Abstract
Acute fibrinous and organizing pneumonia (AFOP) is a very rare pathological entity of lung injury characterized by intra-alveolar fibrin balls.
Hemeoxygenase (HO) -1 is a cytoprotective enzyme against oxidative stress and inflammation. It is known to be expressed in the alveolar macrophages in the healthy adults and overexpressed in other various lung cells of the lung injury patients.
We experienced two cases of subacute form AFOP for these 10 years and reviewed clinico-pathological characteristics. The average age was 62 years old and both were male. The etiology of both cases was idiopathic. The average PaO2/FIO2 ratio was 274.5 ± 84.1. The average levels of C-reactive protein and surfactant protein - A of the serum were elevated to 19.8 ± 6.3 mg/dL and 67.6 ± 15.8 ng/mL, respectively. Serum sialylated carbohydrate antigen levels were normal in both cases. The characteristic radiographic findings were bilateral consolidations and ground glass opacities. Lung biopsy specimens revealed fibrin balls and alveolitis with abundant cellular HO-1 expression. Steroid response was excellent and the pulmonary involvements absolutely disappeared for about 3 months.
Keywords: Acute fibrinous and organizing pneumonia, Corticosteroid, Diffuse alveolar damage, Fibrin balls, Hemeoxygenase-1, Organizing pneumonia, Pulmonary cellular protection
Introduction
Acute fibrinous and organizing pneumonia (AFOP), first described by Beasley, is a very rare histological entity, which is characterized by intra-alveolar deposit of fibrin forming fibrin balls and organizing pneumonia (OP) with patchy distribution without hyaline membrane, eosinophilic infiltrations, extensive abscess formation and granulomatous inflammation [1]. The histological pattern of AFOP is known to be the variant of diffuse alveolar damage (DAD) and OP. AFOP may be idiopathic in nature or associated with infection (Haemophilus influenza, Pneumocystis jiroveci and Chlamydia Pneumoniae and HIV infection), connective tissue disorders (systemic lupus erythematosus and undifferentiated connective tissue disease), drug exposure (avacavir, amiodarone and decitabine), and hematological malignancies (myelodysplastic syndrome and lymphoma). Beasley et al. described two distinct patterns of disease progression: acute form with rapid progression to death or subacute form with recovery [1]. In the literature review of 29 AFOP cases, 19 cases were subacute form and acute form was more critical than subacute form [2]. Beasley et al. noted that 9 out of 17 AFOP cases were dead of the disease and the time from the presentation of symptoms to the death was 6–36 days (average 29 days).
HO-1, 32 kDa heat shock protein, is a cytoprotective enzyme playing a central role in the defense against oxidative stress and inflammation in the lung catalyzing the degradation of heme into biliverdin, iron, and carbon monoxide [3]. In the healthy lung, HO-1 is expressed mainly in the alveolar macrophages [4]. On the injuried lung, HO-1 is known to limit the disease progression potentially by the HO-1 overexpression in the alveolar macrophages, epithelial cells, endothelial cells and fibroblasts, reflecting pulmonary cellular protective reaction [5].
We experienced the rare two cases of subacute form AFOP and examined these clinical characteristics (etiology, serum biomarkers including surfactant protein (SP) -A, SP-D and sialylated carbohydrate antigen (KL-6) that were widely used as the biomarkers for interstitial lung diseases (ILD), radiographic findings and treatment outcome) (case 2 has been already published [6]). Moreover, we assessed the pathophysiology of these AFOP cases using hematoxylin-eosin and HO-1 immunohistichemical staining using monoclonal anti-HO-1 antibody (abcam, Tokyo, Japan; dilution 1:100). This study were approved by the institutional review board of National Defense Medical College.
Case reports
Case 1
A 70-year-old man had complained of fever and dry cough for one week. The chest radiograph at his first visit showed right upper lobe opacity. He developed dyspnea and was admitted to our hospital one week later with tachypnea and fever. Fine crackles were heard in the right chest. White blood cell counts (WBC) was 18700/μL (normal: 4000–8000/μL), C-reactive protein (CRP) was 24.2 mg/dL (normal: ≦0.3 mg/dL). Brain natriuretic peptide, antineutrophil cytoplasmic antibodies (ANCA) and antinuclear antibody (ANA) were normal. Urine antigen testing for Legionella and Streptococcus pneumoniae was negative. Blood gas analysis (BGA) showed hypoxemia with pH 7.43, PaCO2 34 mmHg and PaO2 62 mmHg with oxygen supplementation (2 L) by nasal cannula. High-resolution computed tomography (HRCT) showed ground glass opacities (GGO) in the subpleural upper lobe. His symptoms progressed over a week despite oxygen and antibiotics and repeat HRCT showed air space consolidation with GGO in the right upper and middle lobes with some GGO in the left lung as well (Fig. 1A). The bronchoalveolar lavage fluid (BALF) had a total cell count of 8.4 × 105/mL, with 9% macrophages, 16% lymphocytes, 1% eosinophils, 74% neutrophils and CD4/8 ratio of 1.93. Cytomegalovirus and Pneumocystis jeroveci were negative by polymerase chain reaction (PCR). Transbronchial lung biopsy (TBLB) specimens showed numerous fibrin balls and hyperplasia of type II pneumocytes. Eosinophils, hyaline membranes, and hemosiderin-laden macrophages were absent. Immunohistochemistry of biposy specimens using anti-HO-1 antibody confirmed increased HO-1 in the macrophages within fibrin balls and alveolar walls around fibrin balls (Fig. 2A). Methylpredonisolone pulse therapy was given, 1000 mg/day for 3 days, followed by oral prednisolone (0.5 mg/kg). After two weeks, his symptoms and chest radiograph dramatically improved and pulmonary involvements absolutely disappeared after about 3.7 months without relapse.
Fig. 1.
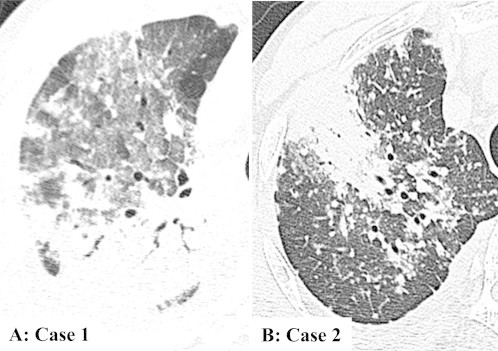
High resolution computed tomography revealed air space consolidation intermingled groung glass opacities in the upper and middle lobes in the right lung (A: case 1) and bilateral patchy consolidation and diffuse small nodules (B: case 2).
Fig. 2.
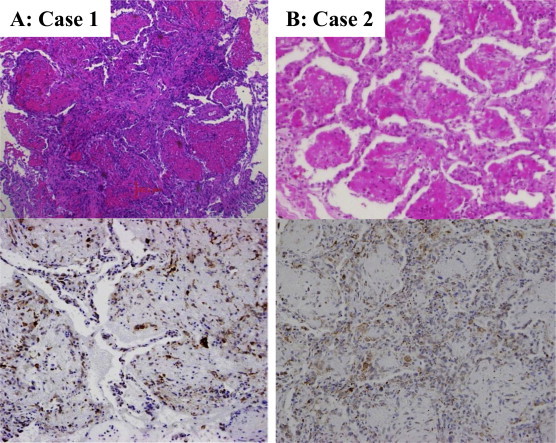
Case 1 (A) was diagnosed by transbronchial lung biopsy and case 2 (B) was by surgical lung biopsy. These specimens revealed the numerous fibrin balls, organizing loose connective tissues and hyperplasia of type II pneumocytes. But the accumulation of eosinophils, the formation of hyaline membrane and hemosiderin-laden macrophages were not visible (hematoxylin-eosin stain, original magnification × 200). Immunohistochemical stain with anti-hemeoxygenase (HO) -1 antibody to the affected lung demonstrated HO-1 overexpression in the macrophages within fibrin balls and type II pneumocytes around fibrin balls (original magnification × 200).
Case 2 [6].
A 55-year-old man had visited a hospital for chronic gromerulonephritis and had been on dialysis for fifteen years. He had complained of cough and dyspnea for two weeks. And the initial chest radiograph revealed a localized nodular lesion. After 3 weeks, the opacities getting worse rapidly and HRCT revealed bilateral patchy consolidation and diffuse small nodules (Fig. 1B). WBC was 4200/μL, CRP was 15.3 mg/dL. ANCA and ANA were normal. Urine antigen testing for Legionella and S. pneumoniae was negative. BGA showed hypoxemia with pH 7.411, PaCO2 34.0 mmHg and PaO2 70.1 mmHg (room air). The BALF had a total cell count of 3.7 × 105/mL, with 93% macrophages, 5% lymphocytes, 2% neutrophils and CD4/8 ratio of 1.94. Cytomegalovirus and P. jeroveci were negative by PCR. Surgical lung biopsy (SLB) specimens revealed numerous fibrin balls and organization with type II pneumocytes hyperplasia without the presence of hyaline membranes, eosinophilic infiltrations and granulomas (Fig. 2B). And HO-1 was overexpressed in the macrophages within fibrin balls and alveolar epithelial cells. Methylpredonisolone pulse therapy was given, 1000 mg/day for 3days, followed by oral prednisolone (0.5 mg/kg). Pulmonary involvements dramatically improved and almost disappeared after 3 months.
Discussion
We experienced the rare two cases of subacute form AFOP for these 10 years and reviewed these clinico-pathological characteristics (Table 1).
Table 1.
The clinico-pathological characteristics of our AFOP cases.
| Characteristic | Case 1 | Case 2 |
|---|---|---|
| Age/Gender | 70yrs/male | 55yrs/male |
| Smoking status | Never smoker | Never smoker |
| Medical history | None | Chronic renal failure |
| Etiology | Idiopathic | Idiopathic |
| Time evolution | 5 weeks | 2 weeks |
| Diagnostic procedure | Transbronchial lung biopsy | Video-assisted thoracoscopic biopsy |
| PaO2/FIO2 | 215 | 334 |
| APACHE II | 12 | 6 |
| CRP (mg/dL) (<0.3) | 24.2 | 15.3 |
| LDH (IU/L) (<225) | 209 | 221 |
| SP-A (ng/mL) (<43.8) | 56.4 | 78.8 |
| SP-D (ng/mL) (<110) | 34.4 | 229 |
| KL-6 (U/mL) (<500) | 343 | 300 |
| The findings of computed tomography | Bilateral air space consolidation and GGO | Bilateral patchy consolidation and diffuse small nodules |
| Pathological findings | Numerous FBs with type II pneumocytes hyperplasia | Numerous FBs and organization with type II pneumocytes hyperplasia |
| Distribution of Hemeoxygenase-1 | FBs and alveolar walls | Alveolar walls dominant |
| Treatment and outcome | Steroid pulse, then taperd for 3 months Without relapse for 3.5 years |
Steroid pulse, then tapered for 3 months Without relapse for 9 years |
APACHE, Acute Physiology and Chronic Health Evaluation; CRP, C-reactive protein; LDH, lactate dehydrogenase; SP, sufactant protein; KL-6, sialylated carbohydrate antigen; GGO, Ground Glass Opacity; FBs, fibrin balls.
The average age was 62.5 years old and both cases were male. Lopez-Cuenca et al. reported in the review of literature of AFOP that the average age was 55.2 years old and male were 19 of 29 cases (66%) [2]. There was no current smoker in our AFOP cases, but the relationship between smoking status and the incidence of AFOP was unclear. The probable etiology of our cases was idiopathic. In the previous reports of AFOP, the most frequent etiology was also idiopathic other than collagen vascular disorders, infection and drug reaction. The average time of presentation of symptoms to starting treatment was 3.4 weeks and all cases were subacute form. Beasley et al., noted that the survival rate of acute form was worse than subacute form [1]. The diagnostic procedure of case 1 and 2 was TBLB, although the most frequent procedure was SLB because of the non-specific reaction adjacent to lesions such as granuloma, abscesses and neoplasm [7]. However, we believe that even if it is impossible to perform SLB due to severe hypoxemia, AFOP should be diagnosed under the careful evaluation of clinical and radiographic information with small biopsy specimens. Actually, the case of AFOP following hematopoietic stem cell transplantation reported by Lee et al. was diagnosed by TBLB [8].
In our two cases, the average PaO2/FIO2 ratio were 274.5 and the average CRP levels were elevated to 19.8 mg/dL. In spite of sever hypoxemia and inflammation, the average acute physiology and chronic health evaluation II score was relatively low and not so critical. We also evaluated serum SP-A, SP-D and KL-6 levels and found that serum SP-A was elevated and serum KL-6 was normal in all three cases. To our knowledge, there were no AFOP case reports which were discussed about serum SP-A levels. According to the report of Takahashi et al., serum SP-A levels elevated due to the accelerated production by type II pneumocytes or destruction of the epithelial-endothelium barrier [9]. And serum KL-6 levels are considered to be associated with increased permeability of alveolar capillary barrier [10] and the regeneration of type II pneumocytes (induced by fibroblast in vitro [11]) [12]. From these, it is suggested that in AFOP patients, serum SP-A levels elevated due to the accelerated production by type II pneumocytes hyperplasia but serum KL-6 levels were normal due to the lack of fibrosis or mild destruction of epithelial-endothelium barrier. In case 1, serum SP-A levels was normalized after steroid therapy (data not shown). However, because serum SP-A is non-specific biomarker for patients with ILD, we need to establish further prospective research whether serum SP-A could become biomarker for patients with AFOP.
Beasley et al. noted that the most common radiographic finding was bilateral patchy infiltrate [1]. The radiographic findings of both two cases revealed bilateral consolidation which were typical findings. When diagnosing of AFOP, we need to evaluate HRCT findings with careful consideration, because it can be difficult to perform SLB under the critical respiratory failure.
AFOP has not any specific treatment. In case 1, steroid therapy was initiated due to the lack of response of antibiotics. In both two cases, steroid pulse therapy was given and then steroid was tapered for 3 months. After the steroid administration, pulmonary involvements absolutely disappeared for a few months. Perhaps, despite of severe hypoxemia and inflammation, the steroid response of subacute form AFOP is good due to the lack of fibrosis and maybe mild destruction of the epithelial-endothelium barrier.
Katzenstein and Beasley classified DAD, AFOP and COP into lung injury pattern [7,13]. And the disease progression of DAD is the most fluminant. HO-1 is a stress-inducible protein, working as a cytoprotective enzyme in the defense against oxidative stress and inflammation in the lung by catalyzing the degradation of heme into biliverdin, iron, and carbon monoxide [3] and is known to limit the progression of lung injury potentially [14]. We evaluated the pulmonary cellular HO-1 expression of our AFOP, COP and DAD cases (COP: 3 cases diagnosed by SLB, DAD: single autopsy case) using the immunohistochemical staining with HO-1 and found that in the AFOP lung, HO-1 was overexpressed mainly in the macrophages within fibrin balls and type II pneumocytes, while in the healthy lung, HO-1 mainly expressed in the alveolar macrophages, and the pulmonary HO-1 expression was apparent from in DAD lung to in COP lung in order (Supplementary data). So it implies that the degree of HO-1 expression in the lung might reflect the severity of lung injury and the pulmonary cellular HO-1 overexpression might contribute to limit the progression of lung injury potentially. Furthermore, in case 1, serum and BALF HO-1 levels on admission measured using enzyme-linked immunosorbent assay method were 4.1 ng/mL and 10.3 ng/mL, respectively and decreased after two weeks to 1.2 ng/mL in serum and undetectable level in BALF (for discussion – previously reported normal range of serum HO-1 is 2.75 ± 0.22 ng/mL (Age: 71.6 ± 1.0 yr) [15]). These suggested that serum and BALF HO-1 levels might reflect pulmonary cellular HO-1 expression in patients with AFOP, however, we need further study about the relationship between severity of lung injury and serum and BALF HO-1 levels.
In conclusion, we examined the clinico-pathological characteristics of subacute form of AFOP cases. The steroid therapy might be the main therapeutic option for subacute form AFOP. Also, we think that the pulmonary overexpression of HO-1 might influence the pathophysiology and clinical outcomes of subacute form AFOP.
This case reports have been presented in American Thoracic Society international conference in May, 2012.
Funding
None.
Acknowledgments
We appreciate Mrs. Yoriko Inoue for her technical assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
The pulmonary HO-1 expression was apparent from in diffuse alveolar damage lung to in cryptogenic organizing pneumonia lung in order (original magnification × 200).
References
- 1.Beasley M.B., Franks T.J., Galvin J.R., Gochuico B., Travis W.D. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126:1064–1070. doi: 10.5858/2002-126-1064-AFAOP. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Cuenca S., Morales-Garcia S., Martin-Hita A., Frutos-Vivar F., Fernandez-Segoviano P., Esteban A. Severe acute respiratory failure secondary to acute fibrinous and organizing pneumonia requiring mechanical ventilation: a case report and literature review. Respir Care. 2012;57:1337–1341. doi: 10.4187/respcare.01452. [DOI] [PubMed] [Google Scholar]
- 3.Choi A.M., Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 4.Lakari E., Pylkas P., Pietarinen-Runtti P., Paakko P., Soini Y., Kinnula V.L. Expression and regulation of hemeoxygenase 1 in healthy human lung and interstitial lung disorders. Hum Pathol. 2001;32:1257–1263. doi: 10.1053/hupa.2001.28937. [DOI] [PubMed] [Google Scholar]
- 5.Mumby S., Upton R.L., Chen Y., Stanford S.J., Quinlan G.J., Nicholson A.G. Lung heme oxygenase-1 is elevated in acute respiratory distress syndrome. Crit Care Med. 2004;32:1130–1135. doi: 10.1097/01.ccm.0000124869.86399.f2. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H., Sugimoto C., Kanoh S., Motoyoshi K., Aida S. Acute fibrinous and organizing pneumonia: initial presentation as a solitary nodule. J Thorac Imaging. 2005;20:291–293. doi: 10.1097/01.rti.0000168600.78213.85. [DOI] [PubMed] [Google Scholar]
- 7.Beasley M.B. The pathologist's approach to acute lung injury. Arch Pathol Lab Med. 2010;134:719–727. doi: 10.5858/134.5.719. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.M., Park J.J., Sung S.H., Kim Y., Lee K.E., Mun Y.C. Acute fibrinous and organizing pneumonia following hematopoietic stem cell transplantation. Korean J Intern Med. 2009;24:156–159. doi: 10.3904/kjim.2009.24.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi H., Kuroki Y., Tanaka H., Saito T., Kurokawa K., Chiba H. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med. 2000;162:258–263. doi: 10.1164/ajrccm.162.1.9903014. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y., Barker E., Daniloff E., Kohno N., Hiwada K., Newman L.S. Pulmonary epithelial cell injury and alveolar-capillary permeability in berylliosis. Am J Respir Crit Care Med. 1997;156:109–115. doi: 10.1164/ajrccm.156.1.9612043. [DOI] [PubMed] [Google Scholar]
- 11.Hirasawa Y., Kohno N., Yokoyama A., Inoue Y., Abe M., Hiwada K. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol. 1997;17:501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- 12.Okada F., Ando Y., Honda K., Tanoue S., Matsumoto S., Mori H. Comparison of pulmonary CT findings and serum KL-6 levels in patients with cryptogenic organizing pneumonia. Br J Radiol. 2009;82:212–218. doi: 10.1259/bjr/72775434. [DOI] [PubMed] [Google Scholar]
- 13.Katzenstein A.L. Acute lung injury patterns: diffuse alveolar damage and bronchiolitis obliterans organizing pneumonia. In: Katzenstein A.L., editor. Katzenstein and Askin's surgical pathology of non-neoplasitic lung disease. 4th ed. WB Saunders; Philadelphia: 2006. pp. 17–49. [Google Scholar]
- 14.Faller S., Hoetzel A. Carbon monoxide in acute lung injury. Curr Pharm Biotechnol. 2012;13:777–786. doi: 10.2174/138920112800399185. [DOI] [PubMed] [Google Scholar]
- 15.Sato T., Takeno T., Honma K., Yamaguchi H., Saito Y., Sasaki T. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am J Respir Crit Care Med. 2006;174:906–914. doi: 10.1164/rccm.200508-1237OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


