Abstract
Voltage gated calcium channels (VGCCs) are multi-subunit membrane proteins present in a variety of tissues and control many essential physiological processes. Due to their vital importance, VGCCs are regulated by a myriad of proteins and signaling pathways. Here we review the literature on the regulation of VGCCs by proteolysis of the pore-forming α1 subunit, Cavα1. This form of regulation modulates channel function and degradation and affects cellular gene expression and excitability. L-type Ca2+ channels are proteolyzed in two ways, depending on tissue localization. In the heart and skeletal muscle, the distal C-terminus of Cavα1 is cleaved and acts as an autoinhibitor when it reassociates with the proximal C-terminus. Relief of this autoinhibition underlies the β-adrenergic stimulation-induced enhancement of cardiac and skeletal muscle calcium currents, part of the “fight or flight” response. Proteolysis of the distal C-terminus of L-type channels also occurs in the brain and is probably catalyzed by a calpain-like protease. In some brain regions, the entire C-terminus of L-type Ca2+ channels can be cleaved by an unknown protease and translocates to the nucleus acting as a transcription factor. The distal C-terminus of P/Q-channel Cavα1 is also proteolyzed and translocates to the nucleus. Truncated forms of the PQ-channel Cavα1 are produced by many disease-causing mutations and interfere with the function of full-length channels. Truncated forms of N-type channel Cavα1, generated by mutagenesis, affect the expression of full-length channels. New forms of proteolysis of VGCC subunits remain to be discovered and may represent a fruitful area of VGCC research.
Keywords: ion channels, calcium signaling, proteolytic processing, modulation, channelopathy, gene expression
Proteolysis as a regulatory mechanism has been reported for a variety of ion channels. Epithelial Na+ channels are targeted by the protease furin, which cleaves a small section of the α1 subunit, releasing this inhibitory segment and activating the channel [1]. The AMPA receptor undergoes a C-terminal cleavage mediated by calpain which alters channel properties [2]. The voltage-gated Na+ channel α1 subunit is also proteolyzed by calpain[3], and its associated β2 subunit is proteolyzed by β- and γ-secretase [4]. This review surveys studies on proteolysis of voltage-gated calcium channels (VGCCs), focusing on L-, N-, and P/Q-type channels.
VGCCs control many critical physiological processes, including hormone and neurotransmitter release, cell migration, gene transcription, and muscle contraction [5]. In response to changes in membrane voltage, and under the regulation of many other proteins, these channels allow the heart to beat, the kidney to function, and neurons to communicate with one another. With such a diverse range of functions, it is of no surprise that VGCCs must be intricately regulated by a variety of mechanisms. For example, the G protein heterodimer, Gβγ, acts to stabilize the channel in a closed conformation and it is only after Gβγ dissociates that the channel can return to a state willing to open [6]. Protein kinase C phosphorylates sites on the I–II linker of VGCC α1 subunit (Cavα1), reversing G-protein inhibition, most likely by interfering with the Gβγ binding domains [7]. The RGK proteins, comprised of Rem, Rem2, Rad and Gem/Kir, are Ras-like small GTP-binding proteins [8] and act as inhibitors of VGCCs [9]. Calmodulin (CaM) assists with Ca2+ dependent inactivation by acting as a Ca2+ sensor to inactivate the channel when intracellular Ca2+ levels reach a certain concentration. Ca2+ dependent inactivation is a rapid process, occurring within milliseconds of Ca2+ influx [10]. CaM binds to the IQ region on the C-terminus, but once the two high-affinity Ca2+ binding sites on CaM are occupied, translocates to a different position on the α1 subunit to inactivate the channel [11]. CaM also plays a role in Ca2+ dependent facilitation, whereby an increase in intracellular Ca2+ levels leads to channel opening [12]. Proteases are yet another subset of proteins that act on VGCCs to modulate their function, modify gene expression, or to prepare the channel for degradation.
1 VGCC subunits
VGCCs consist of an assembly of subunits that together form a functional channel. The main Cavα1 subunit associates with auxiliary subunits β, α2-δ and sometimes γ to form the VGCC complex. Although α1 alone is able to conduct current, the auxiliary subunits allow for proper activation and inactivation properties [13]. Co-expression of β is necessary for α1 trafficking from the endoplasmic reticulum to the plasma membrane; in heterologous systems little α1 is expressed on the cell surface without β present. Cavα1 contains the voltage sensor and the ion conduction pore and is responsible for most of the biophysical and pharmacological properties of VGCCs [5]. Cavα1 consists of four homologous repeats, each containing six transmembrane segments (S1–S6). The cytoplasmic N- and C-termini and the intracellular linkers connecting the repeats contain binding sites for a multitude of proteins responsible for channel modulation and regulation. The pore-lining loops (P loops) between S5 and S6 contain highly conserved glutamic acid residues and are responsible for the high Ca2+ selectivity of VGCCs [14]. The S4 segments contain positively charged lysines and arginines that act as the voltage sensor to open and close the channel [15]. The S6 segment is involved in channel inactivation, whereby the I–II linker connecting the first two repeats presumably docks to the intracellular end of S6 in response to depolarization, essentially blocking the pore [16].
Ten different genes for α1 have been identified [5]. The α1 subunit dictates the type of currents conducted through the channel and whether the channel is high-voltage activated (HVA) or low-voltage activated (LVA). Cav1.1, Cav1.2, Cav1.3, and Cav1.4 form L-type channels, Cav2.1 forms P/Q-type channels, Cav2.2 forms N-type channels, and Cav2.3 forms R-type channels, all of which constitute HVA channels. Cav3.1, Cav3.2 and Cav3.3 form LVA, T-type channels.
The cytosolic β subunit is a multifunctional protein [17]. It acts as a chaperone to α1, trafficking it out of the endoplasmic reticulum and to the plasma membrane. The β subunit also greatly affects activation and inactivation properties of the channel. There are four known β subunit genes, each with splice variants, termed β1, β2, β3 and β4. Each subtype of VGCC associates with one specific β. β directly interacts with α1 on the I–II linker through high-affinity interactions with a binding site called the α1-interaction domain, or AID. This high-affinity binding is responsible for β’s chaperone function. It also enables otherwise intrinsically low-affinity interactions between other regions of α1 and β to fine tune channel biophysical properties.
The α2 and δ subunits are generally considered one subunit as they are encoded by the same gene [18]. However, posttranslational cleavage of the single polypeptide produces a 143-kDa α2 and a 27-kDa δ subunit, which are then linked by a disulfide bond to form the α2-δ complex. Several splice variants of α2-δ are known, many with specific tissue expression. The α2δ subunit assists with α1 trafficking to the plasma membrane, increases currents of α1/β recombinant channels, and influences channel inactivation. The α2-δ subunit is a target for pain and epilepsy drugs such as pregabalin and gabapentin.
The γ subunit is a hydrophobic glycoprotein with four transmembrane domains with cytoplasmic N- and C-termini [17]. There are eight known γ genes. Only the non-neuronal L-type channels associate with the γ subunit. The most robust functional effect of γ on VGCCs is a small reduction of currents.
2 Subtypes of VGCCS
Two types of VGCCs exist, HVA and LVA, and they are further differentiated based on the type of α1 subunit they contain and on their biophysical and pharmacological properties. T-type currents are classified as LVA channels, and L-, R-, N-, and P/Q-type currents are classified as HVA channels. This review focuses on proteolysis of HVA channels.
L-type channels have four different gene transcripts, all signifying different α1 subtypes. These are labeled CaV1.1, CaV1.2, CaV1.3, and CaV1.4, which express α1S, α1C, α1D, and α1F respectively. CaV1.1 is predominately found in skeletal muscle and CaV1.2 is located in cardiac tissue and brain. CaV1.3 is generally in the central nervous system [5] and auditory hair cells [19]. CaV1.4 is expressed in the retina and is involved in phototransduction [20]. Neuronal L-type channels (CaV1.2 and CaV1.3) are found at postsynaptic synapses and in the soma. They are also recruited to microdomains along distal dendrites and spines, which allows for easier synapse-to-nucleus communication [21]. L-type channels play a role in gene transcription in neurons [22] and are involved in hormone secretion in endocrine cells [23]. They are known to be sensitive to phenylalkylamines, benzothiazapines, and dihydropyridines [24] and are blocked by the spider toxin ω-agatoxin IIIA [25]. L-type channels are implicated in many disease states, including Duchenne muscular dystrophy [26], arrhythmias and hypertension [27], Timothy syndrome [28], hypokalemic periodic paralysis and night blindness [29].
P/Q-, R- and N-type Ca2+ channels make up the CaV2 family and are widely expressed in neurons and chromaffin cells [30]. P/Q-type channels are classified as CaV2.1 and express α1A, N-type channels are classified as CaV2.2 and express α1B, and R-type channels are classified as CaV2.3 and express α1E. P- and Q-type channels are derived from alternative splice variants of the same gene [31], and can be distinguished based on their sensitivities to various toxins. P-type channels are more sensitive to ω-AgaIVA, whereas Q-type channels are sensitive to ω-CMVIIC [32]. N-type channels are specifically blocked by the marine snail toxin, ω-conotoxin GVIA [33]. R-type channels are resistant to these toxins, thus termed “R” for resistant. P/Q- and N-type channels are abundant in presynaptic terminals and dendrites and are involved in neurotransmitter release [34]. While CaV2.2 knockouts do not display an adverse phenotype besides higher threshold for pain [35], CaV2.1 knockout mice survive for only a few weeks, and display seizures and ataxia while alive [36]. Also, a lack of N- and R-type channels can be compensated for, whereas deficiencies in P/Q-type channels cannot [35].
P/Q-type channels (CaV2.1) are found throughout the entire brain but are expressed at particularly high levels in the cerebellum [37]. CaV2.1 channels are also found in brain regions associated with the perception of pain, such as the trigeminal ganglia and brainstem nuclei [37]. They play a major role at excitatory synapses (glutamate) and a lesser role at inhibitory synapses (GABA) [38]. There are restrictions on the number of P/Q-type channels that can occupy specific slots at synapses, therefore if some channels are of mutated form, a decrease in activity will result [39].
N-type channels are found in neurons and neuroendocrine cells, and they are predominately involved in neurotransmitter release [40]. They have also been identified in saccular hair cells [41]. In the spinal cord [42] they are located in dorsal root ganglia and at synaptic terminals in dorsal horn neurons that connect to afferent sensory fibers [43]. In the dorsal horn, they are found predominately in substance P containing cells [42, 44]. N-type channels are responsible for the majority of synaptic processing of nociceptive information in dorsal horn neurons [45, 46]. The natural N-type channel antagonists, ω-conotoxins, inhibit CaV2.2 channels and prevent release of pronociceptive neurotransmitters from central nerve terminals of primary afferent neurons [47].
3 L-type channel proteolysis
Two forms of CaV1.2 channel exist in the heart, full length and truncated. Approximately 80% of CaV1.2 channels are truncated in the heart [48]; thus, the truncated channel is the predominant physiological form. This truncated version is produced by a proteolytic cleavage of the full length protein in the C-terminus between residues 1 685 and 1 699, producing a distal C-terminal fragment of ~45 kDa [48]. In skeletal muscle, the C-terminus of CaV1.1 is cleaved between A1664 and A1665, producing a similar size fragment [49]. The C-terminus is an important element for VGCC function, affecting channel properties through interactions with calmodulin [50], the β subunit [51], cAMP-dependent protein kinase (PKA) [52], and by being involved in targeting the channel to the plasma membrane [53]. What purpose would this proteolysis serve if the C-terminus is so important for channel function?
In the heart and skeletal muscle, excitation-contraction coupling is initiated by the opening of L-type channels, whose activity is greatly increased by the stimulation of the sympathetic nervous system [54]. This current potentiation is thought to underlie the “fight or flight” phenomenon. Basically, when faced with a threat, epinephrine and norepinephrine are released and activate the β-adrenergic system, which in turn enhances L-type channel activity allowing cardiac and skeletal muscle contraction to increase and the heart to beat faster [55]. This mechanism has now been parsed out and the cleaved CaV1 C-terminal fragment plays a major role in the “fight or flight” phenomenon.
CaV1 potentiation in both the heart and skeletal muscle is controlled by a rapid phosphorylation by PKA [52, 56]. Two conserved phosphorylation sites (S1575 and T1579 in CaV1.1 and S1700 and T1704 in CaV1.2) are regulated by the β-adrenergic system. S1575 phosphorylation is increased with isoproterenol, a β-adrenergic receptor agonist, and decreased with propranolol, an antagonist [57]. These experiments were more relevant than past in vitro phosphorylation assays [58, 59] because they were done using isolated rabbit skeletal muscle, whereby the animal was injected with these drugs while moving and breathing, and therefore are more physiologically relevant. While PKA has been primarily implicated in the β-adrenergic system, it was found that S1575 can be phosphorylated by both PKA and CAMKII [57]. On the other hand, T1579 is phosphorylated by casein kinase 2 [57]. PKA phosphorylation dramatically increases CaV1 current [59], presumably by relieving the autoinhibition produced by the proteolytically cleaved distal C-terminal fragment, as will be discussed below.
PKA phosphorylation occurs rapidly due to the fact that PKA is stationed very close to the channel. An anchoring protein, AKAP-15, was found to be critical for PKA phosphorylation. When a peptide corresponding to a 24-amino acid segment of a human AKAP protein was applied into myotubes through a patch electrode, it was able to inhibit channel current to the same extent as PKA inhibitors did [56]. AKAP-15 co-immunoprecipitated with CaV1.1 from skeletal muscle [60] and from tsa-201 cells [61], and confocal microscopy revealed it colocalized with CaV1.1 in distinct areas [61]. Yeast two-hybrid screen showed that AKAP-15 bound to the distal C-terminal domain of CaV1.1 (residues 1 774–1 841) [62]. This interaction occurs through a leucine zipper (LZ) motif on AKAP-15 and a LZ-like motif on the CaV1.1 C-terminus. When either of the two regions was mutated to alanine, or a synthetic peptide comprising the LZ motif of AKAP-15 was introduced to compete with AKAP-15, PKA was unable to anchor to the channel, disrupting L-type channel phosphorylation and potentiation [61, 62].
CaV1.2 is targeted by AKAP-15 and PKA and is phosphorylated in the same manner as CaV1.1 is. This mechanism has recently been reconstituted in tsa-201 cells with CaV1.2 [58] after transfection of the necessary molecular players, namely AKAP-15, PKA, CaV1.2 (1–1 800) and the distal C-terminus. Furthermore, mice with CaV1.2 lacking the distal C-terminus developed cardiac hypertrophy and their β-adrenergic pathway was disabled due to an inability of PKA to regulate CaV1.2 function [63]. Cardiac myocytes from these mice also showed decreased surface expression of this truncated CaV1.2, consistent with a role of the C-terminus in trafficking the channel to the plasma membrane. These experiments confirmed the importance of CaV1.2 proteolysis and CaV1.2-S1700 phosphorylation in the β-adrenergic signaling pathway.
It seems strange that the sites of phosphorylation and AKAP-15 interaction occur in the distal C-terminus, a region that is cleaved from the channel under physiological conditions. It turns out that the cleaved C-terminus reassociates with the channel. The distal and proximal C-terminus co-immunoprecipitated when co-expressed in HEK-293 cells and also directly interacted in a yeast-two hybrid screen [49]. Functional studies revealed that when the C-terminus was removed from the channel by mutagenesis, an increase in peak current amplitude resulted [64]. This increase has also been demonstrated using tsa-201 cells with cardiac CaV1.2 channels truncated at 1 821 [65] or 1 905 [66]. When truncated channels were co-expressed with the C-terminal fragment, a 20-fold inhibition occurred; even full-length channels were further inhibited by the C-terminal fragment [65]. Channels missing only their last 147 residues displayed an increase in peak current amplitude compared to full-length channels, which could be reversed by the addition of the missing C-terminal fragment [67]. Arginines 1 696 and 1 697 on the proximal C-terminus interact with negatively charged residues (E2103, E2106, and D2110) on the distal C-terminal fragment to exert this inhibitory effect [65]. Thus, the distal C-terminal fragment cleaved from the α1 subunit can reassociate with the channel and act as an autoinhibitor. Besides an overall decrease in peak current amplitude, the distal C-terminal fragment shifts the voltage dependence of activation causing the channels open at higher voltages [65].
The proteolytic cleavage described above for cardiac and skeletal muscle CaV1 channels also takes place in neurons. In response to NMDA receptor activation, which leads to increases in intracellular Ca2+, CaV1.2 channels in the hippocampus underwent extensive C-terminal cleavage, while other classes of VGCCs were unaffected [68]. Thus, proteolytic cleavage of the distal C-terminus of L-type Ca2+ channels appears to be a widespread phenomenon.
There is evidence suggesting that calpain-like proteases may be responsible for CaV1 proteolysis. In hippocampal neurons, cleavage of CaV1.2 C-terminus is induced by Ca2+ influx through NMDA receptors and is blocked by calpain inhibitors [68]. Purified calpain increased CaV1 C-terminal proteolysis in vitro [68]. Cleavage of CaV1.1 at A1664 [49] is also consistent with the catalytic specificity of calpain. Calpain requires Ca2+ for its catalytic activity and is stimulated by membrane depolarization [69], presumably through depolarization-induced intracellular Ca2+ increase. Calpain-mediated CaV1 proteolysis may thus represent a feedback regulation of CaV1 channel activity.
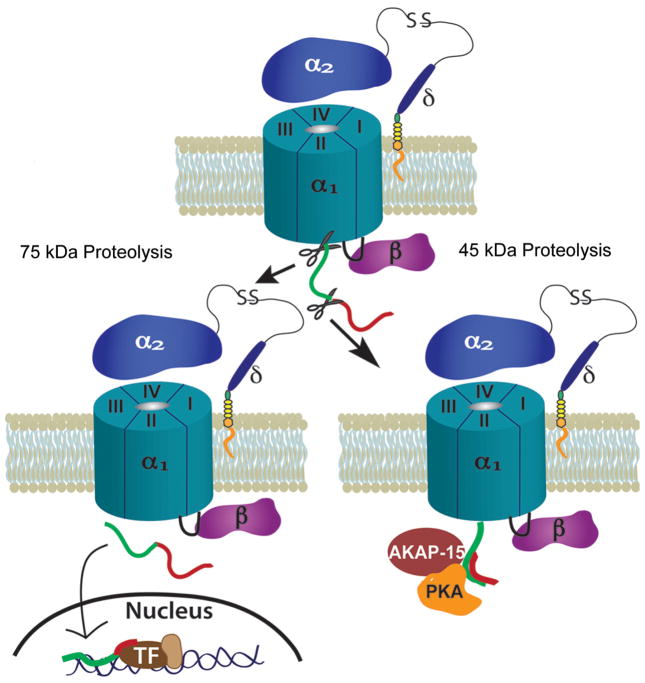
To summarize, under normal physiological conditions, CaV1 is proteolyzed in the C-terminus (see Fig. 1), perhaps by calpain-like proteases. The cleaved distal C-terminus reassociates with the channel to inhibit its function. AKAP-15 binds to the C-terminal fragment and recruits PKA to the scene. In a “fight or flight” scenario the β-adrenergic system is activated, in turn activating PKA. PKA phosphorylates the channel, which dispels the distal C-terminus from the channel, relieving inhibition and greatly enhancing channel activity.
Fig. 1.
Two forms of proteolytic processing of L-type voltage-gated calcium channels. Top: A schematic of an L-type VGCC complex on the plasma membrane. The α1 subunit is the pore-forming subunit and contains four homologous repeats (I–IV). α2δ and β are auxiliary subunits. The red and green regions represent the C-terminus of α1. Two sites of proteolysis are indicated. Bottom left: The 75 kDa cut: the C-terminus of α1 is cleaved from the channel and translocates to the nucleus to interact with transcription factors (TF) and influence gene expression. Bottom right: The 45 kDa cut: the distal C-terminus (red) of α1 is cleaved from the channel and reassocciates with the proximal C-terminus (green) causing inhibition of the channel. AKAP-15 and PKA are docked close to this site. When activated by the β-adrenergic pathway, PKA phosphorylates a site to dislodge the distal C-terminus, relieving the inhibition.
Proteolysis of CaV1.3 has not been reported, but a truncated form of the channel exists naturally due to alternative splicing [70]. The truncated CaV1.3 incorporates an alternative exon that encodes a stop codon after the IQ domain in the C-terminus. Truncated CaV1.3 channels inactivate much faster, due mainly to enhanced calcium-dependent inactivation, and they activate at more negative potentials than full length CaV1.3 does.
It has been well established that one of the many functions of L-type channels is to control gene expression; however, it was always thought to occur through calcium-mediated activation of transcription factors or signaling proteins [22]. Several years ago, the C-terminus of neuronal CaV1.2 itself was found to act as a transcription factor [71]. The C-terminal fragment, termed calcium channel associated transcriptional regulator (CCAT), was found to translocate to the nucleus of mainly inhibitory neurons. CCAT is larger than the cleaved distal C-terminal fragment described above; it appears as a ~75 kDa band in Western blots and comprises virtually the entire C-terminus of CaV1.2 (Fig. 1). The appearance of this fragment is developmentally regulated, gradually becoming more prominent in the nucleus starting at age P1 through adulthood. In addition, another 150 kDa fragment began to appear in rats around P21, suggesting that other forms of L-type channel proteolysis might exist. While it is assumed that the full-length channel is proteolyzed to generate CCAT, neither the cleavage site nor the protease was identified. CCAT translocation to the nucleus appeared to be activity-dependent. When L-type channels were stimulated with either KCl or glutamate, less CCAT was detected in the nucleus. Conversely, chelating intracellular calcium caused a significant increase in the amount of CCAT in the nucleus. The expression of 16 genes was upregulated and 31 genes was downregulated in Neuro2A cells transfected with CCAT, and CCAT itself bound to the enhancer of the Connexin 3.1 gene. In addition, CCAT appeared to increase neurite growth in cerebellar granule neurons.
A ~37 kDa C-terminal fragment of CaV1.2 has also been found to exhibit transcriptional activity in cardiac myocytes [72]. This C-terminal fragment was found to localize to the nucleus, interact with the CaV1.2 promoter, and ultimately act as a repressor, downregulating CaV1.2 levels. It is unclear how this fragment relates to the two C-terminal fragments described above. Nevertheless, it seems that the C-terminus of CaV1.2 is cleaved from the full-length channel and has the ability to enter the nucleus, affecting the expression of various genes, including its own gene.
4 PQ- AND N-type channel proteolysis
Two P/Q-type channels of different sizes, 220 kDa and 170 kDa, have been observed [73, 74]. In addition, a 95 kDa short form of the channel has been discovered that comprises the N-terminus to the middle of the II–III linker [75]. This short form interacts with β and is glycosylated. It has yet to be determined whether this 95 kDa form of CaV2.1 is a splice variant or the result of post-translational proteolysis, although a splice variant causing a truncation in the II–III linker has been reported [76]. However, the 170 kDa fragment could be the result of C-terminal proteolysis. A 60–75 kDa fragment was detected from mouse cerebellar lysates and was confirmed to include the C-terminus of CaV2.1 after staining with two different C-terminal antibodies [74]. The 60–75 kDa fragment appeared in cell nuclei, suggesting that it might also affect gene expression. CaV2.1 C-terminus contains a histidine repeat (11 copies) that is common among transcription factors, serving as a nuclear localization signal [77]. In addition, three other putative nuclear localization signals were identified and when mutated decreased nuclear localization of the 60–75 kDa fragment by almost 60% [74].
Many P/Q-type channel truncations resulting from premature stop codon mutations have been implicated in disease. In episodic ataxia type 2 (EA2) there are over 50 different mutations leading to truncated channels [78]. When co-expressed with full-length channels, CaV2.1 truncation mutants markedly repressed peak current amplitude. This dominant-negative effect was dependent on the AID being present in the truncated construct [79]. One possibility is that the truncated channels compete with full-length channels for the β subunit. The dominant-negative effect of the truncated channels sheds light on a possible pathogenic mechanism for episodic ataxias. It also demonstrates a possible physiological effect of fragment channels that might be produced by proteolysis of CaV2.1.
Two main N-type α1 subunits have been identified, one 240 kDa and the other 210 kDa, that differ in their C-terminus [80]. While they are both substrates for PKA, protein kinase C and c-GMP dependent protein kinase, only the 240 kDaa isoform is phosphorylated by camodulin-dependent protein kinase II, indicating that the two isoforms are independently regulated [81]. A 140 kDa form of CaV2.2 has also been reported [82]. It is unknown whether the smaller forms are due to post-translational proteolysis or are specific splice variants.
While specific regulatory proteolysis has not been identified for CaV2.2 channels, truncated versions of the channel have been generated by mutagenesis and studied. When truncated CaV2.2 channels containing either the I–II repeat or the III–IV repeat were co-expressed with full-length CaV2.2, they markedly inhibited channel currents without changing their biophysical properties [83]. The truncated fragment containing the I–II repeat, CaV2.2(I–II), almost abolished membrane expression of full-length channels. This effect was not due to the sequestration of β since co-expression of the I–II linker alone had no effect and overexpression of β did not rescue full-length membrane expression [83]. Instead, the decreased expression of full-length CaV2.2 was due to CaV2.2(I–II) triggering the unfolded protein response by activating the RNA-dependent kinase PERK and inhibiting CaV2.2 exit from the endoplasmic reticulum [84]. The region of CaV2.2(I–II) responsible for this inhibition and decreased surface expression has now been identified as the first 95 residues of the N-terminus, specifically the arginines at position 52 and 54[85]. When these two residues are mutated to alanines, full-length CaV2.2 protein levels actually surpassed that of wild-type CaV2.2 when coexpressed with α2δ and β1. These experiments further highlight how short forms of VGCCs can interfere or regulate full-length forms.
5 Concluding remarks
It is becoming increasingly apparent that proteolysis of HVA Ca2+ channels may be responsible for specific physiological functions. The proteolysis of the C-terminus of cardiac and skeletal muscle CaV1 channels resulting in autoinhibition and β-adrenergic regulation exemplifies the importance of VGCC proteolysis in channel regulation and in the fight or flight response. The C-terminal proteolysis of neuronal CaV1.2 illustrates the potential importance of VGCC proteolysis in gene expression. Although proteolysis of Cav2 channels has not been extensively observed, many short forms of CaV2 channels are known to exist as a result of alternative splicing or disease-causing mutations. These truncated forms of CaV2 channels markedly change the function and expression of their full-length counterparts. With improved molecular biology tools and finer detection methods, future studies will likely uncover new forms of proteolytic processing of VGCCs. A major challenge is to distinguish regulated proteolysis from non-specific protein degradation. Another challenge is to demonstrate the physiological significance of VGCC proteolysis, as has been done for the proteolysis of cardiac L-type Ca2+ channels.
Acknowledgments
This work was supported by National Institutes of Health, USA grant RO1 NS053494 and by the Top Talents Program of Yunnan Province, China (J. Y.).
References
- 1.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284(31):20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi X, Chang V, Molnar E, McIlhinney RA, Baudry M. The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience. 1996;73(4):903–906. doi: 10.1016/0306-4522(96)00157-1. [DOI] [PubMed] [Google Scholar]
- 3.von Reyn CR, Spaethling JM, Mesfin MN, Ma M, Neumar RW, Smith DH, Siman R, Meaney DF. Calpain mediates proteolysis of the voltage-gated sodium channel alpha-subunit. J Neurosci. 2009;29(33):10350–10356. doi: 10.1523/JNEUROSCI.2339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM. Presenilin/gamma-secretase-mediated cleavage of the voltage-gated sodium channel beta2-subunit regulates cell adhesion and migration. J Biol Chem. 2005;280(24):23251–23261. doi: 10.1074/jbc.M412938200. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 6.Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gbeta subunit. Proc Natl Acad Sci U S A. 1998;95(7):4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel alpha1 subunit. Nature. 1997;385(6615):442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 8.Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20(2):292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58(4):837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 10.Lee KS, Marban E, Tsien RW. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci STKE. 2006;(318):er1. doi: 10.1126/stke.3182006er1. [DOI] [PubMed] [Google Scholar]
- 12.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411(6836):484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Reyes E, Schneider T. Molecular biology of calcium channels. Kidney Int. 1995;48(4):1111–1124. doi: 10.1038/ki.1995.395. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366(6451):158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 15.Bezanilla F. Voltage sensor movements. J Gen Physiol. 2002;120(4):465–473. doi: 10.1085/jgp.20028660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stotz SC, Jarvis SE, Zamponi GW. Functional roles of cytoplasmic loops and pore lining transmembrane helices in the voltage-dependent inactivation of HVA calcium channels. J Physiol. 2004;554(Pt 2):263–273. doi: 10.1113/jphysiol.2003.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buraei Z, Yang J. The beta subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90(4):1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolphin AC. Calcium channel auxiliary alpha(2)delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci. 2012;13(8):542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 19.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 20.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11. 23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19(3):264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 21.Leitch B, Szostek A, Lin R, Shevtsova O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience. 2009;164(2):641–657. doi: 10.1016/j.neuroscience.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 23.Milani D, Malgaroli A, Guidolin D, Fasolato C, Skaper SD, Meldolesi J, Pozzan T. Ca2+ channels and intracellular Ca2+ stores in neuronal and neuroendocrine cells. Cell Calcium. 1990;11(2–3):191–199. doi: 10.1016/0143-4160(90)90070-b. [DOI] [PubMed] [Google Scholar]
- 24.Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 25.Mintz IM, Venema VJ, Adams ME, Bean BP. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin omega-Aga-IIIA. Proc Natl Acad Sci U S A. 1991;88(15):6628–6631. doi: 10.1073/pnas.88.15.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich O, von Wegner F, Chamberlain JS, Fink RH, Rohrbach P. L-type Ca2+ channel function is linked to dystrophin expression in mammalian muscle. PLoS One. 2008;3(3):e1762. doi: 10.1371/journal.pone.0001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triggle DJ. L-type calcium channels. Curr Pharm Des. 2006;12(4):443–457. doi: 10.2174/138161206775474503. [DOI] [PubMed] [Google Scholar]
- 28.Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1. 2 L-type calcium channels. Proc Natl Acad Sci U S A. 2008;105(6):2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Striessnig J, Hoda JC, Koschak A, Zaghetto F, Mullner C, Sinnegger-Brauns MJ, Wild C, Watschinger K, Trockenbacher A, Pelster G. L-type Ca2+ channels in Ca2+ channelopathies. Biochem Biophys Res Commun. 2004;322(4):1341–1346. doi: 10.1016/j.bbrc.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86(4):1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- 31.Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2(5):407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- 32.Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 35.Pietrobon D. Function and dysfunction of synaptic calcium channels: insights from mouse models. Curr Opin Neurobiol. 2005;15(3):257–265. doi: 10.1016/j.conb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin HS. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci U S A. 1999;96(26):15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietrobon D. Migraine: new molecular mechanisms. Neuroscientist. 2005;11(4):373–386. doi: 10.1177/1073858405275554. [DOI] [PubMed] [Google Scholar]
- 38.Timmermann DB, Westenbroek RE, Schousboe A, Catterall WA. Distribution of high-voltage-activated calcium channels in cultured gamma-aminobutyric acidergic neurons from mouse cerebral cortex. J Neurosci Res. 2002;67(1):48–61. doi: 10.1002/jnr.10074. [DOI] [PubMed] [Google Scholar]
- 39.Cao YQ, Piedras-Renteria ES, Smith GB, Chen G, Harata NC, Tsien RW. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2004;43(3):387–400. doi: 10.1016/j.neuron.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Catterall WA, Few AP. Calcium channel regulation and pre-synaptic plasticity. Neuron. 2008;59(6):882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishnan NA, Drescher MJ, Sheikhali SA, Khan KM, Hatfield JS, Dickson MJ, Drescher DG. Molecular identification of an N-type Ca2+ channel in saccular hair cells. Neuroscience. 2006;139(4):1417–1434. doi: 10.1016/j.neuroscience.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 42.Snutch TP. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx. 2005;2(4):662–670. doi: 10.1602/neurorx.2.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perret D, Luo ZD. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics. 2009;6(4):679–692. doi: 10.1016/j.nurt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27(24):6363–6373. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews EA, Dickenson AH. Effects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain. 2001;92(1–2):235–246. doi: 10.1016/s0304-3959(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 46.Hatakeyama S, Wakamori M, Ino M, Miyamoto N, Takahashi E, Yoshinaga T, Sawada K, Imoto K, Tanaka I, Yoshizawa T, Nishizawa Y, Mori Y, Niidome T, Shoji S. Differential nociceptive responses in mice lacking the alpha(1B) subunit of N-type Ca(2+) channels. Neuroreport. 2001;12(11):2423–2427. doi: 10.1097/00001756-200108080-00027. [DOI] [PubMed] [Google Scholar]
- 47.Smith MT, Cabot PJ, Ross FB, Robertson AD, Lewis RJ. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96(1–2):119–127. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- 48.De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991;88(23):10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG, 2nd, Bigelow DJ, Catterall WA. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1. 1 channels in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102(14):5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuhlke RD, Reuter H. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the alpha1C subunit. Proc Natl Acad Sci U S A. 1998;95(6):3287–3294. doi: 10.1073/pnas.95.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao T, Puri TS, Gerhardstein BL, Chien AJ, Green RD, Hosey MM. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272(31):19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- 52.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364(6434):240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 53.Gao T, Bunemann M, Gerhardstein BL, Ma H, Hosey MM. Role of the C terminus of the alpha 1C (CaV1. 2) subunit in membrane targeting of cardiac L-type calcium channels. J Biol Chem. 2000;275(33):25436–25444. doi: 10.1074/jbc.M003465200. [DOI] [PubMed] [Google Scholar]
- 54.Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991;64(5):871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- 55.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 56.Johnson BD, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91(24):11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emrick MA, Sadilek M, Konoki K, Catterall WA. Beta-adrenergic-regulated phosphorylation of the skeletal muscle Ca(V)1. 1 channel in the fight-or-flight response. Proc Natl Acad Sci U S A. 2010;107(43):18712–18717. doi: 10.1073/pnas.1012384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3(141):ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson BD, Brousal JP, Peterson BZ, Gallombardo PA, Hockerman GH, Lai Y, Scheuer T, Catterall WA. Modulation of the cloned skeletal muscle L-type Ca2+ channel by anchored cAMP-dependent protein kinase. J Neurosci. 1997;17(4):1243–1255. doi: 10.1523/JNEUROSCI.17-04-01243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272(10):6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 61.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, 3rd, Scheuer T, Catterall WA, Murphy BJ. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20(5):1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 62.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277(6):4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 63.Fu Y, Westenbroek RE, Yu FH, Clark JP, 3rd, Marshall MR, Scheuer T, Catterall WA. Deletion of the distal C terminus of CaV1. 2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286(14):12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac alpha 1 subunit. J Biol Chem. 1994;269(3):1635–1640. [PubMed] [Google Scholar]
- 65.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1. 2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576(Pt 1):87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the alpha(1C) subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275(12):8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- 67.Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM. C-terminal fragments of the alpha 1C (CaV1. 2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated alpha 1C subunits. J Biol Chem. 2001;276(24):21089–21097. doi: 10.1074/jbc.M008000200. [DOI] [PubMed] [Google Scholar]
- 68.Hell JW, Westenbroek RE, Breeze LJ, Wang KK, Chavkin C, Catterall WA. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci U S A. 1996;93(8):3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roehm PC, Xu N, Woodson EA, Green SH, Hansen MR. Membrane depolarization inhibits spiral ganglion neurite growth via activation of multiple types of voltage sensitive calcium channels and calpain. Mol Cell Neurosci. 2008;37(2):376–387. doi: 10.1016/j.mcn.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A. Modulation of voltage- and Ca2+-dependent gating of CaV1. 3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem. 2008;283(30):20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1. 2 encodes a transcription factor. Cell. 2006;127(3):591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104(12):1373–1381. doi: 10.1161/CIRCRESAHA.108.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15(10):6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM. C-termini of P/Q-type Ca2+ channel alpha1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet. 2006;15(10):1587–1599. doi: 10.1093/hmg/ddl080. [DOI] [PubMed] [Google Scholar]
- 75.Scott VE, Felix R, Arikkath J, Campbell KP. Evidence for a 95 kDa short form of the alpha1A subunit associated with the omega-conotoxin MVIIC receptor of the P/Q-type Ca2+ channels. J Neurosci. 1998;18(2):641–647. doi: 10.1523/JNEUROSCI.18-02-00641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soong TW, DeMaria CD, Alvania RS, Zweifel LS, Liang MC, Mittman S, Agnew WS, Yue DT. Systematic identification of splice variants in human P/Q-type channel alpha1(2. 1) subunits: implications for current density and Ca2+-dependent inactivation. J Neurosci. 2002;22(23):10142–10152. doi: 10.1523/JNEUROSCI.22-23-10142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salichs E, Ledda A, Mularoni L, Alba MM, de la Luna S. Genome-wide analysis of histidine repeats reveals their role in the localization of human proteins to the nuclear speckles compartment. PLoS Genet. 2009;5(3):e1000397. doi: 10.1371/journal.pgen.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pietrobon D. CaV2. 1 channelopathies. Pflugers Arch. 2010;460(2):375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- 79.Raike RS, Kordasiewicz HB, Thompson RM, Gomez CM. Dominant-negative suppression of Cav2.1 currents by alpha(1)2. 1 truncations requires the conserved interaction domain for beta subunits. Mol Cell Neurosci. 2007;34(2):168–177. doi: 10.1016/j.mcn.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992;9(6):1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 81.Hell JW, Appleyard SM, Yokoyama CT, Warner C, Catterall WA. Differential phosphorylation of two size forms of the N-type calcium channel alpha 1 subunit which have different COOH termini. J Biol Chem. 1994;269(10):7390–7396. [PubMed] [Google Scholar]
- 82.Leenders AG, Lin L, Huang LD, Gerwin C, Lu PH, Sheng ZH. The role of MAP1A light chain 2 in synaptic surface retention of Cav2. 2 channels in hippocampal neurons. J Neurosci. 2008;28(44):11333–11346. doi: 10.1523/JNEUROSCI.3078-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raghib A, Bertaso F, Davies A, Page KM, Meir A, Bogdanov Y, Dolphin AC. Dominant-negative synthesis suppression of voltage-gated calcium channel Cav2. 2 induced by truncated constructs. J Neurosci. 2001;21(21):8495–8504. doi: 10.1523/JNEUROSCI.21-21-08495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Page KM, Heblich F, Davies A, Butcher AJ, Leroy J, Bertaso F, Pratt WS, Dolphin AC. Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J Neurosci. 2004;24(23):5400–5409. doi: 10.1523/JNEUROSCI.0553-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Page KM, Heblich F, Margas W, Pratt WS, Nieto-Rostro M, Chaggar K, Sandhu K, Davies A, Dolphin AC. N terminus is key to the dominant negative suppression of Ca(V)2 calcium channels: implications for episodic ataxia type 2. J Biol Chem. 2010;285(2):835–844. doi: 10.1074/jbc.M109.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]