell can be regulated by altering the function and number of its receptors. Changes in postsynaptic signaling are most commonly associated with N-methyl-d-aspartic acid receptor (NMDAR)-dependent LTP and LTD, which are the most analyzed forms of plasticity at excitatory synapses. When astrocytes interact with neuronal synapses, the structure is known as tripartite synapse. Synaptic physiology is usually based on the bidirectional communication between astrocytes and neurons. Since recent evidence has demonstrated that astrocytes integrate and process synaptic information as well as control synaptic transmission and plasticity, astrocytes, being active partners in synaptic function, are cellular elements involved in the processing, transfer, and storage of information by the nervous system. As evidences suggest, H2S is generated from astrocytes and considered as the main source. Therefore, it may affect neuronal function when it comes in contact with the synapse and may influence the synapse function.
6. GLIA AND NEURONS INTERACTIONS
Glial cells have been considered to be the nonexcitable and supportive elements in the nervous system, but they are now regarded as fundamentals for neuronal activity and modulate synaptic activity (Haydon, 2001). Glial cells, such as microglia and astrocytes, have neurotransmitter and hormone receptors and also integrate neuronal functions. The multiple interactions between neurons and glia strongly suggest that glial cells are integral parts of neurons and are referred to as modulatory elements in synaptic transmission (Araque, Parpura, Sanzgiri, & Haydon, 1999). The observation that H2S enhances the induction of hippocampal LTP suggests that H2S may modulate some aspects of synaptic activity. Although H2S enhances the NMDA receptor-mediated responses to glutamate in neurons, the effects of H2S in the absence of glutamate on brain cells are not well understood. Interactions between neurons and glia may modulate synaptic transmission, as neuronal activity can evoke glial function; may inhibit the exocytosis of glutamate or some other factor from nerve terminals when neurons are stimulated by NMDA. H2S released in response to neuronal excitation may increase intracellular Ca2+ and induce Ca2+ waves in neighboring astrocytes. Physiological concentrations of H2S specifically potentiate the activity of NMDA receptors and alter the induction of LTP in the hippocampus, a synaptic model for memory (Abe & Kimura, 1996). H2S can also regulate the release of the corticotrophin-releasing hormone from the hypothalamus (Dello Russo et al., 2000; Walsh et al., 2014). H2S increases intracellular concentrations of Ca2+ in glia and induces Ca2+ waves, which mediate glial signal transmission (Nagai et al., 2004). Given the accumulating evidence for reciprocal interactions between glia and neurons, it has been suggested that glia modulate the synaptic transmission. H2S may regulate the synaptic activity by modulating the activity of both neurons and glia in the brain. Based upon these observations, it has been proposed that H2S may function as a neuromodulator (Abe & Kimura, 1996).
7. EFFECT OF H2S ON NEURONAL REDOX STRESS
H2S has been functioning as an endogenous neuromodulator through the activation of NMDA receptors. NMDA receptors also lead to a sustained rise of neuronal cytosolic calcium ion, and interestingly, NMDA receptors are highly sensitive to oxidative stress (Kamat et al., 2013, Kamat, Tota, Saxena, Shukla, & Nath, 2010; Rai et al., 2013). The reduction potential of H2S is close to that of the thiol group of reduced glutathione, and H2S, like reduced glutathione, inhibits the oxidative stress-induced damage (Tyagi, Mishra, & Tyagi, 2009; Zhou & Freed, 2005). The reports from various experiments showed protective mechanisms of H2S against cellular stress. The protection afforded by H2S against hydrogen peroxide-induced cellular damage (Wang et al., 2013), against protein nitration induced by peroxynitrite (Whiteman et al., 2004), and against oxidative stress-induced death of neurons (Kalani, Kamat, Chaturvedi, Tyagi, & Tyagi, 2014; Kalani, Kamat, Givvimani, et al., 2014; Kamat et al., 2013). These reports further support a role of H2S as a significant cellular antioxidant. However, the molecular mechanisms through which H2S can attenuate the neuronal oxidative stress are still to be settled because H2S can act both as a sacrificial scavenger of ROS and also as an inhibitor of major ROS production in cells. In addition to the well-known inhibition by H2S of the mitochondrial respiration (Wang, Guo, & Wang, 2012), it is to be noted that the expression of gp91phox (ROS generation regulatory protein), a plasma membrane-bound NADPH-dependent oxidase which releases superoxide anion, has been recently shown to be downregulated by H2S level (Dong et al., 2012).
The oxidative stress plays a critical role at the early stages of apoptosis. As we and others have shown in earlier works (Kalani, Kamat, Chaturvedi, et al., 2014; Kalani, Kamat, Givvimani, et al., 2014; Kamat et al., 2010; Tyagi et al., 2009), oxidative stress is largely generated by production of superoxide anions such as reactive oxygen species and nitrogen species. Glutathione (g-glutamylcysteinyl glycine; GSH) is a tripeptide containing cysteine, glutamate, and glycine with the amine group of cysteine forming a peptide bond with the carboxyl group of the side chain found in glutamate. It can exist alone in reduced forms as glutathione or in an oxidized dimer form also known as glutathione disulfide (GSSG) (Monks, Ghersi-Egea, Philbert, Cooper, & Lock, 1999). Glutathione biosynthesis is catalyzed by the enzyme g-glutamylcysteine synthetase and glutathione synthase, while glutathione recovery from GSSG is catalyzed by GSSG reductase (Kimura & Kimura, 2004). Recent studies have also suggested that H2S can antagonize apoptosis through inhibiting the production of ROS and thus promotes neuronal survival (Tang et al., 2013, 2011). H2S can also protect against oxidative stress-induced neuronal damage through increasing the level of intracellular glutathione (Kimura & Kimura, 2004; Yang et al., 2011). Previous evidences also show that the pretreatment of H2S may attenuate neuronal injury/apoptosis through inhibition of cellular apoptosis (Biermann, Lagreze, Schallner, Schwer, & Goebel, 2011; Elrod et al., 2007). Glutathione evenly distributed throughout the brain occurs low in neuronal cells but high in astrocytes and oligodendrocytes, indicating that these glial cells may be the major source of glutathione generated from H2S; which is also an indication of the presence of H2S in astrocyte and microglial cells. Lastly, glutathione is formed by the H2S in the CNS.
As a reducing agent, glutathione protects neuronal as well as non-neuronal cells from free radical species either by direct action or indirectly by promoting the regeneration of other antioxidant systems (Monks et al., 1999). H2S was also reported to inhibit peroxynitrite-induced cytotoxicity, intracellular protein nitration, and protein oxidation in human neuroblastoma SH-SY5Y cells. These data suggest that H2S has the potential to act as an inhibitor of peroxynitrite-mediated processes in vivo and the potential antioxidant action of H2S (Whiteman et al., 2004). Similarly, H2S inhibits cell toxicity due to oxytosis (a novel form of apoptosis), a form of oxidative glutamate toxicity independent of glutamatergic signaling at ionotropic glutamate receptors, in neuronal cells and primary cultured immature cortical neurons (Umemura & Kimura, 2007). In these cells, increased intracellular cysteine levels were observed to correlate to the glutathione levels (Fig. 3). Therefore, H2S protects against the activity of peroxynitrite and other damages of the cells from free radicals, presumably through increased glutathione production in the neuronal cells as well as neuronal supportive cells.
8. EFFECT OF H2S ON GLUTAMATE NEUROTRANSMISSION
NMDA receptor blockers were reported to inhibit H2S-induced cell death in neurons (Cheung, Peng, Chen, Moore, & Whiteman, 2007) and infarct volume in an in vivo rat stroke model (Qu et al., 2006), suggesting that H2S may induce cell death through the opening of NMDA receptors. In summary of the properties of H2S-induced NMDA signaling, H2S may promote excitation and regulate survival/death decisions of the neurons. It was reported that H2S increased glutamate secretion in rat cerebellar granule neurons, which resulted in the neuronal cell death (Garcia-Bereguiain et al., 2008). This was demonstrated by a significant increase in extracellular concentrations of glutamate from physiological concentrations of 2–5 µM (Erecinska, Dagani, Nelson, Deas, & Silver, 1991; Erecinska & Silver, 1990) to supraphysiological (and thus toxic) concentrations of 10–15 µM. This observation was confirmed by blockade of H2S-induced cell death by NMDA blocker MK-801 and glutamate antagonist dl-2-amino-5-phosphonovaleric acid. A recent paper by Whitfield et al. (2008) suggested the amount of H2S derived from the plasma is likely to be negligible compared to endogenous production. On the other hand, H2S is more likely to decay rapidly than persist at micromolar concentrations in vitro (Hu et al., 2009), and this suggests that the observed downstream effects of glutamate release by H2S due to continuous maintenance of high H2S concentrations (Garcia-Bereguiain et al., 2008) may be reflective of a toxicological situation. If so, a toxic exposure of the cells to glutamate due to a constant high level of H2S may lead to excitotoxicity and neuronal cell death, thus leading to the compromised neuronal functions, e.g., memory, in addition to neuropathic pain (Hudspith, 1997).
9. EFFECT OF H2S ON NMDA RECEPTOR REGULATION
The NMDA type of glutamate receptor (NMDAR) plays a key role in neuronal plasticity, learning, and memory in the CNS, most of which is related to its high permeability to Ca2+ (Li & Tsien, 2009). Synaptic stimulation through NMDA receptors is important for the learning and memory functions, but excess glutamate can over stimulate these receptors resulting into excitotoxicity and neurodegeneration (Michaels & Rothman, 1990). Glutamate is an important excitatory amino acid that functions as a neurotransmitter in the mammalian brain. Glutamate plays a role in physiological processes including learning and memory, especially with respect to its central role in induction of LTP, perception of pain, and also in pathological processes such as excitatory neuronal injury (Hudspith & Munglani, 1998). NMDA receptors are a class of receptor-operated glutamate receptors mostly expressed in the nervous system (central and peripheral) (Laezza, Doherty, & Dingledine, 1999). Although there is no direct evidence demonstrating agonist activity of H2S on NMDA receptors, accumulating evidence indicates that H2S may produce physiological or pathological functions via regulating NMDA receptors. It was found that H2S stimulates LTP via potentiation of NMDA receptors. This effect was achieved mainly by H2S-induced activation of cAMP/PKA pathway (Kimura, 2000). Excessive NMDA receptor activation causes calcium overload in the cells leading to cell death (Gagliardi, 2000). Harris, Ganong, and Cotman (1984) reported that hippocampal LTP alone is not facilitated by H2S alone and it needs a stimulation to activate NMDA receptors. It seems that H2S alone does not induce any illusive currents, but significantly increases the NMDA current. The enhancing effect of H2S on the NMDA receptor is also concentration dependent. Therefore, H2S enhances the induction of LTP by activating NMDA receptors. Some reports also suggest that disulfide bonds play a role in modulating the function of many proteins, including NMDA receptors. It is therefore possible that H2S interacts with disulfide bonds or free thiol group (S-H) in NMDA receptors (Fig. 3). Therefore, NMDA receptors have important roles in the brain disease (Myers, Dingledine, & Borges, 1999) and H2S may modulate this disease progression.
10. EFFECT OF H2S ON GABA-MEDIATED NEUROTRANSMISSION
GABA, an inhibitory neurotransmitter, is present at high concentrations in the mammalian brain, especially in the axons. Within the mammalian CNS, GABA is the major inhibitory neurotransmitter: About 20–30% of all synapses in the CNS employ GABA as their neurotransmitter (Kaila, 1994). GABA-mediated inhibition in the CNS is critical as loss of GABAergic inhibition leads to seizures and neuronal hyperexcitability. There are three types of receptors for GABA in the CNS: GABAA, GABAB, and GABAC receptors, and they produce slow, prolonged inhibitory signals and function to modulate the release of neurotransmitters (Chebib & Johnston, 1999). H2S was known to promote amelioration of hippocampal damage induced by recurrent seizures via reversing the loss of GABABR1 and GABABR2 which is caused by febrile seizures (Han et al., 2005). This amelioration was outlined to the increased mRNA and protein levels of these GABA receptors, which may be due to acute increases in the Ca2+ leading to Ca2+-dependent transcription by H2S induction (Clapham, 2007; Lipscombe, Helton, & Xu, 2004; Pietrobon, 2002). This may have an effect on the restoration of the excitation/inhibition balance perturbed and affecting slow, prolonged inhibitory signals and neurotransmitter release. It was inferred from the above study that H2S may have therapeutic use in the treatment of excitatory diseases such as epilepsy (Han et al., 2005).
11. EFFECT OF H2S ON CALMODULIN KINASE
Calcium/calmodulin-dependent protein kinase II (CaM kinase II or CaMKII) is a serine/threonine-specific protein kinase that is regulated by the Ca2+/calmodulin complex. CaMKII is also necessary for Ca2+ homeostasis in the neuronal cells. On the other hand, CBS is the main enzyme for the synthesis of H2S in the brain. In addition, it is also regulated by S-adenosylmethionine which acts as an allosteric activator of CBS. As CBS enzyme is a Ca2+ and calmodulin-dependent enzyme, the biosynthesis of H2S is strongly controlled by the intracellular concentration of the Ca2+ ion. In addition to that, CBS is also regulated by S-adenosyl-l-methionine (SAM) and pyridoxal-5′ -phosphate. It was recently found that Ca2+/calmodulin-mediated pathways are involved in the regulation of CBS activity, which acts as an allosteric activator of CBS. In neurons, H2S stimulates the production of cAMP probably by direct activation of adenylyl cyclase and thus activates cAMP-dependent processes. Cyclic-AMP-mediated pathways may be involved in the modulation of NMDA receptors by H2S (Kimura, 2000). Ko and Chu (2005) showed a novel regulatory mechanism for H2S production by Ca2+/calmodulin. In addition to that, they have also shown that l-glutamate, as well as electrical stimulation, enhances the production of H2S from brain slices and that LTP is altered in CBS knockout mice. The observations by Ko and Chu (2005) also support that endogenous H2S is produced when CBS is activated by the Ca2+ which occurs with neuronal excitation, and that H2S may function as a neuromodulator or neurotransmitter (Baranano, Ferris, & Snyder, 2001). Thus, H2S is produced in response to neuronal excitation and alters hippocampal LTP, a synaptic basis of memory (Fig. 4).
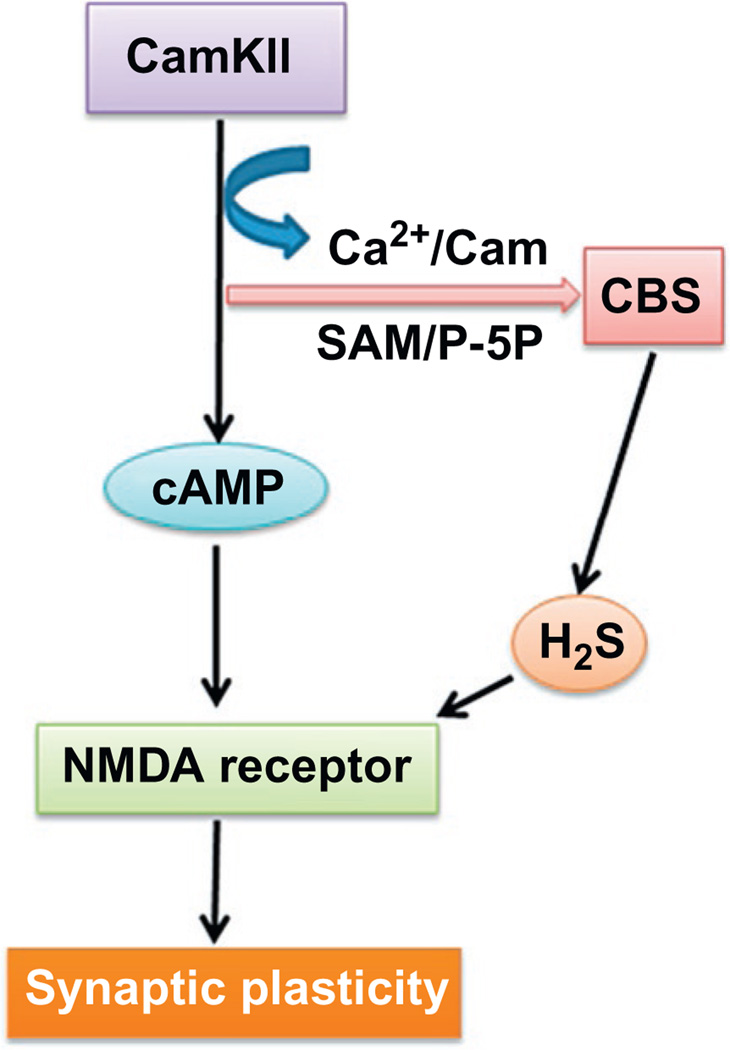
Figure 4.
Flow diagram indicates the interaction and regulation of CBS enzyme with the help of CaMKII, calcium ion, S-adenosyl methionine, and pyridoxal-5′-phosphate. In other ways, CaMKII also potentiate the NMDA receptor via cAMP pathways. Further, H2S produced from different sources with the help of CBS enzyme interacts with NMDA receptor and modulates their function and thereby maintains the synaptic plasticity. P-5P, pyridoxal-5′-phosphate; cAMP, cyclic adenosine monophosphate; CamKIIα, calmodulin kinase II alfa; SAM, S-adenosyl methionine; NMDA, N-methyl-d-aspartate.
12. CONCLUSION
Recent and previous evidences suggest that H2S plays an important role in the maintenance of physiological conditions of neurons by its neuroprotective effects. H2S is originated from both neurons and glial cells in the CNS. It has a potential effect on neurotransmitters such as glutamate, GABA, and AMPA neuronal receptors. This effect of H2S also has an impact on synapse signaling by interacting with neuronal receptor and thus maintains the neuronal function as well as synapse functions. Under pathological condition, H2S acts as an anti-inflammatory and anti-oxidative molecule and hence protects neurons and synapse from abnormal pathology, thereby remodeling the neurons as well as neuronal synapse during or after pathology.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant HL107640-NT.
ABBREVIATIONS
- 3MST
3-mercaptopyruvate sulfurtransferase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- Ca2+/calmodulin
calcium ion-dependent calmodulin kinase
- CBS
cystathionine beta-synthase
- CNS
central nervous system
- CSE
cystathionine gamma-lyase
- GSH
reduced form of glutathione
- GSSG
oxidized form of glutathione
- H2S
hydrogen sulfide
- LTD
long-term depression
- LTP
long-term potentiation
- NMDAR
N-methyl-d-aspartate receptor
- PAG
dl-propargylglycine
- SAM
S-adenosyl-l-methionine
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Alasti N, Mueller AL. Long-term potentiation in the presence of NMDA receptor antagonist arylalkylamine spider toxins. Journal of Neuroscience Research. 2000;62:177–185. doi: 10.1002/1097-4547(20001015)62:2<177::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: A review. Nutrition Journal. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends in Neurosciences. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Barker AJ, Ullian EM. New roles for astrocytes in developing synaptic circuits. Communicative & Integrative Biology. 2008;1:207–211. doi: 10.4161/cib.1.2.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann J, Lagreze WA, Schallner N, Schwer CI, Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Molecular Vision. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Jamain S, Lin CW, Rujescu D, Tseng GC, Sibille E. A conserved BDNF, glutamate- and GABA-enriched gene module related to human depression identified by coexpression meta-analysis and DNA variant genome-wide association studies. PLoS One. 2014;9:e90980. doi: 10.1371/journal.pone.0090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: A brief review. Clinical and Experimental Pharmacology & Physiology. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- Cheung NS, Peng ZF, Chen MJ, Moore PK, Whiteman M. Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons. Neuropharmacology. 2007;53:505–514. doi: 10.1016/j.neuropharm.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Tringali G, Ragazzoni E, Maggiano N, Menini E, Vairano M, et al. Evidence that hydrogen sulphide can modulate hypothalamo-pituitary-adrenal axis function: In vitro and in vivo studies in the rat. Journal of Neuroendocrinology. 2000;12:225–233. doi: 10.1046/j.1365-2826.2000.00441.x. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. The Journal of Pharmacology and Experimental Therapeutics. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Dong XB, Yang CT, Zheng DD, Mo LQ, Wang XY, Lan AP, et al. Inhibition of ROS-activated ERK1/2 pathway contributes to the protection of H2S against chemical hypoxia-induced injury in H9c2 cells. Molecular and Cellular Biochemistry. 2012;362:149–157. doi: 10.1007/s11010-011-1137-2. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2005;19:1854–1856. doi: 10.1096/fj.05-3724fje. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Dagani F, Nelson D, Deas J, Silver IA. Relations between intracellular ions and energy metabolism: A study with monensin in synaptosomes, neurons, and C6 glioma cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1991;11:2410–2421. doi: 10.1523/JNEUROSCI.11-08-02410.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Progress in Neurobiology. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Esechie A, Kiss L, Olah G, Horvath EM, Hawkins H, Szabo C, et al. Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clinical Science. 2008;115:91–97. doi: 10.1042/CS20080021. [DOI] [PubMed] [Google Scholar]
- Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- Gagliardi RJ. Neuroprotection, excitotoxicity and NMDA antagonists. Arquivos de Neuro-Psiquiatria. 2000;58:583–588. doi: 10.1590/s0004-282x2000000300030. [DOI] [PubMed] [Google Scholar]
- Garcia-Bereguiain MA, Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C. Hydrogen sulfide raises cytosolic calcium in neurons through activation of L-type Ca2+ channels. Antioxidants & Redox Signaling. 2008;10:31–42. doi: 10.1089/ars.2007.1656. [DOI] [PubMed] [Google Scholar]
- Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, et al. H2S generated by heart in rat and its effects on cardiac function. Biochemical and Biophysical Research Communications. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: Roles for gliotransmission in health and disease. Trends in Molecular Medicine. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Qin J, Chang X, Yang Z, Bu D, Du J. Modulating effect of hydrogen sulfide on gamma-aminobutyric acid B receptor in recurrent febrile seizures in rats. Neuroscience Research. 2005;53:216–219. doi: 10.1016/j.neures.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Harris EW, Ganong AH, Cotman CW. Long-term potentiation in the hippocampus involves activation of N-methyl-d-aspartate receptors. Brain Research. 1984;323:132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: Listening and talking to the synapse. Nature Reviews. Neuroscience. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hudspith MJ. Glutamate: A role in normal brain function, anaesthesia, analgesia and CNS injury. British Journal of Anaesthesia. 1997;78:731–747. doi: 10.1093/bja/78.6.731. [DOI] [PubMed] [Google Scholar]
- Hudspith M, Munglani R. A role for presynaptic NMDA receptors in central sensitization in the spinal cord dorsal horn? British Journal of Anaesthesia. 1998;81:294–295. doi: 10.1093/bja/81.2.294. [DOI] [PubMed] [Google Scholar]
- Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystathionine beta-synthase is enriched in the brains of Down’s patients. Biochemical and Biophysical Research Communications. 2005;338:1547–1550. doi: 10.1016/j.bbrc.2005.10.118. [DOI] [PubMed] [Google Scholar]
- Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxidants & Redox Signaling. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, et al. Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Progress in Neurobiology. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sciences. 2014;107:1–7. doi: 10.1016/j.lfs.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Givvimani S, Brown K, Metreveli N, Tyagi SC, et al. Nutri-epigenetics ameliorates blood-brain barrier damage and neurodegeneration in hyperhomocysteinemia: Role of folic acid. Journal of Molecular Neuroscience: MN. 2014;52:202–215. doi: 10.1007/s12031-013-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–319. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat PK, Rai S, Swarnkar S, Shukla R, Nath C. Mechanism of synapse redox stress in Okadaic acid (ICV) induced memory impairment: Role of NMDA receptor. Neurochemistry International. 2014;76:32–41. doi: 10.1016/j.neuint.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Tota S, Saxena G, Shukla R, Nath C. Okadaic acid (ICV) induced memory impairment in rats: A suitable experimental model to test anti-dementia activity. Brain Research. 2010;1309:66–74. doi: 10.1016/j.brainres.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Kim B, Lee J, Jang J, Han D, Kim KH. Prediction on the seasonal behavior of hydrogen sulfide using a neural network model. The Scientific World Journal. 2011;11:992–1004. doi: 10.1100/tsw.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochemical and Biophysical Research Communications. 2000;267:129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiology of Aging. 2002;23:787–794. doi: 10.1016/s0197-4580(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Ko TH, Chu H. Spectroscopic study on sorption of hydrogen sulfide by means of red soil. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 2005;61:2253–2259. doi: 10.1016/j.saa.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kubo S, Kajiwara M, Kawabata A. Dual modulation of the tension of isolated gastric artery and gastric mucosal circulation by hydrogen sulfide in rats. Inflammopharmacology. 2007;15:288–292. doi: 10.1007/s10787-007-1590-4. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: Joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim CH, Kim DG, Ahn YS. Zinc inhibits amyloid beta production from Alzheimer’s amyloid precursor protein in SH-SY5Y cells. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2009;13:195–200. doi: 10.4196/kjpp.2009.13.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Li F, Tsien JZ. Memory and the NMDA receptors. The New England Journal of Medicine. 2009;361:302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: The low down. Journal of Neurophysiology. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Lopez A, Prior MG, Reiffenstein RJ, Goodwin LR. Peracute toxic effects of inhaled hydrogen sulfide and injected sodium hydrosulfide on the lungs of rats. Fundamental and Applied Toxicology: Official Journal of the Society of Toxicology. 1989;12:367–373. doi: 10.1016/0272-0590(89)90053-5. [DOI] [PubMed] [Google Scholar]
- Lowicka E, Beltowski J. Hydrogen sulfide (H2S)—The third gas of interest for pharmacologists. Pharmacological Reports: PR. 2007;59:4–24. [PubMed] [Google Scholar]
- McGeer PL, Yasojima K, McGeer EG. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neuroscience Letters. 2002;326:67–69. doi: 10.1016/s0304-3940(02)00300-2. [DOI] [PubMed] [Google Scholar]
- Michaels RL, Rothman SM. Glutamate neurotoxicity in vitro: Antagonist pharmacology and intracellular calcium concentrations. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1990;10:283–292. doi: 10.1523/JNEUROSCI.10-01-00283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi SC. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. American Journal of Physiology. Heart and Circulatory Physiology. 2010;298:H451–H456. doi: 10.1152/ajpheart.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks TJ, Ghersi-Egea JF, Philbert M, Cooper AJ, Lock EA. Symposium overview: The role of glutathione in neuroprotection and neurotoxicity. Toxicological Sciences: An Official Journal of the Society of Toxicology. 1999;51:161–177. doi: 10.1093/toxsci/51.2.161. [DOI] [PubMed] [Google Scholar]
- Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nature Neuroscience. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Myers SJ, Dingledine R, Borges K. Genetic regulation of glutamate receptor ion channels. Annual Review of Pharmacology and Toxicology. 1999;39:221–241. doi: 10.1146/annurev.pharmtox.39.1.221. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radical Biology & Medicine. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, et al. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;295:R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Research Reviews. 2010;63:93–102. doi: 10.1016/j.brainresrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: Astrocytes process and control synaptic information. Trends in Neurosciences. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Calcium channels and channelopathies of the central nervous system. Molecular Neurobiology. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. International Journal of Biological Sciences. 2012;8:430–441. doi: 10.7150/ijbs.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu K, Chen CP, Halliwell B, Moore PK, Wong PT. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke: A Journal of Cerebral Circulation. 2006;37:889–893. doi: 10.1161/01.STR.0000204184.34946.41. [DOI] [PubMed] [Google Scholar]
- Rai S, Kamat PK, Nath C, Shukla R. A study on neuroinflammation and NMDA receptor function in STZ (ICV) induced memory impaired rats. Journal of Neuroimmunology. 2013;254:1–9. doi: 10.1016/j.jneuroim.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Rai S, Kamat PK, Nath C, Shukla R. Glial activation and post-synaptic neurotoxicity: The key events in streptozotocin (ICV) induced memory impairment in rats. Pharmacology, Biochemistry, and Behavior. 2014;117:104–117. doi: 10.1016/j.pbb.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last) Trends in Neurosciences. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Rezessy-Szabo JM, Nguyen QD, Hoschke A, Braet C, Hajos G, Claeyssens M. A novel thermostable alpha-galactosidase from the thermophilic fungus Thermomyces lanuginosus CBS 395.62/b: Purification and characterization. Biochimica et Biophysica Acta. 2007;1770:55–62. doi: 10.1016/j.bbagen.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, et al. Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. American Journal of Physiology. Cell Physiology. 2012;303:C41–C51. doi: 10.1152/ajpcell.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxidants & Redox Signaling. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F, Giudici R, Duy CN, Schelzig H, Oter S, Groger M, et al. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]
- Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, et al. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-thoracic Surgery. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Chen RQ, Dong L, Ren YK, Del Soldato P, Sparatore A, et al. Role of paraoxonase-1 in the protection of hydrogen sulfide-donating sildenafil (ACS6) against homocysteine-induced neurotoxicity. Journal of Molecular Neuroscience: MN. 2013;50:70–77. doi: 10.1007/s12031-012-9862-x. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Chen RQ, Ren YK, Soldato PD, Sparatore A, Zhuang YY, et al. ACS6, a hydrogen sulfide-donating derivative of sildenafil, inhibits homocysteine-induced apoptosis by preservation of mitochondrial function. Medical Gas Research. 2011;1:20. doi: 10.1186/2045-9912-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa K. Production of H2S by 3-mercaptopyruvate sulphurtransferase. Journal of Biochemistry. 2011;149:357–359. doi: 10.1093/jb/mvr018. [DOI] [PubMed] [Google Scholar]
- Tsugane M, Nagai Y, Kimura Y, Oka J, Kimura H. Differentiated astrocytes acquire sensitivity to hydrogen sulfide that is diminished by the transformation into reactive astrocytes. Antioxidants & Redox Signaling. 2007;9:257–269. doi: 10.1089/ars.2007.9.257. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Mishra PK, Tyagi SC. Homocysteine, hydrogen sulfide (H2S) and NMDA-receptor in heart failure. Indian Journal of Biochemistry & Biophysics. 2009;46:441–446. [PubMed] [Google Scholar]
- Tyagi N, Vacek JC, Givvimani S, Sen U, Tyagi SC. Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. Journal of Receptor and Signal Transduction Research. 2010;30:78–87. doi: 10.3109/10799891003614808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura K, Kimura H. Hydrogen sulfide enhances reducing activity in neurons: Neurotrophic role of H2S in the brain? Antioxidants & Redox Signaling. 2007;9:2035–2041. doi: 10.1089/ars.2007.1802. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends in Pharmacological Sciences. 2007;10:501–505. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nature Neuroscience. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Guo Z, Wang S. Cystathionine gamma-lyase expression is regulated by exogenous hydrogen peroxide in the mammalian cells. Gene Expression. 2012;15:235–241. doi: 10.3727/105221613x13571653093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Han A, Wen C, Chen M, Chen X, Yang X, et al. The effects of H2S on the activities of CYP2B6, CYP2D6, CYP3A4, CYP2C19 and CYP2C9 in vivo in rat. International Journal of Molecular Sciences. 2013;14:24055–24063. doi: 10.3390/ijms141224055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochemical Pharmacology. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? Journal of Neurochemistry. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang C, Xiao L, Liao X, Lan A, Wang X, et al. Novel insights into the role of HSP90 in cytoprotection of H2S against chemical hypoxia-induced injury in H9c2 cardiac myocytes. International Journal of Molecular Medicine. 2011;28:397–403. doi: 10.3892/ijmm.2011.682. [DOI] [PubMed] [Google Scholar]
- Zhao H, Chan SJ, Ng YK, Wong PT. Brain 3-mercaptopyruvate sulfurtransferase (3MST): Cellular localization and downregulation after acute stroke. PLoS One. 2013;8:e67322. doi: 10.1371/journal.pone.0067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chen MH, Shen ZM, Kahn PC, Lipke PN. Environmentally induced reversible conformational switching in the yeast cell adhesion protein alpha-agglutinin. Protein Science: A Publication of the Protein Society. 2001;10:1113–1123. doi: 10.1110/ps.41701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. The Journal of Biological Chemistry. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- Zhu W, Song X, Li M, Dao J. CBS gene variations and serum homocysteine level associated with congenital heart defects. Wei Sheng Yan Jiu = Journal of Hygiene Research. 2008;37:463–467. [PubMed] [Google Scholar]