Abstract
Retinal prosthesis have been translated from the laboratory to the clinical over the past two decades. Currently, two devices have regulatory approval for the treatment of retinitis pigmentosa. These devices provide partial sight restoration and patients use this improved vision in their everyday lives. Improved mobility and object detection are some of the more notable findings from the clinical trials. However, significant vision restoration will require both better technology and improved understanding of the interaction between electrical stimulation and the retina. This paper reviews the recent clinical trials, highlights technology breakthroughs that will contribute to next generation of retinal prostheses.
Keywords: retinal prosthesis, medical device, blindness, neural prosthesis
I. Introduction
In a healthy retina, light is detected by photoreceptors (rods and cones) via photosensitive molecules in the outer segments of the photoreceptors [1]. These molecules, once transformed by light, trigger a cascade of neurochemical events that leads to other neural cells in the retina (ganglion cells) sending signals to the downstream visual centers of the brain. Retinal degenerative diseases, like retinitis pigmentosa [2] and age-related macular degeneration [3] affect primarily photoreceptors, leaving the retina unable to sense light, but remaining neurons in the retina (bipolar cells and ganglion cells) can be electrically activated, based on established techniques for nerve stimulation.
Several studies from early scientists demonstrated that electric and magnetic fields could excite neurons in the visual system and create the sensation of light. However, the work of Brindley [4] is often cited as the seminal study that spawned the current efforts in visual prostheses. In this study, a blind patient was implanted with a device that allowed 80 independent stimulus channels interfaced with the visual cortex (occipital pole in the back of the brain). An external system was used to wirelessly drive the internal system. Using this device, the patient could see spots of light when the individual channels were activated. Around the same time Potts and co-workers were using a corneal electrode to stimulate the eye [5]. Although their primarily goal was to characterize the cortical evoked potential resulting from ocular/retinal stimulation, they did include individuals with retinitis pigmentosa in their study. The RP patients showed similar evoked potential patterns as the normally sighted volunteers. Together, this early work demonstrated that the visual system could be electrically stimulated in a potentially useful way and that RP did not eliminate the eye’s ability to respond to electrical stimulation.
The field of retinal prosthesis took a large step forward in the 1990’s, with experiments involving temporary (acute) implantation of a stimulating array in the eye of blind test subjects.[6],[7] In an operating room setting, but under only local anesthesia, a hand-held probe was inserted in the eye. At the end of these probes, were electrodes that could be used to stimulate the retina. The electrodes were placed at different retinal locations and the subjects described what they saw when the current pulses were applied to the electrode. Given the spatial mapping (retinotopic organization), when the electrode was on the right side of the eye, the subject would see something on the left, and vice-versa. These studies demonstrated that potentially useful spots of light could be created, even in a retina that had degenerated due to decades of disease. When multi-electrode arrays were used, simple forms (lines) could be created. It was important to conduct acute studies to demonstrate basic feasibility before undertaking the expensive and challenging effort of making a device for long-term (chronic) implantation.
II. Types of Systems
A typical retinal prosthesis has multiple components each with a specific function (Figure 1). These include an imager to convert light to electrical signal, electronics to process and condition the image and generate electrical stimulus pulses, and an array of microelectrodes to stimulate the retina. Other important components found in one form or another on most implants include hermetic packaging and wireless data/power transmission. However, retinal prostheses are often categorized based on the location of the electrode array, which forms the functional interface with the retina. The locations where an electrode array can be implanted to stimulate the retina include epiretinal (on the top surface of the retina), subretinal (under the retina), and suprachoroidal (between the sclera and choroid). Figure 1 a conceptual diagram of a retinal prosthesis.
Figure 1.

Retinal Prosthesis Concept. An image is converted to an electrical signal by an imaging device. The electrical signal is processed by external and/or implanted circuitry. The circuit produces a pattern of electrical stimulus which is applied to the retina via a microelectrode array positioned near the retina. Image courtesy of Annual Review of Biomedical Engineering.
Several prototype systems of chronic implantable devices have been tested in clinical trials. These fall into two types of implants. The first type of device was designed to show feasibility in humans, but not intended to be a commercially viable medical device. Thus, the main concern for these devices was patient safety and they generally were not refined to the same degree as a commercial product. For example, a number of these early chronic implants could only be activated when the patient visited the clinic. The second type of devices are intended as commercial devices, so they are engineered not only for robustness and safety, but also to have expected longevity of over a decade and with human factors considerations. The Argus II retinal prosthesis (Second Sight Medical Products, Inc.) has received approval in both Europe and the United States to be sold as a treatment for retinitis pigmentosa. The Alpha-IMS system by Retina Implant has received regulatory approval in Europe. A summary of current and past clinical trials of retinal implants can be found at www.clinicaltrials.gov, search “retinal prosthesis”.
A. Epiretinal
Epiretinal implants involved in feasibility studies include the Argus I from Second Sight and devices from Intelligent Medical Implants, GmbH (IMI) and the Epi-Ret Consortium (Epi-Ret). All three of these systems used wireless power and data delivery (to avoid transcutaneous wires).
The Argus I device was implanted in 6 subjects between 2002-2004.[8] Argus I was a modified cochlear implant. As such, the electronics were implanted behind the ear and a cable run along the temple, into the orbit, and, finally, into the eye, terminating in a 4×4 grid of platinum electrodes, either 520 μm or 260 μm diameter. With training, subjects were able to distinguish objects from a small set [9] and detect motion of a moving bar. Subjects have been implanted for as long as 10 years with the device still functional (Arup Roy, personal communication).
The IMI device was implanted in a total of 7 subjects.[10] This device utilized a microfabricated array with a total of 49 electrodes. The implant was entirely in the orbit, with the electronics module on the outside of the eye and the electrode array in the eye (via a cable across the eye wall). This system could only be activated in the clinic as no camera system was available. Using this device, subjects could see spots of light and some simple patterns. The implants were removed after several months, or in one case, 1.5 years, possibly due to the fact that the electronics were encapsulated with a polymer (see section III.A for discussion of packaging).
The Epi-Ret device was implanted in 6 patients.[11] The stimulating array had 25 electrodes and also was miniaturized to the point where the entire device could be implanted in the eye. A unique feature of the stimulating array was protruding electrodes, which improved contact with the retina and resulted in low perceptual thresholds. Like the IMI device, the Epi-Ret system was designed only for semi-chronic implantation (4 weeks) and did not have a camera system so the implant could only be activated in the clinic.
The Argus II is similar to the IMI device, in that the electronics are on the outside of the eye and the electrode array is on the retina, connected by a cable across the eyewall to the electronics.[12] The Argus II however, has a camera system that provides input to the implant, allowing patients to leave the clinic with the system operational. The electronics are protected with a hermetic enclosure and implant longevity is estimated to be greater than 10 years, based on accelerated lifetime testing.[13] 60 electrodes are arranged in a 6×10 format. Figure 2 shows the components of the Argus II system.
Figure 2.
The Argus II Retina Prosthesis. A. The external system has a camera mounted in a pair of glasses. The video processing unit (VPU) processes the camera data, then transmits wireless power and data via the coil. B. The implant coil receives power and data for processing by the electronics (within the silver case). The coil and case are secured with a scleral band that encircles the eye. The electrode cable traverses the eye wall and the electrode array is attached to the array with a retinal tack. Images from [12]
Thirty subjects were enrolled between 2007 and 2009 in the Argus II clinical trial [12]. All subjects were able to perceive light during electrical stimulation. Other tasks on which subjects performed well include object localization (27/28 subjects better with system on vs. system off) and motion detection (16/28 subjects better with system on vs. system off). Letter reading was tested in 22/30 subjects.[14] Six of these subjects were able to identify any letter of the alphabet at a 63.5% success rate (vs. 9.5% with the system off). In all 22 subjects tested, a small set of 8 letters was identified 72.5% correctly, vs. 16.8% with the system off. Subjects were free to take as much time as needed to make a judgment. Adverse events that occurred during the trial include low eye pressure and infection, but such events decreased with in later implants.
B. Subretinal
The second type of experimental implants tested in humans were subretinal implants, which were implanted in the location vacated by degenerated photoreceptors. Optobionics tested passive subretinal implants in 30 subjects.[15] A passive implant relies on incident light to activate photodiodes arrayed on a silicon disk, with the resulting electrical current stimulating the retina via electrodes also patterned on the disk. These devices did not account for the low-level of light that reaches the back of the eye.[16] Ultimately, passive subretinal implants proved to produce inadequate stimulating current, though some patients reported improved vision. Animal studies show a “neurotrophic effect”,[17] which results in improved retinal health due to the presence of the subretinal implant, suggesting a possible mechanism behind improved vision reported by patients.
A more sophisticated device was tested in human by Retina Implant, GmbH (RI).[18] The RI device had a subretinal array of microphotodiodes like the Optobionics device, but in addition included circuitry to amplify the photocurrent to an adequate level, as shown in Figure 3. This additional circuitry required an external power source. In the experimental device, implant power was provided via a percutaneous cable, which crossed the skin behind the ear. The commercial system described below (Alpha IMS) includes a wireless power module in the place of the percutaneous cable. In addition to the microphotodiode array, the experimental system included 16 “direct stimulation (DS)” electrodes, each of which were connected to the percutaneous connector via a separate path, thus allowing access to these electrodes by external test equipment.[19] The DS electrodes were either 50×50 μm or 100×100 μm (larger electrodes in later implants).
Figure 3.
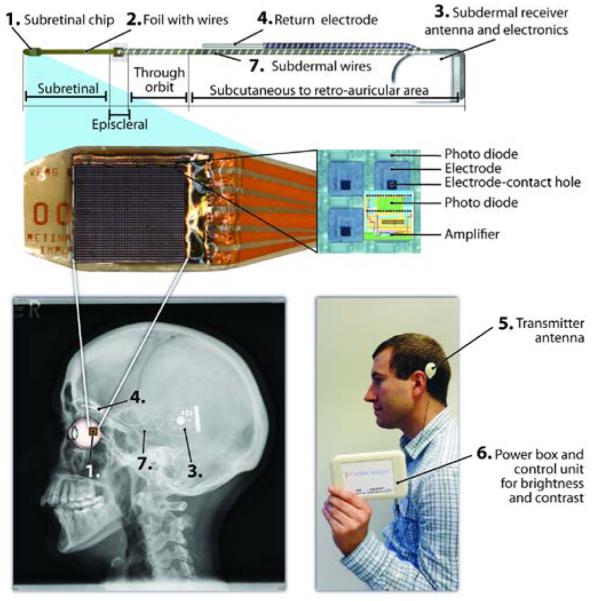
Alpha – IMS system (Retina Implant, GmbH). 1. The subretinal photodiode array (MPDA). The MPDA has 1500 elements, each of which has a photodiode to sense light, circuitry to amplify the signal, and electrode to deliver the stimulus pulse. 2. A micro flex cable holds the MPDA while larger wire connect to 3. the subdermal receiver. 4. The return electrode is outside the eye, under the skin. 5 & 6. The external system provides power to the implant as well as some configuration data. 7. The implant position on the head. Images Courtesy Dr. Eberhart Zrenner.
12 patients were implanted with experimental RI system[18]. One implant failed shortly after surgery and was not tested, so only 11 subjects are considered. Using the DS electrodes, 6 of 11 subjects could perceive light from single electrode stimulation. Two additional subjects could detect light when DS electrodes were used in combination while three subjects could not detect light from DS stimulation. Of the 8 subjects who could detect light, 6 could detect patterns consisting of simple lines or letters formed from the 4×4 array of DS electrodes. The authors note that more success was possible in pattern detection when electrodes were activated sequentially vs. simultaneously.
Detailed test results for MPDA mediated vision are available on the last 3 of the 12 subjects implanted with the experimental system[18]. These subjects received implants with an improved seal and redesigned cable, which allowed for longer implant lifetimes. The average implant length for these three subjects was 126 days. All three subjects could detect light mediated by the MPDA. Two subjects had measurable visual acuity (0.34 and 0.22 cycles per degree). One subject had could successfully complete a number of visually guided tasks, including letter recognition. This subject could recognize letters and correctly report light in a quadrant.
The Alpha IMS Wireless implant has been tested in nine subjects to date[20]. It is identical to the RI implant described above, except the wireless version has a power module under the skin behind the ear (Figure 3). The power module receives power and configuration data from an external system via inductive coupling. 8 of 9 subjects could perceive light using the system. The subjects could also detect and identify objects used in a table setting. 4 subjects could read letters from a reduced set. Three of the implants showed visible chip degradation eventually leading to chip failure after approximately 250 days. The authors attribute this failure to the quality of the chip’s hermetic seal [21]. Recently, the Alpha IMS Wireless implant received CE Marking for commercial sale in Europe.
C. Suprachoroidal
Recently, a supra-choroid transcleral (STS) retinal prosthesis has been tested in humans [22]. The surgical approach for this implant involves cutting a flap in the sclera (the outer layer of the eye) and placing an electrode in the flap, such that the exposed electrode contacts are directed towards the choroid (vascular layer) and retina, which lies on the other side of the choroid. A single return electrode is placed in the vitreous cavity. Thus, stimulus current passes through the choroid to the retina. An STS device with 9 electrodes was implanted in a limited study in two patients. The electronics case was positioned under the skin behind the ear. It was shown that stimulation resulted in light perception and the subjects could reach out and grab an object, using a camera to guide them. This study was limited to 4 weeks implantation.
D. Limitations of current systems
All the systems described above face limitations. The Argus II and the STS systems are relatively low resolution devices. Both of these devices are designed as permanent implants, thus the hermetic packaging (see below) is large. The Alpha-IMS has the highest density of electrodes, but this density is only possible because the system has less robust packaging scheme. As such, the longest Alpha-IMS lifetime reported is less than 1 year. With current packaging technology, there appears to be a trade-off between system lifetime and visual acuity. In Section III, we review engineering research in packaging and other areas that will expand the system design space for retinal implants and may obviate some of these trade-offs.
Even if a high density, long-lasting implant can be made, questions remain about how to deploy such a system to achieve high-resolution vision on a consistent basis. The biological challenges facing retinal implants may be even greater than the engineering challenges. None of the implants tested in patients have produced perceptions of “pixelized vision”, as depicted in numerous studies of simulated vision[23]-[25]. For example, vision produced by the Argus II does not result in a 6×10 array of identical phosphenes that can be precisely controlled by the external camera system. Visual acuity testing demonstrates the challenges remaining for prosthetic vision system. In the Argus II trial, best measured visual acuity was (20/1260)[12]. Measurable visual acuity was obtained in 7 of 30 test subjects. The Alpha-IMS study reports visual acuity in 2 of 9 subjects, with a best measurement of 20/546 [20]. Thus, in both clinical trials, fewer than 25% of patients had measurable visual acuity. A magnifying feature under user control on the Argus II may enable 20/200 vision [26]. However, this approach would be akin to looking through binoculars where even slight movements of the head results in exaggerated shifts in visual field. In both the Argus II and Alpha IMS, the typical patients do not perceive even simple forms in an immediate way, rather they scan their head and/or eyes over objects in order to make a judgment as to what they are viewing. With natural vision one can perceive the letter “T”, for example. One does not consciously think about a horizontal line on top of a vertical line, but simply knows that it is a “T” without conscious thought. Retinal prosthesis subjects have not yet achieved this type of vision. Yet, there exist “high achieving” patients with both systems, where visual acuity can be measured. In those patients with measureable visual acuity, strong evidence exists that the system does provide spatial information that can be used in visual tasks [27]. Thus, it is critical to understand why these subjects perform well while others do not.
III. Engineering Research
Technical challenges exist that must be overcome before a high-resolution, long-lasting retinal prosthesis can be achieved. To protect electronics long-term, new materials and processes are needed to create a robust, yet thin, barrier to water and ions. Efficient circuit architectures must be utilized that allow 100s of channels of stimulation while maintaining power consumption below a safe level. Electrode arrays have a number of areas for improvement. Electrode substrates need to adapt to the curvature of the retina without crushing the soft retinal tissue. Electrode materials must support higher charge density since small electrodes are needed for high-resolution retinal stimulation. Video processing is one area that can contribute to improving the visual performance in patients with limited resolution devices.
A. Packaging
Implantable electronic devices require a protective barrier to ensure that neither moisture nor ions reach incorporated electronic circuits. Donaldson has described two methods to achieve this barrier: encapsulation and hermetic enclosure [28]. Encapsulation refers to the use of a conformal coating to form a protective layer around the electronics. When polymers are used to form this layer, water will penetrate through the polymer, but the conformality of the coating prevents the condensation of water on the surface of the electronics. This method relies heavily on “cleanliness and absence of voids”[28], meaning the chip surface must be free particulates and the conformal coating cannot have voids where water can condense. This is very difficult to achieve reliably in practice, as evidenced by the fact that Donaldson could identify only a single electronically active medical device that uses the encapsulation approach. The 1-2 year lifetimes of the retinal prosthesis devices encapsulated with polymers suggest that more process development is needed to make this a viable approach for long-term encapsulation.
The vast majority of current neurostimulation implants use the hermetic enclosure approach. Traditional methods for forming the hermetic enclosure involve the use of titanium or ceramic cases joined to a feedthrough (a substrate with multiple isolated conductors) that together form a hermetic package for the electronics.[29] (Figure 4) Both the thickness of the case and the conductor pitch (spacing) lead to implant sizes that are much larger than those of the enclosed electronics. The Argus II uses this style of packaging and due to limitations in conductor spacing, is limited to 60 independent stimulus channels. This is not to diminish the engineering accomplishment of a 60 conductor feedthrough. In fact, the Argus II packaging represents a 3X increase in number channel number and a 10X decrease in size, when compared to cochlear implants. Rather, this example demonstrates how much more remains to be accomplished in bioelectronics packaging before a high resolution device with a hermetic enclosure is possible. Typical feedthrough conductor spacing in present day bioelectronic implants is on the order of 1 mm. Contrast that dimension with integrated circuit pad spacing of 150 μm, electrode dimensions of less than 25 μm, and transistor sizes of less than 1 μm, and it is becomes clear that packaging is a major driver of implant size, particularly with implants that are inductively powered and do not have batteries (another major size factor).
Figure 4.

Hermetic Packaging Schemes. Top – An electronics modules is placed inside an enclosure, which includes a feedthrough platform with conductors and a case or lid. The feedthrough and case are sealed together. Bottom – Coating an electronic chip is an ideal protection scheme, but to date no coating technology has proven adequate for long-term implantation.
Increased feedthrough density is being realized with advanced processing techniques. Suaning and co-workers have achieved high feedthrough density by laminating a laser-cut platinum foil between two sheets of alumina (a ceramic used commonly in medical implants).[30] The platinum foil is patterned to form long lines. The gap between the lines is filled with alumina suspended in a viscous liquid. Subsequent baking of the laminated structures promotes crystal regrowth in the alumina suspension, leading to a dense alumina surrounding the platinum lines. Interior package bond pads are created by drilling through one of the alumina sheets to the Pt foil followed by an electrodeposition process to fill the drilled hole. Electronics are placed on the same side of the laminate and connected to the bond pads, to allow signal communication across the package. An alumina cap is then bonded to the laminate structure to seal the electronics. Schuettler et al, describe a screen printing approach to achieving 360 channels in a feedthrough whose overall size is less than 25 mm × 25 mm.[31] Gill et al stack green alumina sheets and pt wires in an alternating pattern, and then heat and compress the structure to densify the alumina around the wires.[32] Cutting sections normal to the wires results in a patterned grid of platinum conductors embedded in alumina (Figure 5). Testing of this substrate showed helium leak rate of less than 8×10−11 mbar-l/s
Figure 5.
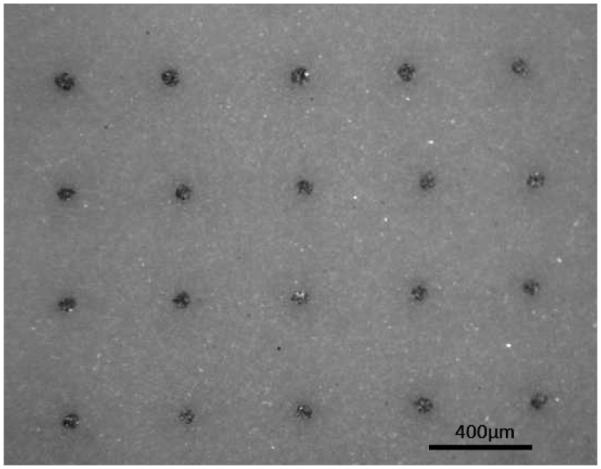
High density feedthrough technology. Front view shows platinum conductors embedded in alumina substrate.
To replace the large metal enclosure, several groups have investigated deposition of thin films to serve as barriers to water and ions. Such an approach can reduce the volume of the implant by 50% or more. However, making pinhole free films is challenging, particularly around three dimensional structures. As discussed earlier, polymers do not provide adequate, long-term protection against water vapor penetration. The IMI device used parylene coating and did not function longer than 2 years. Current research is directed at using diamond like films, nano-crystalline diamond, or other silicon based films (silicon-carbide). Weiland (one of the authors) and co-workers have investigated multi-layer, multi-material films, using both diamond like coatings and metal films, that show good conformality around corners and edges and improved ion barrier properties, though long-term testing has not been completed.[33] Ultra-nanocrystalline diamond films have been deposited in very dense and inert films, however this material may have an unacceptably high conductivity at higher voltages, thus it may need to be insulated from biases by a better dielectric.[34] Recently, amorphous silicon carbide (a-SiCx:H or a-SiC) has been proposed as an encapsulation material for electrodes and electronics by several groups.[35],[36] This material has perhaps the most promise of all materials tested so far, but still has key shortcomings. SiC has excellent dielectric properties and can be deposited, with careful instrumentation, at temperatures below 400°C. It is also highly resistant to degradation. Limitations of SiC include slow deposition rates (0.2 to 0.5 μm/hr) when using low temperature deposition. The a-SiC intrinsic compressive stress is moderate (0.2 to 0.3 GPa), but this level of stress may still limit the maximum film thickness (e.g., < 5 μm) to maintain good adhesion and avoid device distortion.
B. Electronics
There are several electronics components involved in retina prostheses. For those using external cameras, both the camera and supporting hardware are custom electronic systems, but typically use off-the-shelf parts. As such, these require careful engineering, but do not present significant technical challenges. Some of the more innovative approaches to camera/video processing are reviewed in section III.D below.
In contrast, active research is taking place to improve electronics for inductive power and data telemetry as well as implanted electronics. Recent advances in these areas are reviewed below.
1) Telemetry
All retinal prosthetic systems use some form of wireless technology for data and/or power. In contrast to deep brain stimulators which run on batteries with fixed settings, retinal implants have a requirement for small size which preclude batteries and must be constantly updated with new data based on the input to the camera. Conceivably, a retinal prosthesis could be configured with a battery module in the chest with a cable running up to the orbit and a camera in the eye. Such a system would only require occasional wireless access for battery recharging and system diagnostic and configuration. However, this architecture would complicate the surgical approach by requiring placement of a battery module in the chest and connection of this module to the eye, via a subcutaneous lead. Presently, no retinal prosthesis system uses a battery.
The Alpha IMS has an implanted imager chip thus no real-time data link is needed for this system. An earlier prototype has a percutaneous connector behind the ear, but the most recent version has a wireless module behind the ear, with a cable running to the eye. The position of the wireless module has the advantage of ensuring a strong, stable inductive link, since the wireless module can be just under the skin. In contrast, inductive coils on the eye, as is done in the Argus II and IMI implants, mandate additional design considerations for relative movement between the primary and secondary coils. The behind the ear position is very similar to the cochlear implant, so many of the wireless design details have been optimized already. The trade-off for a behind the ear wireless module is the increased surgical complexity needed for running a cable from behind the ear into the eye.
Cochlear implants use a single wireless link to carry both power and data. The signal is modulated to carry data information and the induced current from the AC signal is rectified and regulated to provide DC power. However, a fundamental conflict exists when choosing a wireless frequency. To achieve an adequate data rate, the carrier frequency is typically an order of magnitude higher than the required data rate, which for advanced retinal prosthesis may be megabits/sec. Thus a carrier frequency of greater than 10 MHz may be needed.[37] However, to maintain efficiency of the power link, in terms of signal penetration into tissue and AC-DC conversion, frequency less than 10 MHz is desired.[38]
Multi-band approaches have been proposed to allow efficiency in both the power and data links. This approach separates the frequencies used for power and data.[39] The power signal will be stronger, use a lower frequency and use larger coils, to accommodate the need for transmission of significant power (implants require 10’s of mW). . The data signal is higher frequency, can use much smaller coils since the data signal can be much lower power. However, such systems require careful design since some interference between the links will exist. A 256 channel retinal prosthesis system with dual band telemetry has been demonstrated on the bench top, supporting the technical feasibility of this approach.[39]
Optical data and power links have also been investigated for retinal implants. The optobionics subretinal microphotodiode array was powered by incident light, but this was inadequate to stimulate the retina. Gross et al. have designed a retinal prosthesis with an optical data link combined with an inductive power link.[40] The authors were able to achieve a data rate of 200 kB/s. This prototype evolved eventually into the IMI implant.
2) Integrated circuit
The implanted IC must perform a number of functions. These include AC-DC conversion (if inductive power telemetry is use), data demodulation (if wireless data link), digital control, analog drivers, and reverse telemetry for diagnostics. One of the major design challenges are related to the multiple voltages and negative voltages. Negative voltages are needed since the stimulus driver must supply both anodic and cathodic current.
Liu and colleagues have designed, fabricated, and tested multiple generations of integrated circuits (IC) for retinal stimulation. They designed the initial version of the IC that is now in the Argus II. Their most recent contribution is a complete system with data demodulation, timing controlled rectification, digital control, and 256 independent stimulus drivers.[41] (Figure 6) The timing controlled rectifier does not use diodes and thus eliminates a significant source of power loss. This IC was demonstrated as part of an end-to-end functional system,[39] with the actual analog output displayed on an LED board and wireless power and data. An innovative aspect of this design is the used of Circuit under Pad layout, which takes advantage of the multiple metal layers in the process to place bond pads over the circuitry, thereby reducing the IC area.
Figure 6.
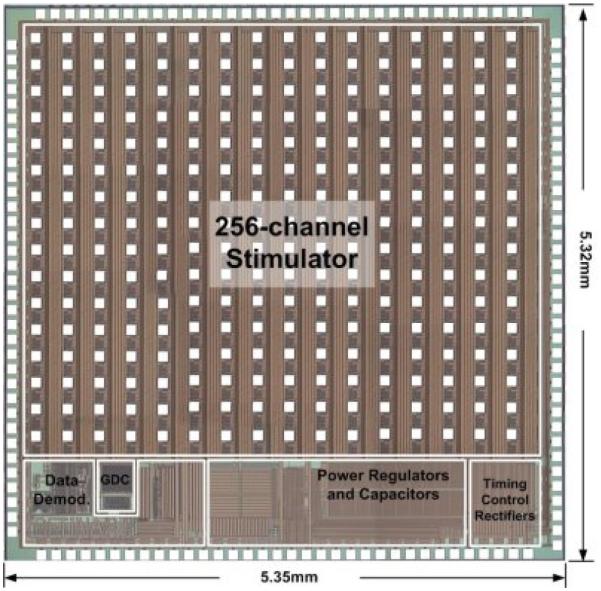
A 256 channel stimulator by Liu and colleagues. The chip has integrated power conditioning and data decode, and 256 independent output channels. Image courtesy of Dr. Wentai Liu.
Ortmanns and colleagues have designed a 232 channel retinal stimulator chip.[42] The chip is fabricated in 0.35 micron CMOS and includes high voltage (+/− 15V) compatible with high stimulus currents through small electrodes. The overall chip size is less than 5×5 mm. An innovative charge balancing scheme is used to reduce accumulated, residual charge on the electrodes due to imbalance in the current source. Electrode voltage is sensed in between pulses and voltages greater than +/− 50 mV trigger balance pulses that reduce the voltage. This active scheme reduces chip size versus standard schemes, which involve blocking capacitors or high-voltage transistors to connect the electrodes to a common point.
A chip for subretinal stimulation has been fabricated and tested.[43] It has 256 channel and was fabricated in 0.18 micron CMOS. High voltage components were included to allow a wide range of stimulus currents. The chip includes power and data telemetry blocks as well as diagnostics for sampling the electrode voltage on any output and transmission of this data via a low-rate, reverse telemetry link. Output drivers can be configured as sources or sinks to allow current steering.
A 96 channel retinal stimulator has been designed to allow current steering between local sources and sinks.[44] The chip included high-voltage drivers (up to 20V) which requires special, high voltage transistors, since the design was done in 0.35 μm process (Austria Microsystems). However, many of the other chip features could be handled by low voltage transistors, resulting in significant space saving. The chip includes switches that allow each electrode to be configured as either a current source or a group of 6 electrodes forming a hexagonal sink surrounding the source. The purpose of having a local sink is to focus current in a narrow area of the retina. It was implemented as a test chip, with 2 current drivers and switching to accommodate 14 electrodes. The chip also includes a feature to short all electrodes together to dissipate accumulated charge on any single electrode.
A 512 channel retinal chip has been implemented in 65 nm CMOS.[45] This chip features novel auto-calibration circuitry on the output, which is used to tune the circuit to improve precision and eliminate charge balance. The data channel can support data rates of 20 MB/s. The small transistor size allows the chip to be 4.5 × 3.1 mm2, so the chip can possibly fit entirely inside the eye, depending on the size added by the packaging scheme. Voltage is +/− 2.5V. While this design choice will allow low power operation, the output current may be limited depending on the impedance of the electrodes.
C. Electrodes
Electrode technology used by the Argus II and Alpha-IMS represents an important step for implantable bioelectronics. Older implants such as cochlear implants and deep brain stimulators used hand-made electrode assemblies. These feature large platinum contacts supported by a silicone substrate. In contrast, retinal implants use photolithography and micromachining to fabricate arrays. This is needed because of the high number and the density of contacts required for vision. Figure 7 shows an example of 1000 electrode epi-retinal array, implanted in the eye of large animal model. Along with the cortical array used in the Braingate study [46], stimulating arrays now implanted in humans build on decades of research and development.[47].[48] Also, both the Argus II and Alpha IMS use advanced electrode materials, platinum gray and titanium nitride, respectively. Both are superior to bulk platinum, which is typically used for neurostimulation, in terms of charge injection capacity. Platinum grey[13] can inject 1 mC/cm2 and titanium nitride[49] can inject 0.9 mC/cm2, versus 0.1-0.35 mC/cm2 for platinum.[50]
Figure 7.
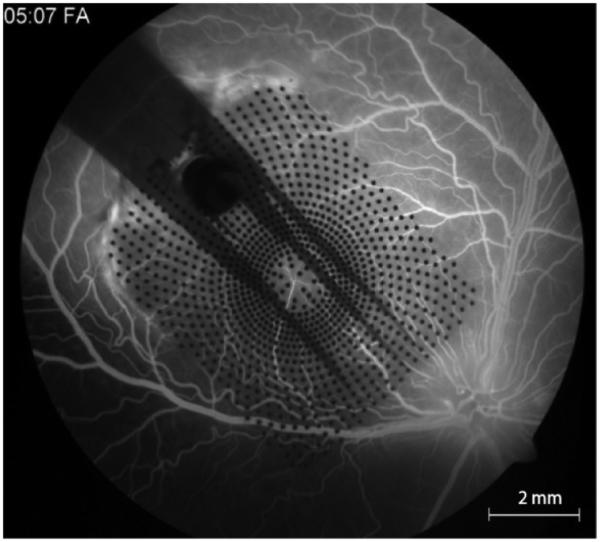
A parylene substrate electrode array with 1000 individual electrode contacts, implanted in the eye of a canine. The image is acquired via a fundus camera, through the dilated pupil. Fluorscein injected into the circulatory systemically shows integrity of the retinal blood vessels under the array.
Mechanical damage to the retina is possible if the electrode substrate materials are too rigid. Polyimide and parylene are traditional substrates for photolithographically patterned electrode arrays, but are rigid relative to silicone. However, patterning metal onto silicone is difficult. Schuettler et al have invented a novel process for fabrication of silicone/platinum arrays.[51] Medical grade silicone is spun on a glass carrier. A platinum foil is placed on top of the layer of silicone. A Nd:YAG laser is used to cut a pattern in the platinum, to form conducting lines, bond pads, and electrodes. Another layer of silicone is deposited on the top to form the final insulation layer. Prototype devices demonstrated a minimal feature size of 30 μm, which is greater than photolithography (typically 2-5 μm), but may be adequate depending on the number of pixels in the array.
A fundamental problem facing retinal prostheses is the visual field that can be addressed. The eye is a 2.5 cm diameter sphere (roughly) and the retina lines the back half of the eye. To completely address the entire retina, the dimension of the array must be π*d/2 or almost 4 cm. Subretinal implants are limited by the fact that the retina must be detached to insert these arrays. Current implants cover less than 10 degrees of visual angle. Increasing the subretinal array size risks detaching the entire retina, which has disastrous consequences for the long term health of the retina. The surgery for the Alpha-IMS includes a silicone oil injection in the eye, which reduces the likelihood of retinal detachment.[20] Epiretinal implant size is limited because of the small incisions that can be safely made in the eyewall (less than 5 mm). Ameri et al describes a foldable array that can be inserted through a small sclerotomy (eye wall incision), then expanded once in the eye.[52] This approach allows 1 cm wide array, thus 45-50 degrees of visual angle. Subsequent development of this idea has demonstrated long-term implantation of these arrays into animal eyes.[53]
D. Cameras and Video processing
The Argus II current uses an off-the-shelf video camera while the Alpha IMS has imaging capability in the eye. Video processing algorithms can potentially improve the performance of retinal prostheses by highlighting important areas and deemphasizing scene elements that are not relevant to the current task. For example, something far in the distance may not be as important as a nearby object. Another aspect of the system design is the position of the camera. The Alpha IMS has a distinct advantage in that the imaging component is inside the eye thus moves with the eye. It is well-known that perception requires not just vision but eye movement and feedback of eye position to integrate visual information into and understanding of a scene. Argus II requires head scanning to integrate visual information. Such motions are not impossible, but less natural. As discussed below, miniaturized implantable cameras are under development that will impart to epiretinal implants the ability to control camera direction with eye position.
1) Machine Vision
The first sophisticated algorithm directed to retinal prosthesis was proposed and developed by Eckmiller and colleagues.[54] The Retinal Encoder predicts the ganglion cell output in response to a given input and can be used to drive the stimulus of an epiretinal device. The encoder approximates the typical primate ganglion cell receptive field properties of primate retinal ganglion cells by means of individually tunable spatiotemporal receptive field filters. The encoder maps visual patterns onto spike trains for a number of contacted ganglion cells. An implementation of this scheme simulated part of the central retina with a hexagonal array of 64 × 64 photosensors / pattern pixels that formed the input to an evenly interlaced distribution of partly superimposed 34 × 34 receptive field (RF) filters of P-On, P-Off, and M ganglion cells. Each ganglion cell filter included one RF-center pixel and six RF-periphery pixels. This algorithm has not been tested in humans with retinal implants.
Others have looked to use software algorithms that process the video to enhance certain relevant parts of the scene. Parikh et. al. used saliency algorithm biased by user input to demonstrate improved object localization with simulated prosthetic vision.[55] The algorithm was trained to selectively highlight a soda can in the user’s peripheral vision (outside the range of simulated prosthetic vision). When simulated phosphenes on the edge of the array where used to guide the user where to look, the user easily located the object of interest. Without these guiding cues, the user struggled to find the soda can.
Enhancement of important areas may be useful for mobility as well. McCarthy et al used a RGB-D sensor (color and depth) to obtain information regarding distance to objects.[56] This information was then processed to extract local surface variation. From this, important objects and nearby objects were highlighted using intensity and presented in a simulated prosthetic vision paradigm. Their results clearly show that this computer vision approach can accentuate nearby, potentially important obstacles such as a low wall next to a sidewalk. Thus, rather than code the brightest objects with the highest intensity, instead determine the importance of objects.
2) Novel camera
As mentioned in the previous section, depth cameras may become important for retinal prostheses if depth perception is not possible due to low resolution or monocular implantation. Depth cameras like those used in commercial products such as the Microsoft Kinnect, project a speckle pattern of infrared dots onto a scene, then detect the relative position of those dots, compared to a calibrated surface. The distortion in the pattern allows depth of the reflecting surfaces to be measured. Depth cameras can be used as input for computer vision algorithms like those described above, but also are used as input to simultaneous localization and mapping (SLAM) algorithms. SLAM can be used to build up a map of the local environment and a person’s position and pose within this environment.[57] Such information can be useful when determining best how to use prosthetic vision to guide someone through a complex environment.[58]
A micro-camera implanted in the place of the crystalline lens can couple gaze with the stimulus pattern. (Figure 8) To fit in this space, a camera has been designed that is a cylinder about 3 mm in diameter and 4 mm long.[59] The design features a hermetic packaged capsule, a custom aspheric lens to focus and the image on the wide-dynamic range CMOS sensor. Power and control circuits are placed behind the sensor. The optical system was designed to have an unusually wide depth of field, to obviate the need for accommodation (i.e. refocusing on close objects). The benefits of an intraocular camera architecture have been explored using simulation, since direct comparison would require a similar system with both external and implanted cameras. Simulation experiments suggest that users can perform tasks in a more natural manner when the images they see are controlled by the direction of eye gaze.
Figure 8.

An intraocular camera positioned in place of the crystalline lens, similar to what is done in cataract surgery when an intraocular lens is implanted. Such a device will allow eye gaze to be in accord with perception, a key to improving the visual capability of implant patients. Image courtesy Dr. Armand R. Tanguay, Jr.
E. Summary
Results from the engineering efforts have made significant advances, but in some areas, much work remains. Integrated circuits have achieved sophistication that meets the requirements of current systems, though more efficient designs are possible. Hermetic packaging has also made significant advances, but hermetic coatings remain a challenge. Electrode arrays can achieve the requisite electrode site density, but must become more adaptable in terms of their shape, such that each site of a multi-electrode array is in close proximity to the retina.
IV. Biological Research
Critical questions remain about the retinal response to electrical stimulation and how the brain interprets this response. The clinical trials of both subretinal and epiretinal implants demonstrate that patients do not experience idealized pixelated vision as depicted in many publications. Each electrode does not produce an identical phosphene that is equidistant from its neighbor. In fact, in the Argus II clinical trial, only 55% of single electrodes could produce a perception within the stimulation safety limits. In the Alpha-IMS device, it is not clear if a single electrode can produce a perception, given its small size (50 microns). In both devices, electrodes grouped together in parallel are used to elicit a response if a single electrode cannot.
In spite of these difficulties in consistently achieving pixelized vision, it is clear that patients do have some spatial information. Patients can detect the direction of movement, which requires multiple pixels that are perceived at different locations. The highest performing test subjects who read letters clearly obtain information from multiple pixels to complete this task and subjects have reported form vision in some cases. But the typical patient experience is not that of an array of independent phosphenes under precise control of the implant. The reasons for this relate to both spatial and temporal irregularity of the phosphenes. The clinical trial results were discussed earlier in Section II. Below, we described more detailed human psychophysics studies that tried to understand which stimulus parameters affect perception. In addition, we discuss recent studies in animal models of retinal disease that are beginning to reveal the basic mechanism of retinal stimulation.
A. Human studies
While all implants have reported basic characteristics of light perception and some have measured visual acuity and object detection, most of the visual psychophysics studies have been published by groups involved with the Argus I and Argus II implants. These implants are long-term, versus, for example, 4 week implants in the Epi-Ret trial. The long term nature of the implant allows in-depth studies with repeated measures in the same subjects, which are needed to build a significantly robust set of data.
1) Psychophysics
Horsager examined channel interaction using several measures in Argus I implant patients.[60] A “same-different” task asked subjects to judge whether two stimuli appeared to be the same or different. If different stimuli generate a “same” response, then the visual system cannot resolve the differences between the two stimuli. A brightness task asked subjects to judge whether or not two stimuli were the same brightness or if the second stimuli was brighter or dimmer than the first. Brightness was used as an indirect measure of strength of activation. Using these measures, subjects were able to detect differences in simultaneous vs. interleaved multi-channel stimulation. Simultaneous stimulation means all electrodes are active at the same time while interleaved stimulation means the pulse are temporally separated so that only one electrode is active. Subjects were able to detect difference when pulses spacing was as low as 3 ms. Thus, the timing of pulses will affect the perception.
Nanduri examined the relationship between perception size and stimulus magnitude and frequency.[60] This is important since increasing the amplitude of the stimulus was found to make perceptions both brighter and larger. However, objects may be brighter, but not bigger. So finding a way to increase the brightness of a perception without increasing its size can potentially improve the quality of the visual experience. It was found the increasing the frequency of stimulation increased perceived brightness without increasing the size. Thus, pulse rate modulation should be considered as a means of intensity coding for future retinal prosthesis.
Neural stimulation can lead to desensitization of nerves, requiring an increasing stimulus to create perception. In retinal prosthesis patients, phosphenes become less bright with continuous stimulation. Nine Argus II implant patients were tested to measure the rapidity of phosphene fading.[62] Pulses were applied continuously on a group of 4 electrodes (simultaneous stimulation). The phosphene was initially bright and distinct in all subjects, but faded in all but one of these subjects. In 4 subjects, the phosphene faded slowly over several seconds, while in the others it faded more rapidly, in less than 1 sec. The residual perception was less distinct and dim. While this is a concern for retinal implants, the natural motion of the head and eye while viewing a scene may act to refresh an electrode. Video processing may be needed to regulate the amount of stimulus applied to single electrode over a given time window.
2) Imaging
Brain imaging studies will be an important part of future retinal prosthesis research, complementing psychophysics. Very little work has been done in this area since implants have only been put in humans in the last decade. One study not involving implants used positron emission tomography (PET) to examine active brain areas when the eye we stimulated with an external electrode.[63] Similar patterns of brain activity were elicited by electrical stimulation in blind subjects when compared to light stimulation in sighted subjects (Figure 9). It is worth noting that the Argus II is labeled as MRI Conditional, which means that patients with implants can undergo MRI procedures within certain limits.[64] Indeed, Argus II implantees have been scanned as part of routine medical care.[65] Functional MRI would require activation of the device in the scanner. Alternatively, PET or EEG can be used, but both are considered less accurate than MRI.
Figure 9.

PET imaging of (left) Light stimulation in subjects with normal vision and (right) Corneal electrical stimulation in blind subjects (with RP). Posterior view shown, arrows indicate posterior pole. Courtesy Dr. John Xie.
B. Animal studies
There are a multitude of reports describing electrical stimulation of the retina in animal models. This work was reviewed by Freeman and the reader is encouraged to use that review to obtain a comprehensive understanding of the prior art.[66] Here, we focus on key results that are of direct consequence to challenges noted in the clinical trials. A distinction made in many of these studies should be defined for clarity. All the studies mentioned below use retinal ganglion cell responses as a measure. This is appropriate, since this represents the output of the retina. Direct stimulation refers to electrical stimulation that depolarizes the retinal ganglion cell until an action potential is initiated. Indirect stimulation refers to electrical stimulation that acts on another cell type in the retina, but through synaptic transmission, causes a retinal ganglion cell to fire an action potential.
1) Increased degeneration leads to higher thresholds
Using animal models of degeneration, multiple studies have demonstrated that degenerated retina has a higher stimulus threshold than normal retina. Jensen et al. showed a consistent elevation in threshold for rd1 mouse retina (vs. normal) up to post-natal day 200.[67] These experiments used subretinal stimulation. Chan et al. examined threshold for epiretinal stimulation in s334ter rat (a common retinal degeneration model) and found threshold elevated, but only in older rats with a greater extent of degeneration.[68] A number of other studies support this finding. However, Sekirnjak et al, found no difference in threshold in old, blind rat retina compared to normal.[69] Experimental differences in this last study include use of a small stimulating electrode (10-20 micron diameter vs. >75 micron diameter in other studies) and explicit direct stimulation. The Chan study of epiretinal stimulation likely included both direct and indirect stimulation. This is a better model of the current clinical situation in patients, but the Sekirnjak study suggests that if individual ganglion cells can be targeted, then threshold differences may not exist. The implications of these findings are that near term retinal implants must be able to supply higher currents, but future implants, with advanced electrode array technology, may be able to use lower stimulus and still achieve perceptions. More detailed studies of retinal ganglion cell properties during retinal degeneration are needed to confirm these results.
2) Axonal stimulation can be avoided with longer pulses
A key challenge facing epiretinal prostheses relates to stimulation of axons. Ideally, each electrode site would stimulate only nearby cell bodies. This will ensure that the perception generated by stimulation will be small (on the order of the electrode) and correlated in visual space with the position of the electrode site on the retina. Images are inverted horizontally and vertically on the retina by the optics of the cornea and lens. Therefore, stimulation in the left half of the retina should generate perceptions in the right visual field. If axons originating from cells in the periphery and passing underneath the electrode site are also stimulated, then the perception will also reflect activation of those peripheral cells and appear as an ellipse or streak. Thus, stimulation of these “axons of passage” should be avoided if possible. This is a challenge, since retinal ganglion cell axons form the top layer of the retina and are closest to epiretinal electrodes.
Axonal stimulation can be avoided by increasing the length of the stimulus pulses. This approach was first explored by Greenberg,[70] then later by Jensen[71] and Fried.[72] Weitz recently demonstrated a calcium imaging technique that allows direct visualization of the intracellular calcium of retinal ganglion cells, which is a reliable and accepted reporter of ganglion cell activity.[73] In all of these studies, it was found that long pulses result in no activation of retinal ganglion cells except by synaptic transmission from bipolar cells. Bipolar cells do respond to long pulses. The mechanism behind this is not clear, but it is thought to be related to the biophysics of the bipolar cell membrane that allows it to integrate stimulus over longer time scales. Freemen proposed that the L-type calcium channel in bipolar cells plays a key role in this behavior.[74]
3) Continuous stimulation
The study of phosphene fading described above can be replicated in animal models. Jensen showed that repetitive stimulation using subretinal stimulation has a desensitizing effect when pulses are spaced up to 400 ms apart.[75] Epiretinal stimulation studies show a similar phenomenon for indirect stimulation. Pulse rates greater than 10 pulses/second result in decreased responses from the retina.[76] In contrast, direct stimulation does not diminish spike responses and each pulse elicits a single action potential at rates up to 500 pulses/second.[77] Desensitization may be related to the adaptive mechanisms that exist in the retina (and in most neural systems). One approach to overcoming this may be to vary the stimulus from pulse to pulse, in both time and space, so that the cells see a new stimulus each time a pulse is applied.
V. Conclusions
Retinal prostheses represent a real, near term hope for blind individuals. The clinical trials demonstrate that tangible benefits can be obtained, specifically improved mobility and object localization. Patients with implants are generally pleased and use the devices in their everyday lives. However, room for improvement exists. When using the prosthesis in the real-world, patients do not report “pixelized vision”, where each electrode creates a spot of light. Creating form vision is a major challenge. Image fading also must be addressed, since patients need to rely on the quality of images throughout the day. Implant patients cannot yet recognize the details of faces or objects. To achieve this important goal, the resolution of implants must be increased, by increasing both the number of electrodes and the density of electrodes. Hermetic packaging is the main engineering challenge limiting the number of independent stimulation channels, which in turn will limit resolution. Significant advances in electrode array and electronic circuits should enable high resolution implants. These challenges must be addressed by interdisciplinary teams of engineers, clinicians, and scientists, working in consultation with industry and patients, to build on the early success of retinal prostheses.
Acknowledgments
The author acknowledge support by the National Science Foundation, Grant No. EEC-0310723 and Office of Science (BER) U.S. Department of Energy, Grant No. DE-FC02-04ER63735, National Eye Institute, Grant No. 5R01EY022931, The W.M. Keck Foundation, and Research to Prevent Blindness. Mark Humayun discloses a financial interest in Second Sight Medical Products, Inc.
VI. References
- [1].Dowling JE. The Retina: an approachable part of the Brain. Bellknap Press; 1987. [Google Scholar]
- [2].Hartong DT, Berson EL, Dryja TP. Retinitis Pigmentosa. Lancet. 2006 Nov;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- [3].Gehrs LM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degneration and a vision of the future. Arch Ophthalmology. 2010 Mar;128(3):349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 1968 May;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Potts AM, Inoue J. The electrically evoked response (EER) of the visual system. II. Effect of adaptation and retinitis pigmentosa. Invest. Ophth. 1969 Jun;8(6):605–12. [PubMed] [Google Scholar]
- [6].Humayun MS, de Juan E, Jr., Weiland JD, Dagnelie G, Katona S, Greenberg RJ, Suzuki S. Pattern electrical stimulation of the human retina. Vision Res. 1999 Jul;39(15):2569–2576. doi: 10.1016/s0042-6989(99)00052-8. [DOI] [PubMed] [Google Scholar]
- [7].Rizzo JF, III, Wyatt J, Loewenstein J, Kelly S, Shire D. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest. Ophth. Vis Sci. 2003 Dec;44(12):5362–5369. doi: 10.1167/iovs.02-0817. [DOI] [PubMed] [Google Scholar]
- [8].Humayun MS, Weiland JD, Fujii GY, Greenberg RJ, Williamson R, Little J, Mech BV, Cimmarusti V, van Boemel G, Dagnelie G, de Juan E., Jr. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 2003 Nov;43(24):2573–2581. doi: 10.1016/s0042-6989(03)00457-7. [DOI] [PubMed] [Google Scholar]
- [9].Yanai DY, Weiland JD, Mahadevappa M, Greenberg RJ, Fine I, Fujii GY, Humayun MS. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am J Ophthalmol. 2007 May;143(5):820–827. doi: 10.1016/j.ajo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- [10].Hornig R, Zehnder T, Velikay-Parel M, Laube T, Feucht M, Richard G. The IMI Retinal Implant System. In: Humayun MS, Weiland JD, Chader G, Greenbaum EX, editors. Artificial Sight: Basic Research, Biomedical Engineering, and Clinical Advances. Springer; New York: 2007. [Google Scholar]
- [11].Roessler G, Laube T, Brockmann C, Kirschkamp T, Mazinani B, Goertz M, Koch C, Krisch I, Sellhaus B, Trieu HK, Weis J, Bornfeld N, Röthgen H, Messner A, Mokwa W, Walter P. Implantation and explantation of a wireless epiretinal retina implant device: observations during the EPIRET3 prospective clinical trial. Invest. Ophth. Vis Sci. 2009 Jun;50(6):3003–3008. doi: 10.1167/iovs.08-2752. [DOI] [PubMed] [Google Scholar]
- [12].Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel J-A, Stanga PE, Cideciyan AV, Duncan JL, Eliott D, Filley E, Ho AC, Santos A, Safran AB, Arditi A, Del Priore LV, Greenberg RJ. Interim results from the international trial of Second Sight's visual prosthesis. Ophthalmology. 2012 Apr;119(4):779–788. doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou DD, Dorn JD, Greenberg RJ. The Argus II Retinal Prosthesis System: An Overview; Proceeding of the MAP4VIP workshop, IEEE ICME conference; San Jose, CA. 2013. [Google Scholar]
- [14].da Cruz L, Coley BF, Dorn JD, Merlini F, Filley E, Christopher P, Chen FK, Wuyyuru F, Sahel J-A, Stanga PE, Humayun MS, Greenberg RJ, Dagnelie G. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. British Journal of Ophthalmology. 2013 May;97(5):632–636. doi: 10.1136/bjophthalmol-2012-301525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chow AY, Chow VY, Packo KH, Pollack JS, Peyman GA, Schuchard R. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol. 2004 Apr;122(4):460–469. doi: 10.1001/archopht.122.4.460. [DOI] [PubMed] [Google Scholar]
- [16].Palanker D, Vankov A, Huie P, Baccus S. Design of a high-resolution optoelectronic retinal prosthesis. J Neural Eng. 2005;2:105–120. doi: 10.1088/1741-2560/2/1/012. [DOI] [PubMed] [Google Scholar]
- [17].Pardue MT, Phillips MJ, Yin H, Fernandes A, Cheng Y, Chow AY, Ball SL. Possible sources of neuroprotection following subretinal silicon chip implantation in RCS rats. J. Neural Eng. 2005 Jan;2(1):S39–S47. doi: 10.1088/1741-2560/2/1/006. [DOI] [PubMed] [Google Scholar]
- [18].Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S, Koch J, Kusnyerik A, Peters T, Stingl K, Sachs H, Stett A, Szurman P, Wilhelm B, Wilke R. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci. 2011 May;278(1711):1489–1497. doi: 10.1098/rspb.2010.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilke R, Gabel V-P, Sachs H, Bartz Schmidt K-U, Gekeler F, Besch D, Szurman P, Stett A, Wilhelm B, Peters T, Harscher A, Greppmaier U, Kibbel S, Benav H, Bruckmann A, Stingl K, Kusnyerik A, Zrenner E. Spatial resolution and perception of patterns mediated by a subretinal 16-electrode array in patients blinded by hereditary retinal dystrophies. Invest. Ophth. and Vis. Sci. 2011 Aug;52(8):5995–6003. doi: 10.1167/iovs.10-6946. [DOI] [PubMed] [Google Scholar]
- [20].Stingl K, Bartz Schmidt K-U, Besch D, Braun A, Bruckmann A, Gekeler F, Greppmaier U, Hipp S, Hörtdörfer G, Kernstock C, Kusnyerik A, Schatz A, Stingl KT, Peters T, Wilhelm B, Zrenner E. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proceedings of the Royal Society B: Biological Sciences . 2013 Apr;280(1757):1–8. doi: 10.1098/rspb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stingl K, Bartz Schmidt K-U, Besch D, Braun A, Bruckmann A, Gekeler F, Greppmaier U, Hipp S, Hörtdörfer G, Kernstock C, Kusnyerik A, Schatz A, Stingl KT, Peters T, Wilhelm B, Zrenner E. Details on the patient cohort and the subretinal implant Alpha IMS (Retina Implant AG, Reutlingen) in the first module of a multicentre clinical trial and video material of patient reports” Supplementary material for “Artificial Vision with Wirelessly Powered Subretinal Electronic Implant Alpha IMS. Proc.R.Soc.B. 2013 Apr;280(1757):1–8. doi: 10.1098/rspb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fujikado T, Morimoto T, Kanda H, Kusaka S, Nakauchi K, Ozawa M, Matsushita K, Sakaguchi H, Ikuno Y, Kamei M, Tano Y. Clinical Trial of Chronic Implantation of Suprachoroidal-Transretinal Stimulation System for Retinal Prosthesis. Sensors and Materials. 2012 Apr;24(4):181–187. [Google Scholar]
- [23].Cha K, Horch K, Normann RA. Simulation of a phosphene-based visual field: visual acuity in a pixelized vision system. Annals of Biomedical Engineering. 1992 Apr;20(4):439–449. doi: 10.1007/BF02368135. [DOI] [PubMed] [Google Scholar]
- [24].Hayes JS, Weiland JD, Humayun MS, Dagnelie G. Visually guided performance of simple tasks using simulated prosthetic vision. Artif. Organs. 2003 Nov;27(11):1016–26. doi: 10.1046/j.1525-1594.2003.07309.x. [DOI] [PubMed] [Google Scholar]
- [25].Thompson RW, Humayun MS, Dagnelie G. Facial Recognition Using Simulated Prosthetic Pixelized Vision. Invest. Ophth. and Vis. Sci. 2003 Nov;44(11):5035–5042. doi: 10.1167/iovs.03-0341. [DOI] [PubMed] [Google Scholar]
- [26].Sahel JA, Mohand-Said S, Stanga PE, Caspi A, Greenberg RJ. Acuboost: enhancing the maximum acuity of the Argus II Retinal Prosthesis System. Invest. Ophthalmol. Vis. Sci. 2013;54 [Google Scholar]
- [27].Caspi A, Dorn JD, McClure KH, Humayun MS, Greenberg RJ, McMahon MJ. Feasibility study of a retinal prosthesis: spatial vision with a 16-electrode implant. Arch Ophthalmol. 2009 Apr;127(4):398–401. doi: 10.1001/archophthalmol.2009.20. [DOI] [PubMed] [Google Scholar]
- [28].Vanhoestenberghe A, Donaldson N. Corrosion of silicon integrated circuits and lifetime predictions in implantable electronic devices. J. Neural Eng. 2013 Mar;10(3):1–13. doi: 10.1088/1741-2560/10/3/031002. [DOI] [PubMed] [Google Scholar]
- [29].Jiang G, Zhou DD. Implantable Neural Prostheses. Vol. 2. Springer; New York: 2010. Technology advances and challenges in hermetic packaging for implantable medical devices; pp. 27–61. [Google Scholar]
- [30].Suaning GJ, Lavoie P, Forrester J, Armitage T, Lovell NH. Microelectronic retinal prosthesis: III. a new method for fabrication of high-density hermetic feedthroughs; Proceedings of the 28th Engineering in Medicine and Biology Society Conference; 2006. [DOI] [PubMed] [Google Scholar]
- [31].Schuettler M, Ordonez JS, Santisteban TS, Schatz A, Wilde J, Stieglitz T. Fabrication and test of a hermetic miniature implant package with 360 electrical feedthroughs; Proceedings of the 32nd Engineering in Medicine and Biology Society Conference; 2010. [DOI] [PubMed] [Google Scholar]
- [32].Gill EC, Antalek J, Kimock FM, Nasiatka PJ, McIntosh BP, Tanguay AR, Jr, Weiland JD. MRS Proceedings. Vol. 1572. Cambridge University Press; 2013. High-Density Feedthrough Technology for Hermetic Biomedical Micropackaging. [Google Scholar]
- [33].Weiland JD, Kimock FM, Yehoda JE, Gill EC, McIntosh BP, Nasiatka PJ, Tanguay AR., Jr. Chip-Scale Packaging for Bioelectronic Implants; Proceedings of the IEEE Neural Engineering Conference; San Diego, CA. Nov, 2013. [Google Scholar]
- [34].Xiao X, Wang J, Liu C, Carlisle JA, Mech BV, Greenberg RJ, Guven D, Freda R, Humayun MS, Weiland JD, Auciello O. In vitro and in vivo evaluation of ultrananocrystalline diamond for coating of implantable retinal microchips. J. Biomed. Mat. Res. Part B. 2006 Feb;77(2):273–281. doi: 10.1002/jbm.b.30448. [DOI] [PubMed] [Google Scholar]
- [35].Cogan SF, Edell DJ, Guzelian AA, Ping Liu Y, Edell R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mat. Res. Part A. 2003 Mar;67(3):856–867. doi: 10.1002/jbm.a.10152. [DOI] [PubMed] [Google Scholar]
- [36].Sharma A, Rieth L, Tathireddy P, Harrison R, Oppermann H, Klein M, Töpper M, Jung E, Normann R, Clark G, Solzbacher F. Evaluation of the packaging and encapsulation reliability in fully integrated, fully wireless 100 channel Utah Slant Electrode Array (USEA): Implications for long term functionality. Sens. and Acts. A: Physical. 2012;188:167–172. doi: 10.1016/j.sna.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhou M, Yuce MR, Liu W. A non-coherent DPSK data receiver with interference cancellation for dual-band transcutaneous telemetries. IEEE J. Solid-State Cir. 2008 Sep;43(9):2003–2012. [Google Scholar]
- [38].Wang G, Liu W, Sivaprakasam M, Zhou M, Weiland JD, Humayun MS. A Dual Band Power and Data Telemetry for Retinal Prosthesis; Proceedings of the 28th IEEE Engineering in Medicine and Biology Conference; 2006. [DOI] [PubMed] [Google Scholar]
- [39].Chen K, Lo Y, Yang Z, Weiland J, Humayun MS, Liu W. A System Verification Platform for High-Density Epiretinal Prostheses. IEEE Trans. on Biomed. Cir. and Systems. 2013 Jun;7(3):326–337. doi: 10.1109/TBCAS.2012.2200103. [DOI] [PubMed] [Google Scholar]
- [40].Gross M, Buss R, Kohler K, Schaub J, Jager D. Optical signal and energy transmission for a retina implant; Proceedings of the 21st IEEE Engineering in Medicine and Biology Conference.1999. [Google Scholar]
- [41].Chen K, Yang Z, Hoang L, Weiland J, Humayun M, Liu W. An integrated 256-channel epiretinal prosthesis. IEEE Journal of Solid-State Circuits. 2010 Sep;45(9):1946–1956. [Google Scholar]
- [42].Ortmanns M, Unger N, Rocke A, Gehrke M, Tietdke HJ. A 0.1 mm2, digitally programmable nerve stimulation pad cell with high-voltage capability for a retinal implant. Digest of Technical Papers from the Solid-State Circuits Conference.2006. [Google Scholar]
- [43].Shire DB, Ellersick W, Kelly SK, Doyle P, Priplata A, Drohan W, Mendoza O, Gingerich M, McKee B, Wyatt JL, Rizzo JF., III ASIC design and data communications for the Boston retinal prosthesis; Proceedings of the 34th IEEE Engineering in Medicine and Biology Conference; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dommel NB, Wong YT, Lehmann T, Dodds CW, Lovell NH, Suaning GJ. A CMOS retinal neurostimulator capable of focussed, simultaneous stimulation. J. Neural Eng. 2009 Mar;6(3):245–252. doi: 10.1088/1741-2560/6/3/035006. [DOI] [PubMed] [Google Scholar]
- [45].Monge M, Raj M, Nazari MH, Chang H-C, Zhao Y, Weiland J, Humayun M, Tai Y-C, Emami A. A Fully Intraocular High-Density Self-Calibrating Epiretinal Prosthesis. IEEE Trans. on Biomed. Circuits and Systems. doi: 10.1109/TBCAS.2014.2298334. In press. [DOI] [PubMed] [Google Scholar]
- [46].Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- [47].Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Barthó P, Wise KD, Buzsáki G. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J. Neurophys. 2003 Feb;90(2):1314–1323. doi: 10.1152/jn.00116.2003. [DOI] [PubMed] [Google Scholar]
- [48].Stieglitz T, Schuettler M, Meyer J-U. Micromachined, polyimide-based devices for flexible neural interfaces. Biomedical Microdevices. 2000 Apr;2(4):283–294. [Google Scholar]
- [49].Weiland JD, Anderson DJ, Humayun MS. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans. on Biomed. Eng. 1999 Dec;49(12):1574–1579. doi: 10.1109/TBME.2002.805487. [DOI] [PubMed] [Google Scholar]
- [50].Rose TL, Robblee LS. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses. IEEE Trans. on Biomed. Eng. 1990 Nov;37(11):1118–1120. doi: 10.1109/10.61038. [DOI] [PubMed] [Google Scholar]
- [51].Schuettler M, Stiess S, King BV, Suaning GJ. Fabrication of implantable microelectrode arrays by laser cutting of silicone rubber and platinum foil. J. Neural Eng. 2005 Jan;2(1):S121–128. doi: 10.1088/1741-2560/2/1/013. [DOI] [PubMed] [Google Scholar]
- [52].Ameri H, Ratanapakorn T, Ufer S, Eckhardt H, Humayun MS, Weiland JD. Toward a wide-field retinal prosthesis. J. Neural Eng. 2009 Mar;6(3):122–128. doi: 10.1088/1741-2560/6/3/035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang Y, Rauen SL, Koss M, Calle A, Diniz B, Swenson S, Markland FS, Ufer S, Eckhardt H, Humayun MS, Weiland JD. Wide-Field Retinal Prosthesis with three dimensional, contoured, silicone/polyimide substrates; Proceedings of the IEEE Neural Engineering Conference; San Diego, CA. Nov, 2013. [Google Scholar]
- [54].Eckmiller R, Neumann D, Baruth O. Tunable retina encoders for retina implants: why and how. J. Neural Eng. 2005 Jan;2(1):45–52. doi: 10.1088/1741-2560/2/1/011. [DOI] [PubMed] [Google Scholar]
- [55].Parikh N, Itti L, Humayun M, Weiland J. Performance of visually guided tasks using simulated prosthetic vision and saliency-based cues. J. Neural Eng. 2013 Feb;10(2):113–121. doi: 10.1088/1741-2560/10/2/026017. [DOI] [PubMed] [Google Scholar]
- [56].McCarthy C, Feng D, Barnes N. Augmenting Intensity to Enhance Scene Structure in Prosthetic Vision; Proceedings of the IEEE MAP4VIP Workshop; San Jose, CA. 2013. [Google Scholar]
- [57].Pradeep V, Medioni G, Weiland JD. Visual loop closing using multi-resolution SIFT grids in metric topological SLAM; Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; Miami, FL. Jun, 2009. [Google Scholar]
- [58].Pradeep V, Medioni G, Weiland JD. A Wearable System for the Visually Impaired; Proceedings of the 32nd IEEE Engineering in Medicine and Biology Conference; 2010. [DOI] [PubMed] [Google Scholar]
- [59].Nasiatka PJ, Stiles NRB, McIntosh BP, Hauer MC, Weiland JD, Humayun MS, Tanguay AR., Jr. An Intraocular Camera for Retinal Prostheses: Restoring Sight to the Blind. In: Serpenguzel A, Poon A, editors. Chapter 20 in Optical Processes in Microparticles and Nanostuctures, Advanced Series in Applied Physics. Vol. 6. World Scientific; Singapore: 2011. pp. 385–429. [Google Scholar]
- [60].Horsager A, Greenberg RJ, Fine I. Spatiotemporal interactions in retinal prosthesis subjects. Invest. Ophth. and Vis. Sci. 2010 Feb;51(2):1223–1233. doi: 10.1167/iovs.09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nanduri D, Fine I, Horsager A, Boynton GM, Humayun MS, Greenberg RJ, Weiland JD. Frequency and amplitude modulation have different effects on the percepts elicited by retinal stimulation. Invest. Ophth. and Vis. Sci. 2012 Jan;53(1):205–214. doi: 10.1167/iovs.11-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fornos AP, Sommerhalder J, Da Cruz L, Sahel JA, Mohand-Said S, Hafezi F, Pelizzone M. Temporal properties of visual perception on electrical stimulation of the retina. Invest. Ophth. and Vis. Sci. 2012 Jun;53(6):2720–2731. doi: 10.1167/iovs.11-9344. [DOI] [PubMed] [Google Scholar]
- [63].Xie J, Wang G-J, Yow L, Humayun MS, Weiland JD, Cela CJ, Jadvar H, Lazzi G, Dhrami-Gavazi E, Tsang SH. Preservation of retinotopic map in retinal degeneration. Experimental Eye Research. 2012 Mar;98:88–96. doi: 10.1016/j.exer.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Weiland JD, Faraji B, Greenberg RJ, Humayun MS, Shellock F. Assessment of MRI issues for the Argus II retinal prosthesis. Magn Reson Imaging. 2012 Apr;30(3):382–389. doi: 10.1016/j.mri.2011.12.005. [DOI] [PubMed] [Google Scholar]
- [65].Luo YH, Davagnanam I, daCruz L. MRI Brain Scans in Two Patients with the Argus II Retinal Prosthesis. Ophthalmology. 2013 Aug;120(8):1711. doi: 10.1016/j.ophtha.2013.04.021. [DOI] [PubMed] [Google Scholar]
- [66].Freeman DK, Rizzo JF, III, Fried SI. Encoding visual information in retinal ganglion cells with prosthetic stimulation. J. Neural Eng. 2011 Mar;8(3):56–68. doi: 10.1088/1741-2560/8/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jensen RJ, Rizzo JF., III Activation of retinal ganglion cells in wild-type and rd1 mice through electrical stimulation of the retinal neural network. Vis Res. 2008 Jun;48(14):1562–1568. doi: 10.1016/j.visres.2008.04.016. [DOI] [PubMed] [Google Scholar]
- [68].Chan LL, Lee EJ, Humayun MS, Weiland JD. Both electrical stimulation thresholds and SMI-32 immunoreactive retinal ganglion cell density correlate with age in s334ter line 3 rat retina. J Neurophys. 2011 Jun;105(6):2687–2697. doi: 10.1152/jn.00619.2010. [DOI] [PubMed] [Google Scholar]
- [69].Sekirnjak C, Hulse C, Jepson LH, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Loss of responses to visual but not electrical stimulation in ganglion cells of rats with severe photoreceptor degeneration. J Neurophys. 2009 Jun;102(6):3260–3269. doi: 10.1152/jn.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Greenberg RJ, Velte TJ, Humayun MS, Scarlatis GN, de Juan E., Jr. A computational model of electrical stimulation of the retinal ganglion cell. IEEE Trans. on Biomed. Eng. 1999 May;46(5):505–514. doi: 10.1109/10.759051. [DOI] [PubMed] [Google Scholar]
- [71].Jensen RJ, Ziv OR, Rizzo JF., III Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Invest. Ophth. and Vis. Sci. 2005 Apr;46(4):1486–1496. doi: 10.1167/iovs.04-1018. [DOI] [PubMed] [Google Scholar]
- [72].Freeman DK, Eddington DK, Rizzo JF, III, Fried SI. Selective activation of neuronal targets with sinusoidal electric stimulation. J Neurophys. 2010 May;104(5):2778–2791. doi: 10.1152/jn.00551.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Weitz AC, Behrend MR, Lee NS, Klein RL, Chiodo VA, Hauswirth WW, Humayun MS, Weiland JD, Chow RH. Imaging the response of the retina to electrical stimulation with genetically encoded calcium indicators. J Neurophys. 2013 Jul;109(7):1979–1988. doi: 10.1152/jn.00852.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Freeman DK, Jeng JS, Kelly SK, Hartveit E, Fried SI. Calcium channel dynamics limit synaptic release in response to prosthetic stimulation with sinusoidal waveforms. J. Neural Eng. 2011 Apr;8(4):322–334. doi: 10.1088/1741-2560/8/4/046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jensen RJ, Rizzo JF., III Responses of ganglion cells to repetitive electrical stimulation of the retina. J. Neural Eng. 2007 Jan;4(1):78–88. doi: 10.1088/1741-2560/4/1/S01. [DOI] [PubMed] [Google Scholar]
- [76].Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J Neurophys. 2010 Jun;95(6):3311–3327. doi: 10.1152/jn.01168.2005. [DOI] [PubMed] [Google Scholar]
- [77].Ahuja AK, Behrend MR, Kuroda M, Humayun MS, Weiland JD. An in vitro model of a retinal prosthesis. IEEE Trans. on Biomed. Eng. 2008 Jun;55(6):1744–1753. doi: 10.1109/tbme.2008.919126. [DOI] [PMC free article] [PubMed] [Google Scholar]



