Abstract
Background
Despite trauma-induced hypothermic coagulopathy being familiar in the clinical setting, empirical experimentation concerning this phenomenon is lacking. In this study we investigated the effects of hypothermia on thrombin generation, clot formation and global hemostatic functions in an in vitro environment using a whole blood model and thromboelastography (TEG) which can recapitulate hypothermia.
Methods
Blood was collected from healthy individuals through venipuncture and treated with corn trypsin inhibitor, to block the contact pathway. Coagulation was initiated with 5pM tissue factor at temperatures 37°C, 32°C, and 27°C. Reactions were quenched over time with soluble and insoluble components of each time point analyzed for thrombin generation, fibrinogen consumption, factor (f)XIII activation and fibrin deposition. Global coagulation potential was evaluated through TEG.
Results
Data showed that thrombin generation in samples at 37°C and 32°C had comparable rates while 27°C had a much lower rate (39.2 ± 1.1 and 43 ± 2.4 nM/min vs 28.6 ± 4.4 nM/min, respectively). Fibrinogen consumption and fXIII activation were highest at 37°C followed by 32°C and 27°C (13.8 ± 2.9 percent/min vs 7.8 ± 1.8 percent/min, respectively). Fibrin formation as seen through clot weights also followed this trend. TEG data showed clot formation was fastest in samples at 37°C and lowest at 27°C. Maximum clot strength was similar for each temperature. Also, percent lysis of clots was highest at 37°C followed by 32°C and then 27°C.
Conclusions
Induced hypothermic conditions directly affect the rate of thrombin generation and clot formation while global clot stability remains intact.
Keywords: hypothermia, trauma, thrombin generation, fibrin formation, lysis
Introduction
Trauma continues to be a leading of cause of death for all ages in the United States and is the major cause of death for ages 15-24 years old.(1) Uncontrolled hemorrhage is the principal cause of death and results from gross vascular injury or disturbances in the coagulation system that leads to a failure to maintain adequate hemostasis. Isolated hypothermia accounts for approximately two deaths per day or 700 deaths per year,(2) and is associated with a 23% mortality rate when the body temperature drops below 32°C.(3) The physiologic responses to hypothermia in the trauma patient are numerous and exacerbate the often already compromised cardiovascular, central nervous, and coagulation systems. These combined effects of severe tissue trauma and hypothermia are deadly, with a reported 100% mortality in trauma patients who present with core body temperatures of less than 32°C.(4)
Isolated hypothermia is generally categorized into mild (32-36°C), moderate (28-32°C), severe (16-27°C), profound (6-15°C) and ultra-profound (<5°C). (5) In the trauma setting, with the combined effect of hypothermia and tissue injury, 36°C to 34°C degrees is considered mild hypothermia, 34°C to 32°C degrees is moderate hypothermia, and severe hypothermia occurs below 32°C degrees.(4, 5) Worsening hypothermia portends greater mortality (4) and increased risk for multiple organ dysfunction syndrome (6).. However, patients presenting with even mild hypothermia have been reported to suffer 40% mortality. Rewarming of hypothermic patients has significant clinical benefit with decreased resuscitative fluid requirements and mortality after major trauma. (7) Hypothermia may have a two-fold effect on the coagulation system. Mild to moderate hypothermia appears to result in primarily platelet dysfunction, while severe hypothermia seemingly leads to both platelet dysfunction and enzyme (clotting factor) activity reduction. (8)
The coagulation system is a complex yet delicate balance of prothrombotic, anticoagulant, and fibrinolytic processes. In trauma patients, tissue damage results in the exposure of massive amounts of tissue factor (Tf) released from the subendothelium into circulation. Membrane-bound Tf binds the circulating enzyme factor (f)VIIa, forming the extrinsic fXase complex which subsequently activates the zymogens fIX and fX to their respective serine proteases, fIXa and fXa.(9-11). Platelet bound fXa subsequently activates a small amount (pM) of the zymogen prothrombin to α-thrombin (12, 13) which in turn activates more platelets (14) and the procofactors fV,(15) and fVIII,(16). Once activated, these cofactors combine with their respective enzymes fIXa and fXa on the surface of activated platelets to form the intrinsic fXase and prothrombinase complexes. The formation of these two essential complexes leads to a massive burst in thrombin generation which simultaneously activates the transglutaminase fXIII,(17) and releases fibrinopeptides A and B to form a stable crosslinked fibrin clot.(14) Interestingly, fibrin clot formation occurs with only 2 nmol/L free thrombin (10 nM TAT) (14, 18) with the vast majority (96%) of thrombin being generated after the initial clot formation has been observed. The subsequent propagation of thrombin generation is necessary to cleave the remaining fibrinogen and fXIII to form a stable crosslinked clot. (19)
The clinical detection of relevant aberrations in clotting factor activation and consumption leading to coagulopathy in trauma patients continues to evolve. For the better part of a century, the standard “bedside” tests of coagulation function have primarily been prothombin time (PT) and activated partial thromboplastin time (aPTT). Despite the fact that they do not measure the vast majority of thrombin generation and may under-appreciate more subtle clinical coagulopathies, these tests are still ubiquitously utilized. Thromboelastography (TEG), which has been in existence for nearly five decades, has gained wider acceptance as a more reliable “bedside” tool for the assessment of coagulopathy in trauma patients. (20-24) Thromboelastrographic profiling has the advantages of providing a comprehensive assessment of blood coagulation and fibrinolysis while utilizing whole blood samples at defined temperatures and controllable environments. Thus, TEG profiling allows for a thorough evaluation of coagulopathy and is developing into a useful clinical tool (25). Specifically, TEG has been shown to provide a more accurate measure of hypothermic and hemodilution effects on traumatic coagulopathy.(20, 25)
Induced hypothermia and hypothermia secondary to hemorrhagic shock are very different physiological states that lead to distinct outcomes (26, 27). The diversity and complexity of the etiology of trauma-induced coagulopathy does not lend itself to the simplistic and reductionist approaches often employed in clinical research. In fact there are no animal models that can account for all of the variables involved in generating trauma-induced coagulopathy in humans.(28) In this study, we explore the potential effects of a trauma-induced hypothermic situation on the coagulation system with particular focus on thrombin generation, fibrinogen consumption and factor fXIII activation using a well-established (18, 29) in vitro whole blood assay and thromboelastography. This study provides a natural history of alterations that occur to blood coagulation when hypothermia is induced from a normal state.
Materials and Methods
Materials
HEPES, Tris-base, ethylenediaminetetraacetic acid (EDTA), trifluoroacetic acid and Benzamidine-HCl were purchased from Fisher (Waltham, MA). 1-palmitoyl-2-oleoylphosphatidyl serine (PS), and 1-palmitoyl-2-oleoyl-phosphatidylcholine (PC) were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). Recombinant Tf was a gift from Drs. Lundblad and Liu (Hyland division, Baxter Healthcare Corp, Duarte, CA) and was relipidated in PCPS (25% PS, 75% PC) vesicles as previously described.(30, 31) Corn trypsin inhibitor (CTI) was prepared as previously described.(32) D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (FPRck) was supplied by Dr. Jenny (Haematologic Technologies, Essex Junction, VT).
Patients
Healthy patients (n=8) with a mean (SD) age of 35.7 ± 10.2 years (range 25.8-58.5 years) were recruited, and advised according to a protocol approved by the University of Vermont Institutional Review board and Human Studies Committee and consent was obtained. Several individuals were studied multiple times. All donors had no history of thrombosis/hemorrhage, regular aspirin use, drug use, or trauma within the past 30 days. No specific limits were provided regarding diet or behavior. Eight individuals were evaluated in the thromboelastography studies and three individuals were evaluated in the whole blood assay with varying temperatures.
Whole blood assay
Tf-initiated whole blood assays were performed as previously described(14, 18) in which 3 temperatures were investigated: normothermia 37°C, moderate hypothermia 32°C, and severe hypothermia 27°C. Experiments were performed in polystyrene tubes placed on a rocking table enclosed in a temperature-controlled glove box at 37°C, 32°C, or 27°C. Contact pathway inhibitor (CTI- 100 μg/ml), which blocks fXIIa, and relipidated Tf at 1:2000 protein/lipid (functionally 5pM) were preloaded into tubes. Blood was collected by venipuncture at either the Fletcher Allen Health Care Clinical Research Center (Burlington, VT) or at the Colchester Research Facility (Colchester, VT) with a 19-3/4 gauge Vacutainer drawn into a 60-ml repeater syringe and the tube was immersed while swirling in a water bath at 37°C, 32°C, or 27°C for a specific time calculated to reach each individual temperature. The blood was then removed from the water bath and 1ml aliquots were placed into tubes at the appropriate temperatures containing the CTI and Tf. A control tube containing CTI and no Tf was used each time. Whole blood was allowed to rock at each temperature during a set time course over 20 minutes. Clot time was determined visually (by two observers: K.B.Z and M.W.). The reaction of dynamic thrombin generation was stopped by the addition of inhibitors to a final concentration 25 mmol/L EDTA and 10 mmol/L benzamidine-HCL in HBS (HEPES [buffered saline], 0.15 mol/L NaCl and 0.02 mol/L HEPES), pH 7.4, and 50 umol/L FPRck in 10 mmol/L HCl at every minute between 0-10 followed by 12, 14, 16 and 20 minutes. The 0 time point contained the inhibitors before the addition of blood. After quenching the coagulation process, samples were centrifuged for 15 minutes at 1100g, and solid and solution phases were stored at −80°C for further analysis.
Thrombin generation
Thrombin generation was measured in complex with antithrombin (TAT, Behring, Westwood, MA) as per the manufacturer's instructions. Assays were duplicated or triplicated using a minimum of five standards as previously described.(32) Results were analyzed on a Vmax microtiter plate reader equipped with Softmax version 2.35 (Molecular Devices, Menlo Park, CA).
Fibrin clot analysis
The insoluble clotted material contained within the whole blood tubes at each time point was analyzed as previously described.(19) Briefly, the insoluble clotted samples were washed 2-3 times with 1 mL of 0.15 mol/L NaCl and then allowed to sit in the salt solution (1 mL) for 12-15 hours so that additional soluble material within the clots could diffuse into solution. The clots were rinsed with H2O to remove salt, lyophilized and weighed.
Fibrinogen consumption and fXIII activation
SDS-PAGE (4-12%) was performed on quenched whole blood serum samples according to the modified Laemmli procedure.(33) High molecular weight standard mixtures (12-200 kDa) were loaded along with fibrinogen standards (300ng/lane), fXIII (50ng/lane), or activated fXIII (fXIIIa) to allow for comparison on the immunoblots. The gels were transferred to nitrocellulose membranes (Bio-Rad) and subjected to semi-dry transfer for 3 h at 250 mAmp as described by Towbin et al. (34) The primary antibodies used were a mouse anti-Fbgn-2E (in house) or a polyclonal rabbit anti-fXIII (D4679, ZymoGenetics, Seattle, WA) for fibrinogen and FXIII detection respectively. The blots were developed as previously described.(18) Comparisons of fXIII conversion to fXIIIa, and fibrinogen levels in solution were analyzed on immunoblots via densitometry. A relative fraction was calculated from fibrinogen or fXIIIa present at each time point divided by total fibrinogen or total fXIII A-chain detectable.
Thromboelastography (TEG)
Thromboelastography studies were performed using phlebotomy blood with 5pM Tf and CTI (100 μg/ml) at 37°C, 32°C, and 27°C. Four TEG units (eight reaction cups; Haemoscope Corporation) were used simultaneously. A single cup containing Tf without CTI was used as control. TEG profiles are analyzed in terms of R, K, alpha, MA, TMA and LY60. “R” is the time from the start of measurement until initial fibrin formation, which again results from fibrinogen cleavage by thrombin. Prolongation of the R-time indicates clotting factor deficiency or enzymatic inhibition. “Alpha” measures the rapidity of fibrin build-up and cross-linking by fXIII, which is the speed of clot strengthening. “K” measures the time necessary to reach 20 mm of clot strength and “MA” or maximal amplitude measures the maximum strength of clot. Together, the K-time and MA measure clot formation and strength, and aberrations more likely represent platelet dysfunction and to a lesser extent fibrin build-up. “TMA” is the time required to reach maximum clot strength. Fibrinolysis is measured by the fibrinolytic index or “LY”. LY60 represents the percent change in clot strength at sixty minutes, respectively.(35, 36)
Statistical analysis
This study is descriptive in nature. The data were evaluated by unpaired t-tests with STATA version 10.0 software (College Station, TX). Significance is indicated as levels of p < 0.05. Data are presented as the mean and standard error of the mean (SEM).
Results
Thrombin generation
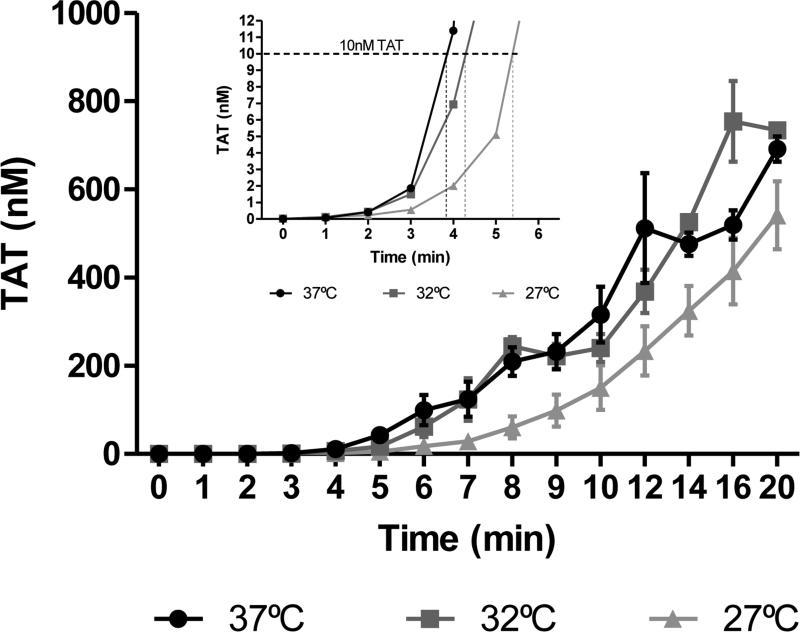
Thrombin generation profiles from whole blood time courses measured with TAT ELISA are displayed in Figure 1. Samples assessed at 37°C produced thrombin at a maximum rate of 39.2 ± 1.1 nM/min, while samples analyzed at 32°C produced thrombin at a maximum rate of 43 ± 2.4 nM/min. Maximum thrombin levels were 727 ± 79 nM for 37°C and 801 ± 67.3 nM for 32°C. Whole blood samples at 27°C reflected both lower rates and levels of thrombin generation, respectively: 28.6 ± 4.4 nM/min and 541 ± 77 nM.
Figure 1. Thrombin generation in whole blood.
Whole blood was drawn into 0.1 mg/mL CTI and activated by 5 pM Tf at t=0. Samples were quenched at varying intervals with EDTA and FPR-chloromethyl ketone prior to being analyzed by TAT ELISA. Main figure. αTAT generation profiles for healthy individuals at 37(●), 32(■) and 27°C (▲) (N=3). Inset. αTAT generation profiles at 37°C (●), 32°C (■) and 27°C (▲) (N=3) for the first 6 minutes of the time course. Data are shown as the mean±SEM.
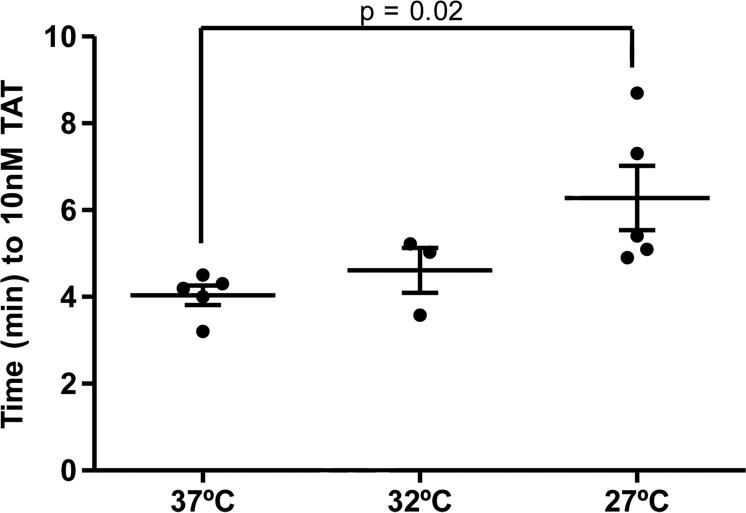
Visual inspection of clot times is an imperfect method for measurement of the clot time and onset of thrombin generation. Therefore, thrombin generation was assessed based on the time to 10 nM TAT which typically coincides with the clot time (Figure 1 inset).(14, 37) At lower temperatures, the time to 10 nM TAT was markedly delayed (37°C: 4.0 ± 0.2 min; 32°C: 4.6 ± 0.5 min; 27°C: 6.3 ± 0.7 min). Samples analyzed at 27°C demonstrated significantly (p=0.02) delayed early thrombin generation when compared to normothermia (Figure 2).
Figure 2. Early thrombin generation in whole blood.
Time (min) to 10nM TAT formation is shown for 37°C, 32°C, and 27 °C (N=3). Data are shown as the mean±SEM.
Fibrinogen consumption and fibrin deposition
Consumption of fibrinogen in healthy individuals is a parallel marker of the onset of thrombin generation (14, 19). Fibrinogen analysis through western blots and densitometry analysis is displayed in Figure 3 and shows a significantly (p=0.01) higher maximum rate of consumption at 37°C (-18.7 ± 2.3 percent/min) when compared to samples at 27°C (-10.7 ± 0.87 percent/min). Whole blood analyzed at 27°C also demonstrated a 57% reduction in initial (5 min) fibrinogen consumption when compared to blood at 37°C (27°C 7.8 ± 1.8 percent/min vs. 37 °C 13.8 ± 2.9 percent/min). While, fibrinogen consumption appeared to be complete between 6 and 20 minutes at 37°C, both the hypothermic time courses (32°C and 27°C) failed to completely deplete their respective stocks of fibrinogen by 20 minutes.
Figure 3. Fibrinogen consumption in whole blood.
Whole blood sera were diluted with 1x SDS-PAGE sample buffer, and run out on 4–20% SDS-PAGE under non-reducing conditions followed by Western blotting probing with an anti-Fibrinogen-2a mouse monoclonal antibody. Densitometry was performed on the blots for the 37°C (●), 32°C (■) and 27°C (▲) time courses (N=3). Data are shown as the mean±SEM of the normalized percent activation.
Alternatively, fibrin clot formation is another important marker of adequate thrombin generation. Fibrin generation as determined through clot mass varied significantly (p<0.003) between normothermic and hypothermic temperatures. Normothermic samples had an average mass of 1.2 ± 0.00. Samples at 32 °C had a mass of 0.88 ± 0.01 mg while samples at 27°C had a mass of 0.82 ± 0.01 mg.
Factor XIII activation
Factor XIII activation (Figure 4) did not vary significantly between mild hypothermic and normothermic temperatures. However, samples analyzed at 27°C (19.1 ± 0.7 percent/min) demonstrated a reduced activation when compared to 32°C (29.9 ± 0.3 percent/min) and 37°C (30.3 ± 0.4 percent/min).
Figure 4. Factor XIII activation in whole blood.
Whole blood sera were diluted with 1x SDS-PAGE sample buffer, and run out on 4–20% SDS-PAGE under non-reducing conditions followed by Western blotting probing with a FXIII polyclonal antibody. Densitometry was performed on the blots for the 37°C (●), 32°C (■) and 27°C (▲) time courses (N=3). Data are shown as the mean±SEM of the normalized percent activation.
Thromboelastography
Clot behavior, determined through TEG assays, reflected a delayed onset of clot formation and clot lysis in hypothermic blood samples (Table 1 and Figure 4). On average, the 32 °C samples displayed shorter R times than the 27 °C with the R time being significantly slower at 32°C (10.1 ± 2.3 min) and 27°C (12.5 ± 2.8 min) when compared to normothermia (8.1 ± 0.6 min). The Alpha or rapidity of fibrin build-up and cross-linking by fXIIIa was also significantly decreased at 32°C (32.5 ± 8.9°) and 27°C (26.6 ± 6.7°) when compared to normothermia (38.2 ± 4.4°). Maximum clot strength or MA was not significantly different at any temperature (37°C: 55.4 ± 4.5mm; 32°C: 55.5 ± 5.6mm; 27°C: 53.6 ± 5.1mm). However, the time to maximum clot formation (TMA) was significantly longer at hypothermic temperatures 32°C (44.0 ± 5.6min) and 27°C (51.2 ± 9.3min) when compared to normothermia (34.5 ± 3.4min). In addition, fibrinolysis was significantly delayed at hypothermic temperatures 32°C (0.8+1.0%) and 27°C (0.2+0.3%) when compared to normothermia (3.8+2.8%).
Table 1.
Summary of thromboelastographic parameters in whole blood.
| TEG Parameter | 27C | 32C | 37C | P<0.05 |
|---|---|---|---|---|
| R (min) | 12.5 (2.8) | 10.1 (2.3) | 8.1 (0.6) | * (32,27 vs 37) |
| K (min) | 8.4 (3.4) | 6.4 (2.5) | 5.0 (1.0) | * (32,27 vs 37) |
| α (deg) | 26.6 (6.7) | 32.5 (8.9) | 38.2 (4.4) | * (32,27 vs 37) |
| MA (mm) | 53.6 (5.1) | 55.5 (5.6) | 55.4 (4.5) | |
| TMA (min) | 51.2 (9.3) | 44.0 (5.6) | 34.5 (3.4) | * (32,27 vs 37) |
| LY60 (%) | 0.2 (0.3) | 0.8 (1.0) | 3.8 (2.8) | * (32,27 vs 37) |
R-time: duration to initial clot formation; K-time: duration from initial clot formation to max clot; Alpha: rapidity of fibrin build up and cross linking; MA: maximum clot strength – fibrin and platelets; TMA: time to maximum clot strength; LY60: percent lysis at 60 minutes. Data are presented as mean (SD).
Discussion
Significant derangements in the coagulation system are known to occur at hypothermic temperatures and effect both enzyme and platelet function. We used our in vitro whole blood model to evaluate how blood coagulation changes from normothermia to hypothermia after a Tf stimulus. Our results show that severe hypothermia (27°C) leads to significant delays in early thrombin generation rate (p=0.02) and maximum rate of fibrinogen consumption (p=0.01). Severe hypothermia also caused a marked 57% reduction in initial (5 min) fibrinogen consumption. Fibrin generation as measured by fibrin clot mass also varied significantly (p<0.003) between normothermic and hypothermic samples. Both severe (27°C) and mild (32°C) hypothermia significantly affected global hemostasis as measured by TEG. In general, hypothermic conditions caused delays in initial thrombin generation, as well as decreases in the rate of clot formation and lysis. Interestingly, hypothermia did not affect the ultimate strength of the formed clot but did prolong the time required to reach the maximum strength.
Thrombin generation occurs in two fundamental steps; initiation and propagation. Using a standard ELISA assay (TAT) on cooled healthy human blood samples activated by a standard 5 pM Tf stimulus in the presence of CTI, we have demonstrated that moderate and severe hypothermia result in decreased early thrombin generation as a consequence of delayed thrombin initiation. Interestingly, we observed no significant effect on thrombin propagation. These findings have also been previously described in a swine hypothermia model, where hypothermia primarily effected thrombin generation by impeding thrombin initiation.(38) Notably, total thrombin generation in our experimental system was not affected by hypothermia. TEG analysis corroborated these findings, as hypothermic samples revealed significantly prolonged R-time, decreased α-angles and no significant difference in MA when compared to normothermic samples. These data further support previous findings, where isolated hypothermic platelets have also been shown to exhibit a temperature dependent decrease in thrombin generation; with hypothermic platelet samples exhibiting 25% (33°C) and 68% (23°C) less thrombin generation when compared to normothermic platelet samples.(8) Therefore, in the setting of hypothermia with traumatic tissue injury (i.e. Tf exposure to circulating blood and activation of the extrinsic pathway), appropriate microvascular hemostasis may be initially delayed from the postponement of the thrombin initiation phase. In the context of beneficial/induced hypothermia as in the case of surgery, we would expect a similar coagulation response to that observed in this study. The lack of traumatic tissue damage combined with the controlled hypothermic environment should yield a proportionate reduction in thrombin generation rate while maintaining adequate clot formation. The effects of anticoagulants under hypothermic conditions, however, remain to be investigated.
In addition to the detrimental effect on thrombin initiation, fibrinogen consumption was also reduced by hypothermia. Fibrinogen is cleaved by thrombin to form fibrin, which is ultimately cross-linked by fXIIIa, which is also activated by thrombin. Therefore, not surprisingly, we found using immunoblotting techniques that early (5 min) fibrinogen consumption and overall fXIII activation were similarly depressed with severe hypothermia when compared to normothermic samples. In particular, our data shows that under mildly hypothermic conditions (32°C), fibrinogen consumption is comparable to that observed under normothermic conditions. However, under severe hypothermic conditions (27°C) fibrinogen consumption was drastically delayed and TAT assay data corroborates these findings. Under mild hypothermic conditions (32°C), thrombin generation is slightly delayed during the initial phase. This delay becomes visible after four minutes (see Figure 1A insert). Note however, that the time to reach 2nM thrombin is approximately 3 minutes in both 37°C and 32°C samples. As mentioned earlier, initial fibrin clot formation occurs and can be detected with this low nanomolar amount of active thrombin. (9, 13) This explains the results for fibrinogen consumption in 37°C and 32°C samples; since both samples were able to generate 2nM thrombin at comparable times, their fibrinogen consumption profiles can be expected to be quite similar during the early stages of coagulation. The 27°C samples did not generate 2nM thrombin until 4 minutes, and thus also show more pronounced delays over the entire 20-minute time course (Figure 1A). This substantiates the significant reduction in fibrinogen consumption for the 27°C samples observed in Figure 2A.
Notably, total fibrinogen consumption and fXIII activation were not appreciably different at the conclusion of our experiments (20 minutes). This suggests that like thrombin generation, hypothermia affects the rate of fibrin clot formation while clot stability and strength are unaltered.This again is supported by our TEG analysis which revealed prolonged R-time and TMA with hypothermia but similar MA amongst all samples. Decreases (up to 60%) in fXIII activation have been previously implicated in post-operative neurosurgical (39) and intra-operative general surgical bleeding. (40) Our data demonstrates a decrease in fXIII activation in the setting of induced hypothermia that may also contribute to these clinical findings.
Clinically, hyperfibrinolysis has been defined as having a maximal clot lysis at 60 minutes of 10% or higher that is reversible by aprotinin. (41)When we assessed the coagulopathy of clot lysis/fibrinolysis in our system, our data demonstrates that hypothermia results in significantly delayed clot lysis. This can be seen in Table 1, as denoted by the percentage of clot lysis after 60 minutes (LY60%). LY60% in the context of thisstudy, is a measurement of the blood's intrinsic ability to activate fibrinolysis. In the absence of a standardized physiologic plasminogen activator (such as tPA) or the addition of aprotinin, conclusions based on these results are speculative at best. However, this finding of delayed clot lysis in combination with delays in the initiation of thrombin generation, fibrin consumption, and fXIII activation is particularly interesting as it describes a phenomenon where thrombin generation is delayed in the early stages (2-6 minutes) but is pathologically insignificant in later stages of clot formation (>60 minutes).
Clinically, these findings may correlate with the uncontrolled bleeding diathesis in the early phases of trauma care, followed by venous thrombosis upon return to therapeutically achieved normothermia. Furthermore, in line with the obvious biophysics of enzymatic reaction mechanisms, these data support the hypothesis that induced hypothermia primarily affects the rate of thrombin generation and clot formation. Although these data are not directly translatable to the case of prophylaxis in the trauma patient, in the context of induced hypothermia, it suggests that reconstitution to a normothermic state will maintain adequate thrombin generation and clot formation.
We acknowledge certain limitations of this study. Namely, we have utilized a small number of “healthy” control samples in a controlled hypothermia trauma-induced environment and this may not reflect the actual changes in blood samples from hypothermic trauma patients. More specifically, in our model we have not accounted for changes in coagulation factors that are likely occurring in the trauma patient (both consumption and increased production). Future studies need to be performed that describe changes in the hemostatic profile of hypothermic trauma patients applying a similar approach. Our whole blood assay is a compromise between biological authenticity and experimental opacity. However, in the current format, it is impractical to perform in a hospital setting. Therefore, in an attempt to align our research with clinically relevant methodologies, we employed the use of TEG analysis. The use of both of these models provides us with multiple experimental approaches to analyzing coagulation dynamics. While both of these techniques are pertinent to our hypothermia study, there are fundamental differences in their respective methodologies which do not allow them to be directly compared. For example, the ubiquitous use of 5 pM Tf in the TEG experiments was chosen to give an approximate clot time where changes and alterations could be easily observed and noted (see Table 1). We have 15 years experience using 5pM Tf in our in vitro assay and have applied the same concentration to our TEG line of experimentation expecting equal results. Interestingly equal Tf activation in separate models resulted in unique coagulation parameter outcomes per model. Significant differences were found in TEG parameters when comparing whole blood coagulation at various temperatures. Lower concentrations of Tf were not pursued in our in vitro model because of the loss of comparative analysis power that would be realized with a divergence from our extremely well described history of whole blood investigation within this model. We speculate the fundamental differences in assay mechanics plays the largest role in the discrepancies found between models. Due to these differences the clot times and other parameter comparisons between these two experimental methods solely involve general trends. In summary, hypothermia causes a multifactorial delay in clot formation and breakdown, which may lead to both initial problems with bleeding in the trauma patient followed by clot stasis and development of thrombosis (DVT or PE). Our results suggest that induced hypothermia produces only temporal changes to thrombin generation and clot formation.
Figure 5. Thromboelastography (TEG) in whole blood.
TEG data are shown for CTI-inhibited whole blood samples collected at 37°C, 32°C, and 27°C. Coagulation was initiated with 5pM Tf. A. R-time (min): time to initial clot formation. B. K-time (min): duration from initial clot formation to maximum clot. C. Alpha (degrees): rapidity of fibrin build up and cross-linking. D. Maximum Amplitude (MA) (mm): maximum clot strength – fibrin and platelets. E. TMA (min): time to max clot strength. F. LY 60: percent of clot lysis at 60 minutes. (N=8) Data are shown as mean ± SEM.
Acknowledgements
The authors would like to thank Lauren Ferris, Chase Haven and Matthew Gissel for their technical assistance and Dr. Kenneth Mann for his insightful discussions regarding trauma based coagulopathies.
Support: This work was supported by the Pilot Project Award Program of the Office of Patient Oriented Research at UVM/Fletcher Allen Health Care (KBZ), by the Training Grant No. PHST32HL07594-22 (A.K.), Program Project Grant No. HL46703 (Project 5) from the National Institutes of Health and ARO W911NF-10-1-0376.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings: Part of this research was presented at the American Association for the Surgery of Trauma-68th Annual Meeting, Pittsburgh, PA, 2009, Poster #77.
References
- 1.National Vital Statistics System NCfHS, CDC. 10 Leading Causes of Death by Age Group,United States - 2006. 2006 [Google Scholar]
- 2.Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87(1):247–67. doi: 10.1016/j.suc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Danzl DF, Pozos RS, Auerbach PS, Glazer S, Goetz W, Johnson E, et al. Multicenter hypothermia survey. Ann Emerg Med. 1987;16(9):1042–55. doi: 10.1016/s0196-0644(87)80757-6. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27(9):1019–24. [PubMed] [Google Scholar]
- 5.Alam HB, Pusateri AE, Kindzelski A, Egan D, Hoots K, Andrews MT, et al. Hypothermia and hemostasis in severe trauma: A new crossroads workshop report. The journal of trauma and acute care surgery. 2012;73(4):809–17. doi: 10.1097/TA.0b013e318265d1b8. [DOI] [PubMed] [Google Scholar]
- 6.Beilman GJ, Blondet JJ, Nelson TR, Nathens AB, Moore FA, Rhee P, et al. Early hypothermia in severely injured trauma patients is a significant risk factor for multiple organ dysfunction syndrome but not mortality. Ann Surg. 2009;249(5):845–50. doi: 10.1097/SLA.0b013e3181a41f6f. [DOI] [PubMed] [Google Scholar]
- 7.Gentilello LM, Jurkovich GJ, Stark MS, Hassantash SA, O'Keefe GE. Is hypothermia in the victim of major trauma protective or harmful? A randomized, prospective study. Ann Surg. 1997;226(4):439–47. doi: 10.1097/00000658-199710000-00005. discussion 47-9. PMCID: 1191057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolberg AS, Meng ZH, Monroe DM, 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56(6):1221–8. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]
- 9.Jesty J, Silverberg SA. Kinetics of the tissue factor-dependent activation of coagulation Factors IX and X in a bovine plasma system. J Biol Chem. 1979;254(24):12337–45. [PubMed] [Google Scholar]
- 10.Nemerson Y. Tissue factor and hemostasis. Blood. 1988;71(1):1–8. [PubMed] [Google Scholar]
- 11.Brummel Ziedins K, Orfeo T, Jenny N, Everse SJ, Mann KG. Blood coagulation and fibrinolysis. In: Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe's Clinical Hematology. 11th Edition. Lippincott, Williams & Wilkins; Philadelphia: 2004. pp. 677–774. Chapter 21. [Google Scholar]
- 12.Orfeo T, Butenas S, Brummel-Ziedins KE, Mann KG. The tissue factor requirement in blood coagulation. J Biol Chem. 2005;280(52):42887–96. doi: 10.1074/jbc.M505506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orfeo T, Brufatto N, Nesheim ME, Xu H, Butenas S, Mann KG. The factor V activation paradox. J Biol Chem. 2004;279(19):19580–91. doi: 10.1074/jbc.M400727200. [DOI] [PubMed] [Google Scholar]
- 14.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100(1):148–52. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 15.Nesheim ME, Mann KG. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979;254(4):1326–34. [PubMed] [Google Scholar]
- 16.Fay PJ. Subunit structure of thrombin-activated human factor VIIIa. Biochim Biophys Acta. 1988;952(2):181–90. doi: 10.1016/0167-4838(88)90114-8. [DOI] [PubMed] [Google Scholar]
- 17.Lorand L, Konishi K. Activation of the Fibrin Stabilizing Factor of Plasma by Thrombin. Arch Biochem Biophys. 1964;105:58–67. doi: 10.1016/0003-9861(64)90235-8. [DOI] [PubMed] [Google Scholar]
- 18.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88(9):3432–45. [PubMed] [Google Scholar]
- 19.Brummel KE, Butenas S, Mann KG. An integrated study of fibrinogen during blood coagulation. J Biol Chem. 1999;274(32):22862–70. doi: 10.1074/jbc.274.32.22862. [DOI] [PubMed] [Google Scholar]
- 20.Kheirabadi BS, Crissey JM, Deguzman R, Holcomb JB. In vivo bleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy than prothrombin time. J Trauma. 2007;62(6):1352–9. doi: 10.1097/TA.0b013e318047b805. discussion 9-61. [DOI] [PubMed] [Google Scholar]
- 21.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–75. doi: 10.1097/TA.0b013e3181ae6f1c. discussion 75-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashuk JL, Moore EE. The emerging role of rapid thromboelastography in trauma care. J Trauma. 2009;67(2):417–8. doi: 10.1097/TA.0b013e3181ac9cdc. [DOI] [PubMed] [Google Scholar]
- 23.Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146(4):764–72. doi: 10.1016/j.surg.2009.06.054. discussion 72-4. [DOI] [PubMed] [Google Scholar]
- 24.Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65(3):535–43. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 25.Ruzicka J, Stengl M, Bolek L, Benes J, Matejovic M, Krouzecky A. Hypothermic anticoagulation: testing individual responses to graded severe hypothermia with thromboelastography. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2012;23(4):285–9. doi: 10.1097/MBC.0b013e328351885a. [DOI] [PubMed] [Google Scholar]
- 26.Wang HE, Callaway CW, Peitzman AB, Tisherman SA. Admission hypothermia and outcome after major trauma. Crit Care Med. 2005;33(6):1296–301. doi: 10.1097/01.ccm.0000165965.31895.80. [DOI] [PubMed] [Google Scholar]
- 27.Takasu A, Norio H, Gotoh Y, Sakamoto T, Okada Y. Effect of induced-hypothermia on short-term survival after volume-controlled hemorrhage in pigs. Resuscitation. 2003;56(3):319–28. doi: 10.1016/s0300-9572(02)00405-7. [DOI] [PubMed] [Google Scholar]
- 28.Parr MJ, Bouillon B, Brohi K, Dutton RP, Hauser CJ, Hess JR, et al. Traumatic coagulopathy: where are the good experimental models? J Trauma. 2008;65(4):766–71. doi: 10.1097/TA.0b013e31818606d2. [DOI] [PubMed] [Google Scholar]
- 29.Brummel-Ziedins KE, Pouliot RL, Mann KG. Thrombin generation: phenotypic quantitation. J Thromb Haemost. 2004;2(2):281–8. doi: 10.1046/j.1538-7933.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 30.Barenholz Y, Gibbes D, Litman BJ, Goll J, Thompson TE, Carlson RD. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977;16(12):2806–10. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 31.Lawson JH, Krishnaswamy S, Butenas S, Mann KG. Extrinsic pathway proteolytic activity. Methods Enzymol. 1993;222:177–95. doi: 10.1016/0076-6879(93)22013-6. [DOI] [PubMed] [Google Scholar]
- 32.Cawthern KM, van 't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91(12):4581–92. [PubMed] [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4. doi: 10.1073/pnas.76.9.4350. PMCID: 411572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44(5):846–54. doi: 10.1097/00005373-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Kettner SC, Kozek SA, Groetzner JP, Gonano C, Schellongowski A, Kucera M, et al. Effects of hypothermia on thrombelastography in patients undergoing cardiopulmonary bypass. Br J Anaesth. 1998;80(3):313–7. doi: 10.1093/bja/80.3.313. [DOI] [PubMed] [Google Scholar]
- 37.Brummel-Ziedins KE, Vossen CY, Butenas S, Mann KG, Rosendaal FR. Thrombin generation profiles in deep venous thrombosis. J Thromb Haemost. 2005;3(11):2497–505. doi: 10.1111/j.1538-7836.2005.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martini WZ. Coagulopathy by hypothermia and acidosis: mechanisms of thrombin generation and fibrinogen availability. J Trauma. 2009;67(1):202–8. doi: 10.1097/TA.0b013e3181a602a7. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 39.Gerlach R, Tolle F, Raabe A, Zimmermann M, Siegemund A, Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke. 2002;33(6):1618–23. doi: 10.1161/01.str.0000017219.83330.ff. [DOI] [PubMed] [Google Scholar]
- 40.Wettstein P, Haeberli A, Stutz M, Rohner M, Corbetta C, Gabi K, et al. Decreased factor XIII availability for thrombin and early loss of clot firmness in patients with unexplained intraoperative bleeding. Anesth Analg. 2004;99(5):1564–9. doi: 10.1213/01.ANE.0000134800.46276.21. table of contents. [DOI] [PubMed] [Google Scholar]
- 41.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma. The journal of trauma and acute care surgery. 2012;73(1):87–93. doi: 10.1097/TA.0b013e3182598c70. [DOI] [PMC free article] [PubMed] [Google Scholar]