Abstract
Accumulating evidences from animal studies have indicated that both endogenous and exogenous IL-27, an IL-12 family of cytokine, can increase antitumor T-cell activities and inhibit tumor growth. IL-27 can modulate Treg responses, and program effector T cells into a unique T-effector stem cell (TSEC) phenotype, which enhances T-cell survival in the tumor microenvironment. However, animal studies also suggest that IL-27 induces molecular pathways such as IL-10, PD-L1 and CD39, which may downregulate tumor-specific T-cell responses. In this review paper, we will discuss the Yin and Yang aspects of IL-27 in the induction of tumor-specific T-cell responses, and the potential impacts of these functions of IL-27 in the design of cancer immunotherapy.
Keywords: cancer immunotherapy, IL-27, IL-12, regulatory T cells, T-effector stem cells
Cancer immunotherapy based on the adoptive transfer of naturally occurring or genetically redirected tumor-reactive T cells provides the best evidence of the therapeutic efficacy of T lymphocytes [1–4]. However, patients with advanced cancer are resistant to cancer immunotherapy. Although the factors that are responsible for the resistance of cancer to T-cell therapy are not fully understood, the inability of an immunotherapy to expand T cells into high numbers, and induce the right type of T-cell response, that is, T cells with the Th1/Tc1 phenotype and the ability to survive and persist in the tumor microenvironment (TME), appear to be a key factor [5–8]. Another important factor could be tumor-induced expansion of suppressor cells such as FoxP3+ regulatory T (Treg) cells that can actively inhibit antitumor T-cell responses [9,10]. Thus, finding strategies to expand and promote T cells into the appropriate phenotypes and simultaneously inhibit Treg cells are pivotal to the development of highly effective T-cell-based immunotherapies. Recent studies by us and others have revealed that IL-27 can program tumor-specific T cells into Th1/Tc1 effector stem cells [6,11] while modulating the number and functions of regulatory T cells [12–14]. Since induction of right T effector cells and reduction of Treg cells are two major factors for the success of T-cell immunotherapy, we therefore believe that IL-27 may represent a unique opportunity to optimize T-cell-based immunotherapy. However, recent studies [15,16] also suggest that IL-27 induces molecular pathways such as PD-L1 and CD39, which can downregulate tumor-specific T-cell responses. In this review paper, we will mainly review animal studies and discuss the Yin and Yang aspects of IL-27 in the induction of cancer-specific T-cell responses, and discuss the potential impacts of these functions of IL-27 in cancer immunotherapy.
Role of IL-27 in tumor immune surveillance & antitumor immunity
IL-27 is a member of the IL-12 cytokine family that consists of an IL-12 p40-related protein subunit, EBV-induced gene 3 (EBI3), and a p35-related subunit, p28 [17]. IL-27 is produced by activated antigen-presenting cells such as dendritic cells (DCs) and macrophages [18–20], and it signals through a heterodimeric receptor (IL-27R) consisting of the WSX-1 and the gp130 subunits in a variety of cell types including T, NK, B cells and myeloid cells [21]. IL-27R signaling enhances the recruitment of several Jak family kinases and activation of STAT family transcription factors 1 and 3 [22,23]. IL-27 has been shown to have potent activity in regulating Th1, Th2, Th17 and FoxP3+ Treg responses [24–26]. Since the first report in 2004 [27,28], the potent antitumor activities of both endogenous and exogenous IL-27 have been verified in various tumor models [11,27–37]. The requirement of endogenous IL-27 in tumor immunity suggests that IL-27 also play important roles in immune surveillance. Indeed, it was recently shown that IL-27 is also required for resistance to carcinogen-induced and in situ spontaneous cancer development [12].
IL-27 mediates its antitumor activity through several mechanisms including direct inhibition of cancer cell growth, proliferation and migration [34,38–40]; inhibition of tumor angiogenesis [32]; enhancement of NK activity [33,35] and, more importantly, activation of tumor-specific cytotoxic T lymphocyte (CTL) responses [27–29,31,41]. The molecular and cellular basis of IL-27-mediated antitumor CTL response is still not fully understood. It is now well established that sustained IL-27 production in the TME results in antitumor CTL responses and tumor rejection [27–29,31,41]. However, tumor cell production of IL-27 does not appear to enhance priming of antitumor T-cell responses. This notion is supported by a study where sequential injection of IL-12 followed by IL-27 expression vectors, but not IL-27 followed by IL-12 expression vectors, induces CTL responses and tumor rejection [41]. In fact, IL-27 may be a negative regulator of T-cell priming, as IL-27 signaling in DCs has been shown to inhibit the induction of antitumor CTL response [42]. More recently, IL-27 is shown to induce CD39 expression by DC, which in turn decreases the concentrations of extracellular ATP and downregulates nucleotide-dependent activation of the NLRP3 inflammasome, leading to inhibition of T-cell responses [16]. This finding could explain why IL-27 signaling in DC inhibits T-cell priming [42]. Thus, it is likely that IL-27 does not promote initial priming of antitumor T cells, but rather amplifies antitumor T-cell responses via increasing T-cell survival. Indeed, we have recently performed phenotype analysis of IL-27-stimulated tumor-antigen-specific CTL cells, and observed that IL-27 significantly enhances CTL survival and programs them into unique T effector phenotype [11].
IL-27 directly stimulates tumor-specific T cells & programs them into a unique T-effector stem cell phenotype
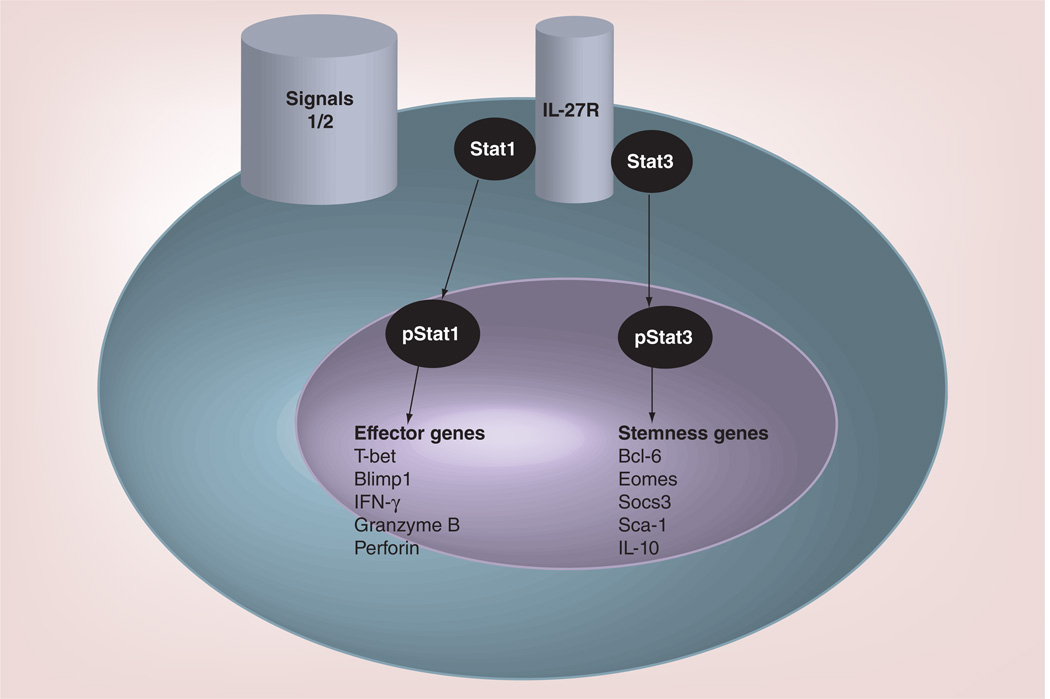
IL-27 activates both Stat1 and Stat3 signaling cascade [22,23]. Activation of Stat1 leads to induction of T-bet expression and Th1 responses [43], and promote CD8+ T cells to express T-bet, Eomes, IL-12Rβ2, granzyme B and Perforin [44,45]. We have recently [11] performed gene array analysis of IL-27-stimulated tumor-antigen-specific CTL cells, and demonstrated that IL-27 not only significantly increases CTL survival, but also programs CTL into stem-cell-like Tc1 effectors, characterized by upregulation of T-bet, Bcl-6, SOCS3, Sca-1 and IL-10, without significantly affecting CTL effector functions. Based on these observations, we propose that IL-27 programs tumor-specific CD8+ T cells into a novel functional subset of CTL in the presence of cognate antigen and co-stimulation signals (Figure 1), which we name it as ‘effector stem T cells’ (TSEC). Based on this model, IL-27-mediated Stat1 stimulation leads to induction of T-bet and Eomes, thereby promoting Th1/Tc1 differentiation [44,45]. Since T cells deficient for both T-bet and Eomes fail to differentiate into terminal effectors [46], IL-27-mediated Stat1 activation should be essential for CTL effector phenotype induction. On the other hand, IL-27-mediated Stat3 activation and induction of Bcl-6, SOCS3, Sca-1 and IL-10 contribute to CTL survival and ‘stemness’.
Figure 1. Role of IL-27 in induction T-effector stem cells.
During T-cell activation, signals through T-cell receptor (signal 1) and co-stimulatory molecules (signal 2) result in tumor-specific T-cell activation and differentiation, while IL-27 signaling activates both Stat1 and Stat3, leading to the expression of gene products that program tumor-specific T cells into a unique effector stem cell phenotype.
Although IL-27-stimulated CTL cells share some common markers such as Sca-1 and Bcl2, the phenotypes of these cells differ from the recently identified T memory stem cells (TSCM), an early stage T memory subset that has robust proliferative potential, long-term survival capacity and ability to mediate superior tumor regression [47,48]. TSCM cells can be generated by programming naive T cells in the presence of small molecules targeting the Wnt/β-catenin pathway, such as GSK-3 β inhibitors [47,48]. In addition, IL-15 [49], IL-7 in combination with IL-15 [49] and IL-21 [50] have also been shown to induce TSCM cells. However, TSCM cells can only be generated from naive cancer antigen-specific T cells [47,48], whose frequencies in cancer patients are low, and it is therefore not feasible to generate large numbers of TSCM cells clinically. In addition, the current protocols for TSCM induction inhibit effector function of tumor-reactive T cells as those protocols prevent T-cell differentiation into full effectors [47–50]. It would be thus not desirable to reduce ongoing immunity to achieve TSCM induction in vivo. At this stage, little is known about the in vivo characteristics and induction mechanisms of CTL effector stem cells. Further investigation of IL-27-mediated ‘stemness’ of T effector cells and its induction mechanisms will be of great importance to the design of T-cell-based cancer therapy.
Role of IL-27 in modulating Treg responses & its implications in cancer immunotherapy
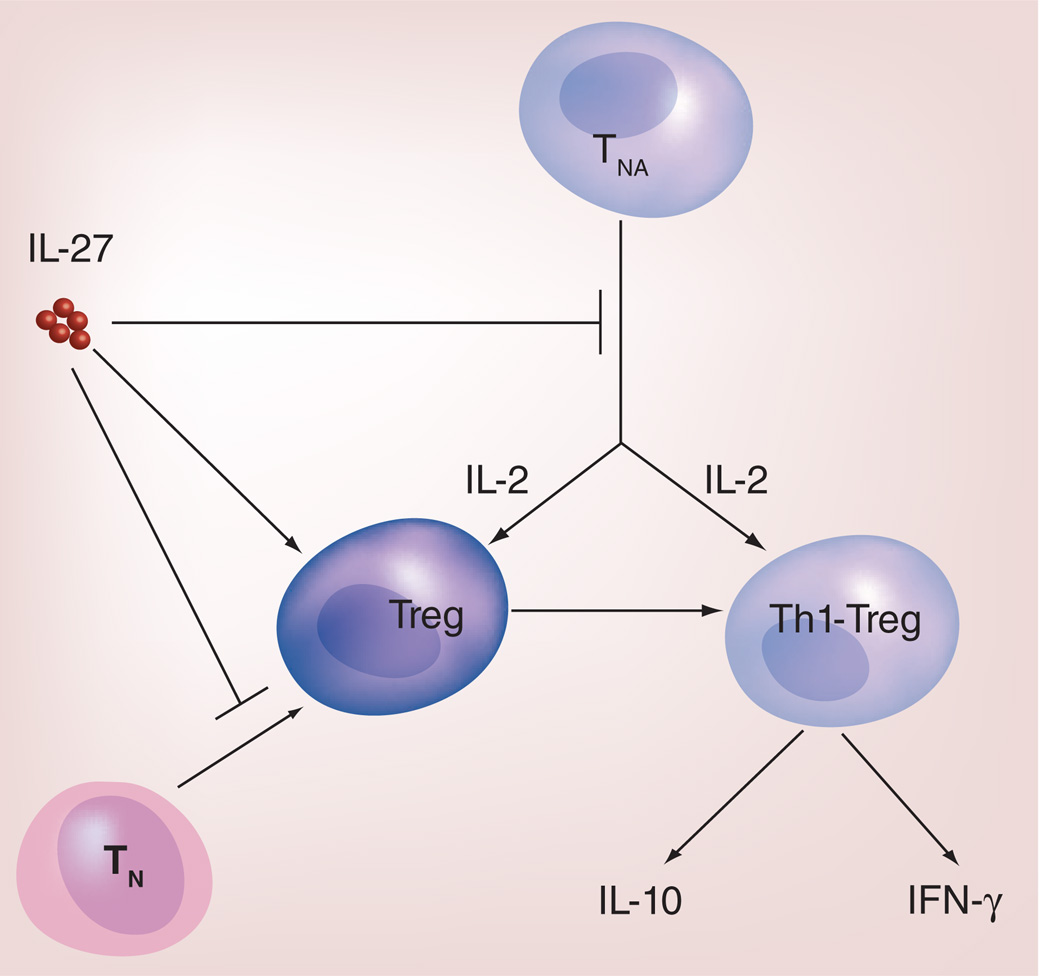
IL-27 has been shown to limit inducible regulatory T-cell (iTreg) responses via directly inhibiting Foxp3 expression [51,52]. However, IL-27 does not downregulate Foxp3 expression in natural Treg (nTreg) cells [13]. In mice overexpressing IL-27, Treg population is severely depleted; bone marrow chimera experiments suggest that the IL-27-mediated depletion of Treg cells is likely due to inhibition of IL-2 production by newly activated T cells [13]. Thus, IL-27 can limit the size of Treg population by inhibiting iTreg conversion and Treg homeostasis. In addition to indirectly regulating Treg homeostasis, recent studies have also shown that IL-27 signaling is required for Treg cell survival [14,53], presumably due to IL-27 activation of Stat3, leading to induction of antiapoptotic molecules [53]. Moreover, IL-27 signaling in Treg cells can program Tregs into a unique T-bet+CXCR3+ phenotype, which produce IL-10 and IFN-γ, specialized for regulating Th1 responses [14]. This latter effect suggests that IL-27 can expand Treg cells and programs Treg cells into Th1/Tc1 suppressor cells. Thus, as summarized in Figure 2, IL-27 has three different roles in regulating Treg response and the overall effect of how Treg cells are regulated by IL-27 may be setting-dependent.
Figure 2. Role of IL-27 in the modulation of Treg responses.
IL-27 can modulate Treg responses in the following three ways: inhibiting IL-2 production by TNA, which affects Treg homeostasis; inhibiting the conversion of TN into inducible Treg cells; and promoting Treg cells (inducible Treg cells and nTreg) to differentiate into Th1-Treg cells that express IL-10 and IFN-γ.
TN: Naive T cells; TNA: Newly activated T cells.
The role of IL-27 in regulating Treg responses in TME remains largely unclear and sometimes controversial. A recent study using IL-27R−/− mice revealed that the number of Treg cells was reduced in TME [12]; while another study using mice with IL-27 conditionally deleted in DC revealed that DC-derived IL-27 recruited Treg into TME [54]. In another study [55], DC-derived IL-27 is shown to induce a Treg population that express CD39 and CD69, which inhibits T-cell responses via generation of adenosine. Since IL-2 plays an important role in Treg homeostasis in TME [56], and IL-27 has potent inhibitory role of IL-2 production by T cells [57], it is expected that IL-27 in TME inhibits Treg homeostasis. Thus, it is likely that IL-27 in the TME plays an overall inhibiting role in Treg expansion. Future studies will elucidate how IL-27 in the TME modulates Treg expansion and function, and the contribution of IL-27-mediated Treg modulation to T-cell response and tumor regression. This information will be critical to know in developing IL-27-based cancer immunotherapy.
Tumor-induced expansion of Treg cells is a significant obstacle to successful cancer immunotherapy [9,10]. Since systemic IL-27 inhibits Treg responses [13], systemic application of IL-27 should be considered to suppress Treg responses during cancer immunotherapy.
IL-27-induced regulatory pathways & their effects on antitumor T-cell responses
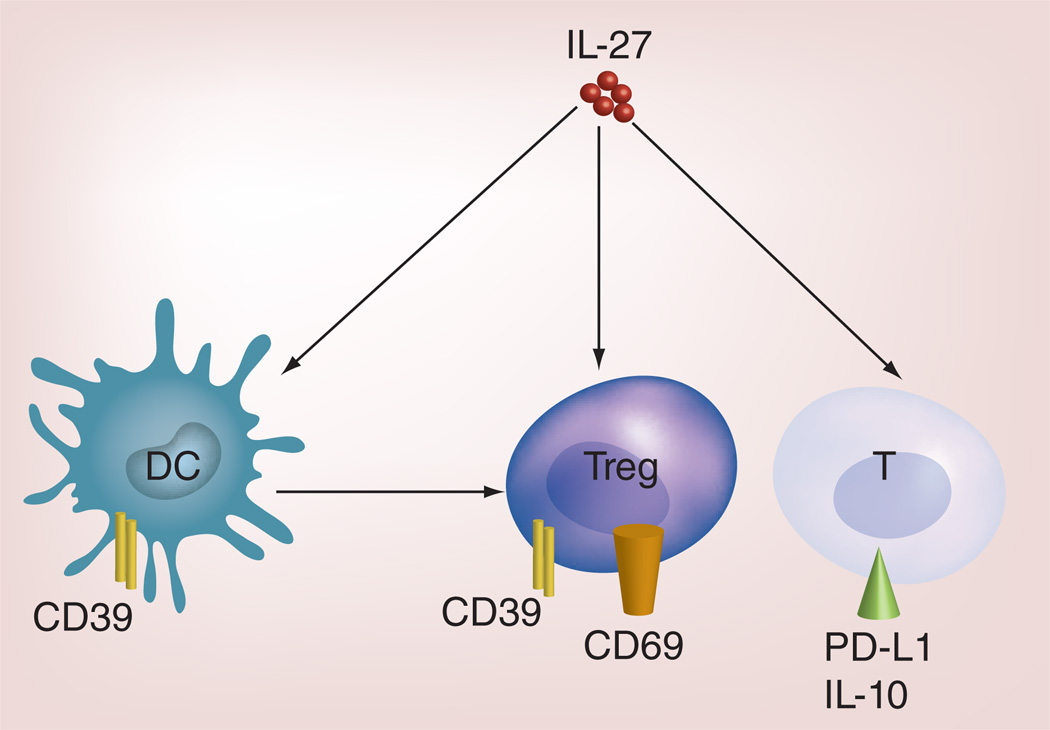
Recently, IL-27 is found to be abundantly expressed by tumor cells in human metastatic tumors but not in primary tumors [58], suggesting that IL-27 may also play a protumor role. As summarized in Figure 3, IL-27 also activates a number of pathways that potentially inhibit tumor-specific T-cell responses.
Figure 3. IL-27-induced pathways that may downregulate T-cell responses.
IL-27 signaling in DC induces CD39 expression, which can induce Treg cells expressing CD69 and CD39; IL-27 signaling in Treg cell promotes Th1-Treg cell development. Finally, IL-27 signaling in T cells promotes T-cell expression of PD-L1 and IL-10.
DC: Dendritic cell.
In addition to the induction of CD39 in DC [16], IL-27 is known to induce T-cell production of IL-10 [59–61] and IL-21 [62–65], and IL-27 has been shown to stimulate CD4+ T cell IL-10 production via induction of IL-21 [62]. However, the roles of T-cell production of IL-10 and IL-21 in IL-27-mediated tumor immunity remain unclear. Although the role of IL-10 in tumor immunity is often controversial, increasing evidence suggests a positive role of IL-10 in the induction of antitumor CTL responses. For instance, in some tumor models, IL-10-deficient mice showed reduced antitumor CTL responses [66] and increased FoxP3+ Treg responses [67], whereas in IL-10 transgenic mice, antitumor CTL responses were primed and shown to be responsible for tumor rejection [66,68]. IL-21 has been demonstrated to have potent activity on the induction of antiviral CTL responses [69–72] and CTL-mediated tumor rejection [50,73–76]. Recently, IL-10/IL-21-induced Stat3 activation has been shown to play a critical role in the survival and memory responses of CD8+ T cells [77,78]. Since IL-27 can strongly induce T-cell production of IL-10 and IL-21, it is anticipated that IL-27 can affect antitumor CTL responses via induction of IL-10/IL-21. Indeed, in our recent study [11], we have found that IL-27-stimulated tumor-antigen-specific T cells produce high levels of IL-10 in vitro and in vivo, and IL-27-induced CTL IL-10 production enhanced CTL survival, which was required for CTL memory responses. Because IL-10 may play different roles in different tumor models [79–81], it is also possible that IL-27-induced IL-10 production may play different roles in T-cell responses in different tumor settings. The roles of IL-27-induced T-cell production of IL-21 in tumor-specific T-cell responses remain unclear. Thus, more experimental data are needed to determine its role in IL-27-induced antitumor immunity.
Recently, IL-27 has been shown to induce the expression of PD-L1 in T cells, which in turn induces T-cell tolerance [15]. However, another study [82] revealed that the PD-L1 expression on tumor-antigen-specific CD8+ T cells is required for CTL survival. Thus, the role of IL-27-mediated induction of PD-L1 expression in T cells in antitumor immunity remains unresolved. At this stage, it remains to be determined if IL-27 also induces PD-L1 expression in cancer cells.
Can IL-27 be used to treat cancer?
Recently, IL-27 gene delivery as a single agent for cancer therapy has been tested in animal models with metastatic prostate cancer and shows promising results [83], suggesting that IL-27-based gene therapy has the potential to be used for cancer therapy. Since IL-27 enhances T effector cell survival [11] and systemic IL-27 reduces numbers of Treg cells [13], IL-27-based gene therapy that leads to systemic production of IL-27 in the blood may present a unique opportunity to optimize T-cell-based immunotherapy.
IL-27 may also be used to enhance cancer vaccine-induced antitumor T-cell responses and cancer vaccine efficacy. IL-27 has been shown to enhance IL-12 vaccination-mediated antitumor efficacy in animals [41]. IL-12 is recognized as a master regulator of Th1/Tc1 responses [84,85]. However, clinical trials with IL-12 have shown limited efficacy in most cases [85,86]. Recent evidence suggests that IL-12 upregulates Tim-3 expression in T cells and induces T-cell exhaustion, and thus IL-12-induced terminal differentiation of T cells could not be sustained for tumor rejection [87]. In this context, IL-27-induced T effector stemness may be suitable to counteract the drawbacks of IL-12-based cancer immunotherapy. Thus, combination of IL-12 vaccine with IL-27 will be an option for enhancing cancer immunotherapy.
A recent study [88] reported that an injection of irradiated IL-27-producing tumor cells resulted in increased CTL response and tumor growth inhibition in mice. However, irradiated B16 melanoma and plasmacytoma J558 cells failed to induce protection to respective live, IL-27-negative tumor cell challenge [Li et al., Unpublished Data]. Our observation is consistent with reports that IL-27 inhibits DC functions [42,89]. Thus, it is unlikely that irradiated IL-27-expressing cancer cells can be used as a cancer vaccine to enhance CTL priming as GM-CSF-expressing cancer vaccine does [90]. However, it remains to be tested if IL-27 in combination with the GM-CSF vaccine can lead to better tumor rejection. The major mechanism of GM-CSF cancer vaccine-mediated tumor immunity is induction of CTL responses via attracting DC to the vaccine injection site and enhances antigen presentation [90,91], therefore, enhancing CTL priming. GM-CSF melanoma vaccine can also induce potent antitumor antibody responses [90], and GM-CSF cancer vaccine has been used in human cancer clinical trials [92]. However, GM-CSF vaccine alone is insufficient to inhibit tumor growth completely [93]. Thus, IL-27 has the potential to amplify the GM-CSF vaccine-induced antitumor T- and B-cell responses, leading to improved cancer immunotherapy. Because IL-27 stimulated T cells exhibit memory precursor/stem cell phenotype [11], IL-27 can potentially enhance cancer vaccine-induced T-cell memory.
IL-27 also has the potential to be used to enhance T-cell adoptive transfer therapy. Our preliminary studies have revealed that IL-27 can enhance tumor antigen-specific T-cell survival and expansion [11]. Thus, IL-27 can be used for ex vivo expansion, and optimal stimulation of CTL cells from tumor-infiltrating T lymphocytes (TILs) for adoptive transfer therapy. Currently, in vitro expansion of TIL T cells involves the use of anti-CD3 and high doses of IL-2, which often lead to the induction of terminal effectors with poor survival potential and expansion of Treg cells [50,94]. Inclusion of IL-27 in the T-cell culture will not only induce expansion of T cells and enhance their survival potential in vivo but also deplete Treg cells from the T-cell population. In addition, in vivo application of IL-27 is likely to enhance T cells engraftment and persistence. Finally, since survival and persistence of chimeric antigen receptor transduced T cells has been a major problem in the clinics [95], in vivo IL-27 delivery should enhance the survival, engraftment of human tumor antigen-specific chimeric antigen receptor T cells, and cause tumor rejection.
Conclusion & future perspective
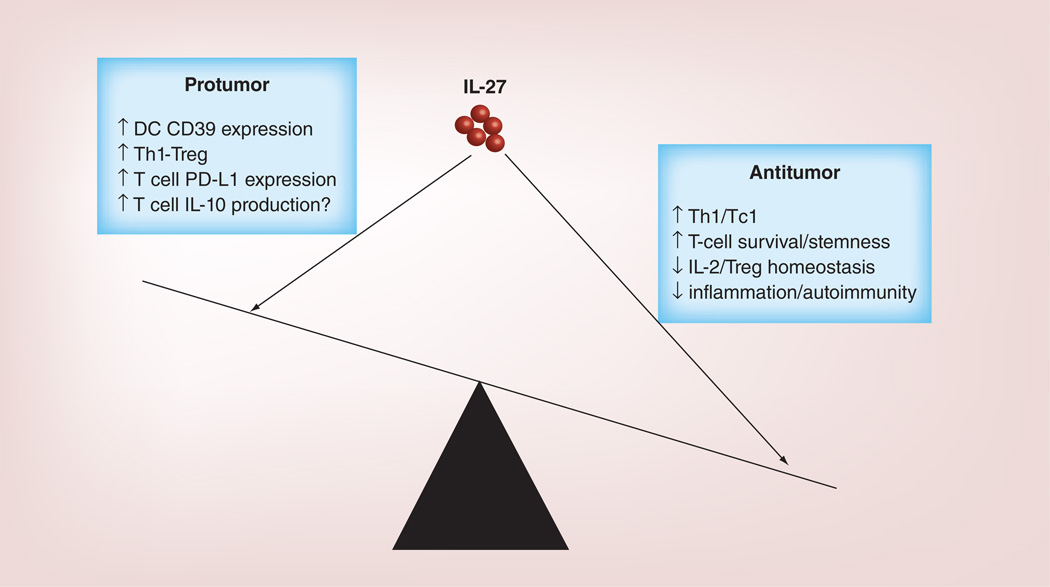
As summarized in Figure 4, IL-27 has both antitumor and protumor activities. The roles of IL-27 in stimulating Th1/Tc1 response, enhancing T-cell survival/expansion, inhibiting Treg homeostasis and inhibiting inflammation represent its antitumor effect; while IL-27 induction of CD39, PD-L1 and IL-10, and promotion of Th1-Treg response may have protumor effect. Studies using various tumor models have revealed that IL-27-mediated antitumor effects are dominant over its protumor effects. However, there is a clear gap in knowledge on some of the antitumor and protumor functions of IL-27. These include: what are the in vivo characteristics of TSEC cells, and how IL-27 induces them? How IL-27 modulates Treg homeostasis and function in the TME and what is its contribution to antitumor T-cell response and tumor regression? What are the contributions of the IL-27-induced regulatory pathways in tumor immunity? These issues should be the targets of future investigation. Although the antitumor immunity of IL-27 has been appreciated for more than 10 years, no IL-27-based immunotherapy has been developed and used in the clinic. In this regard, IL-27 in combination with cancer vaccine or T-cell adoptive transfer therapy may have potential to be developed for future cancer immunotherapy.
Figure 4. The antitumor versus protumor effects of IL-27.
The roles of IL-27 in stimulating Th1/Tc1 response, enhancing T-cell survival/expansion, inhibiting Treg homeostasis and inhibiting inflammation represent its antitumor effect; while IL-27 induction of CD39, PD-L1 and IL-10 and promotion of Th1-Treg response may have protumor effect. Studies using various tumor models have revealed that IL-27-mediated antitumor effects are dominant over its protumor effects.
DC: Dendritic cell.
Executive summary.
Role of IL-27 in tumor immune surveillance & antitumor immunity
IL-27 is required for tumor immune surveillance.
The majority of studies favor a positive role of IL-27 in promoting antitumor T-cell response.
IL-27 directly stimulates tumor-specific T cells & programs them into a unique T effector stem cell phenotype
IL-27 directly stimulates T cells and programs T cells into Th1/Tc1 effector stem cells.
The effector/stem cell phenotype favors T-cell survival.
Role of IL-27 in modulating Treg responses & its implications in cancer immunotherapy
IL-27 is known to regulate size and functions of Treg population.
Systemic IL-27 inhibits Treg response.
IL-27-induced regulatory pathways and its effects on antitumor T-cell response
IL-27 is known to induce some regulatory pathways such as IL-10, PD-L1 and CD39.
The roles of IL-27-induced regulatory pathways in antitumor immunity remain to be evaluated.
Can IL-27 be used to treat cancer?
IL-27 has the potential to be utilized to enhance cancer vaccine efficacy and T-cell adoptive transfer therapy.
Acknowledgements
The authors would like to thank S Basu (Ohio State University) and Y Liu (Children’s National Medical Center) for critical reading of the manuscript.
This study is supported by grants from the National Cancer Institute (R01CA138427 to X-F Bai).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschhorn-Cymerman D, Budhu S, Kitano S, et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J. Exp. Med. 2012;209(11):2113–2126. doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran MA, Geiger TL, Montalvo W, et al. Systemic 4–1 BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J. Exp. Med. 2013;210(4):743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 2004;173(12):7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer. 2007;7(11):880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 10.Curiel TJ. Tregs and rethinking cancer immunotherapy. J. Clin. Invest. 2007;117(5):1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. IL-27 enhances the survival of tumor antigen-specific CD8(+) T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur. J. Immunol. 2013;43(2):468–479. doi: 10.1002/eji.201242930. •• Shows that IL-27 enhances the survival of cytotoxic T-lymphocyte cells and programs them into unique effector stem T cells.
- 12. Natividad KD, Junankar SR, Mohd Redzwan N, et al. Interleukin-27 signaling promotes immunity against endogenously arising murine tumors. PLoS ONE. 2013;8(3):e57469. doi: 10.1371/journal.pone.0057469. • Shows that endogenous IL-27 is required for tumor immune surveillance.
- 13. Wojno ED, Hosken N, Stumhofer JS, et al. A role for IL-27 in limiting T regulatory cell populations. J. Immunol. 2011;187(1):266–273. doi: 10.4049/jimmunol.1004182. •• Shows that systemic IL-27 depletes Treg cells.
- 14.Hall AO, Beiting DP, Tato C, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37(3):511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirahara K, Ghoreschi K, Yang XP, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36(6):1017–1030. doi: 10.1016/j.immuni.2012.03.024. •• Shows that IL-27 induces PD-L1 expression in T cells.
- 16. Mascanfroni ID, Yeste A, Vieira SM, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 2013;14(10):1054–1063. doi: 10.1038/ni.2695. • IL-27 inhibits T-cell priming by dendritic cells (DCs) via induction of CD39 expression on DC.
- 17.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 18.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc. Natl Acad. Sci. USA. 2010;107(25):11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukoc. Biol. 2007;82(5):1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 20.Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur. J. Immunol. 2007;37(12):3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 21.Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004;172(4):2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 22.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J. Interferon Cytokine Res. 2003;23(9):513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 23.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 24.Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv. Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 25.Lucas S, Ghilardi N, Li J, De Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc. Natl Acad. Sci. USA. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diveu C, Mcgeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 2009;182(9):5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 27. Hisada M, Kamiya S, Fujita K, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64(3):1152–1156. doi: 10.1158/0008-5472.can-03-2084. • IL-27 expression in tumor microenvironment enhances antitumor immunity.
- 28.Salcedo R, Stauffer JK, Lincoln E, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J. Immunol. 2004;173(12):7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 29.Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res. 2004;24(6):3763–3767. [PubMed] [Google Scholar]
- 30.Chiyo M, Shimozato O, Yu L, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int. J. Cancer. 2005;115(3):437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 31.Salcedo R, Hixon JA, Stauffer JK, et al. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J. Immunol. 2009;182(7):4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu M, Shimamura M, Owaki T, et al. Antiangiogenic and antitumor activities of IL-27. J. Immunol. 2006;176(12):7317–7324. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 33.Oniki S, Nagai H, Horikawa T, et al. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66(12):6395–6404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto T, Morishima N, Mizoguchi I, et al. Antiproliferative activity of IL-27 on melanoma. J. Immunol. 2008;180(10):6527–6535. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- 35.Matsui M, Kishida T, Nakano H, et al. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009;69(6):2523–2530. doi: 10.1158/0008-5472.CAN-08-2793. [DOI] [PubMed] [Google Scholar]
- 36.Morishima N, Mizoguchi I, Okumura M, et al. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J. Biomed. Biotechnol. 2010;2010:605483. doi: 10.1155/2010/605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murugaiyan G, Saha B. IL-27 in tumor immunity and immunotherapy. Trends Mol. Med. 2013;19(2):108–116. doi: 10.1016/j.molmed.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J. Immunol. 2009;183(10):6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- 39.Cocco C, Giuliani N, Di Carlo E, et al. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin. Cancer Res. 2010;16(16):4188–4197. doi: 10.1158/1078-0432.CCR-10-0173. [DOI] [PubMed] [Google Scholar]
- 40.Chiba Y, Mizoguchi I, Mitobe K, et al. IL-27 enhances the expression of TRAIL and TLR3 in human melanomas and inhibits their tumor growth in cooperation with a TLR3 agonist poly(I:C) partly in a TRAIL-dependent manner. PLoS ONE. 2013;8(10):e76159. doi: 10.1371/journal.pone.0076159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu S, Lee DA, Li S. IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. J. Immunol. 2010;184(5):2348–2354. doi: 10.4049/jimmunol.0902371. •• IL-27 enhances efficacy of IL-12 vaccination.
- 42. Shinozaki Y, Wang S, Miyazaki Y, et al. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int. J. Cancer. 2009;124(6):1372–1378. doi: 10.1002/ijc.24107. • Endogenous IL-27 enhances antitumor T-cell responses but inhibits DC-mediated T-cell priming.
- 43.Chen Q, Ghilardi N, Wang H, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 44.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 2005;175(3):1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 45.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur. J. Immunol. 2011;41(1):47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 46.Li G, Yang Q, Zhu Y, et al. T-Bet and Eomes regulate the balance between the effector/central Memory T cells versus memory stem like T cells. PLoS ONE. 2013;8(6): e67401. doi: 10.1371/journal.pone.0067401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 50.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neufert C, Becker C, Wirtz S, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 2007;37(7):1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 52.Huber M, Steinwald V, Guralnik A, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int. Immunol. 2008;20(2):223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 53.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J. Immunol. 2013;190(4):1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia S, Wei J, Wang J, et al. A requirement of dendritic cell-derived interleukin-27 for the tumor infiltration of regulatory T cells. J. Leukoc. Biol. 2014 doi: 10.1189/jlb.0713371. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 55.Sekar D, Hahn C, Brune B, Roberts E, Weigert A. Apoptotic tumor cells induce IL-27 release from human DCs to activate Treg cells that express CD69 and attenuate cytotoxicity. Eur. J. Immunol. 2012;42(6):1585–1598. doi: 10.1002/eji.201142093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 2007;178(11):6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 57.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, De Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 2006;176(1):237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 58.Gonin J, Carlotti A, Dietrich C, et al. Expression of IL-27 by tumor cells in invascutaneous and metastatic melanomas. PLoS ONE. 2013;8(10):e75694. doi: 10.1371/journal.pone.0075694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T-cell production of interleukin 10. Nat. Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 60.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald DC, Ciric B, Touil T, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179(5):3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 62.Pot C, Jin H, Awasthi A, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batten M, Ramamoorthi N, Kljavin NM, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 2010;207(13):2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007;282(48):34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mittal A, Murugaiyan G, Beynon V, Hu D, Weiner HL. IL-27 induction of IL-21 from human CD8+ T cells induces granzyme B in an autocrine manner. Immunol. Cell Biol. 2012;90(8):831–835. doi: 10.1038/icb.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mumm JB, Emmerich J, Zhang X, et al. IL-10 elicits IFN-gamma-dependent tumor immune surveillance. Cancer Cell. 2011;20(6):781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Tanikawa T, Wilke CM, Kryczek I, et al. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72(2):420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groux H, Cottrez F, Rouleau M, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 1999;162(3):1723–1729. [PubMed] [Google Scholar]
- 69.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 2010;185(8):4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frohlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324(5934):1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 73.Ma HL, Whitters MJ, Konz RF, et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J. Immunol. 2003;171(2):608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 74.Kishida T, Asada H, Itokawa Y, et al. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol. Ther. 2003;8(4):552–558. doi: 10.1016/s1525-0016(03)00222-3. [DOI] [PubMed] [Google Scholar]
- 75.Di Carlo E, Comes A, Orengo AM, et al. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J. Immunol. 2004;172(3):1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 76.Smyth MJ, Hayakawa Y, Cretney E, et al. IL-21 enhances tumor-specific CTL induction by anti-DR5 antibody therapy. J. Immunol. 2006;176(10):6347–6355. doi: 10.4049/jimmunol.176.10.6347. [DOI] [PubMed] [Google Scholar]
- 77.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35(5):792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beissert S, Hosoi J, Grabbe S, Asahina A, Granstein RD. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J. Immunol. 1995;154(3):1280–1286. [PubMed] [Google Scholar]
- 80.Sharma S, Stolina M, Lin Y, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J. Immunol. 1999;163(9):5020–5028. [PubMed] [Google Scholar]
- 81.Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, and enhances NK lysis. Cell. Immunol. 1997;180(1):55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- 82.Pulko V, Harris KJ, Liu X, et al. B7-h1 expressed by activated CD8 T cells is essential for their survival. J. Immunol. 2011;187(11):5606–5614. doi: 10.4049/jimmunol.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zolochevska O, Ellis J, Parelkar S, et al. Interleukin-27 gene delivery for modifying malignant interactions between prostate tumor and bone. Hum. Gene Ther. 2013;24(12):970–981. doi: 10.1089/hum.2013.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 85.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin. Cancer Res. 2007;13(16):4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang ZZ, Grote DM, Ziesmer SC, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J. Clin. Invest. 2012;122(4):1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J, Tian H, Li C, et al. Antitumor effects obtained by autologous Lewis lung cancer cell vaccine engineered to secrete mouse Interleukin 27 by means of cationic liposome. Mol. Immunol. 2013;55(3–4):264–274. doi: 10.1016/j.molimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J. Immunol. 2007;179(10):6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 90.Dranoff G. GM-CSF-based cancer vaccines. Immunol. Rev. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 91.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl Acad. Sci. USA. 1993;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luiten RM, Kueter EW, Mooi W, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. J. Clin. Oncol. 2005;23(35):8978–8991. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 93.Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klapper JA, Thomasian AA, Smith DM, et al. Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy. J. Immunol. Methods. 2009;345(1–2):90–99. doi: 10.1016/j.jim.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kershaw MH, Westwood JA, Parker LL, et al. A Phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]