Abstract
Structural studies on TRP channels, while limited, are poised for a quickened pace and rapid expansion. As of yet, no high-resolution structure of a full length TRP channel exists, but low-resolution electron cryomicroscopy structures have been obtained for 4 TRP channels, and high-resolution NMR and X-ray crystal structures have been obtained for the cytoplasmic domains, including an atypical protein kinase domain, ankyrin repeats, coiled coil domains and a Ca2+-binding domain, of 6 TRP channels. These structures enhance our understanding of TRP channel assembly and regulation. Continued technical advances in structural approaches promise a bright outlook for TRP channel structural biology.
1.1 Introduction
Full understanding of ion channel function requires high-resolution three-dimensional (3D) structures. Structural studies on ion channels entered a new phase in 1998 after the publication of the crystal structure of the bacterial K+ channel, KcsA [1]. Since then, there has been a rapid growth in the number of ion channel structures. To date, there are ~90 crystal structures of full length or near full length ion channels, ~50 electron microscopy structures of full length or near full length ion channels, and ~130 crystal and nuclear magnetic resonance (NMR) structures of ion channel fragments. These structures have led to a quantum leap in our understanding of the molecular and biophysical mechanisms of ion channel assembly, selectivity, conduction, gating and regulation.
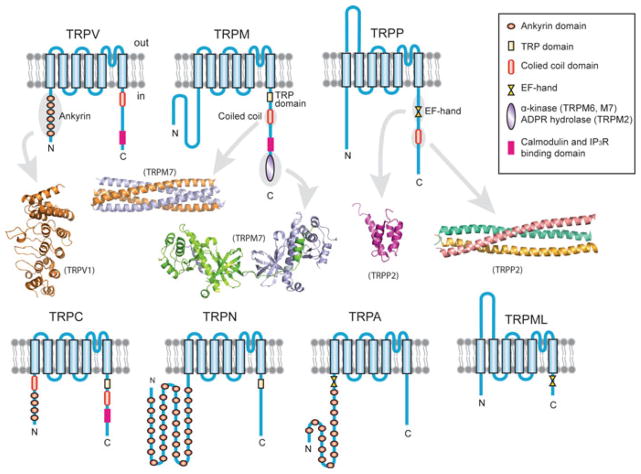
TRP channels constitute a distinct superfamily of ion channels and are distantly related to voltage-gated K+, Na+ and Ca2+ superfamilies. They are expressed and function in diverse organisms, including yeasts, worms, fruit flies, mice and humans. Excluding yeast TRPs, there are seven subfamilies: TRPC, TRPV, TRPM, TRPA, TRPN, TRPP and TRPML, with TRPN absent in mice and humans (Fig. 1.1) [2]. Each subfamily has one or more members. Mice have a total of 28 different members, and humans 27. All TRP channel subunits have six putative transmembrane segments and a pore-forming loop between the last two transmembrane segments (Fig. 1.1). The amino (N) and carboxyl (C) termini are located intracellularly and vary vastly in length (Table 1.1) and amino acid (aa) sequence. These cytoplasmic regions contain various well-recognized domains and motifs that are likely involved in channel assembly, activation and regulation through protein–protein and/or protein–ligand interactions (Fig. 1.1).
Fig. 1.1.
TRP channel subfamilies and the transmembrane topology and domain organization of their subunits. Only commonly present and readily identifiable domains or motifs in the cytoplasmic N and C termini are indicated. Examples of high-resolution structures of some domains or motifs are presented
Table 1.1.
Predicted region and length of the cytoplasmic N and C termini of TRP channel subunits and the number of low-complexity residues in these regions
| Protein | N terminus
|
C terminus
|
||||
|---|---|---|---|---|---|---|
| Channel region | # of residues | # of low-complexity residues | Channel region | # of residues | # of low-complexity residues | |
| TRPC1 | 1–316 | 316 | 14 | 610–759 | 150 | 0 |
| TRPC2 | 1–626 | 626 | 92 | 918–1,172 | 255 | 82 |
| TRPC3 | 1–351 | 351 | 0 | 671–848 | 178 | 0 |
| TRPC4 | 1–327 | 327 | 38 | 618–977 | 360 | 21 |
| TRPC5 | 1–327 | 327 | 41 | 622–973 | 352 | 56 |
| TRPC6 | 1–404 | 404 | 22 | 726–931 | 206 | 7 |
| TRPC7 | 1–351 | 351 | 11 | 671–862 | 192 | 0 |
| TRPV1 | 1–433 | 433 | 0 | 681–839 | 159 | 0 |
| TRPV2 | 1–390 | 390 | 0 | 645–764 | 120 | 0 |
| TRPV3 | 1–438 | 438 | 56 | 675–790 | 116 | 0 |
| TRPV4 | 1–468 | 468 | 26 | 716–871 | 156 | 0 |
| TRPV5 | 1–326 | 326 | 0 | 577–729 | 153 | 22 |
| TRPV6 | 1–326 | 326 | 16 | 577–725 | 149 | 10 |
| TRPM1 | 1–760 | 760 | 84 | 1,053–1,533 | 481 | 27 |
| TRPM2 | 1–750 | 750 | 40 | 1,046–1,503 | 458 | 26 |
| TRPM3 | 1–716 | 716 | 59 | 955–1,554 | 600 | 34 |
| TRPM4 | 1–687 | 687 | 43 | 1,041–1,214 | 174 | 34 |
| TRPM5 | 1–643 | 643 | 0 | 975–1,158 | 184 | 0 |
| TRPM6 | 1–742 | 742 | 15 | 1,075–2,022 | 948 | 34 |
| TRPM7 | 1–756 | 756 | 15 | 1,102–1,864 | 763 | 13 |
| TRPM8 | 1–692 | 692 | 16 | 977–1,104 | 128 | 24 |
| TRPML1 | 1–69 | 69 | 12 | 518–580 | 63 | 13 |
| TRPML2 | 1–61 | 61 | 0 | 508–566 | 59 | 0 |
| TRPML3 | 1–66 | 66 | 13 | 503–553 | 51 | 0 |
| TRPP2 | 1–224 | 224 | 99 | 681–968 | 288 | 87 |
| TRPP3 | 1–104 | 104 | 15 | 558–805 | 248 | 22 |
| TRPP5 | 1–33 | 33 | 0 | 492–613 | 122 | 12 |
| TRPA1 | 1–717 | 717 | 0 | 962–1,119 | 158 | 0 |
All amino acid sequences are from humans except TRPC2, which is from mice, as human TRPC2 is a pseudogene. Transmembrane helices were predicted using the TMHMM Server v. 2.0 at http://www.cbs.dtu.dk/services/TMHMM/. Low-complexity sequences were predicted using the program SEG [80] with the default settings.
All TRP channels are cation selective, with some being highly selective for Ca2+ or Mg2+ [2]. In accord with their amino acid sequence diversity, TRP channels exhibit varied activation and modulatory mechanisms, such as stimulation of G protein coupled receptors, extracellular and intracellular ligands (including H+, Ca2+ and Mg2+), phosphoinositide-4,5pbisphosphate (PIP2), temperature, and mechanical stretch [2]. To fully understand TRP channel diversity, function and regulation, it is necessary to gain structural information on different types of TRP channels.
Of the existing ion channel structures, most come from K+ channels. This is due, in part, to their vast variety and their existence in bacteria, which make them more tractable to structural approaches, especially X-ray crystallography, because they can be more abundantly expressed, are more stable, and hence, are more amicable to purification and crystallization. TRP channels, however, are not endogenously expressed in bacteria. This is perhaps a major contributing factor in the present lack of even a single high-resolution structure of any full length TRP channel. Nevertheless, low-resolution structures have been obtained for 4 full length TRP channels by electron microscopy (EM). Meanwhile, X-ray crystallography and NMR spectroscopy have been employed effectively to garner high-resolution structures of functionally important cytosolic domains of 6 TRP channels (Table 1.2). This chapter describes the existing TRP channel structures and, when available, the mechanistic insights they provide, beginning with a brief overview of structural approaches and considerations. Advances in TRP channel structural biology have been covered in several recent reviews [3–7].
Table 1.2.
High-resolution structures of TRP channel fragments
| Structural description | Channel region | Species | Resolution | Method | PDB code | References |
|---|---|---|---|---|---|---|
| TRPM7 α-kinase | 1,549–1,828 | Mouse | 2.8 Å | X-ray crystallography | 1IAJ | [27] |
| TRPM7 α-kinase, with AMP·PNP | 1,549–1,828 | Mouse | 2.0 Å | X-ray crystallography | 1IA9 | [27] |
| TRPM7 α-kinase, with ADP | 1,549–1,828 | Mouse | 2.4 Å | X-ray crystallography | 1IAH | [27] |
| TRPV1 ankyrin repeats | 101–364 | Rat | 2.7 Å | X-ray crystallography | 2PNN | [39] |
| TRPV2 ankyrin repeats | 75–326 | Rat | 1.65 Å | X-ray crystallography | 2ETB | [37] |
| TRPV2 ankyrin repeats | 69–319 | Human | 1.7 Å | X-ray crystallography | 2F37 | [40] |
| TRPV4 ankyrin repeats | 133–382 | Chicken | 2.3 Å | X-ray crystallography | 3JXI | [38] |
| TRPV6 ankyrin repeats | 44–265 | Mouse | 1.7 Å | X-ray crystallography | 2RFA | [41] |
| TRPM7 coiled coil | 1,230–1,282 | Rat | 2.01 Å | X-ray crystallography | 3E7K | [57] |
| TRPP2 coiled coil, long | 833–895 | Human | 1.9 Å | X-ray crystallography | 3HRN | [58] |
| TRPP2 coiled coil, short | 833–872 | Human | 1.9 Å | X-ray crystallography | 3HRO | [58] |
| TRPP2 E-F hand | 724–796 | Human | NMR | 2KLE | [74] | |
| TRPP2 E-F hand | 720–797 | Human | NMR | 2KQ6 | [75] |
1.2 Structure-Determination Methods and Considerations
When examining the structure of a protein or a protein complex, the first and foremost concern is its resolution. At nanometer-resolutions, certain general features of the protein can be ascertained, including its shape, dimension, subunit stoichiometry and domain organization (Fig. 1.2a). At 4- to 9-Å resolutions, secondary structures can be discerned (Fig. 1.2b). At resolutions below 3.7 Å, amino acid side-chains can be visualized and assigned – the higher the resolution, the higher the precision and confidence (Fig. 1.2c). For example, aromatic side-chains can be identified at 3.5 Å, and individual atoms can be resolved at 1.5 Å [8].
Fig. 1.2.
Examples of membrane protein structures at different resolutions. (a) Side view (left) and top view (right) of a cryo-EM structure of the Drosophila Shaker K+ channel at 25 Å resolution, revealing a fourfold symmetry and a two-layered architecture [76]. (b) Side view of the structure of a monomer of aquaporin 1 obtained by 2D cryo-EM at 6 Å resolution, revealing 6 distinct tilted rods that correspond to membrane-spanning α helices [77]. (c) X-ray crystal structure of the rat Kv1.2 channel at 2.9 Å resolution (left, PDB code 2A79) [78] and (d) The electron density map and side chain assignment of the ion selectivity filter of a rat Kv1.2–Kv2.1 chimeric channel at 2.4 Å resolution (right, PDB code 2R9R) [79]
Three methods are commonly used to determine protein 3D structures – electron cryomicroscopy (cryo-EM), NMR spectroscopy and X-ray crystallography. These methods have different applications, advantages and disadvantages, especially when applied to integral membrane proteins.
Cryo-EM can be used to determine the structure of proteins of various shapes, forms and sizes [9–11]. It is particularly useful for proteins that are too large or too difficult for NMR and X-ray crystallography. Moreover, cryo-EM can probe proteins in their native lipid environment. Cryo-EM can be used to visualize proteins in two-dimensional (2D) sheets or helices or in non-crystal forms. The resolution of single-particle cryo-EM, the most widely used cryo-EM method, generally ranges from 30 to ~6 Å, depending on the quality of protein preparation, protein symmetry, sample size, data processing, and reconstruction. Near atomic resolution can be obtained for highly symmetrical complexes (see e.g., [12]). With 2D crystals, cryo-EM can achieve atomic resolution. For example, the structure of aquaporin-0 in double-layered 2D crystals has been determined at 1.9 Å [13], the highest resolution protein structure solved to date by cryo-EM.
Both NMR and X-ray crystallography allow the determination of protein structures at atomic resolutions. NMR is mainly applicable to relatively small proteins or protein fragments, usually less than 25 kDa, for structural determination, though technical advances allow proteins of up to 900 kDa to be studied [14]. Also, both soluble and membrane proteins can be examined [14, 15]. For partially or wholly unstructured proteins or protein fragments that are resistant to crystallization, NMR is often the only method for structural determination.
X-ray crystallography is by far the most widely used and most effective structure-determination method. As of March 2010, ~86% of the protein structures deposited in the Protein Data Bank and ~88% of the ion channel structures (full length and fragments) are solved by X-ray crystallography. The number of unique structures of membrane proteins solved by X-ray crystallography has been increasing exponentially, from a total of 25 in 1998 when the KcsA structure was published to 212 in 2009. Despite its power, X-ray crystallography has limitations, especially when applied to membrane proteins. Major challenges include maintaining the protein in a soluble form and in its native oligomeric state, crystallizing the protein, and achieving atomic resolution.
An important consideration in protein structure determination is the expression system. Four types of cells have been routinely employed to overexpress membrane proteins: bacteria (Escherichia coli), yeast (Saccharomyces cerevisiae and Pichia pastoris), insect cells (Sf9 cells), and mammalian cells (HEK293 cells and COS7 cells). Obviously, proteins that are endogenously expressed in bacteria are likely to yield better expression in E. coli. There are yet no well-defined guiding principles in choosing an expression system for vertebrate membrane proteins. Trial-and-error seems to be the most effective strategy.
Another key consideration is the choice of detergents. Membrane proteins are embedded in lipids and thus require detergents for solublization, purification and crystallization [16, 17]. Nonionic and zwitterionic detergents are generally less harsh on proteins than ionic detergents and have been much more successfully utilized in structural investigation. Commonly used nonionic and zwitterionic detergents include n-decyl-β-D-maltoside (DM), n-dodecyl-β-D-maltoside (DDM), lauryldimethylamine-N-oxide (LDAO), n-octyl-β-D-glucoside (OG), dodecyl octaethylene glycol ether (C12E8), and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS). In general, detergent concentrations should be significantly higher than the critical micelle concentration (CMC), the concentration at which detergent monomers aggregate to form micelles. Sometimes, different detergents are used for solublization and for purification and crystallization. As with choosing the expression system, there is not a set of rules regarding detergent choice and the concentration to be used; they are largely determined empirically.
Yet another critical consideration is whether to work on full length proteins or smaller fragments. From the functional point of view, it is obviously more desirable to obtain the structure of full length proteins. With cryo-EM, this is usually achievable, even for very large proteins. This is, however, not often feasible with X-ray crystallography. To facilitate protein expression, purification, crystallization, and to improve resolution, it is often necessary to remove parts of a protein. Even with such maneuvers, it is still often unattainable to solve the structure of a membrane protein. In such cases, an alternative is to obtain the structure of the soluble domains of the protein. The extracellular and intracellular regions of membrane proteins usually contain functionally important domains and motifs, which often fold into compact and defined structures. These domains and motifs often can be independently expressed, purified and crystallized, and their structures can provide useful insights into the workings of a protein. Still, the extracellular and intracellular regions of ion channel proteins, including TRP channels, often contain low-complexity sequences (Table 1.1), which are generally detrimental to structural determination by both NMR and X-ray crystallography [18]. Thus, even when working with channel fragments, it is usually necessary to trim them further. Indeed, none of the available high-resolution structures of TRP channels comes from a full length N or C terminus (Table 1.2). Finally, it should be cautioned that the structure of an isolated protein fragment may not always represent its structure in the intact protein. Thus, the validity and usefulness of such a structure needs to be tested in the full length protein.
1.3 EM Structures
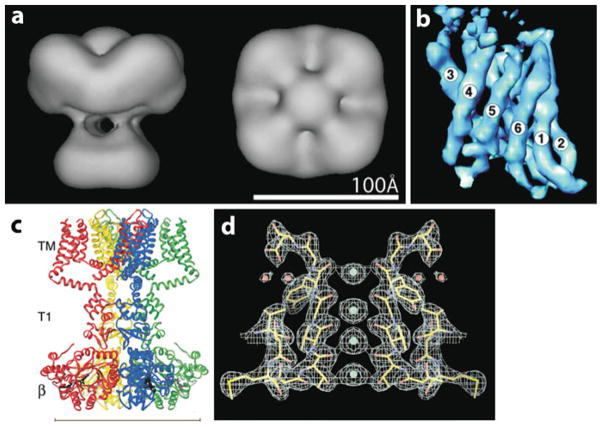
Low-resolution (15–35 Å) EM structures have been obtained for 4 TRP channels from 3 different subfamilies: TRPM2, TRPC3, TRPV1 and TRPV4 (Fig. 1.3) [19–22]. The structures of the latter 3 channels were determined by cryo-EM, but that of TRPM2 was determined by EM with negative staining. A common feature of all four structures is that they exhibit a fourfold rotational symmetry, consistent with the tetrameric subunit stoichiometry that has been demonstrated for several TRP channels by other methods [23, 24]. Strikingly, while the general structure of TRPV1 and TRPV4 is similar, that of TRPM2 and TRPC3 is markedly different (Fig. 1.3).
Fig. 1.3.
TRP channel EM structures. (a) Cryo-EM structure of TRPV1 [21], superimposed with the crystal structure of the Kv1.2 transmembrane domains (maroon; PDB code 2A79) and of the ankyrin repeat domain of TRPV1 (green; PDB code 2PNN). (b) Cryo-EM structure of TRPV4 [22], superimposed with the crystal structure of Mlotik1 (top; PDB code 3BEH) and of the ankyrin repeat domain of TRPV1 (bottom). (c) EM structure of TRPM2 with negative staining [19]. (d) Cryo-EM structure of TRPC3 [20]. All structures are side-views. The white lines mark putative transmembrane regions, so do the blue lines, as presented in [20]. The resolutions of all four structures are based on the 0.5 cutoff criterion in the Fourier shell correlation
The structure of rat TRPV1 was determined by single particle cryo-EM at 19 Å resolution (Fig. 1.3a) [21]. The reconstructed 3D structure stands ~150 Å high and contains two interconnected regions. The small region measures ~60×60 Å, with a height of 40 Å, and accounts for ~30% of the total mass. It likely corresponds to the transmembrane portion of the channel, as suggested by its relative mass and a reasonable fit of the high-resolution structure of the transmembrane domains of the Kv1.2 K+ channel into this region. The large region is shaped like a basket, with a central cavity, and is connected to the small region by 4 bridges. This region, comprising ~70% of the total mass, is ~100 Å wide and 110 Å high and probably corresponds to cytoplasmic N and C termini. Indeed, the 6 ankyrin repeats present in the N terminus of TRPV1 can be comfortably fitted into this region in the vertical orientation. The functional importance of the vacant central chamber is unknown.
The structure of rat TRPV4, reconstructed to 35 Å resolution, is similar to that of TRPV1 and shares the two-layered general architecture (Fig. 1.3b) [22]. This is consistent with the similar size of the two channels (rat TRPV1 and TRPV4 subunits contain 838 and 871 amino acids, respectively). The small region accounts for 30% of the total volume and has a dimension of ~85 Å. The transmembrane domains of Mlotik1, a prokaryotic K+ channel, can be largely superimposed onto this region. The large region is ~112 Å wide, and as in TRPV1, is linked to the putative transmembrane region through 4 short bridges. The N terminus of TRPV4 also contains 6 ankyrin repeats, which can be fitted into the large region, not in the vertical orientation as in TRPV1 but in a tilted orientation (Fig. 1.3b). Despite the similarities, there are some notable differences between the TRPV1 and TRPV4 structures. For example, both the small and large regions of TRPV4 are wider than their counterparts in TRPV1, but the overall height, ~130 Å, is shorter than that of TRPV1. Perhaps the most striking difference is the lack of a vacant cavity in the large region of TRPV4. This may reflect the different arrangement of the cytoplasmic N and C termini of the two channels and their interactions with distinct partners. Furthermore, due to the low resolution, smaller cavities might have not been resolved in the TRPV4 structure.
The 28 Å-resolution EM structure of human TRPM2 was obtained by negatively staining the protein samples with uranyl acetate (Fig. 1.3c) [19]. Instead of directly visualizing protein particles themselves, as in the case of cryo-EM, negative staining reveals the structure of the regions surrounding a protein. As such, this method tends to provide less structural details than single particle cryo-EM. The TRPM2 structure is composed of two main components, a bullet-shaped major component and a prism-shaped minor component. The head of the bullet presumably corresponds to the transmembrane domains, while the rest of the bullet and the prism constitute the cytoplasmic domains. The entire structure is ~250 Å high, with the base of the bullet being ~170 Å wide. The prism has a dimension of ~60 × 60 × 50 Å and is postulated to be formed by the nucleoside diphosphate-linked moiety X-type motif 9 homology (NUDT9-H) domain located in the C-terminus.
The TRPM2 structure is significantly larger than that of TRPV1 and TRPV4. This is expected since the calculated mass of TRPM2 is >1.7 fold higher than that of TRPV1 and TRPV4. Moreover, the total mass estimated from the TRPM2 EM structure is ~30% higher than the calculated mass, presumably due to contributions from attached glycans, lipids and detergents.
Of the four existing EM structures, that of mouse TRPC3 has the highest resolution (15 Å) and is the most unique (Fig. 1.3d) [20]. The TRPC3 structure can also be divided into two components, a dense globular inner core and a sparse outer shell with a mesh-like structure containing many columns and aqueous spaces. Viewing from either the top or bottom, the outer shell columns radiate from the inner core like airport satellite terminals. These antenna-like structures are postulated to function as signal sensing modules for activators and modulators. The overall dimension of the reconstituted TRPC3 structure is ~200 × 200 × 240 Å, which is larger than that of TRPM2, even though the calculated mass of a TRPC3 tetramer is much smaller than that of a TRPM2 tetramer (388 kDa vs. 689 kDa). One reason for this disparity is the presence of many large water-filled cavities in the TRPC3 structure. It is not totally clear why the TRPC3 structure is drastically different from the TRPV1 and TRPV4 structures. It is hypothesized that the simultaneous association of TRPC3 with other protein complexes and its multi-modal activation and modulation mechanisms may underlie its expanded structure [20]. However, the use of an automated particle-selection algorithm might have caused distortions in the data analysis and structural reconstruction of TRPC3, an issue that was also discussed in a recent review [7]. This algorithm was also used for the determination of the TRPM2 structure [19], suggesting another reason (in addition to negative staining) for the significant difference between this structure and the TRPV1 and TRPV4 structures.
1.4 NMR and X-Ray Crystal Structures
1.4.1 TRPM7 α-Kinase Domain
The mammalian TRPM subfamily has 8 members, which display distinct expression, activation, regulation and ion permeation properties [2]. TRPM7 channels and their close relatives TRPM6 channels are unique in several ways. While inhibited by intracellular Mg2+, they are nevertheless highly permeable to Ca2+ and Mg2+, and both are fusion proteins consisting of an ion channel module and an enzymatic module, with an α-kinase domain in the C terminus [2].
α-kinases belong to a family of atypical protein kinases that show little amino acid sequence similarities with classical serine, threonine or tyrosine protein kinases [25]. The substrate residues recognized by classical protein kinases are often located within β-turns, loops or irregular structures [26]. In contrast, the substrate residues targeted by α-kinases are often situated within α helices, although this is not always the case [25].
The α-kinase domain of TRPM7 is located at the distal C terminus, downstream of a coiled coil domain, whose structure was determined recently (see later). The structure of this domain, alone or in complex with AMP·PNP (an ATP analog) or ADP, was solved by X-ray crystallography at 2.8, 2.0 and 2.4 Å resolution, respectively [27]. These structures reveal that the TRPM7α-kinase domain forms a dimer, in which a 27-residue N-terminal segment of one monomer extends out and interacts extensively with the kinase domain of another monomer (Fig. 1.4a). The main component of each monomer contains two lobes (Fig. 1.4b): an N-terminal lobe consisting mainly of a stack of curved β sheets and harboring a phosphate binding P-loop, and a C-terminal lobe consisting of both α helices and β sheets and containing a glycine-rich GXA(G)XXG motif, which is conserved in the α-kinase family and is critical for catalysis. A nucleotide is bound at the interlobe cleft, where both the P-loop and the GXA(G)XXG motif project to (Fig. 1.4b). This overall architecture is highly similar to that of the PKA kinase domain (Fig. 1.4c). Strikingly, despite a lack of overall amino acid sequence conservation, many key residues involved in ATP binding and/or Mg2+ coordination are conserved in TRPM7α-kinase and classical kinases, including (in mouse TRPM7) K1646, D1765, Q1767 and D1775 (Fig. 1.4b).
Fig. 1.4.

Crystal structure of the TRPM7 α-kinase domain. (a) The dimeric structure of the TRPM7 α-kinase domain (PDB code 1IA9). (b) and (c) Comparison of the TRPM7 α-kinase domain (b, PDB code 1IA9) and PKA kinase domain (c, PDB code 1CDK). Regions and residues critical for catalysis are shown; they include the P-loop and the GXA(G)XXG motif in the TRPM7 α-kinase domain (b), and the P-loop, activation loop and catalytic loop in the PKA kinase domain (c) A bound zinc ion is present in the TRPM7 α-kinase domain, and a bound AMP-PNP, an ATP analog, is present in both structures
There are, however, notable differences in the structural details of TRPM7α-kinase and classical kinases. For example, different sets of residues are engaged in contacts with the base and sugar moieties of ATP. As the ATP-binding pocket is an ideal drug target, the intricate differences between the structure of the catalytic site of TRPM7α-kinase and classical kinases might be exploited to develop kinase-specific drugs. Furthermore, in TRPM7α-kinase, the GXA(G)XXG motif replaces the so-called activation loop present in classical kinases (Fig. 1.4), which participates in the recognition of peptide substrates. This difference may underlie, at least partly, the substrate specificity of α-kinases and classical kinases. Finally, the C-lobe of TRPM7α-kinase contains a zinc-binding module (Fig. 1.4a). Zinc coordination maybe important for the structural stability of the kinase domain.
The function of the TRPM7α-kinase domain remains controversial and incompletely understood. Initially, it was reported that replacing the final glycine in the GXA(G)XXG motif severely reduced the kinase activity and channel activity [28]. Likewise, mutating two cysteines in the zinc-binding module produced the same effect [28]. It was subsequently shown that TRPM7 channels remained active when its kinase activity was abolished by point mutations in the α-kinase domain or when the entire α-kinase domain was deleted [29, 30]. On the other hand, point mutations in the nucleotide binding pocket greatly reduced Mg2+- and Mg·ATP-induced suppression of channel activity [29, 30]. Thus, it appears that the α-kinase domain is not essential for TRPM7 channel activation; instead, it contributes to channel regulation by Mg2+ and Mg·ATP. Currently, only a very small number of TRPM7α-kinase substrates are known, including annexin 1 and myosin II [25]. An interesting hypothesis was put forth that the opening of TRPM7 channels affects the activity of its α-kinase, thereby affecting the activity of its substrates [25]; however, this remains to be tested.
1.4.2 TRPV Ankyrin Repeats
Ankyrin repeat is one of the most common amino acid motifs and is present in numerous proteins with diverse structures and functions [4, 31, 32]. It consists of 30–34 amino acids folded into a characteristic helix-turn-helix conformation, with the two helices arranged antiparallely. In most proteins, a string of ankyrin repeats, numbering from 2 to ~30, are stacked in an array. These ankyrin repeat domains (ARDs) are generally thought to play a critical role in mediating protein–protein interactions [4, 31, 32].
The N termini of TRPA, TRPC, TRPN and TRPV contain 4 to ~30 tandem copies of ankyrin repeats. The ARDs in TRPV are highly conserved [6] and functionally important, as their deletion impairs channel activation, assembly or trafficking to the plasma membrane [33–36]. The structure of the ARD of TRPV1, TRPV2, TRPV4 and TRPV6 has been determined by X-ray crystallography at 1.6–2.7 Å resolution [37–41]. All four ARDs contain 6 ankyrin repeats and have highly similar structures (Fig. 1.5a). Each ankyrin repeat consists of 2 antiparallel α helices (named the inner and outer helix, respectively), followed by a hairpin loop (named the finger). The inner helices and fingers form a concave surface, while the outer helices form a convex surface. These surfaces likely constitute interfaces for specific protein–protein or protein–ligand interactions. The amino acid sequence and length of the finger vary among the ankyrin repeats within each ARD and among the ARDs of different TRPV subunits [6]. These differences are evident in the overlay of the 4 TRPV-ARD structures (Fig. 1.5a) and likely contribute to channel-specific functions of each ARD.
Fig. 1.5.
Crystal structures of TRPV ankyrin repeat domains (ARDs). (a) Superposition of the structures of the ARD of TRPV1 (PDB code 2PNN), TRPV2(PDB code 2ETB), TRPV4 (PDB code 3JXI), and TRPV6 (PDB code 2RFA). AR, ankyrin repeat. (b) Structure of the TRPV1-ARD with a bound ATP molecule (red). Side-chains of residues involved in ATP binding are marked in blue. (c) Structure of the TRPV4-ARD, showing the position of the residues that cause diseases when mutated. Residues numbers correspond to human TRPV4
The structure of the TRPV1-ARD unexpectedly revealed a bound ATP molecule, which is cradled in the concave surface of ankyrin repeats 1–3 and interacts with residues in inner helices 1–3 and fingers 1–2 (Fig. 1.5b) [39]. Biochemical studies indicate that Ca2+-calmodulin (Ca2+-CaM) also binds the TRPV1-ARD. Moreover, mutations that eliminate ATP binding also prevent Ca2+-CaM binding. ATP binding to the ARD sensitizes TRPV1 channels to capsaicin (an agonist) and greatly attenuates tachyphylaxis – decreasing responses to repeated applications of cap-saicin. Conversely, Ca2+-CaM binding to the ARD appears to play an opposing role and to be necessary for tachyphylaxis. These findings provide a structural basis for understanding the regulation of TRPV1 channels by Ca2+, CaM and ATP [39]. Whether and to what extent ATP or Ca2+-CaM binding alters the structure of the TRPV1-ARD remains to be investigated.
Amino acid sequence comparison and biochemical studies show that the ATP-and Ca2+-CaM-binding site in the TRPV1-ARD is also present in TRPV3 and TRPV4, but not in TRPV2, TRPV5 and TRPV6 [42]. In accord with these observations, TRPV2 is insensitive to intracellular ATP, while TRPV4 is sensitized by intracellular ATP and this sensitization is eliminated by a binding site mutation. A twist is that the response of TRPV3 to agonists is reduced by intracellular ATP and Ca2+-CaM through their interaction with the ARD [42]. Why ATP and Ca2+-CaM binding to the ARD produce different modulatory effects on TRPV3 vs. TRPV1 and TRPV4 is unclear, but this example illustrates the diversified functions of the ARD.
Recently, a number of mutations in TRPV4 has been linked to several human disorders, including spondylometaphyseal dysplasia (SMD) Kozlowski type (SMDK), metatropic dysplasia, scapuloperoneal spinal muscular atrophy (SPSMA), and Charcot–Marie–Tooth disease type 2C (CMT2C) [43]. Patients with SMDK and nonlethal metatropic dysplasia are characterized by defects in bone development, scoliosis and short stature, and those with SPSMA and CMT2C by distal and proximal muscle weakness and sensory loss. A mutation in the fifth ankyrin repeat was linked to SMDK [44]. This mutation, D333G (Fig. 1.5c), results in increased basal channel activity and increased response to the agonist 4αPDD [44]. Another mutation in the fifth ankyrin repeat, I331F, was tentatively associated with metatropic dysplasia, and its effect on TRPV4 channel activity remains to be determined [44]. Four missense mutations in the third and fourth ankyrin repeats, including R269H, R269C, R315W and R316C (Fig. 1.5c), were linked to SPSMA and CMT2C [38, 45, 46]. The effect of these mutations on TRPV4 channel activity is yet unclear, as both an increase in channel activity [38, 46] and a decrease in channel activity/surface expression [45] in heterologous systems have been reported. It is notable that all the disease-related residues are located on the concave surface formed by the inner helices and fingers. Presumably, the disease-causing mutations alter the interactions of TRPV4 with other proteins or ligands.
An intriguing proposed function of ARDs in TRP channels is that they act as the gating spring of mechanoreceptors [47, 48]. Studies based on molecular dynamics simulations [48] and single molecule measurements by atomic force microscopy [49] suggest that ARDs containing 17–24 ankyrin repeats exhibit proper elastic properties (including stiffness and extension length) that make them fit for mechanotransduction. In this regard, it is of interest to note that several TRP channels with ARDs, including TRPA1, TRPN1 and TRPV4, have been implicated or proposed in a variety of mechanosensory processes in various organisms [50].
1.4.3 TRPM7 Coiled Coil Domain
Coiled coil domains are also one of the most common amino acid motifs found in disparate proteins. They play an important role in the assembly of homomeric and heteromeric protein complexes. Coiled coils are comprised of stretches of α helices with multiple tandem copies of typically heptad (7-residue) repeats. They assemble to form oligomeric complexes, usually containing 2–5 helices, and both homomeric and heteromeric assemblies occur. The α helices in a coiled coil bundle can be parallel (i.e., with the same N and C orientation) or antiparallel (i.e., with opposite N and C orientation). The residues within the heptad, routinely designated as a through g, have characteristic features: the first and fourth residues (i.e., a and d positions) are usually non-polar (commonly Leu, Ile, Val, Phe, and Trp) and project inward to the core of the coiled coil complex; whereas the fifth and seventh residues (i.e., e and g positions) are usually charged and project outward to the outside of the coiled coil complex. The nature of the residues at a, d, e and g positions influences coiled coil stability, oligomeric state, partner selection, and helix–helix orientation, but definitive predictions on coiled coil assembly are difficult based solely on amino acid sequences. Comprehensive discussions on coiled coil structure and design can be found in recent reviews [51–54].
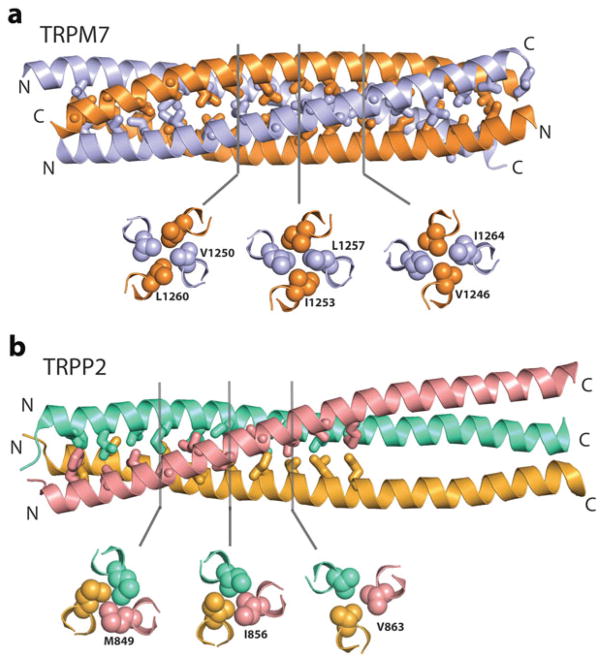
Coiled coil domains are predicted to be present in the cytoplasmic N and/or C termini of TRPC, TRPM, TRPP and TRPV channels and have been reported to be important for channel assembly and function [6, 55, 56]. The crystal structures of a TRPM7 and a TRPP2 C-terminal coiled coil domain have been determined at 2.8 and 1.9 Å resolution, respectively [57, 58]. In agreement with the tetrameric architecture of the full length channel, the TRPM7 coiled coil domain forms a tetramer (Fig. 1.6a). As in other coiled coil complexes, residues at the a and d positions project inward to the core of the complex and engage in extensive hydrophobic interactions. Unexpectedly, the 4 α helices are arranged antiparallely, deviating from the fourfold symmetry of the transmembrane regions. Whether these helices are also arranged in this fashion in the full length channel remains to be seen, but this arrangement fits the dimeric association of the α-kinase domain located ~230 amino acids downstream. Moreover, the bridging sequence of ~100 residues between the end of the last transmembrane segment and the coiled coil domain is sufficiently long to allow the latter to adopt a twofold symmetry. A similar symmetry mismatch has been observed in a crystal structure of a homotetrameric glutamate-gated receptor channel, where the ion channel domain exhibits a fourfold symmetry, whereas the extracellular ligand-binding domain displays a twofold symmetry [59]. As cautioned in a recent review [5], such a symmetry break complicates reconstruction of protein EM structures, where symmetry averaging is routinely used to improve resolution.
Fig. 1.6.
Comparison of the crystal structure of a C-terminal coiled coil domain of TRPM7 (a, PDB code 3E7K) and TRPP2 (b, PDB code 3HRN). Side-chains of residues at the a and d positions of the heptad repeats are shown. Three cross sections, taken at the indicated positions, shows examples of the extensive van der Waals interactions among these residues
The importance of the coiled coil domain of TRPM7 in its assembly and function is not yet clear; meanwhile, deletion and mutagenesis studies on the coiled coil domains of TRPM2, TRPM4 and TRPM8, whose structures have not yet been determined, have produced varied results. In TRPM2, deleting the coiled coil domain or mutating residues at the a and d positions greatly reduced channel assembly and trafficking [60]. In TRPM4, which can be activated by depolarization and intracellular Ca2+, truncation of the coiled coil domain decreased the sensitivity of the channels to Ca2+ and shifted the activation voltage to very positive potentials, but the mutant channels were still able to assemble and traffic properly [61]. In the case of TRPM8, one group reported that deleting the coiled coil domain severely suppressed channel activity but did not compromise assembly and trafficking [62], while another group reported that this deletion prevented TRPM8 surface expression [63]. It was also reported that a single mutation of a key a position residue, L1089P, in the coiled coil domain disrupted channel assembly [64]. Moreover, the coiled coil domain worked as a dominant negative when fused to a transmembrane segment and coexpressed with wild-type TRPM8 [63, 64]. The causes underlying the disparate findings on the function of the TRPM8 coiled coil domain are unclear, but the use of different expression systems and different cell culture temperatures are possible factors [62].
1.4.4 TRPP2 Coiled Coil Domain
TRPP2 forms homomeric channels and also interacts with members of other TRP subfamilies to form heteromeric channels, including TRPV4 and TRPC1 [65]. Furthermore, it associates with PKD1 to form a Ca2+-permeable receptor/ion channel complex that is critical for kidney development and function [65–67]. PKD1 is a member of the polycystic kidney disease family of proteins. It contains 4,302 amino acids, with 11 putative transmembrane segments and a large N-terminus, which harbors several well-recognized domains and motifs involved in protein–protein and protein–carbohydrate interactions; hence, it is generally regarded as a membrane receptor and/or a mechanotransducer. TRPP2 and PKD1 assemble through the direct association of a coiled coil domain in the C terminus of both proteins [65–67]. Mutations in TRPP2 and PKD1 account for the vast majority of autosomal dominant polycystic kidney disease (ADPKD), one of the most common inherited human diseases [66, 67].
A recent study using single channel recording and atomic force microscopy imaging reported that homomeric TRPP2 channels incorporated into lipid bilayers were tetramers [68]. However, another study based on biochemical and single molecule photobleaching experiments reported that TRPP2 tended to form trimers when heterologously expressed in HEK 293 cells and Xenopus oocytes [58], suggesting that the association of the fourth TRPP2 subunit is weaker and that other pore-forming subunits may substitute TRPP2 to form heteromeric complexes. Biochemical studies also indicated that the TRPP2 C terminus formed a trimer complex, so did a coiled coil domain (aa 839–873) within the C terminus [58]. Consistent with these findings, the crystal structure of a TRPP2 C-terminal fragment (G833–G895) encompassing the coiled coil domain shows a 3-stranded complex (Fig. 1.6b). The 3 α helices are arranged in parallel, in accord with the propensity that opposite charges at the e and g positions favor such an orientation.
Single molecule photobleaching showed that the full length PKD1/TRPP2 complex contained 1 PKD1 and 3 TRPP2 [58]. This stoichiometry was preserved in solution for complexes formed by the coiled coil domain of both proteins and by longer C-terminal fragments. The structure of the PKD1/TRPP2 coiled coil domain complex awaits further elucidation.
Mutagenesis studies guided by the structure of the TRPP2 coiled coil domain showed that trimerization of this domain was critical for the assembly and membrane trafficking of homomeric TRPP2 trimer complexes and heteromeric PKD1/TRPP2 complexes [58]. Many ADPKD-causing mutations, including L4224P and R4227X in PKD1 and R742X, R807X, E837X, and R872X in TRPP2 [69], either alter the structure of or altogether delete the coiled coil domains. These mutations further underscore the functional importance of the TRPP2 and PKD1 coiled coil domains.
1.4.5 TRPP2 C-terminal E-F Hand
The EF-hand constitutes yet another one of the most ubiquitous amino acid motifs. It binds Ca2+ and through this binding regulates the activity of proteins with various structures and functions. Structurally, it is defined by a hallmark helix-loop-helix fold and it often exists in pairs [70]. In the so-called canonical EF-hand, Ca2+ is coordinated by 7 residues, which are usually conserved and 5 of them are located in the 9-residue loop. Ca2+ coordination by non-canonical (atypical) EF-hands is more diversified, as the length of the loop and the number and nature of the coordinating residues are variable. The affinity of EF-hands for Ca2+ ranges from nanomolar to millimolar [70].
The activities of many TRP channels are regulated by intracellular Ca2+ [2]. This regulation could be mediated by Ca2+-binding proteins such as CaM or by the direct binding of Ca2+ to the channels. In this regard, it is of interest to note that several TRP channels, including TRPA1, TRPML1 and TRPP2, contain EF-hands in their cytoplasmic regions. TRPP2 channels are permeable to Ca2+ [65], and physiological concentrations of intracellular Ca2+ have been shown to regulate, in a bell-shaped concentration-dependent manner, the activity of TRPP2 channels incorporated into lipid bilayers [71, 72]. Molecular modeling predicted an EF-hand in the C-terminal region from amino acid 720–797 and yielded a structural model similar to the canonical EF-hand [73]. Subsequently, a solution structure of a slightly shorter fragment (aa 724–796) was determined by NMR [74]. This structure shows that, instead of a single EF-hand, this region contains a pair of EF-hands, one atypical and one canonical, both of which are proposed to bind Ca2+ [74]. However, the validity of this structure has been strongly challenged by a recent study that presents a vastly different NMR structure of the 720–797 fragment [75]. This new structure reveals a single E-F hand motif consisting of the helix α3, Ca2+-binding loop, and helix α4 (Fig. 1.7). Paired with this E-F hand is a non-Ca2+-binding helix-loop-helix motif comprising helices α1 and α2; this motif may have evolved from a canonical E-F hand. The 720–797 region binds Ca2+ noncooperatively at a single site (Kd ~214 μM) and undergoes Ca2+-dependent conformational changes [73], and it shows conformational fluctuations even in the Ca2+-bound state [75]. These properties make this region a prime candidate as a Ca2+-sensitive regulator. However, whether and how Ca2+ binding to this region affect TRPP2 channel assembly and function remains to be investigated. The new structure would be very useful for guiding precision mutagenesis experiments.
Fig. 1.7.
NMR structure of an E-F hand in the TRPP2 C terminus. Side-chains of residues involved in Ca2+ binding are indicated. PDB code 2KQ6
It was reported that, in the absence of Ca2+, a TRPP2 C-terminal fragment (aa 680–796) harboring the E-F hand and the non-canonical helix-loop-helix motif forms a dimer, which dissociates upon Ca2+ binding [74]. In contrast, other studies reported that the 720–797 fragment was a monomer either in the absence or presence of Ca2+ [73, 75]. Furthermore, the reported dimeric association of the 680–796 fragment is at variance with the trimeric association of not only the downstream coiled coil domain (aa 839–873) but also a fragment (aa 723–928) encompassing both the EF-hand and the coiled coil domain [58]. Further studies are needed to reconcile this difference.
1.5 Perspectives
Structural study of TRP channels is still in its infancy. However, the few available structures have offered unique mechanistic insights on TRP channel assembly and regulation that would be otherwise difficult to obtain. As X-ray crystallography is increasingly becoming an integral part of the research arsenal in more and more research groups, crystal structures of many more TRP channel fragments will surely be solved in the coming years. It is useful to bear in mind that although protein fragments are easier to express and purify than full length proteins, they are still sometimes difficult to crystallize. In those instances, NMR would be an ideal alternative approach. Nevertheless, although structures of channel fragments undoubtedly enhance our understanding of TRP channel functions, as illustrated above, ultimately high resolution structures of full length channels are needed. With constant technical advances in membrane protein expression, screening, purification, crystallization and data processing, it is hopeful that the goal of solving high resolution structures of full length TRP channels will be achieved in the not too distant future.
Acknowledgments
We thank Kathryn Abele, Ioannis Michailidis and Zafir Buraei for reading and commenting on a draft of this chapter. This work was supported by National Institutes of Health grants NS045383 and GM085234 (to J.Y.).
References
- 1.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 2.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudet R. TRP channels entering the structural era. J Physiol. 2008;586:3565–3575. doi: 10.1113/jphysiol.2008.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst. 2008;4:372–379. doi: 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudet R. Divide and conquer: high resolution structural information on TRP channel fragments. J Gen Physiol. 2009;133:231–237. doi: 10.1085/jgp.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latorre R, Zaelzer C, Brauchi S. Structure-functional intimacies of transient receptor potential channels. Q Rev Biophys. 2009;42:201–246. doi: 10.1017/S0033583509990072. [DOI] [PubMed] [Google Scholar]
- 7.Moiseenkova-Bell VY, Wensel TG. Hot on the trail of TRP channel structure. J Gen Physiol. 2009;133:239–244. doi: 10.1085/jgp.200810123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blow D. Outline of crystallography for biologists. Oxford University Press; Oxford: 2002. [Google Scholar]
- 9.Jonic S, Venien-Bryan C. Protein structure determination by electron cryomicroscopy. Curr Opin Pharmacol. 2009;9:636–642. doi: 10.1016/j.coph.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Chiu W, Baker ML, Jiang W, Dougherty M, Schmid MF. Electron cryomicroscopy of biological machines at subnanometer resolution. Structure. 2005;13:363–372. doi: 10.1016/j.str.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Frank J. Single-particle reconstruction of biological macromolecules in electron microscopy – 30 years. Q Rev Biophys. 2009;42:139–158. doi: 10.1017/S0033583509990059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cong Y, Baker ML, Jakana J, Woolford D, Miller EJ, Reissmann S, Kumar RN, Redding-Johanson AM, Batth TS, Mukhopadhyay A, Ludtke SJ, Frydman J, Chiu W. 4.0-Å resolution cryo-EM structure of the mammalian chaperonin TRiC/CCT reveals its unique subunit arrangement. Proc Natl Acad Sci USA. 2010;107:4967–4972. doi: 10.1073/pnas.0913774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster MP, McElroy CA, Amero CD. Solution NMR of large molecules and assemblies. Biochemistry. 2007;46:331–340. doi: 10.1021/bi0621314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott A. Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys. 2009;38:385–403. doi: 10.1146/annurev.biophys.050708.133719. [DOI] [PubMed] [Google Scholar]
- 16.Linke D. Detergents: an overview. Methods Enzymol. 2009;463:603–617. doi: 10.1016/S0076-6879(09)63034-2. [DOI] [PubMed] [Google Scholar]
- 17.Newby ZE, O‘Connell JD, 3rd, Gruswitz F, Hays FA, Harries WE, Harwood IM, Ho JD, Lee JK, Savage DF, Miercke LJ, Stroud RM. A general protocol for the crystallization of membrane proteins for X-ray structural investigation. Nat Protoc. 2009;4:619–637. doi: 10.1038/nprot.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannen RM, Bingman CA, Phillips GN., Jr Effect of low-complexity regions on protein structure determination. J Struct Funct Genomics. 2007;8:217–226. doi: 10.1007/s10969-008-9039-6. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama Y, Ogura T, Mio K, Kiyonaka S, Kato K, Mori Y, Sato C. Three-dimensional reconstruction using transmission electron microscopy reveals a swollen, bell-shaped structure of transient receptor potential melastatin type 2 cation channel. J Biol Chem. 2007;282:36961–36970. doi: 10.1074/jbc.M705694200. [DOI] [PubMed] [Google Scholar]
- 20.Mio K, Ogura T, Kiyonaka S, Hiroaki Y, Tanimura Y, Fujiyoshi Y, Mori Y, Sato C. The TRPC3 channel has a large internal chamber surrounded by signal sensing antennas. J Mol Biol. 2007;367:373–383. doi: 10.1016/j.jmb.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci USA. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigematsu H, Sokabe T, Danev R, Tominaga M, Nagayama KA. 3.5-nm structure of rat TRPV4 cation channel revealed by Zernike phase-contrast cryoelectron microscopy. J Biol Chem. 2010;285:11210–11218. doi: 10.1074/jbc.M109.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo-and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middelbeek J, Clark K, Venselaar H, Huynen MA, van Leeuwen FN. The alpha-kinase family: an exceptional branch on the protein kinase tree. Cell Mol Life Sci. 2010;67:875–890. doi: 10.1007/s00018-009-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinna LA, Ruzzene M. How do protein kinases recognize their substrates? Biochim Biophys Acta. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell. 2001;7:1047–1057. doi: 10.1016/s1097-2765(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 28.Runnels LW, Yue L, Clapham DE. TRP-PLIKa bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 30.Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006;127:421–434. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Mahajan A, Tsai MD. Ankyrin repeat: a unique motif mediating protein–protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 32.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, Hoenderop JG. Molecular determinants in TRPV5 channel assembly. J Biol Chem. 2004;279:54304–54311. doi: 10.1074/jbc.M406222200. [DOI] [PubMed] [Google Scholar]
- 34.Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem. 2004;279:34456–34463. doi: 10.1074/jbc.M404778200. [DOI] [PubMed] [Google Scholar]
- 35.Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- 36.Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- 37.Jin X, Touhey J, Gaudet R. Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- 38.Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, Choo SS, Phelps CB, Paudel R, Houlden H, Ludlow CL, Caterina MJ, Gaudet R, Kleta R, Fischbeck KH, Sumner CJ. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet. 2010;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 40.McCleverty CJ, Koesema E, Patapoutian A, Lesley SA, Kreusch A. Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Sci. 2006;15:2201–2206. doi: 10.1110/ps.062357206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R. Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry. 2008;47:2476–2484. doi: 10.1021/bi702109w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285:731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers ArchChem. 2010;460:437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- 44.Krakow D, Vriens J, Camacho N, Luong P, Deixler H, Funari TL, Bacino CA, Irons MB, Holm IA, Sadler L, Okenfuss EB, Janssens A, Voets T, Rimoin DL, Lachman RS, Nilius B, Cohn DH. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet. 2009;84:307–315. doi: 10.1016/j.ajhg.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auer-Grumbach M, Olschewski A, Papic L, Kremer H, McEntagart ME, Uhrig S, Fischer C, Frohlich E, Balint Z, Tang B, Strohmaier H, Lochmuller H, Schlotter-Weigel B, Senderek J, Krebs A, Dick KJ, Petty R, Longman C, Anderson NE, Padberg GW, Schelhaas HJ, van Ravenswaaij-Arts CM, Pieber TR, Crosby AH, Guelly C. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42:160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng HX, Klein CJ, Yan J, Shi Y, Wu Y, Fecto F, Yau HJ, Yang Y, Zhai H, Siddique N, Hedley-Whyte ET, Delong R, Martina M, Dyck PJ, Siddique T. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet. 2010;42:165–169. doi: 10.1038/ng.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 48.Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behaviour of ankyrin repeats. Nature. 2006;440:246–249. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- 50.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 51.Lupas AN, Gruber M. The structure of α-helical coiled coils. Adv Protein Chem. 2005;70:37–78. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 52.Parry DA, Fraser RD, Squire JM. Fifty years of coiled-coils and α-helical bundles: a close relationship between sequence and structure. J Struct Biol. 2008;163:258–269. doi: 10.1016/j.jsb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Woolfson DN. The design of coiled-coil structures and assemblies. Adv Protein Chem. 2005;70:79–112. doi: 10.1016/S0065-3233(05)70004-8. [DOI] [PubMed] [Google Scholar]
- 54.Grigoryan G, Keating AE. Structural specificity in coiled–coil interactions. Curr Opin Struct Biol. 2008;18:477–483. doi: 10.1016/j.sbi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lepage PK, Boulay G. Molecular determinants of TRP channel assembly. Biochem Soc Trans. 2007;35:81–83. doi: 10.1042/BST0350081. [DOI] [PubMed] [Google Scholar]
- 56.Schindl R, Romanin C. Assembly domains in TRP channels. Biochem Soc Trans. 2007;35:84–85. doi: 10.1042/BST0350084. [DOI] [PubMed] [Google Scholar]
- 57.Fujiwara Y, Minor DL., Jr X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol. 2008;383:854–870. doi: 10.1016/j.jmb.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, Tong L, Isacoff EY, Yang J. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci USA. 2009;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mei ZZ, Xia R, Beech DJ, Jiang LH. Intracellular coiled-coil domain engaged in subunit interaction and assembly of melastatin-related transient receptor potential channel 2. J Biol Chem. 2006;281:38748–38756. doi: 10.1074/jbc.M607591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 62.Phelps CB, Gaudet R. The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J Biol Chem. 2007;282:36474–36480. doi: 10.1074/jbc.M707205200. [DOI] [PubMed] [Google Scholar]
- 63.Tsuruda PR, Julius D, Minor DL., Jr Coiled coils direct assembly of a cold-activated TRP channel. Neuron. 2006;51:201–212. doi: 10.1016/j.neuron.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem. 2006;281:38396–38404. doi: 10.1074/jbc.M607756200. [DOI] [PubMed] [Google Scholar]
- 65.Tsiokas L. Function and regulation of TRPP2 at the plasma membrane. Am J Physiol Renal Physiol. 2009;297:F1–F9. doi: 10.1152/ajprenal.90277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang P, Luo Y, Chasan B, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, del Cantero MR, Ramos AJ, Goldmann WH, Zhou J, Cantiello HF. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet. 2009;18:1238–1251. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu G, Tian X, Nishimura S, Markowitz GS, D‘Agati V, Park JH, Yao L, Li L, Geng L, Zhao H, Edelmann W, Somlo S. Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet. 2002;11:1845–1854. doi: 10.1093/hmg/11.16.1845. [DOI] [PubMed] [Google Scholar]
- 70.Gifford JL, Walsh MP, Vogel HJ. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 71.Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem. 2004;279:19987–19995. doi: 10.1074/jbc.M312031200. [DOI] [PubMed] [Google Scholar]
- 72.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 73.Celic A, Petri ET, Demeler B, Ehrlich BE, Boggon TJ. Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem. 2008;283:28305–28312. doi: 10.1074/jbc.M802743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schumann F, Hoffmeister H, Bader R, Schmidt M, Witzgall R, Kalbitzer HR. Ca2+-dependent conformational changes in a C-terminal cytosolic domain of polycystin-2. J Biol Chem. 2009;284:24372–24383. doi: 10.1074/jbc.M109.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petri ET, Celic A, Kennedy SD, Ehrlich BE, Boggon TJ, Hodsdon ME. Structure of the EF-hand domain of polycystin-2 suggests a mechanism for Ca2+-dependent regulation of polycystin-2 channel activity. Proc Natl Acad Sci USA. 2010;107:9176–9181. doi: 10.1073/pnas.0912295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sokolova O, Kolmakova-Partensky L, Grigorieff N. Three-dimensional structure of a voltage-gated potassium channel at 2.5 nm resolution. Structure. 2001;9:215–220. doi: 10.1016/s0969-2126(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 77.Walz T, Hirai T, Murata K, Heymann JB, Mitsuoka K, Fujiyoshi Y, Smith BL, Agre P, Engel A. The three-dimensional structure of aquaporin-1. Nature. 1997;387:624–627. doi: 10.1038/42512. [DOI] [PubMed] [Google Scholar]
- 78.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 79.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 80.Wootton JC, Federhen S. Statistics of local complexity in amino acid sequences and sequence databases. Comput Chem. 1993;17:149–163. [Google Scholar]