Abstract
Powering microbes with electrical energy to produce valuable chemicals such as biofuels has recently gained traction as a biosustainable strategy to reduce our dependence on oil. Microbial electrosynthesis (MES) is one of the bioelectrochemical approaches developed in the last decade that could have critical impact on the current methods of chemical synthesis. MES is a process in which electroautotrophic microbes use electrical current as electron source to reduce CO2 to multicarbon organics. Electricity necessary for MES can be harvested from renewable resources such as solar energy, wind turbine, or wastewater treatment processes. The net outcome is that renewable energy is stored in the covalent bonds of organic compounds synthesized from greenhouse gas. This review will discuss the future of MES and the challenges that lie ahead for its development into a mature technology.
Keywords: microbial electrosynthesis, bioelectrochemical systems, electricity, CO2 reduction, electron transfer mechanisms
Introduction
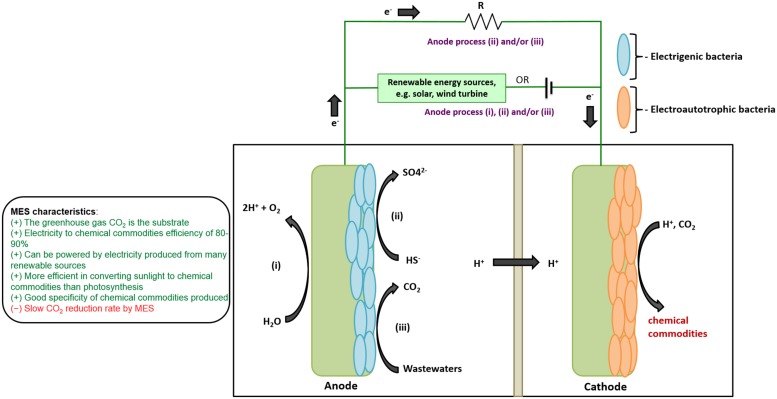
Microbial electrosynthesis (MES) happens when a microbial catalyst reduces CO2 into multicarbon chemical commodities with electrons derived from the cathode of a bioelectrochemical system designed primarily to perform biological reductive reactions (rBES; Rabaey and Rozendal, 2010; Rabaey et al., 2011; Lovley, 2012; Lovley and Nevin, 2013; Wang and Ren, 2013; Hallenbeck et al., 2014; Rosenbaum and Franks, 2014; Figure 1). rBES-driven processes also include electrofermentation, electrorespiration, and electromethanogenesis. Electrofermentation occurs when electrons coming from a cathode are supplied to a fermentative microbial catalyst shifting the fermentation balance toward the production of more reduced products (Rabaey and Rozendal, 2010; Kracke and Krömer, 2014). In the case of electrorespiration, a terminal electron acceptor such as fumarate is reduced by a respiratory microbial catalyst with electrons coming from a cathode (Park et al., 1999; Rabaey and Rozendal, 2010). Electromethanogenesis has similarities with MES since CO2 is the feedstock, but in this case CO2 will be reduced to methane by a methanogenic microbial catalyst using electrons derived from a cathode (Cheng et al., 2009; Villano et al., 2010; Kobayashi et al., 2013).
FIGURE 1.
Principle and flexibility of MES. (i) MES can be coupled with different renewable energy sources such as wind and solar to produce a wide range of chemical commodities. MES can also be coupled to environment-friendly anodic processes such as (ii) sulfide oxidation and (iii) wastewaters treatment.
Besides its capacity of using CO2 directly as feedstock, the two other main qualities of MES are its energetic efficiency and its versatility (Figure 1). The electricity efficiency to chemical commodities of MES processes is ca. 80–90% (Nevin et al., 2010, 2011; Nie et al., 2013; Zhang et al., 2013). Many crop plants have sunlight efficiency to biomass below 3% (MacDonald, 2003), whereas common silicon solar cells are at least six times more efficient at capturing the sun energy (Green et al., 2014). Therefore, powering MES with electricity from solar cells could be a more potent strategy for storing the sun energy into the chemical bonds of multicarbon compounds (Nevin et al., 2010; Lovley and Nevin, 2011).
Microbial electrosynthesis is a versatile technology because the necessary electricity can be generated from multiple renewable sources. Apart from sun energy, MES can be powered with electricity produced by wind turbine. Renewable electricity sources are intermittent by nature and do not harmonize well with the market demand (Jürgensen et al., 2014). In this context, MES becomes a perfect technological fit making possible the direct storage of electricity surplus into value-added chemical commodities (Lovley and Nevin, 2011).
Microbial electrosynthesis can also be coupled with a bioelectrochemical system performing biological oxidation reactions (oBES). Processes driven by oBES are defined by the transfer of electrons from a microbial catalyst metabolizing a given substrate to an electrode collecting electricity. oBESs have been developed for a multitude of applications including wastewater treatments, in situ bioremediation, water desalination, biosensors, electrohydrogenesis, and various types of microbial fuel cells (Cheng and Logan, 2007; Cao et al., 2009; Lovley, 2009, 2012; Logan, 2010; Logan and Rabaey, 2012). Recent studies have shown that electrons coming from oBES-driven processes can be used to supply MES (Figure 1). In sulfide-driven MES, the electricity required for MES is generated by the abiotic oxidation of the toxic contaminant sulfide to sulfur and the subsequent biological oxidation of sulfur to sulfate (Gong et al., 2013). A similar process has also been developed to supply electrons for the electroproduction of methane from CO2 (Jiang et al., 2014). Moreover, MES and electromethanogenesis have been conducted in parallel with the biorecovery of cobalt at the cathode further illustrating the versatility of this technology (Huang et al., 2014).
A vibrant illustration of the significant progress made by MES in a relatively short period of time is that multicarbon compounds production rates by MES have been increased substantially over the last 4 years. For instance, the acetate production rate has been increased 433-fold from ca. 30 mM d-1 m-2 to ca. 1.3 mM d-1 cm-2, whereas the electron transfer rate was enhanced 521-fold from ca. 71 mA m-2 to ca. 3.7 mA cm-2 (Nevin et al., 2010; Marshall et al., 2012, 2013; Nie et al., 2013; Zhang et al., 2013; Jourdin et al., 2014; LaBelle et al., 2014; Table 1). However, the main obstacle for the development of MES as an economically viable technology is still the relatively slow microbial reduction rate of CO2 to multicarbon compounds in scalable rBES reactors. This review will discuss the ongoing efforts to increase MES productivity, stability, long-term efficiency, and versatility by optimizing microbial catalysts and electrochemical hardware and by characterizing the electron transfer mechanisms from cathode to microbe.
Table 1.
Microbial electrosynthesis systems in chronological order of publication.
| Microbial catalyst | Cathode | Comments | Reference |
|---|---|---|---|
| Acidithiobacillus ferrooxidans | -Platinum | -Fe(II)-mediated | Kinsel and Umbreit (1964) |
| A. ferrooxidans | -Platinum mesh | -Fe(II)-mediated | Nakasono et al. (1997) |
| A. ferrooxidans | -Platinum mesh -0.0 V (vs. Ag/AgCl) |
-Fe(II)-mediated | Matsumoto et al. (1999) |
| Leptospirillum ferrooxidans | -Platinum mesh - +0.1 V (vs. Ag/AgCl) |
-Fe(II)-mediated |
Matsumoto et al. (2000) |
| Sporomusa ovata | -Graphite stick - –0.4 V (vs. SHE) |
-Direct electron transfer -Acetate and 2-oxobutyrate produced |
Nevin et al. (2010) |
| A. ferrooxidans | -Graphite felt - –0.0 V (vs. SCE) |
-Direct electron transfer -Current density: 5 A m-2 |
Carbajosa et al. (2010) |
|
Clostridium aceticum Clostridium ljungdahlii Moorella thermoacetica Sporomusa silvacetica Sporomusa sphaeroides |
-Graphite stick - –0.4 V (vs. SHE) |
-Direct electron transfer -Acetate, 2-oxobutyrate and formate produced |
Nevin et al. (2011) |
| Ralstonia eutropha | -Indium foil - –1.6 V (vs. Ag/AgCl) |
-Formate-mediated -Biofuels produced |
Li et al. (2012) |
| Mixed community | -Graphite fiber brush/carbon rod/graphite plate - –0.439 V or –0.539 V (vs. SHE) |
-Current density: 52 mA m-2 (Graphite plate) -Power density: 83 mWm-2 (graphite plate) |
Pisciotta et al. (2012) |
| Mixed community | -Graphite granule - –0.59 V (vs. SHE) |
-Acetate, methane, and H2 produced -Acetate production: >4 mM d-1 |
Marshall et al. (2012) |
| Nitrosomonas europaea | -Nickel, glassy carbon, or copper -Multiple potentials |
-Ammonia-mediated -Multi-reactors system |
Khunjar et al. (2012) |
| Geobacter sulfurreducens | -Stainless steel - –0.6 V (vs. Ag/AgCl) |
-Direct electron transfer -Current density: 30 A m-2 |
Soussan et al. (2013) |
| S. ovata | -Modified carbon cloth - –0.6 V (vs. Ag/AgCl) |
-Direct electron transfer -Best cathode modification: chitosan -Current density: 475 mA m-2 -Acetate production: 229 mM d-1 m-2 |
Zhang et al. (2013) |
| S. ovata | -Graphite plate |
-Powered by sulfide/sulfur bioanode (0.3 V vs. SHE) -Acetate production: 49.9 mmol d-1 m-2 |
Gong et al. (2013) |
| Mariprofundus ferrooxydans | -Graphite —0.076 V (vs. SHE) |
-Direct electron transfer -Cell-normalized electrode oxidation rate: 0.075 pmol electrons cell-1 h-1 |
Summers et al. (2013) |
| Mixed community | -Carbon felt - –1.15 V (vs. Ag/AgCl) |
-Methane and acetate produced -Acetate production: 94.73 mg d-1 |
Jiang et al. (2013) |
| Mixed community | -Graphite granule - –0.59 V (vs. SHE) |
-Acetate, H2, formate, butyrate, and propionate produced -Acetate production: 17.25 mM d-1 |
Marshall et al. (2013) |
| S. ovata | -Nickel nanowires anchored to graphite - –0.6 V (vs. Ag/AgCl) |
-Direct electron transfer -Acetate production: 282 mM d-1 m-2 |
Nie et al. (2013) |
| Mixed community | -Carbon fiber rod - –0.4 V (vs. SHE) |
-Direct electron transfer -Acetate, ethanol, 1-butanol, propionate, butyrate, and H2 produced -Current density: 34 mA m-2 |
Zaybak et al. (2013) |
| Rhodopseudomonas palustris | -Graphite rod -+0.1 V (vs. SHE) |
-Light-driven -Current density: 1.5 μA cm-2 |
Bose et al. (2014) |
| Mixed community | -Graphite felt |
-Cobalt reduction -Methane and acetate produced |
Huang et al. (2014) |
| Mixed community | -Graphite plate modified with NanoWeb-RVC - –0.85 V (vs. SHE) |
-Current density: 3.7 mA cm-2 -Acetate production: 1.3 mM d-1 cm-2 |
Jourdin et al. (2014) |
| R. palustris | -Carbon cloth - –0.22 V (vs. Ag/AgCl) |
-Fe(II)-mediated -Light-driven -Multi-reactor system -Current density: 7.2 μA ml-1 |
Doud and Angenent (2014) |
| Mixed community | -Graphite granule - –0.6 to –0.8 V (vs. SHE) |
-Acidic pH -Acetate, H2, and formate produced -Acetate production: 51.6 mM d-1 (–0.8 V) -High H2 production |
LaBelle et al. (2014) |
The Microbial Catalysts
Mixed Communities
Microbial electrosynthesis can be driven by two major types of microbial catalysts: mixed communities and pure cultures. In the case of mixed communities, the cathodic chamber of the MES system is inoculated with samples from wastewater, sludge, or sediment (Table 1). One of the main advantages of employing a mixed community for MES is that it eliminates the need to work under stringent sterile conditions required with pure culture-driven bioprocesses. Moreover, the MES system with the highest reported acetate production rate to date of 1.3 mM d-1 cm-2 was driven by an uncharacterized mixed community (Jourdin et al., 2014). Mixed community-driven MES systems described until now mainly produce acetate because the microbial population quickly become dominated by acetogenic bacteria like Acetobacterium sp. (Marshall et al., 2012, 2013; LaBelle et al., 2014) and Eubacterium sp. (Pisciotta et al., 2012). There is also simultaneous production of methane due to the coexistent methanogens, unless an inhibitor of methanogenesis is added to the cathode reactor (Marshall et al., 2012, 2013; Pisciotta et al., 2012; Jiang et al., 2013; Huang et al., 2014). Hydrogen and formate produced biologically or abiotically at low cathode potential are other compounds frequently found in mixed community-driven MES reactors (Marshall et al., 2012, 2013; Pisciotta et al., 2012; Zaybak et al., 2013; LaBelle et al., 2014). Furthermore, in a study for the development of a method to facilitate the start-up of autotrophic biocathodes in rBESs, the mixed community microbial catalysts were reported to produce at least six products: butanol, ethanol, hydrogen, acetate, propionate, and butyrate (Zaybak et al., 2013). This study gives a good example of the difficulty generating a single specific product when employing mixed communities to drive MES processes. Other than compromising the purity of the desired product, it also complicates the separation process and reduces the electricity conversion efficiency to a specific multicarbon compound.
Pure Cultures
Acetogenic Bacteria
Diverse autotrophic pure cultures have been employed successfully in the role of microbial catalysts for MES systems. As illustrated by several studies on mixed community-driven MES, acetogens reducing CO2 through the Wood–Ljungdahl pathway (Drake et al., 1997, 2008; Ragsdale and Pierce, 2008) are dominating and efficient electroautotrophs. Pure cultures of Gram negative acetogens like Sporomusa silvacetica and Sporomusa sphaeroides and Gram positive acetogens like Clostridium ljungdahlii, Clostridium aceticum and the thermophile Moorella thermoacetica are all capable of reducing CO2 to multicarbon compounds by MES (Nevin et al., 2011). Among all the tested acetogenic bacteria, Sporomusa ovata DSM-2662 was the most efficient electroautotroph with acetate production rates as high as 282 mM d-1 m-2 and with electricity conversion efficiency to acetate typically above 80% (Nevin et al., 2010; Gong et al., 2013; Nie et al., 2013; Zhang et al., 2013). The production of negligible amount of 2-oxobutyrate and formate in comparison to acetate by S. ovata was also reported (Nevin et al., 2010, 2011). Acetogens like S. ovata have high electricity conversion efficiency to chemical commodities compared to autotrophic bacteria with other types of carbon fixation metabolisms because CO2 is the sole electron acceptor during acetogenesis (Drake et al., 1997, 2008; Ragsdale and Pierce, 2008). Thus, most of the electrons derived from the cathode will end up in reduced multicarbon products. Moreover, a study by Fast and Papousakis (2012) established that the Wood–Ljungdahl pathway is the most energetically efficient non-photosynthetic carbon fixation pathway for the electroproduction of acetate and ethanol (Fast and Papousakis, 2012).
Autotrophic Fe(II) Oxidizing Bacteria
Autotrophic Fe(II) oxidizing bacteria are also capable of reducing CO2 by MES. The acidophilic aerobic Fe(II) oxidizer Acidithiobacillus ferrooxidans (Kinsel and Umbreit, 1964; Nakasono et al., 1997; Matsumoto et al., 1999) and Leptospirillum ferrooxidans (Matsumoto et al., 2000) were able to grow in a bioelectrochemical system by using electrons coming from electrochemically reduced Fe(II). Moreover, A. ferrooxidans (Carbajosa et al., 2010) and the neutrophilic aerobic Fe(II) oxidizer Mariprofundus ferrooxydans (Summers et al., 2013) were shown to draw current directly from a poised cathode in the absence of a redox mediator to grow with CO2 as the source of carbon. The current draw was 5 A m-2 with A. ferrooxidans (Carbajosa et al., 2010) whereas the cell-normalized electrode oxidation rate was 0.075 pmol electrons cell-1 h-1 with M. ferrooxydans (Summers et al., 2013). MES systems driven by these Fe(II) oxidizing bacteria were poised at potential closer to 0 V vs. SHE compared to all the other reported MES systems (Table 1). Potential closer to 0 V at the cathode could translate in lower energy requirements (Lovley and Nevin, 2013). However, in those systems O2 is the final electron acceptor which means that a significant amount of electrons will not be used to reduce CO2 into multicarbon commodities but to reduce O2 and to generate biomass.
Ammonia-Oxidizing Bacteria
Ammonia can be employed as a redox mediator in MES system to promote the growth of ammonia-oxidizing bacteria (Khunjar et al., 2012; Table 1). In the study by Khunjar et al. (2012), nitrite was reduced electrochemically to ammonia in the first reactor and then was fed to a second reactor containing the ammonia-oxidizing bacteria Nitrosomonas europaea. Electrons from ammonia were used by N. europaea to produce biomass from CO2 and to generate nitrite that was then recycled in the first electrochemical reactor. However, like the MES system driven by autotrophic Fe(II)-oxidizer, N. europaea requires O2 as an electron acceptor and thus this system has the same efficiency issue.
Recombinant Microbial Catalyst
The only example to date of a MES process driven by a genetically engineered microbial catalyst was a recombinant strain of the chemolithotrophic bacterium Ralstonia eutropha developed for the electroproduction of biofuels (Li et al., 2012). The recombinant strain of R. eutropha generated 140 mg/ml of biofuels over a period of ca. 100 h by oxidizing formate that was electrochemically produced at the surface of a poised cathode. This MES process required O2 as the final electron acceptor and a cathode poised at very low potential of –1.6 V vs. Ag/AgCl to generate the necessary electron shuttle formate. These two factors are expected to significantly lower the overall efficiency of this MES system due to the energy loss for O2 reduction and formate production. Nevertheless, this study demonstrates that one of the advantages of using a pure culture for MES is that it can be genetically engineered to optimize the metabolism of the microbial catalyst and to increase the range of possible products.
Geobacter sulfurreducens
Evidence suggest that Geobacter sulfurreducens can also reduce CO2 into a multicarbon compound by MES (Soussan et al., 2013; Table 1). G. sulfurreducens is a well-characterized electrigenic bacterium generating power densities in oBESs as high as 3.9 W/m2 (Yi et al., 2009). G. sulfurreducens pre-grown with acetate as an electron donor and carbon source was also shown to have the capacity to accept electrons from a cathode to reduce fumarate (Gregory et al., 2004; Dumas et al., 2008) or uranium(VI; Gregory and Lovley, 2005) after the depletion of acetate. In a study by Soussan et al. (2013), G. sulfurreducens first used electrons derived from the cathode to reduce the final electron acceptor fumarate into succinate. When all the fumarate was depleted, G. sulfurreducens appeared to start producing glycerol by a process combining CO2 possibly reduced electrochemically to bicarbonate with succinate. Interestingly, a previous report demonstrated that G. sulfurreducens cannot grow with CO2 alone (Coppi et al., 2004). Recently, the closely related species Geobacter metallireducens was shown to be capable to grow autotrophically with formate as the sole source of carbon (Feist et al., 2014). G. metallireducens genome encodes two known carbon fixation pathways, the reductive TCA cycle and the dicarboxylate–hydroxybutyrate cycle, both of which are not present in the genome of G. sulfurreducens. It has been suggested that formate could be assimilated by G. sulfurreducens in the presence of a small quantity of acetate via the pyruvate formate lyase (Speers and Reguera, 2012). However, in Soussan et al. (2013) study, the cathode is poised at a potential (–0.6 V vs. Ag/AgCl) too high for CO2 reduction to formate, hence the biochemical mechanism for the assimilation of CO2/bicarbonate and the production of glycerol remains unclear.
Photosynthetic Fe(II)-Oxidizing Bacteria
Microbial electrosynthesis system can also be exposed to light to provide energy for CO2 reduction by anaerobic photosynthetic Fe(II)-oxidizing bacteria. Rhodopseudomonas palustris is the microbial catalyst in two reported photobiocathode-based MES studies. In the first study, R. palustris was accepting electrons from a poised cathode in a one-reactor system to reduce CO2 with an electron transfer rate of 1.5 μA cm-2 (Bose et al., 2014). Electron uptake was stimulated by light exposure, but occurred at a significantly lower rate in the dark. However, it is not clear if R. palustris was accepting electrons directly from the cathode or from Fe(II) reduced electrochemically. The second study described a multi-reactor system similar to the aforementioned ammonia shuttling MES system by Khunjar et al. (2012). The redox mediator Fe(III) was first reduced abiotically by a cathode to Fe(II) in an electrochemical reactor before being fed to a second photobioreactor where R. palustris was growing with CO2 as the sole carbon source, Fe(II) as the electron source, and light (Doud and Angenent, 2014).
Electron Transfer from the Cathode to the Microbial Catalyst
Indirect Electron Transfer
Exogenous Electron Shuttles
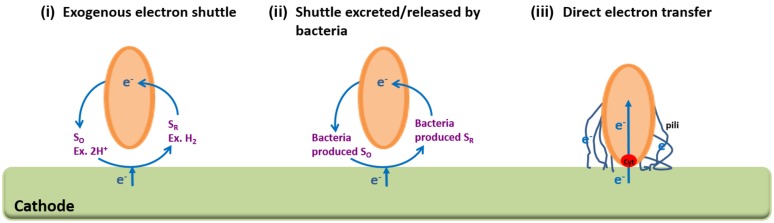
Understanding the electron transfer mechanisms involved in MES could lead to major breakthroughs in the effort to increase the electron transfer rate between the cathode and the microbial catalyst. Electrons can either be transferred directly or indirectly via a shuttle (Patil et al., 2012; Figure 2). H2, formate, Fe(II) and ammonia have all been reported to function as redox mediator in MES systems (Lovley and Nevin, 2013). H2 has been the most prevalent (Table 1) since it requires only that the cathode in the MES reactor is poised at a lower potential than –0.41 V (vs. SHE). Under this condition, significant quantities of H2 are generated from the electrons coming from the cathode and the protons migrating from the anodic chamber. However, employing H2 as a redox mediator for MES is not optimal because its low solubility might cause energy losses.
FIGURE 2.
Possible extracellular electron transfer mechanisms from the cathode to the microbial catalyst. Indirect electron transfer via (i) an exogenous shuttle or (ii) a shuttle released/excreted by the microbial catalyst. (iii) Direct electron transfer via bacterial outer surface components such as c-type cytochromes or pili. SO is oxidized electron shuttle and SR is reduced electron shuttle.
Shuttles Excreted/Released by Bacteria
Another possible indirect electron transfer mechanism is that microbial catalysts could be producing and excreting their own soluble redox mediators to carry electrons from the cathode. Redox mediators either excreted by the bacteria such as phenazine and riboflavin or released after cell death such as vitamin B12 or DNA have all been suggested as possible components involved in extracellular electron transfer (Rosenbaum et al., 2011).
Direct Electron Transfer
Direct electron transfer requires physical contacts between extracellular components of the microbial catalyst involved in the transport of electrons and the cathode. Direct electron transfer from the cathode to the microbial catalyst in the absence of a redox mediator has been shown to occur in MES processes driven by the following electroautotrophic species: A. ferrooxidans (Carbajosa et al., 2010; Rodrigues and Rosenbaum, 2014), M. ferrooxydans (Summers et al., 2013) and a group of acetogenic bacteria (Nevin et al., 2010, 2011; Nie et al., 2013; Zhang et al., 2013). This conclusion is mainly based on the fact that all these MES systems were operated with cathodes poised at potentials too high to produce significant quantity of H2. Moreover, driving MES systems with cathodes poised at lower potentials than the formal potential of the 2H+/H2 couple does not exclude the possibility of direct electron transfer. A study by Marshall et al. (2012) reported evidence that direct electron transfer was occurring at the same time as hydrogen-mediated electron transfer. This suggests that microbial catalysts are capable of acquiring electrons from a cathode through many paths simultaneously.
Electromethanogenic Bacteria
Recently, it was demonstrated that a mutant strain of Methanococcus maripaludis lacking all its catabolic hydrogenases was still capable of performing electromethanogenesis (Lohner et al., 2014) thus providing supplementary significant evidence that electrotrophic bacteria can accept electrons directly from the cathode independently of the redox mediator H2.
The Model Metal-Reducing Bacteria
Two of the most detailed studies about electron transfer mechanisms from the cathode to the microbe have been done with the two model metal-reducing bacteria G. sulfurreducens (Strycharz et al., 2011) and Shewanella oneidensis (Ross et al., 2011). In G. sulfurreducens, the monoheme c-type cytochrome GSU3274 predicted to be localized in the periplasm was found to be specifically required for electron transfer from the cathode demonstrating the importance of c-type cytochromes for electron uptake. Like G. sulfurreducens, S. oneidensis can do electrorespiration accepting electrons from the cathode to reduce fumarate into succinate (Ross et al., 2011). Based on functional genetic studies, Ross et al. (2011) proposed a model where the respiratory pathway normally responsible for transferring electron from the cytoplasmic metabolism to extracellular electron acceptors (Shi et al., 2012) works in reverse. Therefore, the decaheme outer membrane c-type cytochrome MtrC, the decaheme periplasmic c-type cytochrome MtrA, the porin MtrB responsible for connecting MtrC to MtrA, the tetraheme cytoplasmic membrane-associated c-type cytochrome CymA and the menaquinone pool are all critical components of the electron uptake pathway of S. oneidensis.
Electroautotrophic Bacteria
Direct electron transfer pathways are poorly characterized in electroautotrophic bacteria. For A. ferrooxidans, experimental evidence indicated that a Fe species excreted by the cells in the cathode biofilm could be responsible for electron uptake (Carbajosa et al., 2010). It has been speculated that c-type cytochromes which are critical components for the uptake of electrons from extracellular Fe(II) could also be involved in the transport of electron from the cathode (Rosenbaum et al., 2011; Sydow et al., 2014). In support of this hypothesis, metagenomics and metaproteomics of the mixed community populating a self-regenerating biocathode suggest that a member of the Chromatiaceae family reduced CO2 with electrons acquired directly from the cathode via c-type cytochromes and other proteins associated with Fe(II) oxidation (Wang et al., 2015).
Much less information is known about how electrons are acquired by acetogens from the cathode. Recently, a genetic system developed for the Gram positive bacterium C. ljungdahlii (Leang et al., 2013; Banerjee et al., 2014; Ueki et al., 2014) led to the confirmation of the identity of the proton pump responsible for the generation of a proton motive force essential for growth with CO2 as the sole source of carbon (Tremblay et al., 2013). This study provided insights on the energy conservation mechanism involved in the electroautotrophic growth of acetogens. The electron uptake mechanism of C. ljungdahlii is expected to be significantly different compared to other electrotrophic bacteria because it cannot synthesize c-type cytochromes or quinones (Köpke et al., 2010). The availability of a genetic toolbox should accelerate the characterization of the specificities of C. ljungdahlii’s electron transfer pathway and could provide general information about electron uptake by other Gram positive bacteria.
The genome sequence of the Gram negative and acetogenic species S. ovata has recently been made available (Poehlein et al., 2013). Genes coding for c-type cytochromes and type IV pili, two components of bacterial extracellular electron transfer mechanisms, are present in the genome. As mentioned before, c-type cytochromes are critical components of extracellular electron transfer pathways in both electrigenic and electrotrophic bacteria. In Geobacter spp., type IV pili are filaments with metallic-like conductivity facilitating long-range electron transfer (Lovley, 2011, 2012; Malvankar and Lovley, 2012; Vargas et al., 2013; Malvankar et al., 2014) involved in the reduction of insoluble electron acceptors (Reguera et al., 2005; Tremblay et al., 2012). Ubiquinone (Möller et al., 1984), another critical component of electron transport pathway, was also detected in S. ovata and genes coding for enzymes involved in its biosynthesis were found in the genome (Poehlein et al., 2013). S. ovata possesses several well-characterized components of microbial extracellular transfer pathways which indicates that electron uptake by S. ovata could have similarities with other electrigenic or electrotrophic bacteria. Recently, an acetogenic bacteria closely related to S. sphaeroides was shown to do acetogenesis with metallic iron (F(0)) as the sole electron donor suggesting that direct electron transfer could be a useful strategy for Gram negative acetogens in multiple environments (Kato et al., 2015).
The Electrochemical Hardware
Cathode Materials Tested for Current Draw
A lot of effort has been put into optimizing rBESs/MES by selecting or developing more efficient and less expensive components for bioelectrochemical reactors (Krieg et al., 2014). Until now, most of the studies published on this topic are presenting the impact of different types of biocompatible cathodes on the performance of rBESs/MES. Cathodes described in the literatures relied mainly on carbonaceous materials (Table 1). Indium foil (Li et al., 2012) and platinum (Kinsel and Umbreit, 1964; Nakasono et al., 1997; Matsumoto et al., 1999) have also been tried in rBESs but the most efficient cathode material to date for electron transfer is stainless steel with current draw as high as 30 A m-2 with G. sulfurreducens as the microbial catalyst (Soussan et al., 2013).
Conductive materials that will self-assemble in the cathodic biofilm are another approach to enhance electron transfer in rBESs. A S. oneidensis biofilm assembled with embodied graphene oxide could uptake electrons 74 times more efficiently (Yong et al., 2014). Oligoelectrolytes can also facilitate electron transfer from the cathode by inserting itself in the lipid membrane of bacteria to enable transmembrane charge transfer as demonstrated by Thomas et al. (2013).
Cathode Materials Tested for Chemical Production
Cathodes employed for MES are generally made of carbonaceous materials like graphite. These basic cathodes can be treated or coated with other materials resulting in modifications of their surface.
Untreated Surface
When chemical commodities production rate is not normalized to total surface area of cathode, one of the best materials is granular graphite with acetate production from a mixed community catalyst reaching 51.6 mM per day or 3.0 gL-1 d-1 (LaBelle et al., 2014). As a packed structure, the main advantage of granular graphite over other carbonaceous cathodes is the high specific area for bacterial adhesion (Wei et al., 2011).
Treated Surface
Modifications of carbonaceous cathodes have resulted in critical improvements in MES systems driven either by mixed communities or by pure cultures. Recently, Jourdin et al. (2014) coated carbon nanotubes on reticulated vitreous carbon (NanoWeb-RVC) to enhance bacterial attachment in a mixed community-driven MES system resulting in the highest normalized current density and the highest normalized acetate production rate reported for any MES systems until now (Table 1). The authors suggested that this performance improvement was due to the high surface to volume ratio of NanoWeb-RVC responsible for enhanced bacterial adhesion and effective mass transfer within the electrode-biofílm superstructure (Jourdin et al., 2014). The higher reported acetate production rate and current density compared to other MES systems relying on direct electron transfer (Nevin et al., 2010, 2011; Nie et al., 2013; Zhang et al., 2013) can also be attributed in part to the low potential of the cathode (–0.85 V vs. SHE) promoting indirect electron transfer that could be advantageous for the unknown bacterial species populating the reactor. Maintaining the cathode at this potential is possibly responsible for the slightly lower electricity to acetate efficiency of 70% observed with this MES reactor. Although this system is promising, the volumetric acetate production rate of ca. 0.025 gL-1 d-1 is 120-fold lower than that of the MES system also driven by a mixed community developed by LaBelle et al. (2014). Scaling up test for the NanoWeb-RVC biocathode of Jourdin et al. (2014) will indicate its real potential for industrial applications.
A number of modified carbonaceous cathodes have been proposed for MES systems driven by the pure culture catalyst S. ovata with significant success. Functionalization of carbon cloth cathodes with chitosan or other compounds conferring a positive charge to the electrode surface with the aim of increasing interactions with negatively charged bacteria like S. ovata (Zhang et al., 2013), resulted in higher cell density at the cathode surface (up to 9-fold increase), in better electron transfer (up to 6.7-fold increase as 475 mA m-2) and in higher normalized acetate production rates (up to 7.6-fold increase as 229 mM d-1 m-2). In a second approach, carbon cloth cathodes were treated with metal, such as gold, palladium, or nickel nanoparticles to harness their exceptional catalytic activities for MES processes. Significant increases in normalized acetate production rate by MES were recorded with all three metals in the range of 100–200 mM d-1 m-2 (Zhang et al., 2013). Modifying polyester- or cotton-based textile composite cathodes with carbon nanotubes to create a three-dimensional matrix with more surface area available for bacteria also resulted in significant increase in the productivity of MES of ca. 100 mM d-1 m-2 (Zhang et al., 2013). In a later study by the same group, using nickel nanowires anchored to graphite electrode resulted in the highest normalized acetate production rate recorded for a pure culture-driven MES system due to enhanced surface area and the generation of a porous structure (Nie et al., 2013; Table 1).
Concluding Remarks
Microbial electrosynthesis is a young technology that made significant progress in terms of productivity over the last 5 years. Large efforts have been done to optimize known microbial catalysts for MES and to screen new ones with strong electroautotrophic properties (Rodrigues and Rosenbaum, 2014). Meanwhile, cathodes fabricated with novel materials or designed with better spatial arrangement are being explored and developed. Optimization of other parts of the electrochemical hardware such as the ion-exchange membrane and the current-collecting structure for MES processes are also ongoing (Varcoe et al., 2014). For example, it has been suggested that anion-exchange membranes can be exploited in the in situ selective extraction of acetate produced by MES at a lower energetic cost (Andersen et al., 2014). Furthermore, understanding in detail how electrons are transferred from the cathode to the microbial catalyst will help devise better-designed strategies to improve all aspects of MES. If this rate of improvement is maintained in the future, MES could fulfill its promise as an energetically efficient, environment-friendly, and versatile bioproduction strategy.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the Novo Nordisk Foundation.
References
- Andersen S. J., Hennebel T., Gildemyn S., Coma M., Desloover J., Berton J., et al. (2014). Electrolytic membrane extraction enables production of fine chemicals from biorefinery sidestreams. Environ. Sci. Technol. 48 7135–7142 10.1021/es500483w [DOI] [PubMed] [Google Scholar]
- Banerjee A., Leang C., Ueki T., Nevin K. P., Lovley D. R. (2014). A Lactose-Inducible system for metabolic engineering of Clostridium ljungdahlii. Appl. Environ. Microbiol. 80 2410–2416 10.1128/AEM.03666-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Gardel E. J., Vidoudez C., Parra E. A., Girguis P. R. (2014). Electron uptake by iron-oxidizing phototrophic bacteria. Nat. Commun. 5 3391 10.1038/ncomms4391 [DOI] [PubMed] [Google Scholar]
- Cao X., Huang X., Liang P., Xiao K., Zhou Y., Zhang X., et al. (2009). A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 43 7148–7152 10.1021/es901950j [DOI] [PubMed] [Google Scholar]
- Carbajosa S., Malki M., Caillard R., Lopez M. F., Palomares F. J., Martin-Gago J. A., et al. (2010). Electrochemical growth of Acidithiobacillus ferrooxidans on a graphite electrode for obtaining a biocathode for direct electrocatalytic reduction of oxygen. Biosens. Bioelectron. 26 877–880 10.1016/j.bios.2010.07.037 [DOI] [PubMed] [Google Scholar]
- Cheng S., Logan B. E. (2007). Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. U.S.A. 104 18871–18873 10.1073/pnas.0706379104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Xing D., Call D. F., Logan B. E. (2009). Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 43 3953–3958 10.1021/es803531g [DOI] [PubMed] [Google Scholar]
- Coppi M. V., O’Neil R. A., Lovley D. R. (2004). Identification of an uptake hydrogenase required for hydrogen-dependent reduction of Fe(III) and other electron acceptors by Geobacter sulfurreducens. J. Bacteriol. 186 3022–3028 10.1128/JB.186.10.3022-3028.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doud D. F. R., Angenent L. T. (2014). Towards electrosynthesis with uncoupled extracellular electron uptake and metabolic growth: enhancing current uptake with Rhodopseudomonas palustris. Environ. Sci. Technol. Lett. 1 351–355 10.1021/ez500244n [DOI] [Google Scholar]
- Drake H. L., Daniel S. L., Kusel K., Matthies C., Kuhner C., Braus-Stromeyer S. (1997). Acetogenic bacteria: what are the in situ consequences of their diverse metabolic versatilities? Biofactors 6 13–24 10.1002/biof.5520060103 [DOI] [PubMed] [Google Scholar]
- Drake H. L., Gossner A. S., Daniel S. L. (2008). Old acetogens, new light. Ann. N. Y. Acad. Sci. 1125 100–128 10.1196/annals.1419.016 [DOI] [PubMed] [Google Scholar]
- Dumas C., Basseguy R., Bergel A. (2008). Microbial electrocatalysis with Geobacter sulfurreducens biofilm on stainless steel cathodes. Electrochim. Acta 53 2494–2500 10.1016/j.electacta.2007.10.018 [DOI] [Google Scholar]
- Fast A. G., Papousakis E. T. (2012). Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Curr. Opin. Chem. Eng. 1 380–395 10.1016/j.coche.2012.07.005 [DOI] [Google Scholar]
- Feist A. M., Nagarajan H., Rotaru A. E., Tremblay P. L., Zhang T., Nevin K. P., et al. (2014). Constraint-based modeling of carbon fixation and the energetics of electron transfer in Geobacter metallireducens. PLoS Comput. Biol. 10:e1003575 10.1371/journal.pcbi.1003575PCOMPBIOL-D-13-01735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Ebrahim A., Feist A. M., Embree M., Zhang T., Lovley D., et al. (2013). Sulfide-driven microbial electrosynthesis. Environ. Sci. Technol. 47 568–573 10.1021/es303837j [DOI] [PubMed] [Google Scholar]
- Green M. A., Emery K., Hishikawa Y., Warta W., Dunlop E. D. (2014). Solar cell efficiency tables (version 45). Prog. Photovolt. Res. Appl. 23 1–9 10.1002/pip.2573 [DOI] [Google Scholar]
- Gregory K. B., Bond D. R., Lovley D. R. (2004). Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 6 596–604 10.1111/j.1462-2920.2004.00593.x [DOI] [PubMed] [Google Scholar]
- Gregory K. B., Lovley D. R. (2005). Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 39 8943–8947 10.1021/es050457e [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Grogger M., Veverka D. (2014). Recent advances in microbial electrocatalysis. Electrocatalysis 5 319–329 10.1007/s12678-014-0198-x [DOI] [Google Scholar]
- Huang L. P., Jiang L., Wang Q., Quan X., Yang J., Chen L. (2014). Cobalt recovery with simultaneous methane and acetate production in biocathode microbial electrolysis cells. Chem. Eng. J. 253 281–290 10.1016/j.cej.2014.05.080 [DOI] [Google Scholar]
- Jiang Y., Su M., Li D. (2014). Removal of sulfide and production of methane from carbon dioxide in microbial fuel cells-microbial electrolysis cell (MFCs-MEC) coupled system. Appl. Biochem. Biotechnol. 172 2720–2731 10.1007/s12010-013-0718-9 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Su M., Zhang Y., Zhan G., Tao Y., Li D. (2013). Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrogen. Ener. 38 3497–3502. 10.1016/j.ijhydene.2012.12.107 [Google Scholar]
- Jourdin L., Freguia S., Donose B. C., Chen J., Wallace G. G., Keller J., et al. (2014). A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis. J. Mater. Chem. A 2 13093–13102 10.1039/c4ta03101f [DOI] [Google Scholar]
- Jürgensen L., Ehimen E. A., Born J., Holm-Nielsen J. B. (2014). Utilization of surplus electricity from wind power for dynamic biogas upgrading: Nothern Germany case study. Biomass Bioenergy 66 126–132 10.1016/j.biombioe.2014.02.032 [DOI] [Google Scholar]
- Kato S., Yumoto I., Kamagata Y. (2015). Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 81 67–73 10.1128/AEM.02767-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khunjar W. O., Sahin A., West A. C., Chandran K., Banta S. (2012). Biomass production from electricity using ammonia as an electron carrier in a reverse microbial fuel cell. PLoS ONE 7:e44846 10.1371/journal.pone.0044846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsel N. A., Umbreit W. W. (1964). Method for electrolysis of culture medium to increase growth of the sulfur-oxidizing iron bacterium Ferrobacillus sulfooxidans. J. Bacteriol. 87 1243–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Saito N., Fu Q., Kawaguchi H., Vilcaez J., Wakayama T., et al. (2013). Bio-electrochemical property and phylogenetic diversity of microbial communities associated with bioelectrodes of an electromethanogenic reactor. J. Biosci. Bioeng. 116 114–117 10.1016/j.jbiosc.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Köpke M., Held C., Hujer S., Liesegang H., Wiezer A., Wollherr A., et al. (2010). Clostridium ljungdahlii represents a microbial production platform based on syngas. Proc. Natl. Acad. Sci. U.S.A. 107 13087–13092 10.1073/pnas.1004716107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracke F., Krömer J. O. (2014). Identifying target processes for microbial electrosynthesis by elementary mode analysis. BMC Bioinformatics 15:6590 10.1186/s12859-014-0410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg T., Sydow A., Schroder U., Schrader J., Holtmann D. (2014). Reactor concepts for bioelectrochemical syntheses and energy conversion. Trends Biotechnol. 32 645–655 10.1016/j.tibtech.2014.10.004 [DOI] [PubMed] [Google Scholar]
- LaBelle E. V., Marshall C. W., Gilbert J. A., May H. D. (2014). Influence of acidic pH on hydrogen and acetate production by an electrosynthetic microbiome. PLoS ONE 9:e109935 10.1371/journal.pone.0109935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leang C., Ueki T., Nevin K. P., Lovley D. R. (2013). A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen. Appl. Environ. Microbiol. 79 1102–1109 10.1128/AEM.02891-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Opgenorth P. H., Wernick D. G., Rogers S., Wu T. Y., Higashide W., et al. (2012). Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335:1596 10.1126/science.1217643 [DOI] [PubMed] [Google Scholar]
- Logan B. E. (2010). Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 85 1665–1671 10.1007/s00253-009-2378-9 [DOI] [PubMed] [Google Scholar]
- Logan B. E., Rabaey K. (2012). Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337 686–690 10.1126/science.1217412 [DOI] [PubMed] [Google Scholar]
- Lohner S. T., Deutzmann J. S., Logan B. E., Leigh J., Spormann A. M. (2014). Hydrogenase-independent uptake and metabolism of electrons by the archaeon Methanococcus maripaludis. ISME J. 8 1673–1681 10.1038/ismej.2014.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R. (2009). Future shock from the microbe electric. Microb. Biotechnol. 2 139–141 10.1111/j.1751-7915.2009.00090_9.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R. (2011). Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 4 4896–4906 10.1039/C1ee02229f [DOI] [Google Scholar]
- Lovley D. R. (2012). Electromicrobiology. Annu. Rev. Microbiol. 66 391–409 10.1146/annurev-micro-092611-150104 [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Nevin K. P. (2011). A shift in the current: new applications and concepts for microbe-electrode electron exchange. Curr. Opin. Biotechnol. 22 441–448 10.1016/j.copbio.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Nevin K. P. (2013). Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr. Opin. Biotechnol. 24 385–390 10.1016/j.copbio.2013.02.012 [DOI] [PubMed] [Google Scholar]
- MacDonald M. (2003). Photobiology of Higher Plants. New York, NY: Wiley. [Google Scholar]
- Malvankar N. S., Lovley D. R. (2012). Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. ChemSusChem 5 1039–1046 10.1002/cssc.201100733 [DOI] [PubMed] [Google Scholar]
- Malvankar N. S., Yalcin S. E., Tuominen M. T., Lovley D. R. (2014). Visualization of charge propagation along individual pili proteins using ambient electrostatic force microscopy. Nat. Nanotechnol. 9 1012–1017 10.1038/nnano.2014.236 [DOI] [PubMed] [Google Scholar]
- Marshall C. W., Ross D. E., Fichot E. B., Norman R. S., May H. D. (2012). Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 78 8412–8420 10.1128/AEM.02401-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. W., Ross D. E., Fichot E. B., Norman R. S., May H. D. (2013). Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ. Sci. Technol. 47 6023–6029 10.1021/es400341b [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Nakasono S., Ohmura N., Saiki H. (1999). Extension of logarithmic growth of Thiobacillus ferrooxidans by potential controlled electrochemical reduction of Fe(III). Biotechnol. Bioeng. 64 716–721 [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Yoshinaga H., Ohmura N., Ando A., Saiki H. (2000). High density cultivation of two strains of iron-oxidizing bacteria through reduction of ferric iron by intermittent electrolysis. Biotechnol. Bioeng. 70 464–466 [DOI] [PubMed] [Google Scholar]
- Möller B., Ossmer R., Howard B. H., Gottschalk G., Hippe H. (1984). Sporomusa, a new genus of gram-negative anaerobic-bacteria including Sporomusa sphaeroides spec. nov and Sporomusa ovata spec. nov. Arch. Microbiol. 139 388–396 10.1007/Bf00408385 [DOI] [Google Scholar]
- Nakasono S., Matsumoto N., Saiki H. (1997). Electrochemical cultivation of Thiobacillus ferrooxidans by potential control. Bioelectrochem. Bioenerg. 43 61–66 10.1016/S0302-4598(97)00001-9 [DOI] [Google Scholar]
- Nevin K. P., Hensley S. A., Franks A. E., Summers Z. M., Ou J., Woodard T. L., et al. (2011). Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 77 2882–2886 10.1128/AEM.02642-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin K. P., Woodard T. L., Franks A. E., Summers Z. M., Lovley D. R. (2010). Microbial electrosynthesis: feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 1 e00103-10. 10.1128/mBio.00103-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H., Zhang T., Cui M., Lu H., Lovley D. R., Russell T. P. (2013). Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys. Chem. Chem. Phys. 15 14290–14294 10.1039/c3cp52697f [DOI] [PubMed] [Google Scholar]
- Park D. H., Laivenieks M., Guettler M. V., Jain M. K., Zeikus J. G. (1999). Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl. Environ. Microbiol. 65 2912–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S. A., Hägerhäll C., Gorton L. (2012). Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal. Rev. 4 159–192 10.1007/s12566-012-0033-x [DOI] [Google Scholar]
- Pisciotta J. M., Zaybak Z., Call D. F., Nam J. Y., Logan B. E. (2012). Enrichment of microbial electrolysis cell biocathodes from sediment microbial fuel cell bioanodes. Appl. Environ. Microbiol. 78 5212–5219 10.1128/AEM.00480-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlein A., Gottschalk G., Daniel R. (2013). First insights into the genome of the gram-negative, endospore-forming organism Sporomusa ovata strain H1 DSM 2662. Genome Announc. 1 e00734-13 10.1128/genomeA.00734-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaey K., Girguis P., Nielsen L. K. (2011). Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 22 371–377 10.1016/j.copbio.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Rabaey K., Rozendal R. A. (2010). Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 8 706–716 10.1038/nrmicro2422 [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Pierce E. (2008). Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 1784 1873–1898 10.1016/j.bbapap.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G., Mccarthy K. D., Mehta T., Nicoll J. S., Tuominen M. T., Lovley D. R. (2005). Extracellular electron transfer via microbial nanowires. Nature 435 1098–1101 10.1038/nature03661 [DOI] [PubMed] [Google Scholar]
- Rodrigues T. D., Rosenbaum M. A. (2014). Microbial electroreduction: screening for new cathodic biocatalysts. Chemelectrochem 1 1916–1922 10.1002/celc.201402239 [DOI] [Google Scholar]
- Rosenbaum M., Aulenta F., Villano M., Angenent L. T. (2011). Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 102 324–333 10.1016/j.biortech.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M. A., Franks A. E. (2014). Microbial catalysis in bioelectrochemical technologies: status quo, challenges and perspectives. Appl. Microbiol. Biotechnol. 98 509–518 10.1007/s00253-013-5396-6 [DOI] [PubMed] [Google Scholar]
- Ross D. E., Flynn J. M., Baron D. B., Gralnick J. A., Bond D. R. (2011). Towards electrosynthesis in Shewanella: energetics of reversing the mtr pathway for reductive metabolism. PLoS ONE 6:e16649 10.1371/journal.pone.0016649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Rosso K. M., Clarke T. A., Richardson D. J., Zachara J. M., Fredrickson J. K. (2012). Molecular underpinnings of Fe(III) oxide reduction by Shewanella oneidensis MR-1. Front. Microbiol. 3:50 10.3389/fmicb.2012.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussan L., Riess J., Erable B., Delia M. L., Bergel A. (2013). Electrochemical reduction of CO2 catalysed by Geobacter sulfurreducens grown on polarized stainless steel cathodes. Electrochem. Commun. 28 27–30 10.1016/j.elecom.2012.11.033 [DOI] [Google Scholar]
- Speers A. M., Reguera G. (2012). Electron donors supporting growth and electroactivity of Geobacter sulfurreducens anode biofilms. Appl. Environ. Microbiol. 78 437–444 10.1128/AEM.06782-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strycharz S. M., Glaven R. H., Coppi M. V., Gannon S. M., Perpetua L. A., Liu A., et al. (2011). Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 80 142–150 10.1016/j.bioelechem.2010.07.005 [DOI] [PubMed] [Google Scholar]
- Summers Z. M., Gralnick J. A., Bond D. R. (2013). Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes. mBio 4 e00420–e00412 10.1128/mBio.00420-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow A., Krieg T., Mayer F., Schrader J., Holtmann D. (2014). Electroactive bacteria-molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 98 8481–8495 10.1007/s00253-014-6005-z [DOI] [PubMed] [Google Scholar]
- Thomas A. W., Garner L. E., Nevin K. P., Woodard T. L., Franks A. E., Lovley D. R., et al. (2013). A lipid membrane intercalating conjugated oligoelectrolyte enables electrode driven succinate production in Shewanella. Energy Environ. Sci. 6 1761–1765 10.1039/C3ee00071k [DOI] [Google Scholar]
- Tremblay P. L., Aklujkar M., Leang C., Nevin K. P., Lovley D. (2012). A genetic system for Geobacter metallireducens: role of the flagellin and pilin in the reduction of Fe(III) oxide. Environ. Microbiol. Rep. 4 82–88 10.1111/j.1758-2229.2011.00305.x [DOI] [PubMed] [Google Scholar]
- Tremblay P. L., Zhang T., Dar S. A., Leang C., Lovley D. R. (2013). The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio 4 e00406–e00412 10.1128/mBio.00406-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T., Nevin K. P., Woodard T. L., Lovley D. R. (2014). Converting carbon dioxide to butyrate with an engineered strain of Clostridium ljungdahlii. mBio 5 e01636–e01614 10.1128/mBio.01636-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcoe J. R., Atanassov P., Dekel D. R., Herring A. M., Hickner M. A., Kohl P. A., et al. (2014). Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 7 3135–3191 10.1039/C4ee01303d [DOI] [Google Scholar]
- Vargas M., Malvankar N. S., Tremblay P. L., Leang C., Smith J. A., Patel P., et al. (2013). Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. mBio 4 e00105–e00113 10.1128/mBio.00105-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villano M., Aulenta F., Ciucci C., Ferri T., Giuliano A., Majone M. (2010). Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 101 3085–3090 10.1016/j.biortech.2009.12.077 [DOI] [PubMed] [Google Scholar]
- Wang H., Ren Z. J. (2013). A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 31 1796–1807 10.1016/j.biotechadv.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Wang Z., Leary D. H., Malanoski A. P., Li R. W., Hervey W. J. T., Eddie B. J., et al. (2015). A previously uncharacterized, nonphotosynthetic member of the Chromatiaceae is the primary CO2-fixing constituent in a self-regenerating biocathode. Appl. Environ. Microbiol. 81 699–712 10.1128/AEM.02947-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Liang P., Huang X. (2011). Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 102 9335–9344 10.1016/j.biortech.2011.07.019 [DOI] [PubMed] [Google Scholar]
- Yi H., Nevin K. P., Kim B. C., Franks A. E., Klimes A., Tender L. M., et al. (2009). Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens. Bioelectron. 24 3498–3503 10.1016/j.bios.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Yong Y. C., Yu Y. Y., Zhang X., Song H. (2014). Highly active bidirectional electron transfer by a self-assembled electroactive reduced-graphene-oxide-hybridized biofilm. Angew. Chem. Int. Ed. Engl. 53 4480–4483 10.1002/anie.201400463 [DOI] [PubMed] [Google Scholar]
- Zaybak Z., Pisciotta J. M., Tokash J. C., Logan B. E. (2013). Enhanced start-up of anaerobic facultatively autotrophic biocathodes in bioelectrochemical systems. J. Biotechnol. 168 478–485 10.1016/j.jbiotec.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Zhang T., Nie H. R., Bain T. S., Lu H. Y., Cui M. M., Snoeyenbos-West O. L., et al. (2013). Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 6 217–224 10.1039/C2ee23350a [DOI] [Google Scholar]