Abstract
Lysine demethylases (KDMs) are epigenetic regulators whose dysfunction is implicated in the pathology of many human diseases including various types of cancer, inflammation and X-linked intellectual disability. Particular demethylases have been identified as promising therapeutic targets, and tremendous efforts are being devoted toward developing suitable small-molecule inhibitors for clinical and research use. Several high-throughput screening strategies have been developed to screen for small-molecule inhibitors of KDMs, each with advantages and disadvantages in terms of time, cost, effort, reliability and sensitivity. In this Special Report, we review and evaluate the high-throughput screening methods utilized for discovery of novel small-molecule KDM inhibitors.
Keywords: high-throughput screening, JmjC, KDM, LSD1, lysine demethylase, small-molecule inhibitor
Lysine demethylases (KDMs) and their counterparts lysine methyltransferases (KMTs) dynamically regulate lysine methylation in cells, of which the most studied form is histone lysine methylation. The methylation states of particular lysines in histone tails contribute significantly to transcriptional regulation. In general, methylation at histone H3 lysine 4 (H3K4) and H3K36 is associated with active transcription, while methylation at H3K9, H3K27 and H4K20 is thought to repress transcription [1]. Changes in methylation at these sites can have important biological consequences. In addition to histones, increasing numbers of proteins have been found to be mono-, di- and tri-methylated on lysine residues [2,3], and certain KDMs have been discovered to act on these residues, often with significant cellular outcomes. For instance, demethylation of p53 by LSD1 represses p53 function through blocking interaction between p53 and 53BP1 [4] and demethylation of RelA by KDM2A inhibits NF-κB activity [5,6].
About 20 mammalian KDMs have been discovered so far, each exhibiting specificity for particular histone and/or nonhistone substrates (reviewed here [7]). These KDMs fall into two classes: flavin adenine dinucleotide-dependent oxidases, discovered in 2004 [8] and Jumonji-C (JmjC) domain-containing enzymes, first identified in 2006 [9]. LSD1 and related enzymes function through flavin adenine dinucleotide oxidation of the methylated amine. In contrast, the JmjC domain-containing KDMs demethylate through a hydroxylation reaction with cofactors Fe2+ and α-ketoglutarate [10].
Mutations or changes in expression of particular KDMs are associated with many types of cancer, inflammation and X-linked intellectual disability [11–14]. For this reason, small-molecule modulators of KDM function are now in high demand for use in research to better understand the functions of KDMs and for development of anti-cancer therapies. Several inhibitors of select KDMs have already been developed. In fact, two inhibitors of LSD1 are currently in clinical trials for small-cell lung carcinoma (GlaxoSmithKline, clinicaltrials.gov identifier: NCT02034123) and acute myeloid leukemia (ORYZON, Barcelona, Spain). Specific and potent inhibitors of certain JmjC domain-containing KDMs have also been discovered, despite similarities in their catalytic sites in which Fe2+ is coordinated by α-ketoglutarate and three conserved residues: two histidines and one aspartic or glutamic acid [15]. Though small-molecule inhibitors whose mechanism of action involves general disruption of the active site, for instance iron chelators, may inhibit multiple JmjC domain-containing KDMs, unique traits of these enzymes have allowed for development of a number of relatively specific inhibitors. For extensive reviews of existing KDM inhibitors, see [10] and [16].
Many research teams have used structure-based virtual modeling and screening, medicinal chemistry and focused campaigns to identify inhibitors of KDMs (e.g., see [17–21]). However, this is not amenable for all KDMs and there are likely numerous undiscovered chemotypes that can specifically inhibit activity of particular KDMs. Therefore, high-throughput screening (HTS) has been employed to search for small-molecule inhibitors of these enzymes. Several different HTS platforms have been utilized for KDMs, each with particular strengths and weaknesses. In order to compare the quality of these different platforms, the statistical parameter Z′ (Z prime) factor [22] is used, with a Z′ value over 0.5 indicating a robust assay and a Z′ value over 0.7 indicating an excellent assay. Many assays can be utilized for detection of methyltransferase and demethylase activity [23], but we have limited discussion in this Special Report to techniques used or optimized recently in HTS campaigns for lysine demethylases.
Coupled enzyme-based assays
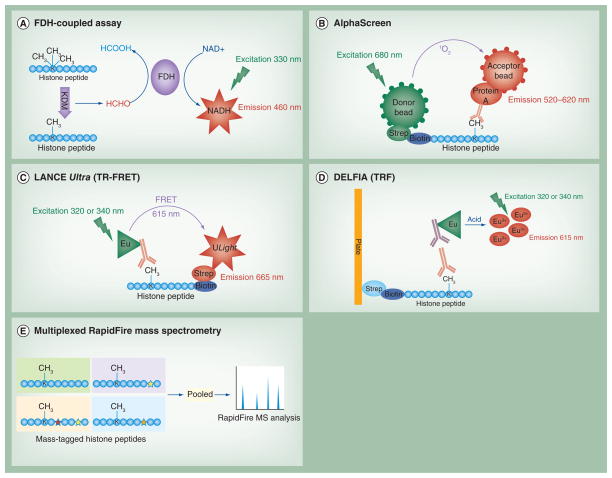
Both LSD1 and JmjC domain-containing demethylases generate byproducts as a result of the demethylation reaction that can be quantified as a proxy of demethylase activity. For instance, LSD1 activity can be measured by hydrogen peroxide reaction with horseradish peroxidase and a fluorophore [24,25]. Recent high-throughput screens have favored measuring demethylase activity by the detection of the formaldehyde byproduct generated by both LSD1 and JmjC demethylases. This assay couples formation of formaldehyde with the formation of reduced nicotinamide adenine dinucleotide (NADH), which is easily detected spectroscopically. Formaldehyde dehydrogenase (FDH) is used to oxidize formaldehyde into formic acid through the coupled reduction of NAD+ into NADH. NADH formation is monitored by absorbance at 340 nm [26] or more precisely by fluorescence intensity with excitation at 330 nm and emission at 460 nm (Figure 1A) [27,28]. Based on this reaction, Simeonov and colleagues developed a HTS platform [29], which was used to screen approximately 236,000 compounds for inhibitory activity against the catalytic domain of JMJD2E [27]. This assay has also been shown to work for LSD1 using native bulk histones as substrate instead of specific peptides [28]. FDH-coupled assays can measure demethylase activity of any KDM and are not limited by the availability of histone methylation-specific antibodies. However, artifacts from the coupled enzyme reaction can lead to false positives and negatives, which require a thorough counterscreen to eliminate.
Figure 1. High-throughput screening methods for inhibitors of lysine demethylases.
(A) FDH-coupled assays detect formaldehyde formation as a proxy of demethylation. (B–E) display methods capable of detecting demethylated product or substrate.
(B) AlphaScreen is a bead-based assay to detect demethylation. (C) LANCE Ultra, a form of TR-FRET, is a homogeneous assay to detect demethylation. (D) DELFIA is a heterogeneous TRF assay to detect demethylation. (E) Multiplexed RapidFire mass spectroscopy pools multiple mass-labeled peptides for detection after demethylation. Stars indicate amino acid substitutions.
AlphaScreen: Amplified luminescent proximity homogeneous assay; DELFIA: Dissociation-enhanced lanthanide fluorescent immunoassay; Eu: Europium; FDH: Formaldehyde dehydrogenase; FRET: Fluorescence resonance energy transfer; KDM: Lysine demethylase; LANCE: Lanthanide chelate excite; NADH: Reduced nicotinamide adenine dinucleotide; Strep: Streptavidin; TR-FRET: Time-resolved fluorescence resonance energy transfer; TRF: Time-resolved fluorescence.
Radiation-based assays are considered the gold standard to measure methyltransferase activity [23], and therefore were adapted for use in detecting demethylase activity by radiolabeling substrate methyl groups and detecting tritiated formaldehyde (demonstrated here [30]). Recently, Yu et al. established coupled scintillation-proximity assays (SPAs) to detect demethylase activity in a high-throughput format for LSD1, JMJD1A and JMJD2A [31]. In these assays, a biotinlabeled peptide was demethylated by the target KDM and subsequently remethylated by a paired KMT using 3H-S-(5′-adenosyl)-L-methionine (3H-SAM). Peptides were bound to streptavidin/scintillant-coated plates prior to detection using a scintillation counter specialized for high-throughput SPAs [31]. While SPA assays are robust, the coupled assays described above require significant efforts in preparation of enzyme and optimization of conditions for two enzymatic reactions. Similar to the FDH-coupled assays, compounds that affect the coupled KMTs can lead to false positives and negatives.
Antibody-based assays
As highly specific antibodies against the particular methylation states of lysine residues have been generated, HTS methods for KDMs have evolved to incorporate new highly sensitive technologies. Antibody-based screens fall into two categories: homogeneous or heterogeneous, each with distinct benefits and drawbacks. Homogeneous assays generally require few steps and very small quantities of enzyme and substrate, which make them especially suitable when reaction components such as enzymes are limited. However, they are subject to compound interference with readout signals and require counterscreens to rule out any artificial signal production or quenching. Heterogeneous assays separate the demethylation reaction from the readout by incorporating several wash steps, thereby eliminating compound interference with the final detection signal. However, the additional washes require time and larger quantities of peptide substrate. Still, both antibody assay types offer great flexibility and sensitivity.
Homogeneous assays
Homogeneous antibody-based screens for inhibitors of KDMs have used either amplified luminescent proximity homogeneous assay (Alpha) technology or time-resolved fluorescence resonance energy transfer (TR-FRET). Alpha is a bead-based system in which ‘donor’ beads excited by a laser transfer energy in the form of singlet oxygen to ‘acceptor’ beads within 200 nm, inciting emission of a luminescent signal [32]. AlphaScreen (PerkinElmer, MA, USA) assays to screen for KDM inhibitors have used donor beads coated by streptavidin to bind to a biotinylated peptide substrate, combined with rubrene-based acceptor beads coated by protein A that bind to an antibody against the demethylated product [33,34]. Laser excitation at 680 nm results in emission of a luminescent signal between 520 and 620 nm, typically detected at 570 nm (Figure 1B). As the wavelength for emission is lower than that for excitation, these assays have very low background fluorescence signal. Sayegh et al. used this assay to screen approximately 15,000 compounds for inhibitors of full length JARID1B [34]. AlphaLISA (PerkinElmer) utilizes the same donor beads as AlphaScreen, but its europium-based acceptor beads narrow the emission spectrum to center around 615 nm. In addition, antibodies are covalently conjugated to the acceptor beads. Gauthier et al. optimized conditions of AlphaLISA screening for LSD1 inhibitors [35]. While AlphaLISA provides a more precise signal that is less vulnerable to compound interference, the beads used are considerably more expensive than those for AlphaScreen.
TR-FRET technology has also been established for HTS campaigns of KDMs. Gauthier et al. optimized conditions for LANCE (lanthanide chelate excite) Ultra (PerkinElmer) screening for LSD1 inhibitors. LANCE is a TR-FRET technology that uses a europium-labeled antibody against the substrate or the demethylated product as a donor, and another fluorophore, such as ULight in LANCE Ultra, as the acceptor. When the donor europium chelate is excited at 320 or 340 nm, energy in the form of FRET elicits fluorescence emission at 665 nm from a nearby streptavidin-tagged ULight acceptor bound to biotinylated peptide substrate (Figure 1C). Though an excellent Z′ factor was obtained, it is of note that the signal to background ratio was more than a magnitude lower than the ratio for the AlphaLISA assay optimized by this research group [35]. A similar TR-FRET assay was optimized by Wang et al. and used to screen approximately 14,000 compounds against LSD1 [36]. Two TR-FRET assays, LANCE and homogeneous time resolved fluorescence (HTRF, Cisbio Bioassays, Codelet, France), were optimized for the catalytic domain of JMJD2C and LSD1, respectively, by Yu et al. [37]. These assays both use europium-labeled antibodies, but europium chelate is used in LANCE and europium cryptate in HTRF. In both assays, streptavidin-tagged fluorophore acceptors bind biotinylated peptide substrate. If the methylation state recognized by the europium-labeled antibody is in close proximity to the acceptor, in this case allophycocyanin (APC), FRET occurs upon donor excitation. These authors used a loss of signal to indicate demethylation by LSD1, but a gain of signal to indicate demethylation by JMJD2C, highlighting the flexibility of antibody-based assays [37].
While many aspects of Alpha and TR-FRET assays are similar, singlet oxygen in the Alpha assays can transfer energy over longer distances (approximately 200 nm) than FRET (approximately 7 nm), allowing use of bulkier substrates that may be more biologically relevant [38]. However, Alpha assays can be more sensitive to light and high quantities of demethylase reaction components such as ascorbate and Fe2+. Compounds that scavenge singlet oxygen can interfere with AlphaScreen results, while compounds that quench fluorescence or autofluoresce can interfere with TR-FRET results.
Heterogeneous assays
Time-resolved fluorometry (TRF) using a europium-labeled secondary antibody has been the method of choice for heterogeneous antibody-based screens of KDMs. These screens secure substrate to the surface of the plate and wash out compounds and unbound antibody before the detection step, thereby eliminating compound interference with the fluorescent signal. Dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA, PerkinElmer) was optimized by Schmitt et al. to screen for inhibitors of LSD1. In their optimized assay, the demethylation of the biotinylated peptide substrate was performed in solution, and the peptide product was bound to streptavidin-coated plates before antibody detection by a specific primary antibody followed by a europium-labeled secondary antibody (Figure 1D). Incubation with an acidic enhancement solution released Eu3+ that was detected by laser excitation at 365 nm and emission at 380–620 nm [25]. Hauser et al. developed a similar assay for LSD1 without the requirement for manufactured biotinylated peptides and streptavidin-coated plates. Instead, native histones were secured using plates with a high protein-binding capacity. Europium-labeled secondary antibodies were used, and after addition of enhancement solution, released Eu3+ was detected by laser excitation at 340 nm and emission at 615 nm [28]. The heterogeneous antibody-based assays above have large signal to noise ratios and high sensitivity, but require several washes between steps that may make them cumbersome for use in HTS campaigns.
Mass spectrometry-based assays
Several screening campaigns for KDMs have relied on mass spectrometry, a method that allows direct detection of the demethylated product by the 14 Da change in mass that occurs with loss of each methyl group. This method does not require specific antibodies and escapes the nuisances of compound interference with the readout signal. Mass spectrometry has conventionally been used in combination with other approaches to identify novel KDM inhibitors (for example [39]), as the traditional assays were not suitable for HTS. However, recent screens for demethylase inhibitors have incorporated the RapidFire chromatography system (Agilent Technologies, Santa Clara, California) combined with a triple stage quadrupole mass spectrometer. This system robotically prepares and injects samples, and allows fast detection (6–8 s per well) of multiple label-free peptides at the same time, greatly increasing throughput from traditional mass spectrometry assays for KDMs. Plant et al. used this system to monitor di-, mono- and un-methylated H3K4 analytes after reaction of LSD1 with dimethylated H3K4 peptide in the presence of almost 58,000 compounds. Hits from this screen were then used to identify structurally related compounds for use in subsequent rounds of focused screening to identify potent inhibitors [40]. Hutchinson et al. also used this system to screen over 100,000 compounds for inhibition of the catalytic domain of JMJD2C. They detected tri-, di- and mono-methylated H3K9 analytes after JMJD2C reaction with an optimized tri-methylated H3K9 peptide [41].
The compound throughput of this assay was increased fourfold by Leveridge et al. through a multiplexing technique using ‘mass-tagged’ peptides as substrates. Four peptides were developed for use in the screen that differed by one or two residues in the protein sequence to change the peptide mass, but were demethylated at H3K9 with equal affinity by JMJD2D. Four compound plates were assayed with JMJD2D in parallel, each plate using a distinct peptide. The peptides were then pooled together for analysis of tri- and di-methylated H3K9 for each peptide (Figure 1E). This enabled four compounds to be analyzed at a time in each well, as opposed to one compound analyzed per well in the assays above. This method was tested by screening about 1400 compounds for their ability to inhibit JMJD2D and yielded an average Z′ score only slightly less than that of the single peptide control assay [42].
In vivo assays
In order to discover small-molecule inhibitors of KDMs that can enter cells and operate in vivo, recent HTS campaigns have used live cells to weed out inhibitors that do not show in vivo activity. Mulji et al. developed a high-content imaging platform to detect changes in methylation levels using immunofluorescence. 87,500 compounds were screened against JMJD3 by detecting changes in H3K27 trimethylation levels in human HEK293 cells transiently overexpressing Flag-JMJD3. Transient transfection of JMJD3 depleted the trimethylated H3K27 signal, but inhibition of JMJD3 rescued the signal. Signal intensity was determined by quantitative imaging analysis. By surveying KDMs in their native environment, this assay allows exploration of chemical space potentially untapped in in vitro assays and selects for molecules with good physicochemical properties. However, complications in signal readout can occur from cell sensitivity to particular compounds [43].
Mannironi et al. screened for inhibitors of the JARID1/KDM5 family using a genetically altered strain of Saccharomyces cerevisiae that requires JHD2 demethylase activity to grow in the presence of rapamycin. JHD2 is the yeast homolog of the human JARID1 family of lysine demethylases, and has been shown to demethylate tri- and di-methylated H3K4. The yeast strain used for the screen deleted endogenous JHD2 and NOT4 and artificially re-expressed JHD2 through a plasmid. This re-expression of JHD2 offered rapamycin resistance, and cell death in the presence of rapamycin was used to indicate inhibition of the demethylase in a screen of 45 compounds [44].
Although not developed to screen for KDM inhibitors specifically, the in vivo screen using locus derepression in mammary adenocarcinoma cells performed by Wang et al. is worth noting as it led to the discovery of an inhibitor of JmjC demethylases. Here, derepression of a green fluorescent protein (GFP)-estrogen receptor transgene construct was used to signal epigenetic modulation in a screen of approximately 2000 compounds [45].
In vivo screens are often less specific than in vitro screens, and overexpressing an enzyme has caveats. For instance, the overexpressed enzyme may not be localized in the same way as the endogenous enzyme. Despite these drawbacks, in vivo screens can be extremely useful in identifying cell permeable, active compounds.
Conclusion
As seen above, several different HTS approaches have been developed to probe for potent small-molecule inhibitors of KDMs. Choosing the most suitable method for use depends on several factors including the desired size of the compound library, existing knowledge about the target KDM and the particular goals and resources of the research team. Our lab selected the AlphaScreen platform to search for small-molecule inhibitors of JARID1B [34] and JARID1A [Unpublished Data, Gale M, Sayegh J, & Yan Q] because it offered high sensitivity and reliability at a reasonable cost in terms of time, effort and money. However, counterscreening in other assay formats was required to eliminate false positives and hits that did not have suitable physicochemical properties. Thus, each research team must weigh the pros and cons of the available methods (Table 1) before selecting the most appropriate platform(s) for their needs
Table 1.
Evaluation of the platforms used in recent high-throughput screening campaigns for inhibitors oflysine demethylases.
| Assay type | Method | Target KDM | Average Z′ factor | Pros | Cons | Study (year) |
|---|---|---|---|---|---|---|
| Coupled enzyme | FDH-coupled | JMJD2E | 0.85 [27]; 0.93 [29] | Enzyme independent, high sensitivity, label-free | Artifacts from off-target inhibition of the coupled enzyme | King et al. (2010); Sakurai et al. (2010) [27,29] |
| Coupled SPA | LSD1, JMJD1A, JMJD2A | 0.72, 0.7, and 0.8 | Not susceptible to fluorescence interference | Requires preparation of two enzymes and optimization of two reactions, artifacts from off-target inhibition of the coupled enzyme | Yu et al. (2014) [31] | |
|
| ||||||
| Homogeneous antibody | AlphaScreen | JMJD2E; JARID1B | 0.8 [34] | Highest sensitivity, no wash steps | Requires specific antibodies, susceptible to quenching of singlet oxygen by compounds | Kawamura et al. (2010); Sayegh et al. (2013) [33,34] |
| AlphaLISA | LSD1 | 0.8 | Highest sensitivity, no wash steps | Requires specific antibodycoated beads, susceptible to quenching of singlet oxygen by compounds | Gauthier et al. (2012) [35] | |
| TR-FRET | LSD1; LSD1; LSD1 and JMJD2C | 0.88 [35]; 0.85 [36]; 0.76 and 0.77 [37] | High sensitivity, no wash steps | Requires specific labeled antibodies, susceptible to autofluorescence and/or quenching by compounds | Gauthier et al. (2012); Wang et al. (2012); Yu et al. (2012) [35–37] | |
|
| ||||||
| Heterogeneous antibody | TRF | LSD1 | 0.7 [25] | High sensitivity, not susceptible to autofluorescence and/or quenching by compounds | Requires specific antibodies, multiple wash steps | Schmitt et al. (2014); Hauser et al. (2012) [25,28] |
|
| ||||||
| Mass spectrometry | RapidFire mass spectrometry | LSD1; JMJD2C | 0.76 [40]; 0.81 [41] | Direct readout of product formation, no counterscreen required, label-free | Low throughput | Plant et al. (2011); Hutchinson et al. (2012) [40,41] |
| Multiplexed RapidFire mass spectrometry | JMJD2D | 0.72 (multiplexed), 0.85 (single peptide) | Direct readout of product formation, no counterscreen required, label-free | Medium throughput | Leveridge et al. (2014) [42] | |
|
| ||||||
| In vivo | High-content imaging of human cells | JMJD3 | 0.7 (median) | All hits are cell permeable | Requires specific antibodies, compounds may inhibit KDM indirectly, artifacts from cell sensitivity to compounds | Mulji et al. (2012) [43] |
| Yeast requiring demethylase activity for survival | JHD2(yeast homologue of human JARID1 family) | – | Hits are likely to be cell permeable in human cells | Low throughput, compounds may inhibit KDM indirectly | Mannironi et al. (2014) [44] | |
| Locus derepression generating a GFP signal in human cancer cells | JmjC family | – | All hits are cell permeable | Not specifically designed for KDM inhibitor screen, compounds may inhibit KDM indirectly | Wang et al. (2013) [45] | |
Alpha: Amplified luminescent proximity homogeneous assay; FDH: Formaldehyde dehydrogenase; GFP: Green fluorescent protein; JmjC: Jumonji C; KDM: Lysine demethylase; SPA: Scintillation proximity assay; TR-FRET: Time-resolved fluorescence resonance energy transfer; TRF: Time-resolved fluorometry.
Future perspective
The study of KDMs has only grown since their discovery a decade ago and will continue to expand. In order to fully understand the functions and downstream effects of KDMs, small-molecule modulators of KDM activity are extremely useful and in demand. Further optimization of existing assays and development of new assays will lead to sensitive, cost–effective, reliable and robust HTS methods in the future. Combined with structure-based virtual screening and medicinal chemistry optimization, these assays will lead to potent and specific small-molecule inhibitors suitable for use in laboratory studies and in the clinic.
Executive summary.
Coupled enzyme-based assays
Formaldehyde dehydrogenase can be used to detect the formaldehyde byproduct of demethylation reactions through the coupled formation of reduced nicotinamide adenine dinucleotide, which is detectable by absorbance and fluorescence.
Coupled scintillation-proximity assay reactions in which a biotinylated peptide is demethylated and subsequently remethylated by a paired methyltransferase in the presence of 3H-S-(5′-adenosyl)-L-methionine can be used to detect demethylase activity.
Homogeneous antibody-based assays
AlphaScreen and AlphaLISA are bead-based assays in which laser excitation generates a luminescent signal if an acceptor bead bound to a specific antibody targeting a particular methylation state is in close proximity to a donor bead bound to a tagged substrate.
Time-resolved fluorescence resonance energy transfer measures a fluorescent signal generated by laser excitation when a europium-labeled antibody targeting a specific methylation state is in close proximity to a fluorophore acceptor bound to the substrate.
Heterogeneous antibody-based assays
Time-resolved fluorescence assays such as dissociation-enhanced lanthanide fluorescent immunoassay separate the demethylation reaction and detection steps by binding the substrate to the reaction plate and washing. Europium-labeled secondary antibodies are used to generate a fluorescent signal.
Mass spectrometry-based assays
RapidFire mass spectrometry assays allow detection of multiple label-free analytes per well.
Pooling of mass-tagged peptides after separate demethylation reactions increases throughput and permits analysis of multiple compounds per well.
In vivo assays
High content imaging of methylation changes using immunofluorescence in human cell lines overexpressing a lysine demethylase of interest can be used to screen for cell permeable small-molecule inhibitors.
Yeast and human cell lines can be genetically engineered to have a particular detectable phenotype in response to inhibition of a lysine demethylase.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by American Cancer Society Research Scholar Grant RSG-13-384-01-DMC, Connecticut Department of Public Health Biomedical Research Grant 2013-0201, Department of Defense Peer Reviewed Cancer Research Program Career Development Award W81XWH-13-1-0235 and Department of Defense Breast Cancer Research Program Breakthrough Award W81XWH-14-1-0308 to Q Yan, and NIH grant CA16359 (to the Yale Comprehensive Cancer Center). M Gale is supported by NSF Graduate Research Fellowship DGE-1122492. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 2.Bremang M, Cuomo A, Agresta AM, Stugiewicz M, Spadotto V, Bonaldi T. Mass spectrometry-based identification and characterisation of lysine and arginine methylation in the human proteome. Mol Biosyst. 2013;9(9):2231–2247. doi: 10.1039/c3mb00009e. [DOI] [PubMed] [Google Scholar]
- 3.Cao XJ, Arnaudo AM, Garcia BA. Large-scale global identification of protein lysine methylation in vivo. Epigenetics. 2013;8(5):477–485. doi: 10.4161/epi.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Sengupta R, Espejo AB, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449(7158):105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 5.Lu T, Jackson MW, Singhi AD, et al. Validation-based insertional mutagenesis identifies lysine demethylase FBXL11 as a negative regulator of NFkappaB. Proc Natl Acad Sci USA. 2009;106(38):16339–16344. doi: 10.1073/pnas.0908560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu T, Jackson MW, Wang B, et al. Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc Natl Acad Sci USA. 2010;107(1):46–51. doi: 10.1073/pnas.0912493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol. 2012;13(5):297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- 8••.Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. Discovery of the first lysine demethylase (KDM), LSD1. [DOI] [PubMed] [Google Scholar]
- 9••.Tsukada Y, Fang J, Erdjument-Bromage H, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 439(7078):811–816. doi: 10.1038/nature04433. Discovery of the Jumonji C (JmjC) domain as a KDM motif. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Miyata N. Lysine demethylases inhibitors. J Med Chem. 2011;54(24):8236–8250. doi: 10.1021/jm201048w. [DOI] [PubMed] [Google Scholar]
- 11•.Hojfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nat Rev Drug Discov. 2013;12(12):917–930. doi: 10.1038/nrd4154. Review of KDMs in human disease and recent progress in targeting KDMs therapeutically. [DOI] [PubMed] [Google Scholar]
- 12.Blair LP, Cao J, Zou MR, Sayegh J, Yan Q. Epigenetic regulation by lysine demethylase 5 (KDM5) enzymes in cancer. Cancers (Basel) 2011;3(1):1383–1404. doi: 10.3390/cancers3011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Jensen LR, Amende M, Gurok U, et al. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet. 2005;76(2):227–236. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou H, Yu H. Structural insights into histone lysine demethylation. Curr Opin Struct Biol. 2010;20(6):739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thinnes CC, England KS, Kawamura A, Chowdhury R, Schofield CJ, Hopkinson RJ. Targeting histone lysine demethylases: progress, challenges, and the future. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagrm.2014.05.009. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X, Liu Y, Kubicek S, et al. A selective inhibitor and probe of the cellular functions of Jumonji C domain-containing histone demethylases. J Am Chem Soc. 2011;133(24):9451–9456. doi: 10.1021/ja201597b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruidenier L, Chung CW, Cheng Z, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488(7411):404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorna V, Theisen ER, Stephens B, et al. High-throughput virtual screening identifies novel N′-(1-phenylethylidene)-benzohydrazides as potent, specific, and reversible LSD1 inhibitors. J Med Chem. 2013;56(23):9496–9508. doi: 10.1021/jm400870h. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Lu F, Ren Q, et al. Novel histone demethylase LSD1 inhibitors selectively target cancer cells with pluripotent stem cell properties. Cancer Res. 2011;71(23):7238–7249. doi: 10.1158/0008-5472.CAN-11-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willmann D, Lim S, Wetzel S, et al. Impairment of prostate cancer cell growth by a selective and reversible lysine-specific demethylase 1 inhibitor. Int J Cancer. 2012;131(11):2704–2709. doi: 10.1002/ijc.27555. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high-throughput screening assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 23•.Quinn AM, Simeonov A. Methods for activity analysis of the proteins that regulate histone methylation. Curr Chem Genomics. 2011;5(Suppl 1):95–105. doi: 10.2174/1875397301005010095. Describes many assays to detect lysine methylation and demethylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spannhoff A, Hauser AT, Heinke R, Sippl W, Jung M. The emerging therapeutic potential of histone methyltransferase and demethylase inhibitors. ChemMedChem. 2009;4(10):1568–1582. doi: 10.1002/cmdc.200900301. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt ML, Ladwein KI, Carlino L, et al. Heterogeneous antibody-based activity assay for lysine specific demethylase 1 (LSD1) on a histone peptide substrate. J Biomol Screen. 2014;19(6):973–978. doi: 10.1177/1087057114529156. [DOI] [PubMed] [Google Scholar]
- 26.Lizcano JM, Unzeta M, Tipton KF. A spectrophotometric method for determining the oxidative deamination of methylamine by the amine oxidases. Anal Biochem. 2000;286(1):75–79. doi: 10.1006/abio.2000.4782. [DOI] [PubMed] [Google Scholar]
- 27••.King ON, Li XS, Sakurai M, et al. Quantitative high-throughput screening identifies 8-hydroxyquinolines as cell-active histone demethylase inhibitors. PLoS ONE. 2010;5(11):e15535. doi: 10.1371/journal.pone.0015535. First published lysine demethylase high-throughput inhibitor screen using a large compound library. Screened approximately 236,000 compounds against JMJD2E using a formaldehyde dehydrogenase-coupled assay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauser AT, Bissinger EM, Metzger E, et al. Screening assays for epigenetic targets using native histones as substrates. J Biomol Screen. 2012;17(1):18–26. doi: 10.1177/1087057111423968. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai M, Rose NR, Schultz L, et al. A miniaturized screen for inhibitors of Jumonji histone demethylases. Mol Biosyst. 2010;6(2):357–364. doi: 10.1039/b912993f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhang H, Chen Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138(4):660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Yu W, Eram MS, Hajian T, et al. A scintillation proximity assay for histone demethylases. Anal Biochem. 2014;463:54–60. doi: 10.1016/j.ab.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Ullman EF, Kirakossian H, Singh S, et al. Luminescent oxygen channeling immunoassay: measurement of particle binding kinetics by chemiluminescence. Proc Natl Acad Sci USA. 1994;91(12):5426–5430. doi: 10.1073/pnas.91.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamura A, Tumber A, Rose NR, et al. Development of homogeneous luminescence assays for histone demethylase catalysis and binding. Anal Biochem. 2010;404(1):86–93. doi: 10.1016/j.ab.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Sayegh J, Cao J, Zou MR, et al. Identification of small-molecule inhibitors of Jumonji AT-rich interactive domain 1B (JARID1B) histone demethylase by a sensitive high-throughput screen. J Biol Chem. 2013;288(13):9408–9417. doi: 10.1074/jbc.M112.419861. Screened approximately 15,000 compounds against JARID1B using AlphaScreen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauthier N, Caron M, Pedro L, et al. Development of homogeneous nonradioactive methyltransferase and demethylase assays targeting histone H3 lysine 4. J Biomol Screen. 2012;17(1):49–58. doi: 10.1177/1087057111416659. [DOI] [PubMed] [Google Scholar]
- 36•.Wang C, Caron M, Burdick D, et al. A sensitive, homogeneous, and high-throughput assay for lysine-specific histone demethylases at the H3K4 site. Assay Drug Dev Technol. 2012;10(2):179–186. doi: 10.1089/adt.2011.0395. Screened approximately 14,000 compounds against LSD1 using time-resolved fluorescence resonance energy transfer (TR-FRET) [DOI] [PubMed] [Google Scholar]
- 37.Yu V, Fisch T, Long AM, et al. High-throughput TR-FRET assays for identifying inhibitors of LSD1 and JMJD2C histone lysine demethylases. J Biomol Screen. 2012;17(1):27–38. doi: 10.1177/1087057111418228. [DOI] [PubMed] [Google Scholar]
- 38.Glickman JF, Wu X, Mercuri R, et al. A comparison of ALPHAScreen, TR-FRET, and TRF as assay methods for FXR nuclear receptors. J Biomol Screen. 2002;7(1):3–10. doi: 10.1177/108705710200700102. [DOI] [PubMed] [Google Scholar]
- 39.Rose NR, Woon EC, Kingham GL, et al. Selective inhibitors of the JMJD2 histone demethylases: combined nondenaturing mass spectrometric screening and crystallographic approaches. J Med Chem. 2010;53(4):1810–1818. doi: 10.1021/jm901680b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Plant M, Dineen T, Cheng A, Long AM, Chen H, Morgenstern KA. Screening for lysine-specific demethylase-1 inhibitors using a label-free high-throughput mass spectrometry assay. Anal Biochem. 2011;419(2):217–227. doi: 10.1016/j.ab.2011.07.002. Screened approximately 58,000 compounds against LSD1 using RapidFire mass spectrometry. [DOI] [PubMed] [Google Scholar]
- 41•.Hutchinson SE, Leveridge MV, Heathcote ML, et al. Enabling lead discovery for histone lysine demethylases by high-throughput RapidFire mass spectrometry. J Biomol Screen. 2012;17(1):39–48. doi: 10.1177/1087057111416660. Screened approximately 100,000 compounds against JMJD2C using RapidFire mass spectrometry. [DOI] [PubMed] [Google Scholar]
- 42•.Leveridge M, Buxton R, Argyrou A, et al. Demonstrating enhanced throughput of RapidFire mass spectrometry through multiplexing using the JmjD2d demethylase as a model system. J Biomol Screen. 2014;19(2):278–286. doi: 10.1177/1087057113496276. Pooled mass-tagged peptides to increase throughput of RapidFire mass spectrometry. [DOI] [PubMed] [Google Scholar]
- 43•.Mulji A, Haslam C, Brown F, et al. Configuration of a high-content imaging platform for hit identification and pharmacological assessment of JMJD3 demethylase enzyme inhibitors. J Biomol Screen. 2012;17(1):108–120. doi: 10.1177/1087057111418229. Screened approximately 87,500 compounds against JMJD3 using quantitative immunofluorescence. [DOI] [PubMed] [Google Scholar]
- 44.Mannironi C, Proietto M, Bufalieri F, et al. An high-throughput in vivo screening system to select H3K4-specific histone demethylase inhibitors. PLoS ONE. 2014;9(1):e86002. doi: 10.1371/journal.pone.0086002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Chang J, Varghese D, et al. A small-molecule modulates Jumonji histone demethylase activity and selectively inhibits cancer growth. Nat Commun. 2013;4:2035. doi: 10.1038/ncomms3035. [DOI] [PMC free article] [PubMed] [Google Scholar]