Summary
Despite years of research and efforts to translate stroke research to clinical therapy, ischemic stroke remains a major cause of death, disability, and diminished quality of life. Primary and secondary preventive measures combined with improved quality of care have made significant progress. However, no novel drug for ischemic stroke therapy has been approved in the past decade. Numerous studies have shown beneficial effects of activated protein C (APC) in rodent stroke models. In addition to its natural anticoagulant functions, APC conveys multiple direct cytoprotective effects on many different cell types that involve multiple receptors including protease activated receptor (PAR) 1, PAR3, and the endothelial protein C receptor (EPCR). Application of molecular engineered APC variants with altered selectivity profiles to rodent stroke models demonstrated that the beneficial effects of APC primarily require its cytoprotective activities but not its anticoagulant activities. Extensive basic, preclinical, and clinical research provided a compelling rationale based on strong evidence for translation of APC therapy that has led to the clinical development of the cytoprotective-selective APC variant, 3K3A-APC, for ischemic stroke. Recent identification of non-canonical PAR1 and PAR3 activation by APC that give rise to novel tethered-ligands capable of inducing biased cytoprotective signaling as opposed to the canonical signaling provides a mechanistic explanation for how APC-mediated PAR activation can selectively induce cytoprotective signaling pathways. Collectively, these paradigm-shifting discoveries provide detailed insights into the receptor targets and the molecular mechanisms for neuroprotection by cytoprotective-selective 3K3A-APC, which is currently a biologic drug in clinical trials for ischemic stroke.
Keywords: stroke, activated protein C, PAR1, PAR3, EPCR
Introduction
Stroke is a major cause of death and disability worldwide. In 2010, stroke caused 5.8 million deaths worldwide, which is 11.1% of all deaths globally (1). Ischemic stroke is highly prevalent (87%), whereas intracranial hemorrhage and subarachnoid hemorrhage strokes account for 10% and 3%, respectively. In the United States, someone suffers a stroke approximately every 40 seconds, resulting in a death every 4 min (2,3). The current mortality rate ~16% points to the true health burden of stroke, as most strokes are not fatal (2–4). Surviving stoke is the most common cause of neurological disability. One third of stroke survivors remain functionally disabled after one year and require ongoing support with significant adverse impact on the quality of life thereafter (2,5). Serious long-term disability is the major socio-economic burden of stroke, e.g., it costs the US an estimated $36.5 billion each year of which 56% ($20.6 billion) is in direct medical costs (2). Improving the quality of life after stroke is therefore an unmet medical and socio-economic need. Here we take note of thrombolytic therapy for ischemic stroke and review the promising possibility of cytoprotective-selective activated protein C (APC) for ischemic stroke therapy.
Tissue-type plasminogen activator therapy for ischemic stoke
Therapeutic treatment options for ischemic stroke are limited and focus mainly on achieving early reperfusion by either thrombolytic or endovascular therapy (6). Recombinant tissue-type plasminogen activator (tPA; Alteplase) was approved over a decade ago for thrombolytic therapy and remains the only approved therapeutic (6). Because tPA therapy is effective only within an early window after ischemic stroke (<4.5 h), therapy is limited to ~5% of stroke patients plus concerns for hemorrhagic conversion and tPA’s neurotoxicity have limited its widespread application (2,7–10).
Development of ischemic stroke and its consequent damages are temporally and spatially variable. Early reperfusion reduces mortality and promotes improved functional outcome (6,11). Surrounding the infarct core macroscopically, the penumbra initially escapes acute cell death due to some collateral blood flow but is at risk of irreversible damage when hypoperfusion persists and is prone to reperfusion-associated injury (12). The time-limited benefit of tPA therapy is consistent with the concept that the ischemic damage to the penumbra is reversible for a limited time (6,13). Although more aggressive thrombolytic therapy might seem beneficial, tPA conveys increased risk for hemorrhagic transformation and for neurotoxicity. Hence, there is a delicate balance between tPA’s beneficial and harmful effects (9). Despite the risk for hemorrhagic conversion, an extension of the window for tPA therapy from 3h to 4.5h has been approved in many countries and recommended by the American Heart Association although not yet supported by the FDA (13). To increase the efficacy and safety of tPA, neuroprotective adjunctive agents that blunt tPA’s hemorrhagic conversion and tPA’s neuronal toxicity could have major potential advantages. Indeed, multifunctional APC is a remarkable neuroprotectant and its multiple actions plus its cellular and molecular mechanisms are reviewed below.
Protein C and APC in stroke patients
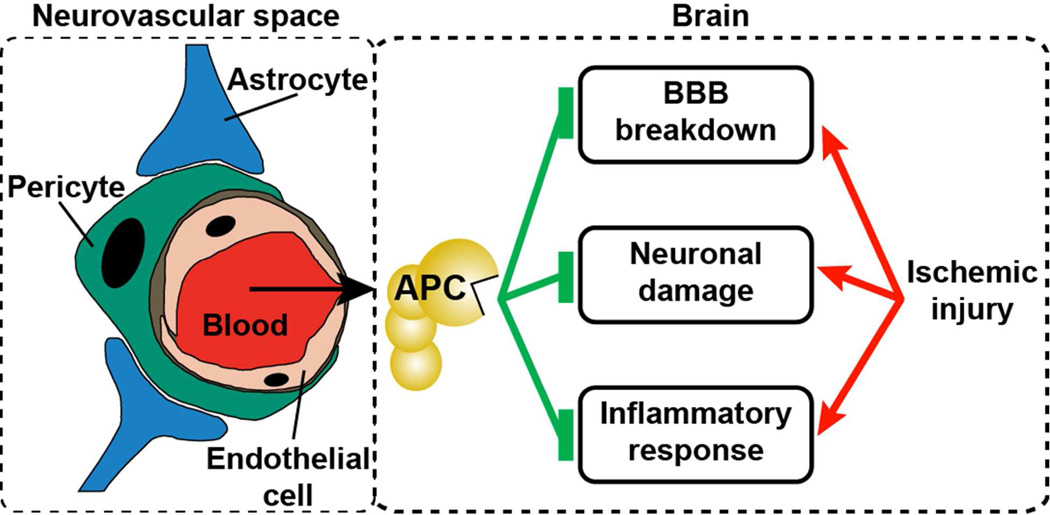
Protein C a vitamin K-dependent plasma glycoprotein and is one of the body’s natural anticoagulants circulating at 70 nmol/L as an inactive protease zymogen (14,15). Physiologic generation of APC occurs on the endothelial surface by binding of thrombin to thrombomodulin and recruitment of protein C to the surface via binding to the EPCR (Figure 1) (14,16). APC has potent anticoagulant activity due to its ability to inactivate factors Va and VIIIa, as well as multiple direct effects on vascular cells that are independent of its anticoagulant activity (14,17). These direct effects on cells that include cell-signaling effects are collectively referred to as APC cytoprotective activities and generally include anti-apoptotic and anti-inflammatory activities, alterations of gene expression profiles and protection of endothelial barrier function. Most studies support the prevailing paradigm for APC’s direct cytoprotective actions on cells that are initiated by APC-mediated PAR1 activation when APC binds to EPCR localized in caveolin-1 enriched lipid rafts (14,17–19). Depending on cell type, additional receptors may contribute to APC-initiated signaling such as sphingosine-1-phosphate receptor 1 (S1P1), apolipoprotein E receptor 2 (ApoER2), glycoprotein Ib, CD11b/CD18 (αMβ2; Mac-1), PAR3, and Tie2 (17,20).
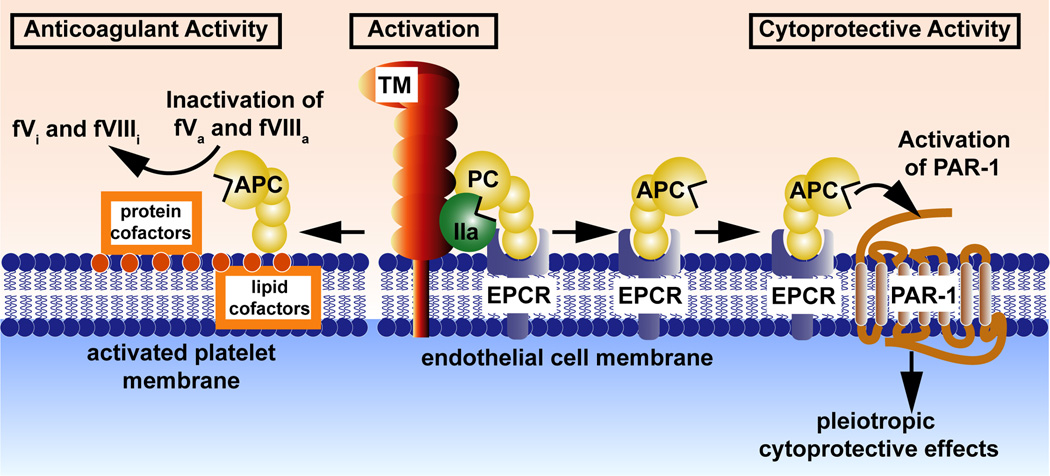
Figure 1. The protein C system.
Depicted are protein C activation (middle) and the anticoagulant (left) and cytoprotective (right) protein C pathways. Efficient activation of protein C zymogen (yellow) (middle of scheme) by thrombin (IIa) (green) requires thrombomodulin (TM) (red) and the endothelial protein C receptor (EPCR) (blue). Retention of APC bound to EPCR allows APC to express multiple cytoprotective activities (right side of scheme) that involve PAR-1. These cytoprotective activities include APC-mediated anti-inflammatory and anti-apoptotic activities, alterations of gene expression profiles, and protection of endothelial barrier functions. Dissociation of APC from EPCR allows binding of APC to negatively charged cell membrane surfaces and expression of APC’s anticoagulant activity (left side of scheme) that involves limited proteolysis of the activated cofactors Va (fVa) and VIIIa (fVIIIa) to yield the inactivated cofactors, fVi and fVIIIi.
Multiple lines of evidence suggest that the brain is particular sensitive to the activities of the APC anticoagulant and cytoprotective pathways. Infants born with severe protein C deficiency are often blind and develop mental retardation (21). Mice with genetically engineered protein C deficiency demonstrate selective fibrin deposition in brain blood vessels (22). Similar characteristic vasculature-specific local thrombosis is observed in mice carrying Factor VLeiden (R506Q) but not in mice with a deficiency of ATIII, which form fibrin deposits in the heart and liver, but not in the brain (23,24). Thus, characteristic brain-specific lesions seem to be related to a loss of specific, possibly APC-mediated cytoprotective functions in the brain rather than a general loss of natural anticoagulant activity.
Endogenous APC is part of a systemic anticoagulant and anti-inflammatory surveillance system and small levels of circulating levels of APC (~40 pmol/L) are normally found in the circulation. APC is also generated in the human brain, e.g. during the short ischemic phase of routine carotid endarterectomy, consistent with a neuroprotective role for APC during ischemia (25–28). However, circulating APC levels are decreased in stroke patients (26). In prospective epidemiologic studies of stroke, protein C levels were inversely associated with the incidence of stroke in the Atherosclerosis Risk in Communities (ARIC) study (29). In this prospective population-based study of 13,879 participants with 613 ischemic strokes after a median 16.9 years follow-up, protein C levels in the lowest quintile (<2.6 µg/ml) was associated with a 1.5 higher incidence of ischemic stroke. Thus, observational data for humans is consistent with a neuroprotective function for APC.
APC’s neuroprotective effects in stroke
Therapeutic application of APC demonstrates protective effects in multiple injury and disease models, especially in models of inflammation, infection, and ischemia/reperfusion injury including ischemic stroke (14,30–32). In rodent models of focal ischemia after middle cerebral artery occlusion (MCAO), APC improves survival via anti-apoptotic, anti-inflammatory, antithrombotic and neuroprotective effects (33–35). After MCAO, APC’s anti-thrombotic effects enhanced restoration of cerebral blood flow and reduced fibrin-positive cerebral vessels. APC’s anti-inflammatory effects inhibited ICAM-1 expression on ischemic cerebral blood vessels, and eliminated infiltration of the brain by neutrophils, whereas APC’s neuroprotective effects inhibited neuronal apoptosis. Collectively, these activities of APC reduced brain infarct volume, diminished edema formation, and improved motor neurological score (33,34,36).
The neuroprotective effects of APC, defined as APC’s cytoprotective effects on cells of the neurovascular unit, were studied in conjunction with tPA therapy in rodent stroke models. Despite promoting restoration of cerebral blood flow and reducing fibrin deposition, tPA treatment increased hemorrhage after MCAO, increased injury volume, and decreased motor scores (37–39). When administered with or following tPA, APC reduced the tPA-mediated increases in injury volume, edema, and neurologic motor scores, but also brought out the tPA’s reperfusion benefits as the tPA-APC combination was superior compared to either agent alone (37–39). Remarkably, APC also decreased tPA-induced brain microhemorrhages (Figure 2). Thus, it appears that APC’s neuroprotective effects integrate well with reperfusion effects of tPA to improve outcome in ischemic stroke models.
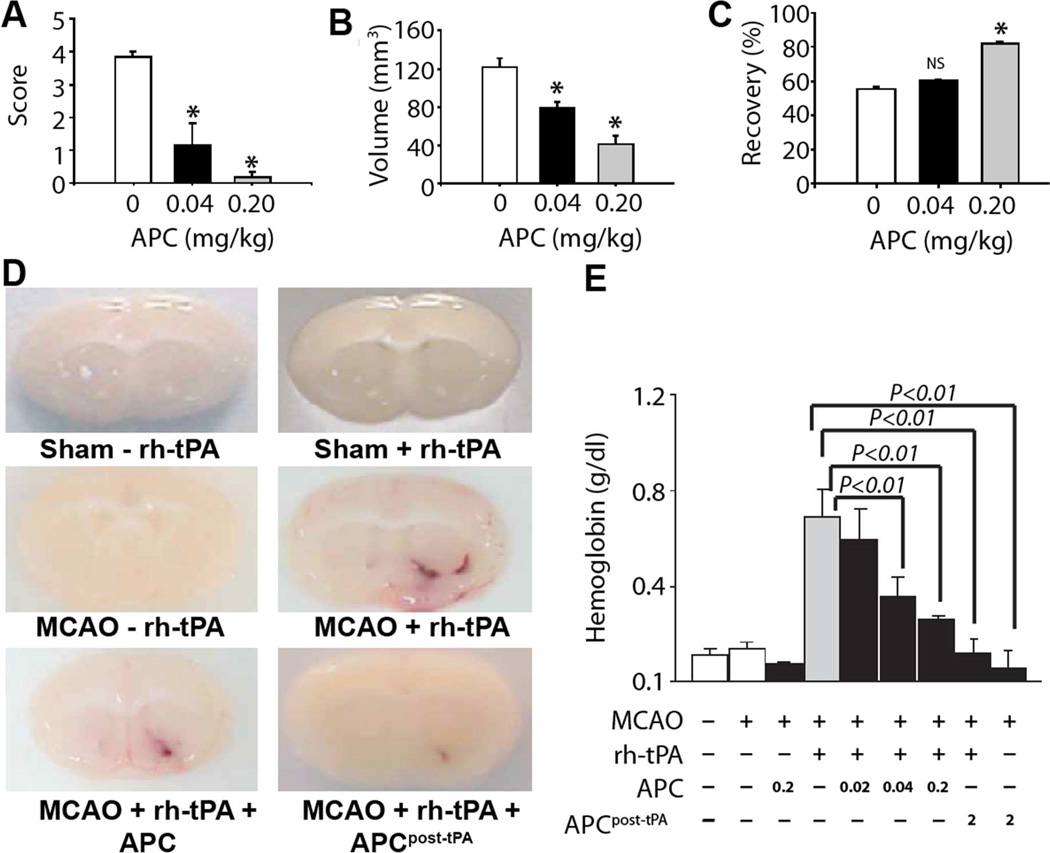
Figure 2. APC neuroprotective effects and tPA-induced hemorrhage during murine ischemic stroke.
in vivo neuroprotective effects of recombinant mouse wt-APC in C57Bl6 mice during focal ischemic stroke. APC or vehicle was given 10 min after MCAO. Motor neurological score (A), total brain injury volume (B) and post-ischemic cerebral blood flow (C). Brain hemorrhage mediated by tPA after transient ischemia in mice was determined 24 h after transient MCAO for mice receiving the indicated treatments (D), where the bottom two images show brains for mice receiving either 0.2 mg/kg APC with tPA or 2.0 mg/kg APC at 3h after tPA. Bleeding in the ischemic brain was quantified by hemoglobin after treatment with or without rh-tPA (0.2 mg/kg) and with or without simultaneous infusion or infusion 3 h post-tPA of mouse wt-APC (0–2 mg/kg) (E). Panels A-C are from Cheng et al. Nat Med 2003 (34), panels D–E are from Cheng et al. Nat Med 2006 (38).
APC activities and activity-selective APC variants
Prevention of tPA-induced bleeding by APC required PAR1 since APC did not prevent tPA-induced bleeding in PAR1−/− mice (38). In addition to PAR1, APC neuroprotective effects also required EPCR and PAR3 (34,36,37). Although genetically modified mice and receptor blocking antibodies clearly indicate important contributions of the cytoprotective pathway for neuroprotective effects of APC, activity-selective APC mutants provided additional answers to the question of the relative contribution of APC’s anticoagulant versus cytoprotective activities.
Engineering approaches for APC exploited the premise that the enzymatic substrates (factors Va and VIIIa versus PAR1/PAR3) and the cofactors (phospholipids/protein S versus EPCR) for APC’s anticoagulant and cytoprotective activities differ in structure and function (Figure 1). A positively charged extended surface involving multiple polypeptide loops on APC is essential for interactions with its substrate, factor Va (Figure 3) (14,40,41). Since at least part of this factor Va exosite on APC is not involved in interactions with PAR1, targeting specific positive residues for mutation to alanine decreased anticoagulant activity yet retained normal cytoprotective activities (Table 1). Cytoprotective-selective APC mutants include RR229/230AA-APC, 3K3A-APC (KKK191-193AAA), 5A-APC (the combination of 3K3A-APC with RR229/230AA-APC), R193E-APC, and Cys67-Cys82-APC (R222C/D237C) (42–46). Other cytoprotective-selective APC mutants targeted the interaction with protein S to reduce anticoagulant activity (L38D-APC) and omitted the glycosylation site at Asn229 to optimize interactions of APC with PAR1 (L38D/N229Q-APC) (47,48). Anticoagulant-selective APC mutants have targeted EPCR binding by the Gla domain (L8W, L8V) (43,46,49), an exosite for PAR1 on APC (E330A and E333A) (50) and the C-terminus of the APC light chain (E149A-APC) (51), which for reasons not yet understood lacks APC cytoprotective activities.
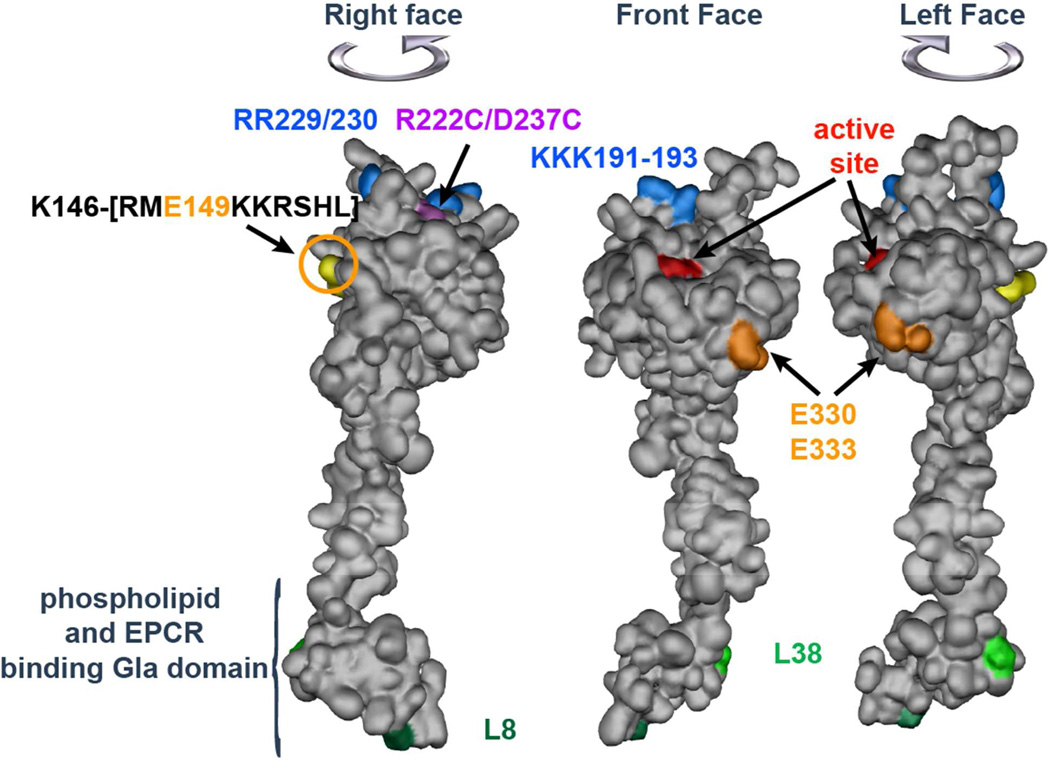
Figure 3. APC space-filling model showing amino acid residues of activity-selective APC mutants.
The model of APC depicts the N-terminal Gla domain at the bottom which binds EPCR and phospholipid membranes, the EGF-1 and EGF-2 domains in the middle and the protease domain at the top with the “active site” residues noted in red. On the top of the model, blue highlights five basic residues (KKK191-193 and RR229/230) which form a large positively charged exosite that recognizes factor Va; mutations of these residues reduce anticoagulant activity but not cytoprotective activity. Purple highlights two residues (R222 and D237) which when mutated to Cys can form a disulfide bond, causing loss of most anticoagulant activity but retention of cytoprotective activity. Light green highlights the L38D mutation that reduces anticoagulant activity due to reduced protein S enhancement. Anticoagulant-selective APC mutants include mutations of E330 and E333 to Ala (orange) that selectively reduce PAR1 signaling, the E149A mutation (yellow) in the C terminus of the light chain, and mutation of L8 in the GLA-domain (dark green) that selectively disrupts APC binding to EPCR but not to negatively charged phospholipids. The model is based on the x-ray crystallographic structure of APC (1AUT) (90).
Table 1.
Activity-selective APC mutants
| Name | Mutation(s) | Target | Anticoagulant activity |
Cytoprotective activity |
Selectivity ratio |
Ref. |
|---|---|---|---|---|---|---|
| Cytoprotective-selective APC | ||||||
| 3K3A-APC | KKK191-193AAA¶ | FVa | 4.6% (25%)§ | 114% (100%)† | 25× (4×) | (44) |
| 229/230-APC | RR229/230AA | FVa | 13% (33%) | 94% (100%) | 7× (3×) | (44) |
| 5A-APC | KKK191-193AAA + RR229/230AA |
FVa | <3% (<8%) | 100% (100%) | >33× (>13×) | (45) |
| Cys67-Cys82-APC | RD222/237CC | FVa | <1% | ~50% | >50× | (42) |
| K193E | K193E | FVa | 4% | 200% | 50× | (46) |
| APC-L38D | L38D | PS | <5%‡ | 100% | >20× | (47) |
| APC-L38D\N229Q | L38D/N229Q | PS+PAR1 | <5%‡ | 500% | >100× | (48) |
| Anticoagulant-selective APC | ||||||
| E149A-APC | E149A | PS+? | 420% (400%) | <6% (<6%) | >70× (>67×) | (51) |
| APC-E167A | E330A | PAR1 | 100% | <1% | >100× | (50) |
| APC-E170A | E333A | PAR1 | 100% | <1% | >100× | (50) |
| L8W-APC | L8Q | EPCR | ~175%‡ | ~10%‡ | ~18× | (46) |
Numbering based on mature protein C. For conversion to chymotrypsin numbering, see Mather et al. (90).
Denoted are values for human APC in human plasma systems and in parentheses the corresponding values for the homologous murine APC mutants in murine plasma systems if data are available.
The percentages reflect the activity of APC mutants based on changes in the dose-response to achieve equal effects compared to wt-APC.
Based on extrapolated values.
PS is an abbreviation for protein S.
Although human APC is neuroprotective in the mouse (33,34,36), effective concentrations are much higher compared to effective murine APC concentrations (52). 3K3A-APC, 5A-APC, and E149A-APC were made as homologous mouse APC variants for in vivo proof of concept studies (Table 1) (51,53). Murine cytoprotective-selective 5A-APC reduced infarct volume and edema, and improved motor score after MCAO, whereas murine anticoagulant-selective E149A-APC worsened outcomes and increased brain hemorrhage after MCAO (54). Murine 3K3A-APC with 80% reduced anticoagulant activity provides 1.5-fold to 2-fold enhanced neuroprotective effects compared to mouse wt-APC (55,56). Human cytoprotective-selective APC mutants, as well as the murine variants, are neuroprotective in ischemic stroke (57–59). Thus, the cytoprotective effects of APC provide neuroprotection in ischemic stroke, whereas the anticoagulant effects of APC exacerbate injury and cause bleeding. These conclusions are consistent with results in experimental sepsis models where murine cytoprotective-selective APC variants (229/230, 3K3A, and 5A) reduce mortality, whereas E149A-APC can increase mortality (51,53,60).
The PAR paradox: APC versus thrombin
The neuroprotective effects of APC require EPCR-assisted activation of the GPCRs, PAR1 and PAR3 (36,56). Yet thrombin activates these receptors much more efficiently, thereby stimulating neuronal damage and severe neurovascular injury (61,62). These discordant effects of PAR1 activation by thrombin versus APC raises the question how PAR1 activation by different proteases can result in opposite effects? The PARs are unique in that they carry their own encrypted ligand encoded in the extracellular N-terminal tail (63). Proteolysis creates a new N-terminus that acts as a tethered-ligand for activation of the PAR. Thrombin activates PAR1 by proteolysis at canonical Arg41, whereas APC but not thrombin can activate PAR1 by proteolysis at non-canonical Arg46 (63,64). Proteolysis at canonical versus non-canonical sites gives rise to different N-terminal sequences, i.e., different tethered ligand agonists, which begin at residue 42 (SFLLRN…) or at residue 47 (NPNDKYE…). Similar to the N-terminal tethered agonists, synthetic peptides such as S/TFLLRN… (aka TRAP) or NPNDKYE… (aka TR47) elicit cell-signaling effects that resemble thrombin or APC effects, respectively.
Emerging insights into mechanisms for biased signaling of GPCRs, the requirement for β-Arrestin 2 for APC-induced cytoprotective PAR1 signaling, and PAR1 cleavages at Arg41 or Arg46 were integrated to provide a new paradigm for PAR1-mediated biased signaling (17,64–68). As indicated (Figure 4), canonical and non-canonical PAR1 activation by different proteases generate biased tethered-ligands that differentially induce distinct active receptor conformations linked to unique signaling pathways. Cleavage by thrombin or a synthetic TRAP stabilizes PAR1 conformations that preferentially associate with G-proteins and induce MAPK phosphorylation, RhoA activation, and endothelial barrier disruptive effects. Cleavage by APC or a synthetic TR47 peptide stabilizes a different subset of PAR1 conformations that preferentially employ biased β-Arrestin-mediated signaling that results in phosphorylation of Akt, activation of Rac1 and endothelial barrier protective effects. The functional selectivity between the PAR1 biased ligands, TRAP and TR47, on endothelial cells is remarkable as exemplified by the difference in MAPK phosphorylation versus Akt phosphorylation and RhoA versus Rac1 activation elicited by TRAP and TR47, respectively (64).
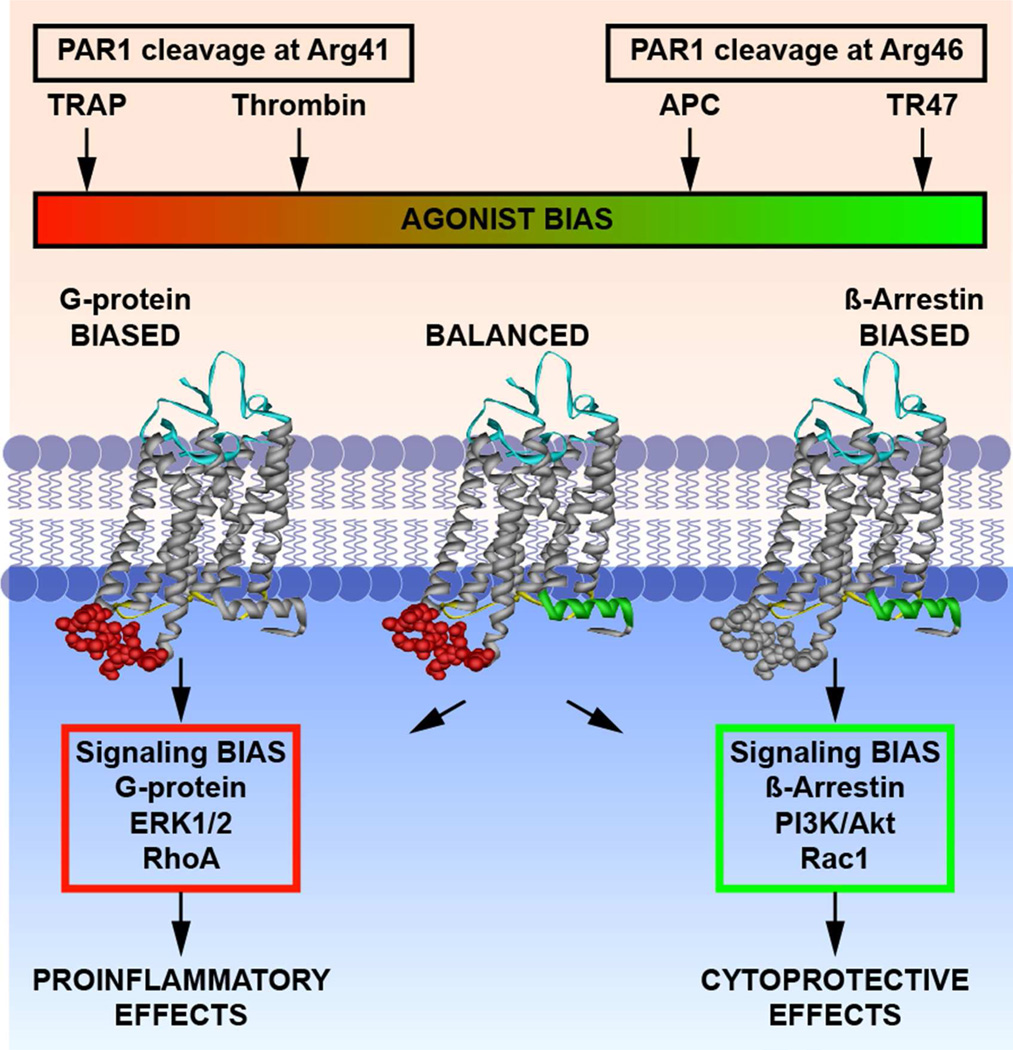
Figure 4. Functional selectivity and biased agonism due to canonical and non-canonical PAR1 activation.
Thrombin and APC display functional selectivity for PAR1 activation that results in either proinflammatory effects or cytoprotective effects, respectively. Thrombin cleaves PAR1 at Arg41 (“canonical cleavage”) and APC cleaves PAR1 at Arg46 (non-canonical cleavage”). The N-terminal tethered-ligands with the new N-terminal sequences as represented by the TRAP peptide that exists after cleavage at Arg41 or the TR47 peptide that exists after cleavage at Arg46 can accordingly cause activation of different signaling pathways, and is termed ‘biased agonism.’ The conformer subsets of PAR1 that are stabilized by TRAP or TR47 peptides preferentially promote signaling mediated by G-proteins or by β-arrestin. The agonist bias is thus directly related to the differing PAR1 cleavage sites. This figure was originally published in Blood (64). © the American Society of Hematology.
By mechanisms that are not yet entirely clear, PAR3 may modulate PAR1-dependent signaling and APC’s neuroprotective actions (36,56,69–72). Proteolysis of human PAR3 by APC occurs at non-canonical Arg41 thereby generating the PAR3 tethered ligand sequence GAPPNS… as opposed to thrombin generating the tethered ligand sequence TFRGAP… by proteolysis at canonical Lys38 (70). The different N-terminal peptide sequences generated from non-canonical cleavages of PAR1 and PAR3 by APC are biased peptide agonists that promote vascular integrity (64,70). Collectively, new paradigms for biased PAR1 and PAR3 signaling due to non-canonical activation provide mechanisms for how different proteases mediate functional selectivity and why APC conveys neuroprotection via PAR1, whereas thrombin mediates neuronal damage and severe neurovascular injury via PAR1 (30,61,62).
Mechanisms of neuroprotection by APC
To provide neuroprotective effect on neurons and other brain cells inside and beyond the neurovascular unit, EPCR enables APC transport across the blood-brain-barrier (BBB) (73,74). Inside the neurovascular unit, APC conveys multiple beneficial effects directly on neurons that include inhibition of apoptosis, anti-inflammatory effects, and alteration of gene expression profiles. As depicted in Figure 5, APC neuroprotective effects and its ability to prevent tPA-induced neurotoxicity in the neurovascular unit include: (i) protection of the BBB integrity, (ii) anti-inflammatory and anti-apoptotic effects on brain endothelial cells, and (iii) anti-apoptotic effects on neurons and other brain cells.
Figure 5. Multiple neuroprotective effects of APC on the neurovascular unit following ischemic injury.
APC neuroprotective effects and its ability to prevent tPA-induced neurotoxicity in the neurovascular unit include (i) protection of the BBB integrity, (ii) anti-inflammatory and anti-apoptotic effects on brain endothelial cells, and (iii) anti-apoptotic effects on neurons and other brain cells. This figure was modified from Zlokovic and Griffin, Trends Neuroscience 2011 (30).
Protection of vascular integrity of the BBB by APC likely involves EPCR-dependent non-canonical activation of PAR1 and PAR3 that results in β-Arrestin 2-mediated biased signaling involving scaffolding by disheveled-2, and activation of Akt and Rac1. Activation of Rac1 in endothelial cells preserves cytoskeleton-mediated cell-cell interactions and prevents actin stress fiber formation (17,64,65). Additional contributions are possibly provided by APC-mediated transactivation of the Angiopoietin/Tie2 system and transactivation of the sphingosine-1-phosphate (S1P) receptor-1 (S1P1) involving APC-mediated activation of sphingosine kinase 1 and generation of S1P (14,17). Furthermore, APC inhibits hypoxia-induced apoptosis in brain endothelial cells by blunting hypoxia-induced up regulation of pro-apoptotic p53 and Bax and preventing loss of anti-apoptotic Bcl-2 (34).
Prevention of tPA-induced hemorrhagic transformation by APC involves the entire neurovascular unit with multiple targets on multiple cells. Exact mechanisms for hemorrhagic transformation by tPA remain unknown but likely relate to non-thrombolytic functions of tPA that encompass: (i) disruption of the BBB, (ii) activation of matrix metalloproteases (MMP) especially MMP9, (iii) excitotoxic stimulation of N-methyl-D-aspartate (NMDA) receptors on neurons, and (iv) dilatation of the cerebral vasculature in non-ischemic areas thereby decreasing blood flow to the penumbra (7,75,76). A prominent role for MMP9 is implicated based on association of elevated levels in stroke patients with worsened outcomes and supported by studies demonstrating deterioration of the BBB by MMP9 due to degradation of vascular basement membranes (76). Moreover, MMP9 increases BBB permeability and degrades neuronal laminin resulting in neuronal apoptosis (76). Hypoxia sensitizes brain endothelial cells to tPA-induced NFκB activation and MMP9 upregulation. Anti-inflammatory effects of APC include prevention of NFκB activation and nuclear translocation, thereby abrogating tPA-induced MMP9 expression (38). Other anti-inflammatory effects of APC inhibit expression of endothelial adhesion molecules that facilitate infiltration of the neurovascular unit by neutrophils, which are another source of MMP9 (33,76). Thus, MMP9 is a major target for prevention of tPA-induced hemorrhagic transformation by APC.
Inside the neurovascular unit, APC is shown to protect murine and rat neurons, astrocytes and other brain cells from neuronal apoptosis induced by N-methyl-D-aspartate (NMDA), glutamate, and hypoxia (36,37,52,56,77,78). These neuronal anti-apoptotic activities of APC require PAR1, PAR3 and EPCR and involve inhibition of nuclear localization of apoptosis-inducing factor (AIF) and NFκB, inhibition of p53 induction, and inhibition of caspase 8 and 9 activation (36,56). Transport of tPA across the BBB by low-density lipoprotein receptor related protein (LRP) results in neurotoxic effects on cells within the neurovascular unit (37,75). APC’s anti-apoptotic activities inhibited augmentation of hypoxia- or NMDA-induced neuronal apoptosis by tPA that involved both mitochondria-mediated (caspase 9-dependent) intrinsic apoptotic pathways as well as extrinsic (caspase 8-dependent) apoptotic pathways (37). In summary, major neuroprotective effects of APC are centered on the triad of: (i) barrier protective effect on endothelial BBB cells, (ii) anti-inflammatory effects on multiple cells that prevent MMP9-mediated BBB breakdown, and (iii) anti-apoptotic effects on neurons and other cells inside the neurovascular unit (Figure 5) (30).
The window of opportunity for benefits from neuroprotective agents may be limited and generally coincides with the window of reperfusion benefit after stroke onset unless neuroprotective agents also have neuro-regenerative activities (13). In addition to APC neuroprotective effects, several lines of evidence suggest that APC promotes neuro-regeneration by inducing neovascularization and neurogenesis (79,80), stimulating production of neuronal progenitor cells (81), and promoting hippocampal metaplasticity associated with long term potentiation (82). These activities of APC may help explain its ability to improve neurological outcome when given beginning as late as 12h to 7days after onset of ischemia (79, 80). Thus, APC provides neuroprotective effects during the reperfusion phase, prevents hemorrhagic conversion and neurotoxic effects caused by tPA, and promotes vascular and neuronal repair in the postischemic brain.
3K3A-APC: preclinical and clinical development
The extensive studies on the effects of APC in the brain formed the impetus for translation of 3K3A-APC as adjuvant neuroprotective therapy to tPA in acute ischemic stroke. Translation of promising preclinical neuroprotectants into stroke therapy has been proven difficult and unsuccessful thus far. The Stroke Therapy Academic Industry Roundtable (STAIR) formulated criteria to increase chances of success of drug development for stroke that were adapted to a STAIR quality scoring system (83). Development of human 3K3A-APC adheres to the STAIR recommendations and scores a perfect 10 for neuroprotective monotherapy and 9 out of 10 for adjuvant therapy with tPA in ischemic stroke (30,58).
Some insights for APC-based therapies for stroke follow from experiences for recombinant wild-type APC (DrotAA; Xigris®) therapy for the treatment of adult severe sepsis that was FDA- and EAMA-approved and used for thousands of patients until it was withdrawn due to lack of efficacy in a second phase 3 trial conducted 10 years after the first very successful trial (84,85). DrotAA therapy employed a 96 hr-infusion of low dose APC (24 µg/kg/hr) based on the incorrect assumption that APC’s anticoagulant actions would protect against sepsis. Similar to stroke preclinical studies, data from in vivo studies using receptor-deficient mice and engineered activity-selective APC mutants imply that APC’s cytoprotective effects are the essential activities for reducing mortality in sepsis. Thus, the ultimate failure of wt-APC infusions in sepsis patients may have been exacerbated by both the wrong hypothesized mechanism of action and by its dosing regimen, which failed to deliver doses high enough to trigger cytoprotective cell signaling. Furthermore, the 96 hr-infusion of low dose APC in sepsis was associated with an increased risk of serious bleeding (86).
3K3A-APC therapy for ischemic stroke is fundamentally different from previous wt-APC therapy in human adult severe sepsis because 3K3A-APC’s mode of action targets APC’s cytoprotective activities and is based on different concepts than DrotAA therapy in sepsis that targeted anticoagulant activities (Table 2). The FDA approved high-dose repeated bolus dosing of ischemic stroke patients using 3K3A-APC following tPA standard-of-care therapy in clinical trials (phase 2). High-dose bolus dosing is consistent with the therapeutic mechanisms of 3K3A-APC inducing PAR1 and PAR3-mediated cytoprotective cell signaling. Many successful in vivo preclinical studies for APC therapy for ischemic stroke employed bolus dosing. Interestingly, one study using continuous infusion failed to demonstrate beneficial effects (87). Bolus dosing also limits APC-mediated, ongoing systemic proteolysis of FV and FVIII that may promote bleeding due to consumption of these clotting factors (88).
Table 2.
Differences in clinical trial designs for two APC therapies.
| Clinical Trial | ||
|---|---|---|
| Adult severe sepsis | Acute Ischemic Stroke | |
| Therapy | wt-APC | tPA + 3K3A-APC |
| Pathway | Anticoagulant | Cytoprotective |
| Target | Factors Va & VIIIa | PAR1 & PAR3 |
| Activity | Anticoagulant: 100% Cytoprotective: 100% |
Anticoagulant: <10% Cytoprotective: 100% |
| Dosing | Low dose continuous 24 µg/kg/hr |
High dose bolus 5× /12 hr |
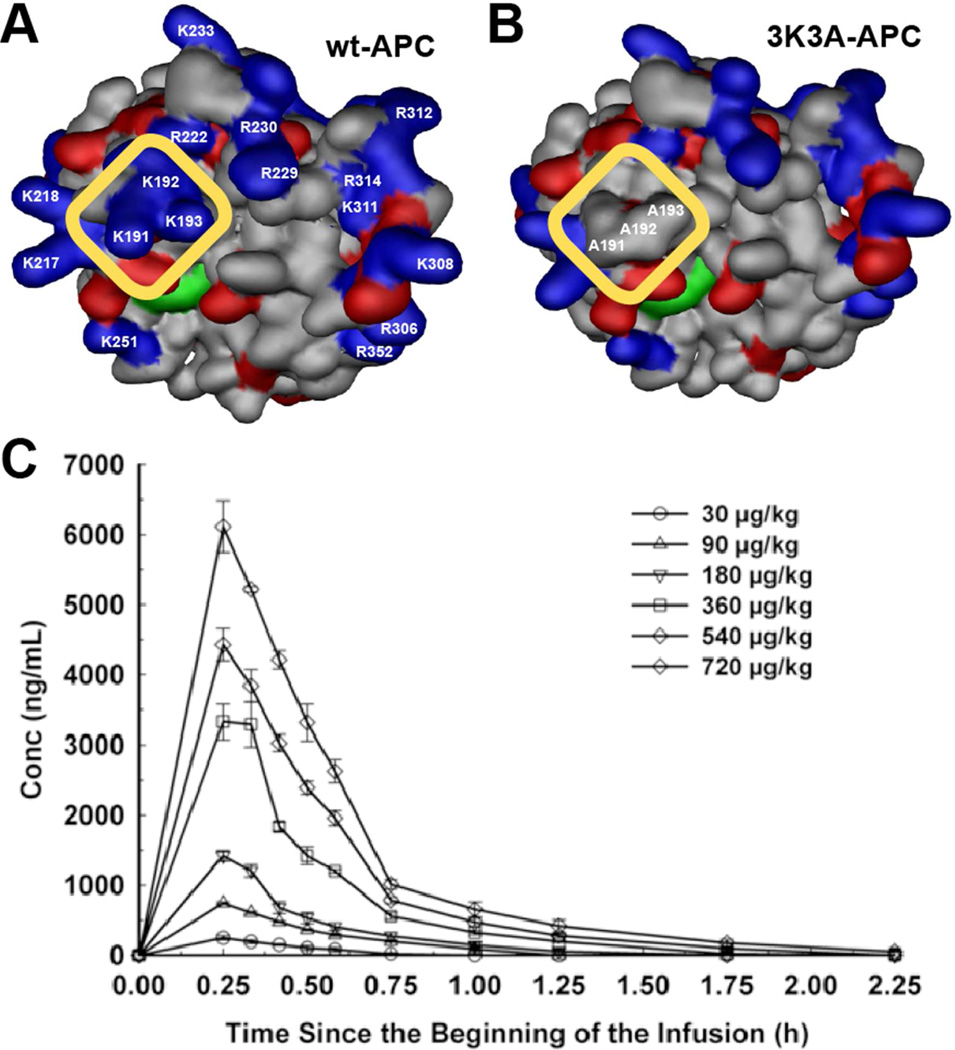
A successful phase 1 study showed that 3K3A-APC was well tolerated at multiple, very high doses (up to 540 µg/kg) every 12h that gave peak plasma levels of ~4,500 ng/ml (Figure 6), which is ~100-times higher than levels of circulating wt-aPC (<50 ng/ml) achieved in the PROWESS or PROWESS-SHOCK trials with 96 h low dose infusions (84,85,89). The phase 1 clinical data for 3K3A-APC support the concept that cytoprotective-selective APC with reduced anticoagulant activity can be dosed significantly higher than wt-APC while causing minimal perturbations to clotting times. The safety, tolerability, and activity of 3K3A-APC, following the use of tissue plasminogen activator (tPA) in subjects with ischemic stroke will remain to be determined and this will be the subject of a phase 2 clinical trial initiated this year. In this trial, 3K3A-APC will be administered after tPA as a 15 minute infusion every 12 hours for up to 5 infusions and at four increasing dose levels in approximately one hundred adult participants who will be followed for 90 days (http://www.neuronext.org/neuronext-pleased-announce-funding-our-fourth-approved-trial-safety-evaluation-3k3a-apc-ischemic).
Figure 6. Dosing of cytoprotective-selective 3K3A-APC in healthy adult volunteers.
The surface contours for the APC protease domain showing positive (blue) and negative (red) side-chains for wt-APC (A) and 3K3A-APC (B). The yellow rectangle highlights the comparison of the protease domain for 3K3A-APC (containing 3 Ala substitutions at Lys191, Lys192, and Lys193) with wt-APC to indicate the loss of three positively charged surface residues from an exosite that is required for normal recognition of factor Va. Loss of this factor Va-binding site results in reduced anticoagulant activity (~5% of wt-APC) but not any loss of cytoprotective actions, hence the designation “cytoprotective-selective” for the 3K3A-APC variant. (C) As the result of its reduced anticoagulant activity, 3K3A-APC can reach high plasma concentrations without adverse effects in healthy human subjects after a brief i.v. infusion of a single dose (data in panel C) or after five repeated high doses every 12 h (data not shown). Panel C is from Lyden et al. Curr Pharm Design 2013 (89).
Summary
Research on the cytoprotective cell signaling actions of the protein C system has made great progress in the last decade. Activity-selective APC mutants have firmly established important roles of APC’s cytoprotective activities for beneficial effects in models of inflammatory and ischemic injury including ischemic stroke. The integration of non-canonical PAR activation by APC and PAR-mediated biased signaling provides a paradigm shift in our understanding why and how different proteases display functional selectivity for cytoprotective versus proinflammatory effects. Collectively, these major advances and the extensive studies on the effects of APC in the brain resulted in the translation of 3K3A-APC to clinical trials for acute ischemic stroke. In spite of the withdrawal of wt-APC therapy from the market in 2011, the 3K3A-APC phase 2 ischemic stroke clinical trial provides a beacon of hope for therapies using APC variants in the future.
Acknowledgements
We apologize to our colleagues whose work could not be cited due to space limitations. We gratefully acknowledge helpful discussions and productive interactions with Drs. Patrick Lyden and Jose A. Fernandez.
Grant support: This manuscript was supported by National Institutes of Health (NHLBI) grants HL104165 (L.O.M.), HL063290 (B.V.Z), HL031950 and HL052246 (J.H.G.).
Abbreviations
- BBB
blood-brain-barrier
- APC
activated protein C
- PAR
protease activated receptor
- EPCR
endothelial protein C receptor
- tPA
tissue-type plasminogen activator
- MMP
matrix metalloprotease
- NMDA
N-methyl-D-aspartate
- MCAO
middle cerebral artery occlusion
Footnotes
Author Statement
LO Mosnier, BV Zlokovic, and JH Griffin wrote the manuscript
Conflict of Interest
LO Mosnier, JH Griffin, and BV Zlokovic are inventors for subject matters related to cytoprotective, neuroprotective APC variants. JH Griffin is a consultant for ZZBiotech LLC. BV Zlokovic is the scientific founder of ZZBiotech LLC, a biotechnology company with a focus to develop activated protein C and its functional mutants for stroke and other neurological disorders.
Reference List
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports. 2013;61:1–118. [PubMed] [Google Scholar]
- 4.Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke. 2014;9:6–18. doi: 10.1111/ijs.12245. [DOI] [PubMed] [Google Scholar]
- 5.Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81:1588–1595. doi: 10.1212/WNL.0b013e3182a9f45f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossman AW, Broderick JP. Advances and challenges in treatment and prevention of ischemic stroke. Ann Neurol. 2013;74:363–372. doi: 10.1002/ana.23993. [DOI] [PubMed] [Google Scholar]
- 7.Bivard A, Lin L, Parsonsb MW. Review of Stroke Thrombolytics. J Stroke. 2013;15:90–98. doi: 10.5853/jos.2013.15.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lees KR, Bluhmki E, von KR, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 9.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 10.Lackland DT, Roccella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazighi M, Chaudhry SA, Ribo M, et al. Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013;127:1980–1985. doi: 10.1161/CIRCULATIONAHA.112.000311. [DOI] [PubMed] [Google Scholar]
- 12.Manning NW, Campbell BC, Oxley TJ, et al. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke. 2014;45:640–644. doi: 10.1161/STROKEAHA.113.003798. [DOI] [PubMed] [Google Scholar]
- 13.Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke. 2013;44:2942–2950. doi: 10.1161/STROKEAHA.113.000731. [DOI] [PubMed] [Google Scholar]
- 14.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 15.Castellino FJ, Ploplis VA. The protein C pathway and pathologic processes. J Thromb Haemost. 2009;7(Suppl 1):140–145. doi: 10.1111/j.1538-7836.2009.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32:49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 17.Bouwens EA, Stavenuiter F, Mosnier LO. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost. 2013;11:242–253. doi: 10.1111/jth.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the PAR-1 dependent signaling specificity of coagulation proteases. IUBMB Life. 2011;63:390–396. doi: 10.1002/iub.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo A, Soh UJ, Trejo J. Proteases Display Biased Agonism at Protease-Activated Receptors: Location matters! Mol Interv. 2009;9:87–96. doi: 10.1124/mi.9.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiler H. Multiple receptor-mediated functions of activated protein C. Hamostaseologie. 2011;31:185–195. doi: 10.5482/ha-1166. [DOI] [PubMed] [Google Scholar]
- 21.Knoebl PN. Severe congenital protein C deficiency: the use of protein C concentrates (human) as replacement therapy for life-threatening blood-clotting complications. Biologics. 2008;2:285–296. doi: 10.2147/btt.s1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalbert LR, Rosen ED, Moons L, et al. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102:1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishiguro K, Kojima T, Kadomatsu K, et al. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest. 2000;106:873–878. doi: 10.1172/JCI10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui J, Eitzman DT, Westrick RJ, et al. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96:4222–4226. [PubMed] [Google Scholar]
- 25.Macko RF, Killewich LA, Fernández JA, et al. Brain-specific protein C activation during carotid artery occlusion in humans. Stroke. 1999;30:542–545. doi: 10.1161/01.str.30.3.542. [DOI] [PubMed] [Google Scholar]
- 26.Macko RF, Ameriso SF, Gruber A, et al. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–2011. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Tran ND, Kittaka M, et al. Thrombomodulin expression in bovine brain capillaries. Anticoagulant function of the blood-brain barrier, regional differences, and regulatory mechanisms. Arterioscler Thromb Vasc Biol. 1997;17:3139–3146. doi: 10.1161/01.atv.17.11.3139. [DOI] [PubMed] [Google Scholar]
- 28.Malek AM, Jackman RW, Rosenberg RD, et al. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res. 1994;74:852–860. doi: 10.1161/01.res.74.5.852. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Ohira T, Yamagishi K, et al. Low protein C and incidence of ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost. 2009;7:1774–1778. doi: 10.1111/j.1538-7836.2009.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danese S, Vetrano S, Zhang L, et al. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010;115:1121–1130. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bock F, Shahzad K, Vergnolle N, et al. Activated protein C based therapeutic strategies in chronic diseases. Thromb Haemost. 2014;111:610–617. doi: 10.1160/TH13-11-0967. [DOI] [PubMed] [Google Scholar]
- 33.Shibata M, Kumar SR, Amar A, et al. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 34.Cheng T, Liu D, Griffin JH, et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 35.Griffin JH, Zlokovic BV, Fernández JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Liu D, Gelbard H, et al. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Cheng T, Guo H, et al. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 38.Cheng T, Petraglia AL, Li Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 39.Zlokovic BV, Zhang C, Liu D, et al. Functional recovery after embolic stroke in rodents by activated protein C. Ann Neurol. 2005;58:474–477. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]
- 40.Wildhagen KC, Lutgens E, Loubele ST, et al. The structure-function relationship of activated protein C. Lessons from natural and engineered mutations. Thromb Haemost. 2011;106:1034–1045. doi: 10.1160/TH11-08-0522. [DOI] [PubMed] [Google Scholar]
- 41.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17:2059–2069. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bae JS, Yang L, Manithody C, et al. Engineering a disulfide bond to stabilize the calcium binding loop of activated protein C eliminates its anticoagulant but not protective signaling properties. J Biol Chem. 2007;282:9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 43.Han MH, Hwang SI, Roy DB, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 44.Mosnier LO, Gale AJ, Yegneswaran S, et al. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1745. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 45.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 46.Gupta A, Gerlitz B, Richardson MA, et al. Distinct Functions of Activated Protein C Differentially Attenuate Acute Kidney Injury. J Am Soc Nephrol. 2009;20:267–277. doi: 10.1681/ASN.2008030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harmon S, Preston RJ, Ainle FN, et al. Dissociation of activated protein C functions by elimination of protein S cofactor enhancement. J Biol Chem. 2008;283:30531–30539. doi: 10.1074/jbc.M802338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni Ainle F, O'Donnell JS, Johnson JA, et al. Activated protein C N-linked glycans modulate cytoprotective signaling function on endothelial cells. J Biol Chem. 2011;286:1323–1330. doi: 10.1074/jbc.M110.159475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preston RJ, Ajzner E, Razzari C, et al. Multifunctional specificity of the protein C/activated protein C GLA domain. J Biol Chem. 2006;281:28850–28857. doi: 10.1074/jbc.M604966200. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Bae JS, Manithody C, et al. Identification of a specific exosite on activated protein C for interaction with protease activated receptor 1. J Biol Chem. 2007;282:25493–25500. doi: 10.1074/jbc.M702131200. [DOI] [PubMed] [Google Scholar]
- 51.Mosnier LO, Zampolli A, Kerschen EJ, et al. Hyper-antithrombotic, non-cytoprotective Glu149Ala-activated protein C mutant. Blood. 2009;113:5970–5978. doi: 10.1182/blood-2008-10-183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo H, Wang Y, Singh I, et al. Species-dependent neuroprotection by activated protein C mutants with reduced anticoagulant activity. J Neurochem. 2009;109:116–124. doi: 10.1111/j.1471-4159.2009.05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Sinha RK, Mosnier LO, et al. Neurotoxicity of the anticoagulant-selective E149A-activated protein C variant after focal ischemic stroke in mice. Blood Cells Mol Dis. 2013;51:104–108. doi: 10.1016/j.bcmd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker CT, Marky AH, Petraglia AL, et al. Activated protein C analog with reduced anti-coagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Res. 2010;1347:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo H, Singh I, Wang Y, et al. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Wang Y, Thiyagarajan M, Chow N, et al. Differential neuroprotection and risk for bleeding from activated protein C with varying degrees of anticoagulant activity. Stroke. 2008;40:1864–1869. doi: 10.1161/STROKEAHA.108.536680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Zhao Z, Chow N, et al. Activated protein C analog protects from ischemic stroke and extends the therapeutic window of tissue-type plasminogen activator in aged female mice and hypertensive rats. Stroke. 2013;44:3529–3536. doi: 10.1161/STROKEAHA.113.003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Zhang Z, Chow N, et al. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43:2444–2449. doi: 10.1161/STROKEAHA.112.658997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bir N, Lafargue M, Howard M, et al. Cytoprotective-selective activated protein C attenuates Pseudomonas aeruginosa-induced lung injury in mice. Am J Respir Cell Mol Biol. 2011;45:632–641. doi: 10.1165/rcmb.2010-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen B, Friedman B, Whitney MA, et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J Neurosci. 2012;32:7622–7631. doi: 10.1523/JNEUROSCI.0369-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen B, Cheng Q, Yang K, et al. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–2352. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- 63.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 64.Mosnier LO, Sinha RK, Burnier L, et al. Biased agonism of protease-activated receptor 1 by activated protein C caused by non-canonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soh UJ, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A. 2011;108:E1372–E1380. doi: 10.1073/pnas.1112482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canto I, Soh UJ, Canto I, et al. Allosteric modulation of protease-activated receptor signaling. Mini Rev Med Chem. 2012;12:804–811. doi: 10.2174/138955712800959116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reiter E, Ahn S, Shukla AK, et al. Molecular mechanism of β-Arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wisler JW, Xiao K, Thomsen AR, et al. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27C:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A. 2007;104:5662–5667. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burnier L, Mosnier LO. Novel mechanisms for activated protein C cytoprotective activities involving non-canonical activation of protease-activated receptor 3. Blood. 2013;122:807–816. doi: 10.1182/blood-2013-03-488957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madhusudhan T, Wang H, Straub BK, et al. Cytoprotective signaling by activated protein C requires protease activated receptor-3 in podocytes. Blood. 2012;119:874–883. doi: 10.1182/blood-2011-07-365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae JS, Rezaie AR. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb Haemost. 2008;100:101–109. doi: 10.1160/TH08-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deane R, LaRue B, Sagare AP, et al. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J Cereb Blood Flow Metab. 2009;29:25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhong Z, Ilieva H, Hallagan L, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abu FR, Nassar T, Yarovoi S, et al. Blood-brain barrier permeability and tPA-mediated neurotoxicity. Neuropharmacology. 2010;58:972–980. doi: 10.1016/j.neuropharm.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38:376–385. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorbacheva LR, Storozhevykh TP, Pinelis VG, et al. Activated protein C via PAR1 receptor regulates survival of neurons under conditions of glutamate excitotoxicity. Biochemistry (Mosc) 2008;73:717–724. doi: 10.1134/s0006297908060138. [DOI] [PubMed] [Google Scholar]
- 78.Gorbacheva L, Pinelis V, Ishiwata S, et al. Activated protein C prevents glutamate- and thrombin-induced activation of nuclear factor-kappaB in cultured hippocampal neurons. Neuroscience. 2010;165:1138–1146. doi: 10.1016/j.neuroscience.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 79.Thiyagarajan M, Fernandez JA, Lane SM, et al. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Zhao Z, Chow N, et al. Activated protein C analog promotes neurogenesis and improves neurological outcome after focal ischemic stroke in mice via protease activated receptor 1. Brain Res. 2013;1507:97–104. doi: 10.1016/j.brainres.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo H, Zhao Z, Yang Q, et al. An activated protein C analog stimulates neuronal production by human neural progenitor cells via a PAR1-PAR3-S1PR1-Akt pathway. J Neurosci. 2013;33:6181–6190. doi: 10.1523/JNEUROSCI.4491-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maggio N, Itsekson Z, Ikenberg B, et al. The anticoagulant activated protein C (aPC) promotes metaplasticity in the hippocampus through an EPCR-PAR1-S1P1 receptors dependent mechanism. Hippocampus. 2014 doi: 10.1002/hipo.22288. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.O’Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 84.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 85.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin Alfa (Activated) in Adults with Septic Shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 86.Bernard GR, Macias WL, Joyce DE, et al. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Crit Care. 2003;7:155–163. doi: 10.1186/cc2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruckner M, Lasarzik I, Jahn-Eimermacher A, et al. High dose infusion of activated protein C (rhAPC) fails to improve neuronal damage and cognitive deficit after global cerebral ischemia in rats. Neurosci Lett. 2013;551:28–33. doi: 10.1016/j.neulet.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 88.Williams PD, Zlokovic BV, Griffin JH, et al. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215–4222. doi: 10.2174/138161212802430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lyden P, Levy H, Weymer S, et al. Phase 1 Safety, Tolerability and Pharmacokinetics of 3K3A-APC in Healthy Adult Volunteers. Curr Pharm Des. 2013;19:7479–7485. doi: 10.2174/1381612819666131230131454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mather T, Oganessyan V, Hof P, et al. The 2.8 Å crystal structure of Gla-domainless activated protein C. EMBO J. 1996;15:6822–6831. [PMC free article] [PubMed] [Google Scholar]