Abstract
The current study explored the picture naming performance of patients with Alzheimer’s disease (AD). First, we evaluated the utility of the Multilingual Naming Test (MINT; Gollan et al., 2011), which was designed to assess naming skills in speakers of multiple languages, for detecting naming impairments in monolingual AD and amnestic mild cognitive impairment (MCI). If the MINT were sensitive to linguistic impairment in AD, using it in clinical practice might have advantages over using tests exclusively designed for English monolinguals. We found that the MINT can be used with both monolinguals and bilinguals: A 32-item subset of the MINT is best for distinguishing monolingual patients from controls, while the full MINT is best for assessing degree of bilingualism and language dominance in bilinguals. We then investigated the cognitive mechanisms underlying naming impairment in AD. To this end, we explored which MINT item characteristics best predicted performance differences between monolingual patients and controls. We found that contextual diversity and imageability, but not word frequency (nor words’ number of senses), contributed unique variance to explaining naming impairments in AD. These findings suggest a semantic component to the naming impairment in AD (modulated by names’ semantic richness and network size).
Keywords: Picture naming, Bilingualism, Bilingual index scores, Language dominance, Frequency, Imageability, Contextual diversity, Number of senses
INTRODUCTION
Fourteen percent of people over age 71 in the United States have dementia (Alzheimer’s Association Report, 2008), and Hispanics are at increased risk (16.3%; Alzheimer’s Association Report, 2004). These numbers obviate a pressing need for standardized measures suited to a variety of different populations, and more research aimed at a better understanding of cognitive impairment in Alzheimer’s disease (AD). In this study, we report an investigation of linguistic impairments in AD, and specifically of picture naming performance. We address two goals. On the practical side, we ask if a picture naming test designed to assess bilingualism (the Multilingual Naming Test, or MINT; Gollan, Weissberger, Runnqvist, Montoya, Cera, 2012) could be used to measure naming performance in AD. On the theoretical side, we examine which picture name characteristics best predict performance differences between patients and controls for each picture name, to shed light on the nature of linguistic impairments in AD.
Although the hallmark of early AD is memory impairment, language skills are also affected as the disease progresses. Picture naming can distinguish AD from other related diseases (Hodges, Salmon, & Butters, 1991) and is commonly administered in neuropsychological assessment (Lezak, Howieson, & Loring, 2004; Spreen & Strauss, 1991), although it does not add incremental diagnostic utility (see Testa et al., 2004). Since AD might affect language skills differentially in the two languages of bilinguals (e.g., Gollan, Salmon, Montoya, & Da Pena, 2010), picture naming is also useful in clinical settings as an objective measure of language dominance and degree of bilingualism (i.e., if bilinguals are about equally proficient in the two languages or proficient in one more than in the other).
One of the most frequently used picture naming tests is the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983). However, this test was designed for monolingual English speakers and is not optimal for assessing the naming performance of monolinguals in other languages, or of multilinguals (e.g., Gollan, Fennema-Notestine, Montoya, & Jernigan, 2007; Kohnert, Hernandez, & Bates, 1998; Ransdell & Fischler, 1987; Roberts, Garcia, Desrochers, & Hernandez, 2002). This is because item difficulty in the BNT increases with item progression, but an item difficult in English may be easy in another language and vice versa (e.g., Allegri et al., 1997; Kohnert et al., 1998).
Recently, Gollan and colleagues introduced the MINT (Gollan et al., 2012), a picture-naming test designed to assess degree of bilingualism and naming skills in speakers of multiple languages (Spanish, English, Mandarin, and Hebrew). Gollan et al. (2012) tested young and older healthy Spanish-English bilinguals with the MINT, and also with oral proficiency interviews, and demonstrated agreement between measures of language dominance and degree of bilingualism. Although performance on the MINT and the BNT was strongly correlated, the BNT underestimated Spanish proficiency, and did not accurately reflect degree of bilingualism and language dominance. Thus, the MINT seems more appropriate than the BNT for assessing bilingual language proficiency. However, the clinical utility of the MINT is currently unknown, and so we investigated this issue here.
Theoretically, picture naming ability can also be used to gain insights into the nature of linguistic impairments in AD. Picture names vary on many characteristics, such as frequency of occurrence, or the ease with which they evoke a mental image (imageability). Thus, one can look into whether any of these characteristics successfully predicts naming accuracy differences between patients and controls for individual pictures. In other words, if it turned out that, for example, frequency successfully predicted patient-control differences, this would mean that these differences were smaller for high-frequency words than for low-frequency words. Such analyses can shed light on the linguistic impairments in AD. For example, a current debate in the field concerns whether naming deficits in AD are primarily caused by permanent loss of semantic knowledge (e.g., Hodges, Salmon, & Butters, 1992), or by difficulties with lexical access and retrieval with intact semantic knowledge (Balota & Duchek, 1991). If naming difficulties—and specifically differences in naming accuracy between patients and controls—are predicted by semantic properties of the objects to be named (such as imageability: Plaut & Shallice, 1993), this would provide evidence of semantic degradation in AD. Conversely, if naming deficits in AD are (also or only) predicted by aspects known to affect name retrieval (such as word frequency: Jescheniak & Levelt, 1994; Kittredge, Dell, Verkuilen, & Schwartz, 2008), this would provide evidence of a retrieval deficit.
Previous studies, however, have produced mixed evidence about the influence of picture name properties on naming in AD. In some studies, word frequency predicted naming success of patients with probable AD (together with age of acquisition and familiarity: Cuetos, Gonzalez-Nosti, & Martinez, 2005; Cuetos, Rosci, Laiacona, & Capitani, 2008; Kemmerer & Tranel, 2000; Kremin et al., 2001; but see Astell & Harley, 1998). However, in one study, frequency affected the performance of AD patients disproportionally relative to controls (Thompson-Schill, Gabrieli, & Fleischman, 1999), while in others this was not the case (Gale, Irvine, Laws, & Ferrissey, 2009; Kemmerer & Tranel, 2000). Some of the same studies that reported robust frequency effects found no influence of imageability on naming performance in AD (Astell & Harley, 1998; Cuetos et al., 2005, 2008).
THE PRESENT STUDY
The present study is a detailed exploration of the performance differences between AD patients and healthy controls, as indicated by a naming test (the MINT), which has not been used before in clinical settings. Our first goal was to establish to what extent the MINT could reflect the naming differences between monolinguals and bilinguals with probable AD and controls, as well as between patients with amnestic mild cognitive impairment (MCI) and controls, while at the same time assessing bilingual language characteristics. Using the MINT in clinical settings could have several benefits. First, it might help reduce the length of neuropsychological testing batteries for bilinguals by providing information both about possible disease effects and about bilinguals’ language profile. Second, if the MINT could also be used with monolingual English speakers, this might facilitate research by enabling comparisons across different populations and testing centers. Third, the MINT could potentially be used with monolingual speakers of languages other than English (specifically Spanish, Mandarin Chinese, and Hebrew), which makes its potential application broader than that of picture naming tests designed only for monolingual English speakers.
We predicted, however, that the full set of MINT items would be sub-optimal for detecting dementia-related naming deficits, as this goal would require items of different difficulty level than assessment of bilingualism. For assessing bilingual language proficiency, items of a broad range of difficulty are needed as low-proficiency bilinguals might not know very difficult items in their non-dominant language. In contrast, for detecting subtle naming deficits associated with the initial stages of AD, more difficult items are best as even impaired patients would be able to name medium-difficulty and easy items. Hence, we then determined a subset of the full MINT comprised of those items that are optimally sensitive to naming deficits in AD.
Our second goal was to investigate which picture name properties best predicted naming differences between monolingual patients and controls for each of the MINT items, to shed light on the underlying cause of naming deficits in AD. To this end, we performed linear regression analyses with the MINT item properties as independent variables, and accuracy differences between patients and controls for each item as the dependent variable. Specifically, we asked whether lexical frequency (Barry, Morrison, & Ellis, 1997; Oldfield & Wingfield, 1965), imageability (Coltheart, 1981; Ellis & Morrison, 1998), contextual diversity (Adelman, Brown, & Quesada, 2006), and number of senses (Fellbaum, 2005) would predict which MINT items produce the largest difference between patients and controls. We explored these specific variables for several reasons. First, given the mixed results in the literature, we wanted to know whether naming differences between patients and controls would be predicted by word frequency, which would point to a retrieval deficit in AD. Alternatively, naming differences might be predicted by imageability, which would point to deficits in semantic knowledge in AD. Indeed, imageability has not yet been found to have an influence on naming in AD (Astell & Harley, 1998; Cuetos et al., 2005, 2008). However, we consider that these studies do not provide conclusive evidence for the role of imageability because they tested a small number of patients (at most 12), focused on error types rather than accuracy (Astell & Harley, 1998), or included only highly imageable picture names (Cuetos et al., 2005: 6.19 to 7.00 on a 7-point scale), which might not have been sufficiently sensitive to the influence of this variable.
Contextual diversity is a measure of the number of different contexts in which a word occurs. We included contextual diversity in our analyses for the following reason. It has long been known that word frequency exerts an influence on both naming speed and naming accuracy, such that high-frequency words are named faster and more accurately than low-frequency words (Jescheniak & Levelt, 1994; Kittredge et al., 2008; Oldfield & Wingfield, 1965). However, it has recently been proposed (Adelman et al., 2006) that it is contextual diversity, not word frequency, that ultimately determines naming speed in young and elderly healthy adults. Here, we considered the possibility that contextual diversity and not frequency might also account for naming accuracy, and, specifically, the accuracy differences between patients and controls for individual picture names. If so, this would have implications about the nature of naming impairments in AD. Contextual diversity is a complex measure that reflects the range of concepts associated with a word’s semantic representation through contextual co-occurrence. Thus, if contextual diversity better predicts naming deficits than frequency, this would point to a greater importance of semantic networks than of simple frequency counts (“ignorant” of semantic representations) for patients’ performance.
Finally, we also included the number of senses (meanings) of MINT items as a predictor in our analyses because of a possible confound of this variable with contextual diversity: The more senses a word has, the more contexts it will occur in (e.g., hand can appear in a scientific context in its sense of “one of two sides of an issue”, in a card-playing context in its sense of “the cards held in a card game”, etc.). Additionally, number of senses might be of interest here in its own right: Given its semantic character, its potential influence on AD patients’ naming performance might shed further light on the nature of patients’ linguistic deficit.
METHOD
Participants
Participants were tested on the MINT during their annual evaluation, which forms part of their participation in a longitudinal study of cognitive impairments in AD at the University of California, San Diego (UCSD) Alzheimer’s Disease Research Center (ADRC). Patients are recruited to the ADRC mostly via San Diego neurologists who refer patients with possible memory deficits to the center. We included 130 monolingual English speakers in our analyses: 68 with a diagnosis of probable AD, 18 with amnestic mild cognitive impairment (MCI), and 44 cognitively healthy controls. We also included 29 Spanish-English bilinguals: 18 with probable AD and 11 cognitively healthy. There were no sampling differences between monolingual and bilingual participants. Diagnoses were made using criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA; McKhann et al., 1984). Twenty-six monolinguals and 5 bilinguals were in a mild stage of the disease (DRS scores between 115 and 137), 38 monolinguals and 2 bilinguals were in a moderate stage (DRS scores between 95 and 123), and 4 monolinguals and 4 bilinguals were in a severe stage (DRS scores between 83 and 106). Participants with amnestic MCI were classified according to criteria developed by Petersen et al. (1999).
Table 1 summarizes the participants’ characteristics. Within each language group, patients were matched with the controls for age and education (anchored by degree level completed, e.g., 12 years for high school, 16 for a Bachelor’s degree, 18 for a Master’s degree; for t-tests see Table 1). All bilinguals reported being exposed to Spanish from birth and were classified into language dominance groups using self-report.
Table 1.
Means, standard deviations and comparisons for all participants’ characteristics
| Monolingual | Bilingual | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| AD (n =68) | MCI (n =18) | NC (n = 44) | F/t | p | AD (n =11) | NC (n =18) | t | p | ||||||
|
|
|
|||||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | |||||
| Age | 77.3 | (8.8) | 75.2 | (7.9) | 78.3 | (8.6) | <1 | .45 | 78.3 | (8.1) | 80.2 | (7.7) | <1 | .52 |
| Education | 15.2 | (2.4) | 16.1 | (2.3) | 15.9 | (2.6) | 1.55 | .22 | 12.1 | (4.1) | 13.3 | (3.6) | <1 | .40 |
| Sex (% Females) | 50% | — | 28% | — | 44% | — | — | — | 64% | — | 61% | — | — | — |
| DRS | 114 | (12.3) | 129.7 | (7.9) | 140.1 | (3.3) | NC vs. MCI: 5.37 | <.001 | 112.8 | (15.5) | 135.4 | (5.1) | 4.68 | <.001 |
| MCI vs. AD: 6.59 | <.001 | |||||||||||||
| MMSE | 22.6 | (3.7) | 27.4 | (2.2) | 29.2 | (0.9) | NC vs. MCI: 3.30 | <.01 | 21.2 | (4.1) | 29.4 | (0.9) | 6.52 | <.001 |
| MCI vs. AD: 6.91 | <.001 | |||||||||||||
| Age of regular English use | — | — | — | — | — | — | — | — | 6.8 | (8.3) | 9.4a | (9.7)a | <1 | .49 |
| % daily English use | — | — | — | — | — | — | — | — | 66% | (32%) | 55% | (40%) | <1 | .44 |
| English proficiencyb | — | — | — | — | — | — | — | — | 5.9 | (1.5) | 5.6 | (1.5) | <1 | .69 |
| Age of regular Spanish use | — | — | — | — | — | — | — | — | 0.7 | (1.3) | 0.2 | (0.6) | 1.31 | .21 |
| Spanish proficiency | — | — | — | — | — | — | — | — | 5.4 | (1.5) | 5.9 | (1.2) | 1.06 | .30 |
| % Spanish-dominantc | — | — | — | — | — | — | — | — | 36% | — | 39% | — | — | — |
| Dom. lang. proficiency | — | — | — | — | — | — | — | — | 6.47 | (0.64) | 6.71 | (0.47) | <1 | .45 |
| Non-dom. lang. proficiency | — | — | — | — | — | — | — | — | 4.70 | (1.71) | 4.72 | (1.15) | <1 | .85 |
AD =patients with Alzheimer’s disease; MCI =patients with amnestic Mild Cognitive Impairment; NC =normal controls; DRS =the Dementia Rating Scale (Mattis, 1988); MMSE =the Mini Mental State Examination (Folstein et al., 1975); Dom. lang. =dominant language; Non-dom. lang. =non-dominant language.
There were three missing data points for the age of regular English use for the Spanish-dominant controls.
Proficiency level is averaged across self-ratings for four types of language use (speaking, comprehension of spoken speech, reading, writing) using a scale of 1–7 (1 means “little to no knowledge” and 7 means “like a native speaker”).
Dominant language was established on the basis of preferred language of testing.
The study procedures conformed to Federal guidelines for the protection of human subjects and were approved by the UCSD Institutional Review Board. Informed consent was obtained from cognitively intact controls and from patients and caregivers (usually the next of kin) before neuropsychological testing and after the procedures of the study had been fully explained.
Materials
The MINT (Gollan et al., 2012) consists of 68 black-and-white line drawings. Since the test was designed to assess both dominant and non-dominant language performance, it includes a greater proportion of medium-difficulty items than is typical for naming tests designed for monolinguals (e.g., the BNT; Kaplan et al., 1983). (For a list of MINT items, see Appendix. For a full description of the MINT item characteristics, see Table 2 and Gollan et al., 2012.) Participants were tested on a 30-item version of the BNT (for a full description, see Kaplan et al., 1983) as part of their annual neuropsychological evaluation at the ADRC.
APPENDIX.
List of the items comprising the MINT, and item scores by group
| Item | Monolingual | Bilingual | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Item# | English name | Spanish name | AD | MCI | NC | AD | NC | ||||||
|
| |||||||||||||
| English | Spanish | English | Spanish | ||||||||||
|
| |||||||||||||
| Dom | Ndom | Dom | Ndom | Dom | Ndom | Dom | Ndom | ||||||
| 1 | hand | mano | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | dog | perro | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.83 | 1.00 | 1.00 | 1.00 | 0.91 |
| 3 | tree | árbol | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.83 | 1.00 | 1.00 | 1.00 | 1.00 |
| 4 | bed | cama | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 5 | door | puerta | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6 | sun | sol | 0.97 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 0.83 | 1.00 | 1.00 | 1.00 | 1.00 |
| 7 | book | libro | 0.97 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 0.83 | 1.00 | 0.86 | 1.00 | 1.00 |
| 8 | butterfly* | mariposa | 0.87 | 1.00 | 1.00 | 0.71 | 0.75 | 1.00 | 0.33 | 1.00 | 0.57 | 1.00 | 0.55 |
| 9 | scissors | tijeras | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.86 | 1.00 | 0.91 |
| 10 | key | llave | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 11 | chair | silla | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 12 | moon | luna | 0.99 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13 | airplane | avión (aeroplano) | 0.96 | 0.94 | 0.98 | 1.00 | 1.00 | 1.00 | 0.67 | 1.00 | 0.57 | 1.00 | 0.91 |
| 14 | apple | manzana | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.91 |
| 15 | fish | pescado (pez) | 0.94 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.91 |
| 16 | grapes | uvas | 0.94 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 1.00 | 1.00 | 0.86 | 1.00 | 0.91 |
| 17 | horse | caballo | 1.00 | 1.00 | 1.00 | 1.00 | 0.75 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18 | drum | tambor (tambora) | 0.97 | 1.00 | 1.00 | 1.00 | 0.75 | 1.00 | 0.50 | 1.00 | 0.43 | 1.00 | 0.73 |
| 19 | glove* | guante | 0.91 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.83 | 1.00 | 0.86 | 1.00 | 0.91 |
| 20 | lightbulb* | foco | 0.85 | 1.00 | 0.93 | 0.86 | 1.00 | 1.00 | 0.33 | 1.00 | 0.43 | 1.00 | 0.55 |
| 21 | cake | pastel | 0.96 | 1.00 | 1.00 | 1.00 | 0.75 | 0.50 | 0.50 | 1.00 | 0.86 | 1.00 | 0.64 |
| 22 | watch* | reloj | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.91 | 1.00 | 1.00 | 0.91 |
| 23 | bear | oso | 0.94 | 1.00 | 1.00 | 1.00 | 0.75 | 0.75 | 0.67 | 1.00 | 0.71 | 1.00 | 1.00 |
| 24 | fork | tenedor | 0.96 | 1.00 | 1.00 | 1.00 | 0.50 | 1.00 | 0.83 | 1.00 | 0.71 | 0.86 | 1.00 |
| 25 | hat | sombrero | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.83 | 1.00 | 1.00 | 1.00 | 1.00 |
| 26 | leaf | hoja | 0.96 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 0.83 | 0.91 | 0.57 | 1.00 | 0.82 |
| 27 | tie | corbata | 0.87 | 1.00 | 0.93 | 0.71 | 0.75 | 1.00 | 0.60 | 0.73 | 0.86 | 1.00 | 0.91 |
| 28 | candle* | vela (candela, veladora) | 0.91 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.67 | 1.00 | 0.71 | 1.00 | 0.82 |
| 29 | basket | canasta (canasto) | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.50 | 1.00 | 0.71 | 0.86 | 0.91 |
| 30 | clown* | payaso | 0.69 | 1.00 | 0.93 | 1.00 | 0.75 | 1.00 | 0.67 | 0.91 | 0.43 | 1.00 | 0.73 |
| 31 | kite* | papalote (cometa) | 0.91 | 0.94 | 1.00 | 0.71 | 0.75 | 1.00 | 0.00 | 0.91 | 0.43 | 0.57 | 0.45 |
| 32 | rainbow* | arco iris | 0.82 | 1.00 | 1.00 | 0.57 | 0.25 | 1.00 | 0.17 | 1.00 | 0.71 | 1.00 | 0.55 |
| 33 | witch* | bruja | 0.88 | 1.00 | 0.98 | 0.86 | 1.00 | 1.00 | 1.00 | 1.00 | 0.57 | 1.00 | 0.91 |
| 34 | seesaw* | subibaja (columpio) | 0.43 | 0.61 | 0.75 | 0.29 | 0.33 | 1.00 | 0.00 | 0.64 | 0.00 | 0.71 | 0.27 |
| 35 | flashlight* | linterna | 0.82 | 0.94 | 0.95 | 0.71 | 0.50 | 0.25 | 0.00 | 1.00 | 0.43 | 0.43 | 0.09 |
| 36 | cloud | nube | 0.96 | 1.00 | 1.00 | 0.86 | 0.75 | 1.00 | 0.67 | 1.00 | 0.43 | 1.00 | 0.91 |
| 37 | iron | plancha | 0.94 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 1.00 | 1.00 | 0.57 | 1.00 | 0.91 |
| 38 | feather | pluma | 0.94 | 1.00 | 0.98 | 0.71 | 0.75 | 1.00 | 0.67 | 1.00 | 0.57 | 1.00 | 0.82 |
| 39 | peacock* | pavo real | 0.47 | 0.56 | 0.80 | 0.14 | 0.25 | 0.50 | 0.50 | 0.73 | 0.43 | 0.71 | 0.45 |
| 40 | bridge | puente | 1.00 | 1.00 | 1.00 | 0.86 | 1.00 | 1.00 | 0.67 | 1.00 | 0.43 | 1.00 | 0.91 |
| 41 | bone | hueso | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.71 | 1.00 | 0.91 |
| 42 | snail* | caracol | 0.62 | 0.89 | 0.93 | 0.29 | 0.00 | 1.00 | 0.17 | 0.91 | 0.29 | 1.00 | 0.40 |
| 43 | zipper | zíper (cierre) | 0.96 | 1.00 | 1.00 | 1.00 | 0.75 | 1.00 | 1.00 | 1.00 | 0.71 | 0.86 | 0.91 |
| 44 | lock | candado | 0.87 | 1.00 | 0.89 | 0.57 | 0.25 | 1.00 | 0.50 | 0.91 | 0.43 | 1.00 | 0.70 |
| 45 | whale* | ballena | 0.79 | 1.00 | 1.00 | 0.29 | 0.50 | 1.00 | 0.50 | 1.00 | 0.43 | 0.86 | 0.73 |
| 46 | nurse | enfermera | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 0.75 | 0.67 | 1.00 | 0.57 | 1.00 | 0.73 |
| 47 | cage* | jaula | 0.88 | 1.00 | 1.00 | 0.86 | 0.75 | 1.00 | 0.40 | 1.00 | 0.29 | 1.00 | 0.64 |
| 48 | arrow | flecha | 0.94 | 1.00 | 1.00 | 0.71 | 0.75 | 0.75 | 0.33 | 0.91 | 0.43 | 1.00 | 0.73 |
| 49 | rake | rastrillo | 0.93 | 1.00 | 0.98 | 0.71 | 0.00 | 0.75 | 0.33 | 1.00 | 0.29 | 0.43 | 0.55 |
| 50 | saw | serrucho (sierra) | 0.96 | 1.00 | 1.00 | 0.86 | 0.50 | 1.00 | 0.50 | 1.00 | 0.29 | 1.00 | 0.73 |
| 51 | nest* | nido | 0.76 | 1.00 | 0.98 | 0.86 | 0.75 | 1.00 | 0.50 | 1.00 | 0.43 | 1.00 | 0.82 |
| 52 | plug* | enchufe | 0.88 | 0.94 | 0.95 | 0.86 | 0.50 | 0.75 | 0.00 | 0.91 | 0.14 | 0.57 | 0.36 |
| 53 | wig* | peluca | 0.65 | 0.94 | 0.93 | 0.57 | 0.75 | 0.75 | 0.00 | 1.00 | 0.29 | 0.86 | 0.45 |
| 54 | screw* | tornillo | 0.90 | 1.00 | 0.98 | 1.00 | 0.75 | 1.00 | 0.50 | 1.00 | 0.43 | 1.00 | 0.91 |
| 55 | king | rey | 0.93 | 1.00 | 0.98 | 0.71 | 1.00 | 1.00 | 0.83 | 1.00 | 0.57 | 1.00 | 0.91 |
| 56 | scarf* | bufanda | 0.75 | 0.94 | 1.00 | 0.71 | 0.25 | 0.75 | 0.33 | 0.91 | 0.29 | 1.00 | 0.55 |
| 57 | well* | pozo | 0.79 | 1.00 | 0.98 | 0.71 | 0.75 | 0.50 | 0.00 | 1.00 | 0.29 | 0.86 | 0.55 |
| 58 | dustpan* | recogedor | 0.62 | 0.67 | 0.98 | 0.71 | 0.50 | 1.00 | 0.00 | 0.91 | 0.14 | 1.00 | 0.27 |
| 59 | parachute* | paracaídas | 0.66 | 0.78 | 0.95 | 0.57 | 0.50 | 0.75 | 0.00 | 0.91 | 0.14 | 0.86 | 0.55 |
| 60 | blind* | persiana | 0.68 | 0.89 | 0.98 | 0.29 | 0.50 | 0.75 | 0.00 | 0.80 | 0.29 | 0.43 | 0.27 |
| 61 | hinge* | bisagra | 0.56 | 0.72 | 0.95 | 0.57 | 0.00 | 0.50 | 0.00 | 0.82 | 0.14 | 0.43 | 0.18 |
| 62 | funnel* | embudo | 0.62 | 0.94 | 0.98 | 0.57 | 0.00 | 0.75 | 0.33 | 0.64 | 0.14 | 1.00 | 0.55 |
| 63 | gauge (manometer, barometer)* | medidor | 0.54 | 0.72 | 0.82 | 0.71 | 0.00 | 0.25 | 0.17 | 0.91 | 0.14 | 0.14 | 0.27 |
| 64 | porthole* | portilla | 0.34 | 0.72 | 0.82 | 0.29 | 0.00 | 0.00 | 0.00 | 0.73 | 0.00 | 0.00 | 0.00 |
| 65 | anvil* | yunque | 0.43 | 0.72 | 0.75 | 0.00 | 0.00 | 0.25 | 0.17 | 0.60 | 0.00 | 0.43 | 0.09 |
| 66 | mortar* | molcajete (mortero) | 0.15 | 0.50 | 0.61 | 0.00 | 0.00 | 0.25 | 0.00 | 0.36 | 0.14 | 0.57 | 0.36 |
| 67 | pestle* | mano de mortero (mano) | 0.33 | 0.67 | 0.67 | 0.14 | 0.00 | 0.00 | 0.00 | 0.55 | 0.00 | 0.00 | 0.00 |
| 68 | axle* | eje | 0.56 | 0.72 | 0.95 | 0.57 | 0.25 | 0.50 | 0.17 | 0.91 | 0.29 | 0.14 | 0.36 |
Items most sensitive to differences between patients and controls (comprising the 32-item subset of the MINT).
Note: AD =patients with probable Alzheimer’s disease; MCI =patients with amnestic Mild Cognitive Impairment; NC =normal controls; Dom =dominant language; Ndom =non-dominant language.
Table 2.
MINT picture name characteristics
| Frequencya | Log frequency | Imageabilityb | Cont. diversitya | Log cont. diversity | No. sensesc | |
|---|---|---|---|---|---|---|
| Mean | 4686 | 2.84 | 583 | 1144 | 2.56 | 4 |
| SD | 18680 | 0.89 | 67 | 1703 | 0.79 | 3 |
| Max | 152523 | 5.18 | 662 | 8146 | 3.91 | 15 |
| Min | 0 | 0 | 341 | 0 | 0 | 1 |
From the SUBTLEXus corpus (Brysbaert & New, 2009; http://expsy.ugent.be/subtlexus/), which contains 51 million words.
From N-Watch (Davis, 2005). Values were available for 55 out of the 68 MINT items.
From WordNet (Fellbaum, 2005).
Procedure
Participants were tested either at the ADRC or in their homes by a proficient Spanish-English bilingual psychometrist. They were instructed to name pictures first in their dominant language and then, for the bilingual group, in their non-dominant language. This was the first time these participants were tested on the MINT (whereas they had been tested on the BNT before as part of their annual examination in the ADRC longitudinal study). A 30-item version of the BNT (odd items for English-dominant bilinguals in both languages, and a combination of odd and even items for Spanish-dominant bilinguals in both languages) was administered in the middle of a 2- to 3-hour neuropsychological battery, as part of the Uniform Data Set (UDS; Morris et al., 2006). The MINT was administered at the end of the battery (thus the BNT and the MINT were separated by approximately 1 hour). Naming trials for both tests were administered according to the standardized BNT instructions (testing was discontinued after six failed naming trials, which included semantic or phonetic cueing for pictures not named spontaneously). Naming accuracy was recorded during testing. Participant demographics and language history had been obtained on previous testing sessions.
Scoring and Data Analyses
Subject analyses
We calculated the proportion of pictures named correctly for each participant, including pictures named spontaneously and those requiring a semantic (but not a phonetic) cue. For bilinguals, we also calculated bilingual index scores and language dominance scores. Bilingual index scores were obtained by dividing the lower of the scores for the two languages by the higher score. Bilingual index scores (see also Gollan et al., 2012) range from 0 to 1 and provide a measure of the extent to which knowledge of the two languages is balanced. For example, a bilingual who named 60 pictures in one language and only 30 in the other would obtain a bilingual index score of 0.5, indicating that they are a relatively unbalanced (50%) bilingual. In contrast, a bilingual who named the same number of pictures in both languages would have a bilingual index score of 1, indicating that they are completely balanced (100% bilingual). Language dominance measures were obtained by subtracting the Spanish from the English score (thus, a positive number indicates English dominance, and a negative number, Spanish dominance).
Items analysis
For each of the MINT items, we calculated the proportion of patients and controls who correctly named this item, and a difference score (e.g., if .80 of the patients and 1.00 of the controls produced the name for butterfly, the difference score for this item would be .20). Because we had a relatively small number of bilinguals, and because bilingualism could introduce additional mechanisms underlying naming deficits in AD, we excluded bilinguals from these analyses. We then performed regression analyses with the difference scores as the dependent variable, and log frequency, log contextual diversity, number of senses, and imageability of the MINT items, as predictor variables. Since imageability values were missing for 13 MINT items, we first performed analyses without including this variable. We report the variance explained when each variable was entered alone in a model, as well as the unique variance explained by each variable (the additional variance explained when a variable was entered in the second step of a hierarchical regression model, with all other variables entered in the first step). When calculating logarithm fits, we increased all counts by 1, to avoid problems from zero counts.
Item characteristics were obtained from the following sources. Frequency and contextual diversity values were extracted from the SUBTLEXus corpus (Brysbaert & New, 2009; http://expsy.ugent.be/subtlexus/), a corpus of American film subtitles consisting of 51 million words. We chose this corpus because it is more recent and better accounts for reaction times and accuracy rates than the traditionally used Kucera & Francis and Celex corpora. Number of senses counts were obtained from WordNet (Fellbaum, 2005), a lexical database of English consisting of 155,287 words organized into sets of cognitive synonyms (synsets) and containing a total of 206,941 word-sense pairs. Imageability ratings were obtained from N-Watch (Davis, 2005). They consist of combined ratings from the Bristol Norms (Stadthagen-Gonzalez & Davis, 2006), for which each of 1526 words was rated for Age of Acquisition, Familiarity, and Imageability by 20 undergraduates from the University of Bristol; and ratings from the MRC database (Coltheart, 1981), which in turn were formed by merging three smaller databases (Gilhooly & Logie, 1980; Paivio, Yuille, & Madigan, 1968; Toglia & Battig, 1978) and provide measures of imageability for 4334 words.
RESULTS
Subjects analysis
Full MINT
Table 3a reports the scores on all tests of the three groups of monolingual participants (AD, MCI, controls), and pairwise comparisons between groups. On the full MINT, there was a significant difference between the scores of patients with probable AD and controls, but not between the scores of MCI patients and controls. Table 3b reports the scores on all tests of bilingual patients with probable AD and controls broken down by test language (dominant, non-dominant), and pairwise comparisons between groups. On the full MINT, there was a significant difference between patients and controls in the dominant, but not in the non-dominant language.
Table 3a.
Means (proportion correct), standard deviations and pairwise comparisons of monolingual participants’ scores on the full MINT, the 32-item subset of the MINT, and a 30-item version of the BNT
| AD | MCI | NC | Difference | tb | pb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| M | SD | M | SD | M | SD | |||||
| full MINT | .83 | (.14) | .94 | (.05) | .96 | (.04) | NC – AD | .13 | 7.22 | <.001 |
| NC – MCI | .02 | 1.72 | .09 | |||||||
| 32-item MINT | .69 | (.22) | .87 | (.12) | .92 | (.08) | NC – AD | .23 | 8.05 | <.001 |
| NC – MCI | .05 | 2.03 | <.05 | |||||||
| 30-item BNT | .63 | (.22) | .84 | (.10) | .94 | (.07) | NC – AD | .31 | 10.67 | <.001 |
| NC – MCI | .10 | 4.45 | <.001 | |||||||
| Difference full MINT – 32-item MINT | .14 | .07 | .04 | |||||||
| tb | 12.90 | 4.69 | 5.95 | |||||||
| pb | .001 | <.001 | .001 | |||||||
AD =patients with probable Alzheimer’s disease; MCI =patients with amnestic Mild Cognitive Impairment; NC =normal controls.
This group included the four patients in a severe stage of the disease.
Values were corrected in cases of unequal variance between groups.
In sum, the MINT seems sensitive to naming impairments in monolinguals with probable AD (but not MCI), and bilinguals with probable AD when tested in their dominant language. An examination of Table 3 further indicates that the difference between patients and controls was larger for BNT scores than for MINT scores, as we predicted given that the MINT contains more easy and medium-difficulty items than the BNT. However, note that the majority of our participants had been tested with the BNT on multiple occasions (as part of the longitudinal study at the ADRC), whereas this was the first time they were tested with the MINT. Patients and controls may be differentially affected by prior testing sessions. For example, controls may be more likely to subsequently learn items they were not able to name on a particular testing session, and this would artificially inflate group differences. Therefore, we caution against a direct comparison of performance on the two tests in the present study.
32-item MINT
To improve the potential clinical utility of the MINT, we compared the naming scores of patients with probable AD to those of monolingual controls, to select the 32 MINT items which produced the largest differences between patients and controls (see Appendix). As predicted, these items tended to be more difficult MINT items. We then tested the utility of this 32-item subset by comparing naming scores of monolinguals with MCI to controls, and bilinguals with probable AD to controls in both languages. The 32-item subset produced a significant difference between monolinguals with MCI and controls, and between bilinguals with probable AD and controls in the dominant, but not in the non-dominant language (see Tables 3a and 3b). Thus, the 32-item MINT subset captured larger differences between these groups than the full MINT (and none of the tests reflected a difference between patients and controls in the non-dominant language).
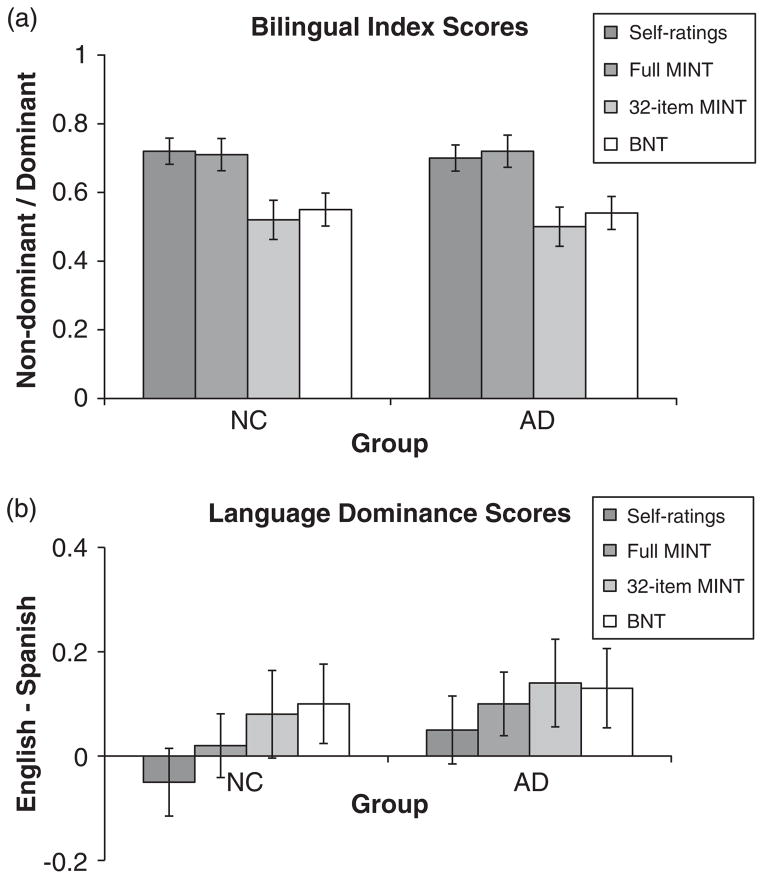
Bilingual index scores and language dominance
Having selected a 32-item subset of the MINT that is more sensitive to naming impairments than the full MINT, we then asked whether this same subset could be useful for assessing the bilingual language profile. For this purpose, we compared language dominance and bilingual index scores derived from the 32-item MINT, the full MINT, and self-rated proficiency in each language (see Figure 1; BNT scores are included for completeness). We hypothesized, however, that different items would be needed to capture naming impairments and language profile, and, thus, that the full MINT would be better suited to the latter goal than the 32-item MINT.
Fig. 1.
(a) Bilingual index scores derived from self-ratings, the full MINT, the 32-item MINT and the BNT. (b) Language dominance scores derived from self-ratings, the full MINT, the 32-item MINT and the BNT. MINT =Multilingual Naming Test; BNT =Boston Naming Test.
This hypothesis was confirmed. Bilingual index scores (Figure 1a) indicated that the 32-item MINT underestimated the degree of bilingualism of both patients with probable AD and controls relative to self-ratings [AD: t(9)1 =2.34, p <.05; normal controls (NC): t(18) =2.98, p <.01] and the full MINT [AD: t(9) =8.23, p <.001; NC: t(18) =7.40, p <.001]. Conversely, bilingual index scores for the full MINT and self-ratings agreed with one another for both patients and controls [both ts <1, both ps >.50] (and both of these were previously found to agree with an objective measure of language proficiency, oral proficiency interviews: Gollan et al., 2012). Also in accord with Gollan et al., the BNT, like the 32-item MINT, seemed to underestimate degree of bilingualism (see Figure 1a).
Language dominance scores (Figure 1b) indicated that the 32-item MINT (as well as the BNT) presented controls, but not patients with probable AD, as slightly more English-dominant than self-ratings [AD: t <1; NC: t(17) =2.00, p =.06], while the full MINT agreed with self-ratings for both groups [AD: t <1; NC: t(17) =1.43, p =.17]. The 32-item MINT and the full MINT did not differ from each other for either patients or controls [AD: t <1; NC: t(17) =1.64, p =.12].
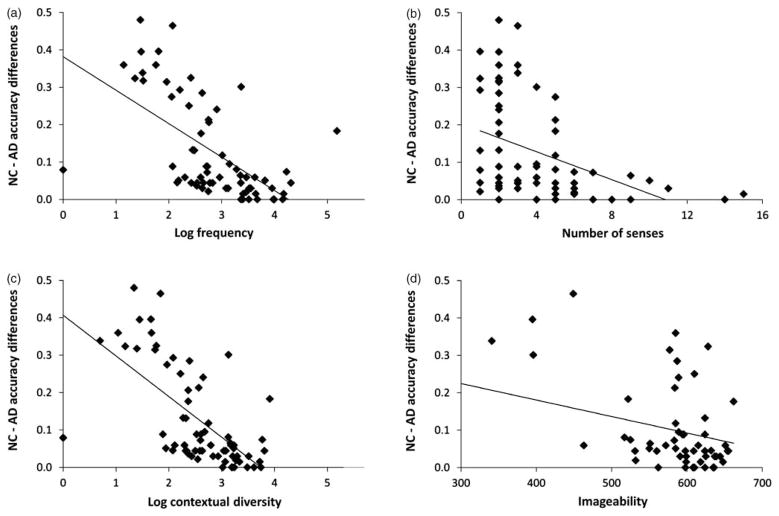
Items analysis: predicting naming deficits in AD
Having shown that the MINT is sensitive to naming differences between AD patients and controls, we next investigated which MINT item characteristics best explained these differences. The results of the regression analyses are reported in Table 4 and Figure 2, and the correlations between the different characteristics, in Table 5. All four variables were highly robust predictors of naming deficits when entered alone. However, when entered together, only contextual diversity (for all 68 items) and contextual diversity and imageability (for 55 items) were significant predictors of the magnitude of the difference between patients and controls in naming scores. Interestingly, when contextual diversity was entered as a predictor, the effect of frequency was only marginally significant or not significant, and actually went in the opposite direction (bigger differences between patients and controls for higher-frequency words). We discuss these findings below.
Table 3b.
Means (proportion correct) standard deviations and pairwise comparisons of bilingual participants’ scores on the full MINT, the 32-item subset of the MINT, and a 30-item version of the BNT
| AD | NC | Difference | ta | pa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Dom | Ndom | Dom | Ndom | |||||||||
|
| ||||||||||||
| M | SD | M | SD | M | SD | M | SD | |||||
| Full MINT | .80 | (.13) | .60b | (.13) | .91 | (.06) | .64 | (.22) | NC – AD (Dom) | .11 | 2.96 | <.01 |
| NC – AD (Ndom) | .04 | <1b | .53b | |||||||||
| 32-item MINT | .66 | (.17) | .36b | (.17) | .82 | (.12) | .43 | (.27) | NC – AD (Dom) | .16 | 3.09 | <.01 |
| NC – AD (Ndom) | .07 | <1b | .38b | |||||||||
| 30-item BNT | .62 | (.22) | .34 | (.18) | .85 | (.15) | .47 | (.23) | NC – AD (Dom) | .23 | 3.33 | <.01 |
| NC – AD (Ndom) | .13 | 1.61 | .12 | |||||||||
| Difference full MINT – 32-item MINT | .14 | .24 | .09 | .21 | ||||||||
| ta | 10.43 | 13.42b | 6.02 | 11.22 | ||||||||
| pa | <.001 | <.001b | .001 | <.001 | ||||||||
AD =patients with probable Alzheimer’s disease; NC =normal controls; Dom =dominant language scores; Ndom =non-dominant language scores.
Values were corrected in cases of unequal variance between groups.
MINT scores in the non-dominant language were missing for one AD participant.
Fig. 2.
Correlations between the accuracy differences (in proportions) between AD patients and controls for each of the MINT items, and the word frequency (a), contextual diversity (b), number of senses (c) and imageability (d) of the items. AD – patients with probable Alzheimer’s disease; NC – normal controls.
Table 4.
Results of multiple regression analyses for the difference in naming performance between patients with probable AD and controls
| Variable | β | t | R2 | ΔR2(%) |
|---|---|---|---|---|
| All items | ||||
| Log Freq | −.598 | −6.06*** | .357 | |
| Log CD | −.645 | −6.86*** | .416 | |
| No. Senses | −.413 | −3.68*** | .170 | |
| Log Freq unique | .914 | 1.95† | .453 | 3.2 |
| Log CD unique | −1.49 | −3.18** | “ | 8.6 |
| No. Senses unique | −.097 | −.875 | “ | 0.6 |
| 55 items | ||||
| Imageability | −.543 | −4.71*** | .295 | |
| Imageability unique | −.372 | −3.77*** | .608 | 11.2 |
Note. Log Freq, Log CD, No. senses, and Imageability rows report the variance explained when each variable was entered alone in a regression model. “Unique” rows report the additional variance explained when a variable was entered in the second step of a hierarchical regression model, with all other variables entered in the first step.
p <1;
p <.01;
p <.001.
DISCUSSION
In this study, we examined the naming performance of individuals with probable AD relative to healthy controls on a picture naming test (the MINT), originally designed to assess naming skills and degree of bilingualism in speakers of multiple languages. Our first goal was to evaluate the potential clinical utility of this test. We found that the full MINT captured differences between AD patients and controls, but not between MCI patients and controls. A specially selected 32-item subset of the MINT, on the other hand, successfully captured performance differences between all patient and control groups (note that neither of the tests distinguished between bilingual patients and controls in their non-dominant language, in accord with Gollan et al., 2010). As we predicted, however, the 32-item MINT was less accurate than the full MINT in assessing bilingual language characteristics. This is because the 32-item MINT comprises many of the most difficult items from the MINT (see Appendix), which are optimal for distinguishing patients from controls but not for accurately assessing degree of bilingualism.
The second goal of this study was to shed more light on the nature of naming deficits in AD. For this purpose, we explored which of several MINT item characteristics (frequency, imageability, contextual diversity, number of senses) best predicted differences in naming scores between monolinguals with AD and controls. We found that the extent of naming impairment was significantly predicted by each of these factors alone, but, when analyzed together, only by contextual diversity and imageability, but not by number of senses or frequency. Specifically, consistently with the findings of Adelman et al. (2006), when controlling for frequency, contextual diversity had explanatory power, but when controlling for contextual diversity, frequency had no explanatory power, or had an inhibitory effect. Additionally, we showed that the contextual diversity effects we observed were not an artifact of words’ number of senses.
These results highlight the importance of meaning for object naming success in AD. Names of high contextual diversity (which elicited smaller differences between patients and controls) might be associated with a larger number of concepts, through their contextual co-occurrence, than items with names of low contextual diversity. Arguably, through their associations with other concepts, high contextual diversity items would have more subtle aspects of meaning and, hence, more semantic features. Likewise, high imageability items (which also elicited smaller differences between patients and controls) are the ones evoking a mental image with greater ease; hence, they would have conceptual representations with more semantic features than low imageability items (since more semantic features would make a mental image more defined). Importantly, contextual diversity and imageability accounted for different portions of the variance of naming deficits, perhaps because they entail different kinds of semantic information: relational, in the case of contextual diversity, and experientially grounded, in the case of imageability.
Our results support the proposal that naming deficits in AD arise because of damage to the integrity of semantic memory (Hodges et al., 1992; Salmon, Heindel, & Lange, 1999), insofar as we found an influence on patients’ naming success of variables associated with semantic information (contextual diversity and imageability) but not of a variable associated with retrieval (frequency) in the presence of the other variables. Indeed, our findings might also be compatible with a retrieval deficit account (Balota & Duchek, 1991; Balota et al., 1999; see also Nebes, 1989). According to such a view, words of high contextual diversity, whose conceptual representations are linked to a large number of concepts (e.g., door) could be retrieved through many different routes (by accessing any of those words whose concepts are linked to the concept for that word, e.g., house, car, restaurant, etc). What our analyses suggest, however, is that the existence of semantic networks (and, therefore, alternative access routes) is more important than the sheer number of “semantically ignorant” frequency counts for naming success in AD.
In any case, it is important to note that contextual diversity effects have not been studied extensively in the context of language production or naming impairments in AD. Thus, the locus of processing at which contextual diversity might affect naming performance is not known. What we have shown here is that the measure traditionally associated with lexical retrieval in models of language production (frequency) does not retain its explanatory power when entered in a regression together with a variable that captures the number of different types of circumstances that surround a word’s occurrence (contextual diversity). We suggest that these effects are probably better construed as stemming from a semantic than from a retrieval deficit, but additional research is needed to support this conclusion. Importantly, without controlling for contextual diversity, naming performance deficits in AD were predicted by both frequency and imageability (see Figure 2)—and both of these variables explain unique variance when entered together in a hierarchical regression model (without contextual diversity). Thus, although we suggest that a deficit to the semantic system is an important factor contributing to AD naming impairments, such impairments are likely caused by a host of different factors, and are unlikely to be captured by a unidimensional explanation.
Our interpretation of the items analyses reported here seems to conflict with the proposal of Gollan et al. (2010) that the dominant language might be more sensitive to cognitive decline in AD than the non-dominant language because it has richer semantic representations than the non-dominant language. Here, however, we suggested that words with richer semantic representations (in monolingual speakers) seem to be less affected by the disease.
One way to reconcile the two studies would be to assume that, although the bilinguals in Gollan et al. (and the present study) could name relatively few pictures in their non-dominant language, the names they did know in this language were richly and robustly represented (more so than some rarely used words from the dominant language). This might be for various reasons, for example, because the relatively “easy” (frequent and familiar) words they know in the non-dominant language are used in diverse contexts, and hence are associated with many concepts. Also, assuming that words in the two languages have common (core) semantic representations (De Bot, 1992; Green, 1986, 1998; Poulisse & Bongaerts, 1994), the easy non-dominant-language words might “inherit” robust representations from the dominant language. These explanations are consistent with the performance of the highly proficient bilinguals in Costa et al. (2012), who showed a similar deterioration of both languages. However, due to the relative paucity of studies speaking to this issue, these accounts are at present fully speculative. The study by Gollan et al. (2010) is to date the only one to examine how AD affects the two languages of non-balanced bilinguals, and additional work is needed to confirm the pattern of dual language decline for such bilinguals.
The results of the current study demonstrate the potential power inherent in tools for collecting measures of performance which are aimed at speakers of more than one language. Evaluating the potential use of a multilingual test with monolinguals also allowed us to draw broader conclusions about the nature of cognitive impairment in Alzheimer’s disease.
Table 5.
Pearson bivariate correlations between the different MINT item characteristics
| Log Freq | Log CD | No. Senses | |
|---|---|---|---|
| Log CD | .98 (p <.001)1 | ||
| No. Senses | .55 (p <.001) | .55 (p <.001) | |
| Imageability | .16 (p =.25) | .25 (p =.07) | .11 (p =.43) |
The high correlation between log frequency and log contextual diversity should not pose a problem for such analyses. First, in instances of high collinearity, estimated coefficients are unbiased, only subject to higher error. This reduces power but does not inflate Type I error rates. Furthermore, Adelman et al. (2006) showed that, when holding log contextual diversity constant, there was little or no effect of log frequency on lexical decision and picture naming reaction times, while holding log frequency constant evidenced a consistent facilitatory effect. In the present study, we were not able to carry out such analyses due to the relatively small number of MINT items (68).
Acknowledgments
This research was supported by an R01 from NICHD (HD050287) and an R01 from NIDCD (R01 DC011492) awarded to Tamar H. Gollan, and by a P50 (AG05131) from NIH/NIA to the University of California. Big thanks go to Sarah Espinoza, Sandra Jerkins and Chi Kim for data collection, and to Rosa I. Montoya for data coding.
Non-exclusive permission is granted to use extracts from the paper: “Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals,” by Tamar H. Golan, Gali H. Weissberger, Elin Runnqvist, Rosa I. Montoy and Cynthia M. Cera Bilingualism: Language and Cognition, Volume 15, Issue 03 (July 2012), pp. 594–615, to be used in the paper “The Multilingual Naming Test in Alzheimer’s Disease: Clues to the Origin of Naming Impairments,” by Iva Ivanova, David P. Salmon, and Tamar H. Gollan, in the Journal of the International Neuropsychological Society. Copyright © 2012 Cambridge University Press.
Footnotes
MINT scores in the non-dominant language were missing for one AD participant.
The authors declare no conflict of interest.
References
- Adelman JS, Brown GDA, Quesada JF. Contextual diversity, not word frequency, determines word-naming and lexical decision times. Psychological Science. 2006;17:814–823. doi: 10.1111/j.1467-9280.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Allegri RF, Mangone CA, Fernández-Villavicencio A, Rymberg S, Taragano F, Baumann D. Spanish Boston Naming Test norms. Clinical Neuropsychology. 1997;11:416–420. [Google Scholar]
- Alzheimer’s Association. Hispanics/ Latinos and Alzheimer’s disease. 2004 Retrieved from http://www.alz.org/national/documents/report-hispanic.pdf.
- Alzheimer’s Association. Alzheimer’s disease facts & figures. Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association. 2008;4:110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Astell AJ, Harley TA. Naming problems in dementia: Semantic or lexical? Aphasiology. 1998;12:357–374. [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, III, McDermott KB, Yerys BE. Veridical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Balota DA, Duchek JM. Semantic priming effects, lexical repetition effects, and contextual disambiguation effects in healthy aged individuals and individuals with senile dementia of the Alzheimer type. Brain and Language. 1991;40:181–201. doi: 10.1016/0093-934x(91)90124-j. [DOI] [PubMed] [Google Scholar]
- Barry C, Morrison CM, Ellis AW. Naming the Snodgrass and Vanderwart pictures: Effects of age of acquisition, frequency, and name agreement. Quarterly Journal of Experimental Psychology. 1997;50A:560–585. [Google Scholar]
- Brysbaert M, New B. Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behavior Research Methods. 2009;41:977–990. doi: 10.3758/BRM.41.4.977. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Costa A, Calabria M, Marne P, Hernández M, Juncadella M, Gascón-Bayarri J, Reñé R. On the parallel deterioration of lexico-semantic processes in the bilinguals’ two languages: Evidence from Alzheimer’s disease. Neuropsychologia. 2012;50:740–753. doi: 10.1016/j.neuropsychologia.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Cuetos F, Gonzalez-Nosti M, Martínez C. The picture-naming task in the analysis of cognitive deterioration in Alzheimer’s disease. Aphasiology. 2005;19:545–557. [Google Scholar]
- Cuetos F, Rosci C, Laiacona M, Capitani E. Different variables predict anomia in different subjects: A longitudinal study of two Alzheimer’s patients. Neuropsychologia. 2008;46:249–260. doi: 10.1016/j.neuropsychologia.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Davis CJ. N-Watch: A program for deriving neighborhood size and other psycholinguistic statistics. Behavior Research Methods. 2005;37:65–70. doi: 10.3758/bf03206399. [DOI] [PubMed] [Google Scholar]
- De Bot K. A bilingual production model: Levelt’s speaking model adapted. Applied Linguistics. 1992;13:1–24. [Google Scholar]
- Ellis AW, Morrison CM. Real age-of-acquisition effects in lexical retrieval. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1998;24:515–523. doi: 10.1037//0278-7393.24.2.515. [DOI] [PubMed] [Google Scholar]
- Fellbaum C. WordNet and wordnets. In: Brown K, editor. Encyclopedia of language and linguistics. 2. Oxford: Elsevier; 2005. pp. 665–670. [Google Scholar]
- Gale TM, Irvine K, Laws KR, Ferrissey S. The naming profile in Alzheimer patients parallels that of elderly controls. Journal of Clinical and Experimental Neuropsychology. 2009;31:565–574. doi: 10.1080/13803390802360542. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. Age of acquisition, imagery, concreteness, familiarity and ambiguity measures for 1944 words. Behaviour Research, Methods, and Instruments. 1980;12:395–427. [Google Scholar]
- Gollan TH, Fennema-Notestine C, Montoya RI, Jernigan TL. The bilingual effect on Boston Naming Test performance. The Journal of the International Neuropsychological Society. 2007;13:197–208. doi: 10.1017/S1355617707070038. [DOI] [PubMed] [Google Scholar]
- Gollan TH, Salmon DP, Montoya RI, Da Pena E. Accessibility of the nondominant language in picture naming: A counterintuitive effect of dementia on bilingual language production. Neuropsychologia. 2010;48:1356–1366. doi: 10.1016/j.neuropsychologia.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish-English bilinguals. Bilingualism: Language and Cognition. 2012;15:594–615. doi: 10.1017/S1366728911000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW. Control, activation and resource: A framework and a model for the control of speech in bilinguals. Brain and Language. 1986;27:210–223. doi: 10.1016/0093-934x(86)90016-7. [DOI] [PubMed] [Google Scholar]
- Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism: Language and Cognition. 1998;1:67–81. [Google Scholar]
- Hodges JR, Salmon DP, Butters N. The nature of the naming deficit in Alzheimer’s and Huntington’s disease. Brain. 1991;114:1547–1558. doi: 10.1093/brain/114.4.1547. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Jescheniak JD, Levelt WJM. Word frequency effects in speech production: Retrieval of syntactic information and of phonological form. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20:824–843. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Kemmerer D, Tranel D. Verb retrieval in brain-damaged subjects: 1. Analysis of stimulus, lexical, and conceptual factors. Brain and Language. 2000;73:347–392. doi: 10.1006/brln.2000.2311. [DOI] [PubMed] [Google Scholar]
- Kittredge AK, Dell GS, Verkuilen J, Schwartz MF. Where is the effect of frequency in word production? Insights from aphasic picture-naming errors. Cognitive Neuropsychology. 2008;25:463–492. doi: 10.1080/02643290701674851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnert KJ, Hernandez AE, Bates E. Bilingual performance on the Boston Naming Test: Preliminary norms in Spanish and English. Brain and Language. 1998;65:422–440. doi: 10.1006/brln.1998.2001. [DOI] [PubMed] [Google Scholar]
- Kremin H, Perrier D, De Wilde M, Dordain M, Le Bayon A, Gatignol P, Arabia C. Factors predicting success in picture naming in Alzheimer’s disease and primary progressive aphasia. Brain and Cognition. 2001;46:180–183. doi: 10.1006/brcg.2000.1270. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Kukull WA. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Disease and Associated Disorders. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Nebes RD. Semantic memory in Alzheimer’s disease. Psychological Bulletin. 1989;106:377–394. doi: 10.1037/0033-2909.106.3.377. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, Wingfield A. Response latencies in naming objects. Quarterly Journal of Experimental Psychology. 1965;17:273–281. doi: 10.1080/17470216508416445. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. Journal of Experimental Psychology Monograph. 1968;76:1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Shallice T. Deep dyslexia: A case study of connectionist neuropsychology. Cognitive Neuropsychology. 1993;10:377–500. [Google Scholar]
- Poulisse N, Bongaerts T. First language use in second language production. Applied Linguistics. 1994;15:36–57. [Google Scholar]
- Ransdell SE, Fischler I. Memory in a monolingual mode: When are bilinguals at a disadvantage? Journal of Memory and Language. 1987;26:392–405. [Google Scholar]
- Roberts PM, Garcia LJ, Desrochers A, Hernandez D. English performance of proficient bilingual adults on the Boston Naming Test. Aphasiology. 2002;16:635–645. [Google Scholar]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: Implications for the integrity of semantic memory. Journal of the International Neuropsychological Society. 1999;5:692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. Oxford, England: Oxford University Press; 1991. [Google Scholar]
- Stadthagen-Gonzalez H, Davis CJ. The Bristol norms for age of acquisition, imageability, and familiarity. Behavior Research Methods. 2006;38:598–605. doi: 10.3758/bf03193891. [DOI] [PubMed] [Google Scholar]
- Testa JA, Ivnik RJ, Boeve B, Petersen RC, Shane Pankratz V, Knopman D, Smith GE. Confrontation naming does not add incremental diagnostic utility in MCI and Alzheimer’s disease. Journal of the International Neuropsychological Society. 2004;10:504–512. doi: 10.1017/S1355617704104177. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Gabrieli JD, Fleischman DA. Effects of structural similarity and name frequency on picture naming in Alzheimer’s disease. Journal of the International Neuropsychological Society. 1999;5:659–667. doi: 10.1017/s1355617799577084. [DOI] [PubMed] [Google Scholar]
- Toglia MP, Battig WR. Handbook of Semantic word norms. New York: Lawrence Erlbaum; 1978. [Google Scholar]