Abstract
Objective
To measure neurodevelopment at 3 years in children with single right ventricle anomalies and to assess its relationship to Norwood shunt type, neurodevelopment at 14 months, and patient and medical factors.
Study design
All subjects in the Single Ventricle Reconstruction Trial who were alive without cardiac transplant were eligible for inclusion. The Ages and Stages Questionnaire (ASQ, n=203) and other measures of behavior, and quality of life (QOL) were completed at age 3 years. Medical history, including measures of growth, feeding, and complications, was assessed through annual record review and phone interview. The Bayley Scales of Infant Development-II (BSID-II) scores from age 14 months were also evaluated as predictors.
Results
Scores on each ASQ domain were significantly lower than normal (p<0.001). ASQ domain scores at 3 years varied nonlinearly with 14-month BSID-II. More complications, abnormal growth, and evidence of feeding, vision, or hearing problems, were independently associated with lower ASQ scores, although models explained < 30% of variation. Shunt type was not associated with any ASQ domain score, or with behavior or QOL measures.
Conclusion
Children with SV have impaired neurodevelopment at 3 years. Lower ASQ scores are associated with medical morbidity, and lower BSID-II scores, but not with shunt type. However, because only a modest percentage of variation in 3-year neurodevelopmental outcome could be predicted from early measures, all children with SV should be followed longitudinally to improve recognition of delays.
Keywords: congenital heart disease, hypoplastic left heart syndrome, Single Ventricle Reconstruction Trial
Despite improvements in survival, children with hypoplastic left heart syndrome (HLHS) and related single ventricle abnormalities treated with staged palliation have a high prevalence of developmental and behavioral abnormalities.1–3 Based on studies of children who have undergone neonatal cardiac surgery, potential risk factors for adverse outcome in these children include underlying genetic conditions,4,5 low birth weight,4,5 premature gestational age,5,6,7 prolonged hospital course,5,8 intraoperative perfusion approaches,9 and lower socioeconomic status.5,10 Most findings on neurodevelopment in this high-risk group of children have derived from either single-center or cross-sectional studies. To date, the relationship between early developmental assessments and measures of development for pre-school age children with single ventricle anomalies has been only preliminarily explored.11
The Single Ventricle Reconstruction Trial Extension Study (SVR-II) was designed to prospectively follow children with HLHS and other forms of single right ventricle malformation who were enrolled as neonates in the Single Ventricle Reconstruction (SVR) trial, a randomized trial comparing the Blalock-Taussig shunt (MBTS) to the right ventricle-to-pulmonary artery shunt (RVPAS) at the time of the Norwood procedure.12, 13 Evaluation with the Bayley Scales of Infant Development-2nd edition (BSID-II) at 14 months showed no difference between shunt groups.5 The SVR-II study continues contact with the SVR cohort and includes developmental assessment at annual intervals. The aims of the current analyses were to measure development, behavior, functional status, and quality of life (QOL) at three years of age in children with HLHS and related single ventricle anomalies, and to determine whether shunt type at the time of the Norwood procedure was related to these outcomes. We also sought to assess the predictive value of developmental testing with the BSID-II at age 14 months to identify developmental delays at three years of age. Finally, we aimed to identify the demographic, surgical, and perioperative factors that were associated with three-year neurodevelopmental status.
Methods
The SVR trial design and results of neurodevelopmental evaluation at age 14 months have been previously published.5,12, 13 In brief, patients were eligible for the SVR trial if they had HLHS or another related single right ventricle abnormality and a Norwood procedure was planned. Exclusion criteria included cardiac anatomy that prohibited either the MBTS or the RVPAS or a major extra-cardiac abnormality that could independently affect the likelihood of transplant-free survival. Subjects were randomized to the Norwood operation with either a MBTS or RVPAS. Prospective observation through 14 months of age was included in the SVR trial. At all SVR participating centers, usual surgical management for children in this cohort included three stages: the Norwood operation in the first 1–2 weeks of life; a stage II operation typically by 6 months of age to take down the shunt and direct blood from the superior vena cava to the branch pulmonary arteries; and the Fontan operation usually by 4 years of age to direct systemic venous return from both venae cavae to the branch pulmonary arteries.
All SVR trial survivors without cardiac transplantation were invited to participate in the SVR-II study, a prospective longitudinal study aimed at observing medical and developmental outcomes. Parents or guardians completed annual questionnaires on development, behavior, QOL, and functional status. Research coordinators reviewed the medical record and contacted parents/guardians to obtain interim medical history annually and following the Fontan operation. Data related to growth, feeding modality, participation in developmental therapies, surgical and catheterization interventions, and the incidence of major complications were collected. Sociodemographic factors included race, ethnicity, percentage below the poverty level living within the participants’ census block at birth per the US Census, 200014,15 and a multifactorial US Census 2000 based score derived from six measures and validated by Diez-Roux.16 Demographic and medical factors related to the perioperative Norwood and Stage II course, were included as candidate predictors. Cumulative exposure to complications, serious adverse events (SAEs), post-operative hospital days, surgical and catheterization procedures, and intraoperative perfusion times were calculated. As not every patient had undergone the Fontan by three years, Fontan factors such as complications and intraoperative perfusion times were included only as part of these cumulative variables. A complete list of candidate predictors is included in Table I (available at www.jpeds.com).
Development at three years was measured with the parent-completed Ages and Stages Questionnaire (ASQ) within the instrument-validated window from 34 months, 16 days to 38 months, 30 days of age.17 Five domains are assessed with the ASQ: a) communication, b) gross motor, c) fine motor, d) problem solving, and e) personal-social. The 36-month ASQ instrument is composed of 30 items written at the 4th–6th grade level with illustrations to facilitate parent understanding. Each item is scored depending upon whether the child performs the item consistently (10 points), sometimes (5 points) or not yet (0 points). Scores for each area are then summed. Domain scores were analyzed as continuous variables based on the normative means and standard deviations defined for each area. In addition, based on the published means and standard deviations, thresholds have been established for each tested area to define a child’s score as below the normal range (>2 SD below the normal mean)17 and representing delay.
Behavior was measured with the Parent Report Behavior Assessment System for Children-2nd Edition (BASC-2)18, designed to measure adaptive and problem behaviors present in the home and at school. It includes 14 subscales which compose four composite scores: Externalizing Problems, Internalizing Problems, Behavioral Symptoms Index, and Adaptive Skills. Scoring of the BASC-2 was completed at the Data Coordinating Center. Each of the composite scores is calculated based on the population mean of 50 points and a standard deviation of 10 points, using the combined-sex norms scores.18 In addition, for the adaptive scales, scores are considered at risk or clinically significant if they were between 31–40 or less than 31 respectively. For scales measuring behavioral problems, scores are considered at risk or clinically significant if 60–69 or greater than 69 respectively.18
The Pediatric Quality of Life Inventory (PedsQL) for 2–4 year olds was employed to measure health-related QOL in study subjects. This parent-report instrument is designed to assess QOL in both healthy and acutely or chronically ill children.19 The PedsQL system addresses physical, emotional, social, and school functioning. In addition, the cardiac disease-specific module was used to assess issues of QOL specific to children with cardiac disease.20 For each of these modules, scores are reported as a percent and higher scores represent better health-related quality of life.
Functional health status was assessed with the Functional Status II(R) (FSII-R)21, which is a parent-report questionnaire designed to assess functional health status in children with chronic health conditions. This instrument provides a total score based on common elements across all ages, in addition to age-specific domain scores. Higher scores represent evidence of better functional status.
The protocol was approved by each center’s institutional review board, and written informed consent was obtained from a parent or guardian.
Statistical Analyses
Descriptive statistics presented include median with interquartile range for skewed variables, mean ± standard deviation (SD) for other continuous variables, and frequency with percentage for categorical variables.
The mean domain scores for the SVR-II patients for the 3-year ASQ, PedsQL, FSII-R, and BASC-2 T scores were compared with those of normal children using a one-sample t-test. The Wilcoxon rank sum test was used to compare all measures of 3-year neurodevelopmental and functional status outcomes by randomly-assigned shunt type.
Based on a nonparametric (LOESS, locally weight scatterplot smoothing) fit demonstrating nonlinear association, we constructed piecewise linear regression models to most accurately estimate the association between 3-year ASQ domain scores and 14-month BSID-II summary scores. We also examined the outcome of delayed ASQ domain, defined as a score >2 SD below the normal mean. Simple logistic regression analyses were performed to identify associations between a delayed ASQ score and clinical factors. All clinical factors with a univariate p-value of less than 0.15 were entered simultaneously into a stepwise selection. Final multivariable logistic regression models include all predictors with P values <0.05. The p-values reported are unadjusted for comparison of the two shunt groups with respect to multiple outcomes. All analyses were conducted using SAS version 9.2 (Statistical Analysis System, Cary, NC) and R 2.13.0 version.
Results
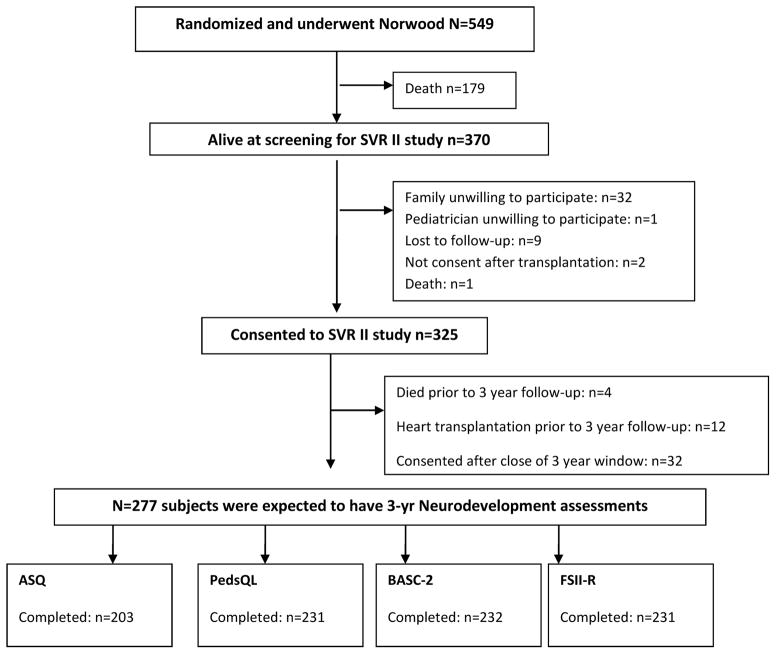
Among the 325 subjects enrolled in the SVR-II study, four died prior to three-year follow-up, 12 underwent heart transplantation, and 32 subjects consented after the close of their 3-year window (Figure 1; available at www.jpeds.com). Of the remaining 277 subjects, 203 (73%) completed the ASQ, the primary outcome instrument, within the study window. The group that completed the ASQ did not differ significantly from those eligible who did not complete the instrument with respect to race, measures of socioeconomic status, genetic syndrome, birth weight, gestational age, or participation in neurodevelopmental therapies. The proportion with a genetic syndrome or other anomalies was lower among those who completed at least one instrument compared with non-participants (29% vs 22%; p=0.03) (Table II; available at www.jpeds.com). Among the secondary outcomes, the BASC-2 was completed by 232 (84%), the PedsQL by 231 (83%) and the FSII-R by 231 (83%) participants within the required windows (Figure 1).
Figure 1.
Single Ventricle Reconstruction Extension Trial Neurodevelopmental Assessment at 3 Years
ASQ scores and the Relationship to BSID-II
Mean scores for each ASQ domain were lower than those of the normative population (Table III). The percentage of subjects with delayed scores (>2 SD below the mean) was 20% for the Communication Scale, 30% for the Gross Motor Scale, 35% for the Fine Motor Scale, 24% for the Problem Solving Scale and 17% for the Personal-Social Scale. Delay was detected in at least one area for 51% of the cohort. Scores on each ASQ scale did not differ by shunt type (Table IV; available at www.jpeds.com).
Table 3.
Neurodevelopmental Test Scores Compared to Normative Sample Values
| Normative Sample Mean±SD |
SVR II Cohort Mean±SD |
P-Value* | |
|---|---|---|---|
| ASQ | |||
| Communication | 54.3±7.8 | 47.0±14.7 | <.001 |
| Gross Motor | 54.7±9.5 | 43.7±16.9 | <.001 |
| Fine Motor | 52.5±10.9 | 38.9±18.5 | <.001 |
| Problem Solving | 55.0±8.2 | 45.4±16.3 | <.001 |
| Personal Social | 53.5±7.4 | 47.0±13.7 | <.001 |
| PEDS QL | |||
| Physical Functioning | 89.8±15.4 | 79.7±19.6 | <.001 |
| Emotional Functioning | 84.3±14.2 | 79.6±16.2 | <.001 |
| Social Functioning | 88.5±15.6 | 83.8±16.1 | <.001 |
| School Functioning | NA | 73.6±23.2 | |
| Psychosocial Health | 86.6±12.3 | 81.3±13.9 | <.001 |
| Peds QL Total Score | 87.9±12.2 | 80.6±15.0 | <.001 |
| BASC-2 T scores | |||
| Hyperactivity | 50±10 | 48.6±8.3 | 0.02 |
| Aggression | 50±10 | 46.8±8.7 | <.001 |
| Anxiety | 50±10 | 49.1±9.8 | 0.210 |
| Depression | 50±10 | 48.4±9.0 | 0.018 |
| Somatization | 50±10 | 53.3±11.0 | <.001 |
| Atypicality | 50±10 | 48.7±8.4 | 0.012 |
| Withdrawn | 50±10 | 48.1±9.7 | 0.004 |
| Attention Problems | 50±10 | 49.7±9.6 | 0.58 |
| Adaptability | 50±10 | 50.8±10.9 | 0.29 |
| Social Skills | 50±10 | 52.2±10.2 | 0.003 |
| Activities of Daily Living | 50±10 | 47.9±9.6 | <.001 |
| Functional Communication | 50±10 | 49.1±9.5 | 0.11 |
| FSII-R | |||
| General Health | 94.7±8.8 | 92.4±11.9 | 0.52 |
| Activity Score | 98.6±2.4 | 91.9±13.3 | <.001 |
| Total Score | 98.4±2.8 | 92.5±12.2 | <.001 |
ASQ-Ages and Stages Questionnaire; BASC-2-Parent Report Behavior Assessment System for Children-2nd Edition; BSID-II-Bayley Scales of Infant Development-II; FSII-R-Functional Status II R.
One-sample t-test or Wilcoxon sign rank test comparing the SVR trial data to the normative mean.
We next analyzed the relationship of the BSID-II administered at 14 months to pre-school age development as measured by the ASQ scales at 3 years. Of the 203 subjects who had a completed ASQ within the window, 188 had a mental development index (MDI) and psychomotor development index (PDI) calculated as part of the 14-month BSID-II. Both the mean PDI and MDI scores of 76±19 and 90±17, respectively, were significantly lower than the population means of 100±15 (p<0.001). Participants with a PDI score <70 at age 14 months were more likely to have abnormally low ASQ domain scores (odds ratios [ORs] 3.4–6.9 depending on domain). Similarly, when MDI scores were <70, ASQ domain scores were more likely to be abnormally low (ORs 8.8–21.0 depending on domain).
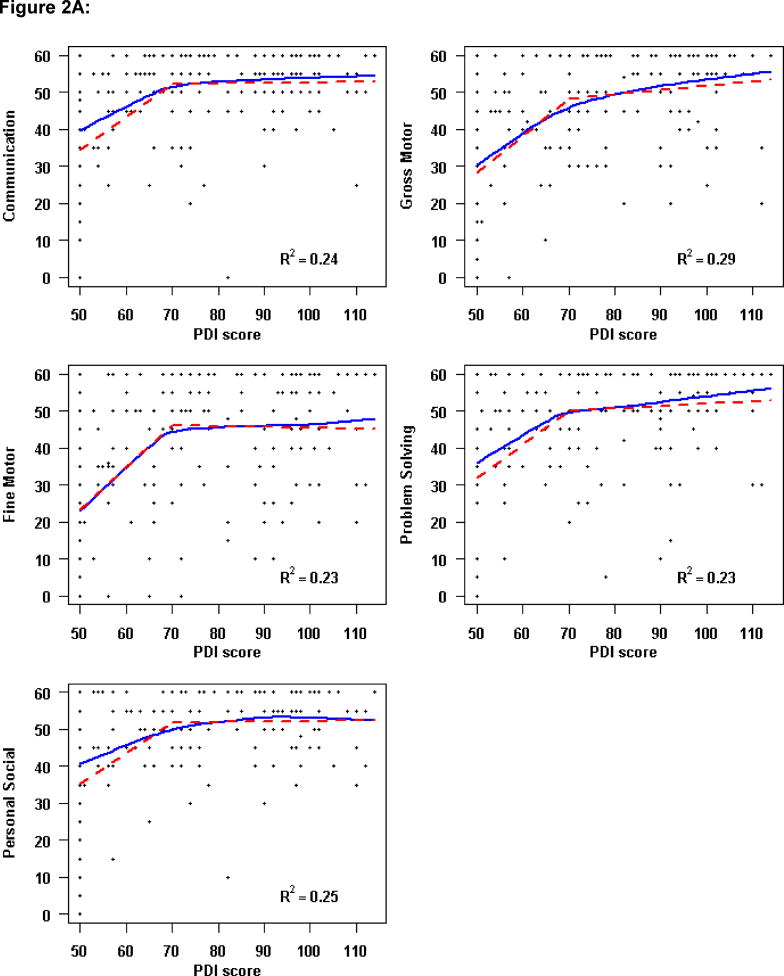
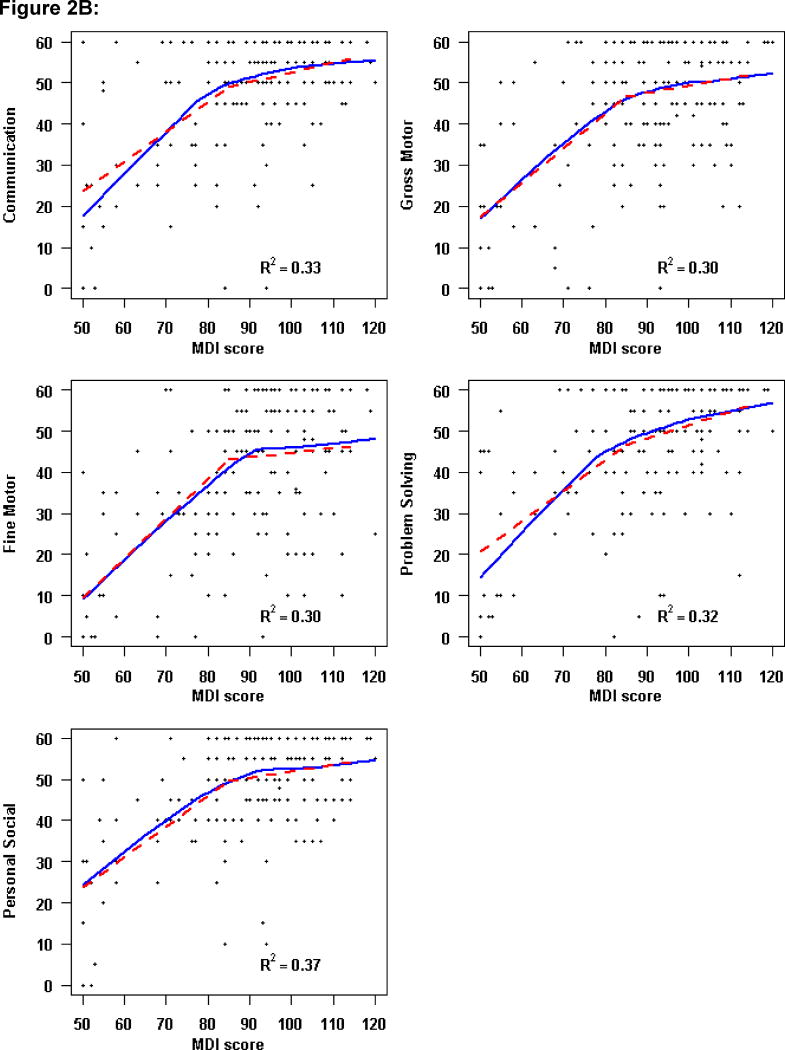
However, the relationship of the 14-month BSID-II to 3-year ASQ scores was not linear. Locally-weighted scatterplot smoothing curves (LOESS) were used to estimate the relationship of BSID-II scales to the ASQ domains (Figure 2; available at www.jpeds.com). An increase of one point in the PDI score up to a PDI of 70 was associated with an increase of 1.2–1.4 points in each ASQ domain. Above a PDI score of 70, an increase in the PDI score was not associated with the ASQ domain score (Figure 2, A). Similarly, among lower MDI scores, a one-unit change in MDI was associated with a 0.7–0.9 increase in ASQ Domain score up to an MDI of approximately 85. Beyond an MDI score of 85, higher MDI scores were not correlated with higher ASQ domain scores (Figure 2, B).
Figure 2. Relationship of Ages and Stages Domain Scores at 3 Years to 14-Month Bayley Scales of Infant Development-II.
Three-year ASQ domains versus A, 1-month Psychomotor Development Index (PDI) and B, 14-month Mental Development Index (MDI). The solid line represents the locally weighted scatterplot smoothing curve. The dashed line denotes the fitted piecewise linear regression model, using a cut-point of A, PDI=70 and B, MDI=85. The R2 assesses the percentage of variability in the ASQ domain explained by PDI score modeled with a piecewise linear regression fit.
Predictors of ASQ Domains
Univariate predictors of a delayed score in each ASQ domain are summarized in Table I. More complications and serious adverse events, and longer post-operative hospital length of stay, were associated with delayed scores on each of the five ASQ domains. The presence of either vision or hearing problems within the first two years was reported for 13% of participants and these were also associated with delayed scores for each ASQ domain. Compared with other subjects, those with vision or hearing problems were more likely to have a genetic syndrome or extracardiac anomaly (44% vs. 17%, p=0.009) and birth weight below 2500 grams (24% vs. 10%, p=0.047).
Multivariable models were constructed to identify the independent risk factors for low scores (>2 SD below the normal mean) in each of the 5 domains of the ASQ (Table V). Factors that were independent predictors of more than one abnormal ASQ domain included living in a census block with a greater percentage below the federal poverty level (Problem Solving, Personal Social); the presence of vision or hearing problems (Communication, Problem Solving, and Personal Social); male sex (Gross Motor and Fine Motor), the need for feeding therapy at 2 years (Gross Motor and Fine Motor); and more total serious adverse events and complications between the Norwood procedure and 3-year neurodevelopmental assessment (Gross Motor and Personal Social). The percentage of variance explained by the final models for each ASQ domain ranged from 18 to 27%. Of note, neither shunt type nor perfusion approach at the time of the Norwood was significantly associated with increased odds of an abnormal ASQ score in any domain in univariate or multivariable analysis.
Table 5.
Final Multivariable Models Predicting Ages and Stages Questionnaire (ASQ) Domain Scores > 2 SD Below Normative Mean
| ASQ Domain | Lower Height for age z-score 14 mos | Lower Head circumference for age z-score 14 mos | Older age at Stage II, mos | Higher Poverty Level Percent | Vision/Hearing Problems by age 2 yrs | Male | Feeding Therapy at age 2 yrs | More Total Serious Adverse Events/Complications | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Communication | 0.26 | ||||||||
| Odds ratio (95% CI) | 1.37 (1.01–1.9) | 1.37 (1.04, 1.80) | 6.48 (2.35–18.3) | ||||||
| P-value | P=0.05 | P=0.03 | P<.001 | ||||||
|
| |||||||||
| Gross Motor | 0.27 | ||||||||
| Odds ratio (95% CI) | 2.68 (1.16–6.18) | 6.49 (2.46–17.1) | 1.16 (1.06–1.26) | ||||||
| P-value | P=0.02 | P<.001 | P<.001 | ||||||
|
| |||||||||
| Fine Motor | 0.24 | ||||||||
| Odds ratio (95% CI) | 1.40 (1.08–1.86) | 4.82 (2.10–11.1) | 3.89 (1.43–10.6) | ||||||
| P-value | P=0.01 | P<.001 | P=0.008 | ||||||
|
| |||||||||
| Problem Solving | 0.18 | ||||||||
| Odds ratio (95% CI) | 1.04 (1.01–1.1) | 6.49 (2.60–16.2) | |||||||
| P-value | P=0.01 | P<.001 | |||||||
|
| |||||||||
| Personal-Social | 0.24 | ||||||||
| Odds ratio (95% CI) | 1.04 (1.01–1.07) | 4.22 (1.57–11.4) | 1.12 (1.04–1.21) | ||||||
| P-value | P=0.03 | P=0.004 | P=0.003 | ||||||
Behavior, Quality of Life, and Functional Status by Shunt Type
The primary measure of behavior was the BASC-2. Depending on the scale, 7.8–21.9% of children in the SVR-II cohort were reported to have behaviors in the at-risk or clinically abnormal range, with scores specifically in the clinically abnormal range 0.4–7.4 % of the time. There were no differences in the percent at risk or clinically abnormal between shunt groups. Compared with normative values, the SVR-II participants were described as having behaviors, on average, more consistent with somatization, and to have lower scores for the Activity of Daily Living domain. The observed differences in these areas are small and may not be clinically meaningful though scores in the lower range could portend the need for greater future societal resources. Mean scores were within the normal range for the other behavior areas. (Table III), Health-related QOL was measured with both the Generic and Cardiac Modules of the PedsQL; compared with healthy norms, children in the SVR-II cohort had a lower mean score in each of the PedsQL Generic domains. Functional status, measured by the FSII-R, demonstrated lower total scores (p<0.001) and activity scores (p<0.001) at three years compared with the general population. Shunt group was not significantly associated with any of the measured behavior, QOL, or functional status outcomes (Table III).
Discussion
We previously demonstrated that PDI and MDI scores on the BSID-II were lower at 14 months in children with HLHS and other forms of single right ventricle enrolled in the SVR trial.5 The predictive factors for developmental delay at 14 months were not related to shunt type, but rather to innate patient factors (e.g., birth weight, the presence of a genetic syndrome) and to measures of medical morbidity (e.g., longer duration of Norwood hospitalization, more complications after the Norwood discharge).5
In the current follow-up study of the SVR cohort to 3 years, development as measured by the ASQ, PedsQL, and parent-reported functional status was lower than that of the general population. The type of shunt at the Norwood operation did not influence developmental, behavioral, functional status, or QOL outcomes. In contrast, Atallah et al did find a difference in PDI at 2 years between those treated with an RVPAS (mean PDI=78) and those with a MBTS (mean PDI=67).22 This finding however was based on an observational cohort, with the shunt type changing by era, so that secular changes in other practices may have confounded this study finding.
Furthermore, we found that in these high-risk cardiac infants, developmental assessment at 14 months was significantly but modestly predictive of developmental domains on the ASQ, consistent with findings in premature infants.23 Scores on the ASQ varied nonlinearly with earlier BSID-II scores, with increasing ASQ scores up to PDI <70 and MDI <85, and a plateau effect beyond these thresholds. Moreover, only 25–30% of the variation in the 3-year ASQ domains was predicted by either the 14-month PDI or MDI. Our analyses suggest that even when 14-month BSID-II scores are within the normal range, children with single ventricle malformations may be at risk for developmental impairment at pre-school age and beyond.
The predictive validity of 14-month BSID-II scores for 3-year developmental and behavioral questionnaires may be limited for several reasons. The ASQ is a screening test, designed to detect those who may require further testing and possible services, whereas the BSID-II measures the full range of ability for infants and toddlers. Furthermore, children with single ventricle malformations are exposed to new events between the 14-month and 3-year assessments, including cardiac operations, cardiac catheterizations and complications. Even when the BSID-II and ASQ have been administered simultaneously in other high-risk patient groups, the correlation between these measures has been limited. Woodward et al found that, among extremely low birth weight infants to whom the BSID-II and the ASQ were concomitantly administered at 18–22 months of age, a delayed score on any ASQ domain had a sensitivity of 73%, a specificity of 65%, a positive predictive value of 52%, and a negative predictive value of 82% for scoring more than 2 SD below the normal mean on either the MDI or PDI.24 Creighton et al did observe a strong correlation (r=.92, p<.001) between the BSID–II at age 2 years and the full scale IQ at 5 years among 14 children who had undergone the Norwood procedure.11 The Creighton study was different from our analysis in that the initial testing was performed at 2 years rather than 14 months, and later testing was at age 5 years with a Wechsler IQ test rather than at 3 years with the ASQ.
Our multi-center design, together with the comprehensive prospective measurement of medical and socio-demographic variables, provided a unique opportunity to identify factors associated with developmental impairment in children with single ventricle lesions. Risk factors for adverse developmental outcome included a more complex medical course, growth impairment, feeding problems, and vision and hearing problems. Although the diagnosis of a genetic syndrome was not an independent predictor of low ASQ domain scores, it was correlated with vision and hearing difficulties, in turn an independent predictor of three ASQ domains (Communication, Problem Solving and Personal Social). Perfusion approach and other aspects of intraoperative management were not associated with 3-year developmental outcome. Whereas some investigators have suggested that regional perfusion may protect the brain from insult during circulatory arrest, our data do not support this hypothesis and are consistent with other studies specifically addressing this topic in children with HLHS.25–29 For all ASQ domains, a relatively small percentage of the variance was explained by the multivariable models.
The SVR-II cohort is a large group of children with HLHS and related single right ventricle malformations studied prospectively for neurodevelopmental impairment. Despite this, not all children eligible for participation completed the developmental and behavioral questionnaires at three years. Some children died or underwent cardiac transplantation between study screening and 3-year questionnaire completion. Children who did not complete the developmental questionnaires had a higher rate of genetic and other non-syndromic abnormalities, potentially causing our outcomes to be somewhat more favorable than those among all single ventricle patients undergoing staged palliation. Eligible subjects who did and did not complete assessments were otherwise similar in measured patient and medical characteristics. The SVR-II study relies in large part on parent report of medical events in the outpatient setting. For SES measures utilizing census block data, information from Census 2000 was used as Census 2010 data were not yet available at the end of the trial in 2009 when the SES measure was calculated for all trial participants. Our association of early measures of development with the 3-year ASQ is based on the 14-month evaluation with the BSID-II, which since has been superseded by the BSID-III. Finally, many medical variables are highly collinear, therefore some pairs of variables significant in univariate analysis may have nearly equal importance as independent predictors despite only one or the other remaining in the model.
Children with HLHS and other single right ventricle anomalies have impairment in multiple developmental domains at three years of age. Significant predictors of adverse neurodevelopmental outcome include measures of medical complexity and impaired feeding and growth. Lower ASQ scores at 3 years were associated with worse 14-month BSID-II scores when the PDI was >2 SD and the MDI >1 SD below the population means. However, only approximately 25% of the variation in the ASQ scores was explained by any of the multivariable models. Consistent with the recently published American Heart Association Scientific Statement on neurodevelopmental outcomes in children with congenital heart disease,30 we recommend that all children with HLHS and related single ventricle malformations be followed longitudinally to improve recognition of delays and potential for intervention.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (NHLBI; HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057, HL109781, HL109737). The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or the National Institutes of Health.
Abbreviations
- ASQ
Ages and Stages Questionnaire
- BASC-2
Parent Report Behavior Assessment System for Children-2nd Edition
- BSID-II
Bayley Scales of Infant Development-II
- FSII-R
Functional Status II R
- HLHS
Hypoplastic left heart syndrome
- MBTS
Modified Blalock Taussig shunt
- MDI
Mental Development Index
- PDI
Psychomotor Development index
- QOL
Quality of life
- RVPAS
Right ventricle to pulmonary artery shunt
- SAE
Serious adverse event
- SVR
Single Ventricle Reconstruction
- SVR-II
Single Ventricle Reconstruction Trial Extension
Appendix
Pediatric Heart Network Investigators include:
National Heart, Lung, and Blood Institute--Gail Pearson, Victoria Pemberton, Rae-Ellen Kavey, Mario Stylianou, Marsha Mathis.
Network Chair--University of Texas Southwestern Medical Center: Lynn Mahony.
Data Coordinating Center--New England Research Institutes: Lynn Sleeper (PI), Sharon Tennstedt (PI), Steven Colan, Lisa Virzi, Patty Connell, Victoria Muratov, Lisa Wruck, Minmin Lu, Dianne Gallagher, Anne Devine, Thomas Travison, David F. Teitel.
Core Clinical Site Investigators--Children’s Hospital Boston: Jane W. Newburger (PI), Peter Laussen, Pedro del Nido, Roger Breitbart, Jami Levine, Ellen McGrath, Carolyn Dunbar-Masterson; Children’s Hospital of New York: Wyman Lai (PI), Beth Printz (currently at Rady Children’s Hospital), Daphne Hsu (currently at Montefiore Medical Center), William Hellenbrand (currently at Yale New Haven Medical Center), Ismee Williams, Ashwin Prakash (currently at Children’s Hospital Boston), Ralph Mosca (currently at New York University Medical Center), Darlene Servedio, Rozelle Corda, Rosalind Korsin, Mary Nash; Children’s Hospital of Philadelphia: Victoria L. Vetter (PI), Sarah Tabbutt (currently at the University of California, San Francisco), J. William Gaynor (Study Co-Chair), Chitra Ravishankar, Thomas Spray, Meryl Cohen, Marisa Nolan, Stephanie Piacentino, Sandra DiLullo, Nicole Mirarchi; Cincinnati Children’s Hospital Medical Center: D. Woodrow Benson (PI), Catherine Dent Krawczeski, Lois Bogenschutz, Teresa Barnard, Michelle Hamstra, Rachel Griffiths, Kathie Hogan, Steven Schwartz (currently at the Hospital for Sick Children, Toronto), David Nelson; North Carolina Consortium (Duke University, East Carolina University, Wake Forest University): Page A. W. Anderson (PI; deceased), Jennifer Li (PI), Wesley Covitz, Kari Crawford, Michael Hines, James Jaggers, Theodore Koutlas, Charlie Sang, Jr, Lori Jo Sutton, Mingfen Xu; Medical University of South Carolina: J. Philip Saul (PI), Andrew Atz, Girish Shirali, Scott Bradley, Eric Graham, Teresa Atz, Patricia Infinger; Primary Children’s Medical Center and the University of Utah, Salt Lake City, Utah: L. LuAnn Minich (PI), John Hawkins (deceased), Michael Puchalski, Richard Williams, Linda Lambert, Jun Porter, Marian Shearrow; Hospital for Sick Children, Toronto, Canada: Brian McCrindle (PI), Joel Kirsh, Chris Caldarone, Elizabeth Radojewski, Svetlana Khaikin, Susan McIntyre, Nancy Slater; University of Michigan: Caren S. Goldberg (PI), Richard G. Ohye (Study Chair), Cheryl Nowak; Children’s Hospital of Wisconsin: Nancy Ghanayem (PI), James Tweddell, Kathy Mussatto, Michele Frommelt, Lisa Young-Borkowski.
Auxiliary Sites--Children’s Hospital Los Angeles: Alan Lewis (PI), Vaughn Starnes, Nancy Pike; The Congenital Heart Institute of Florida: Jeffrey P. Jacobs, MD (PI), James A. Quintessenza, Paul J. Chai, David S. Cooper, J. Blaine John, James C. Huhta, Tina Merola, Tracey Cox; Emory University: Kirk Kanter, William Mahle, Joel Bond, Leslie French, Jeryl Huckaby; Nemours Cardiac Center: Christian Pizarro, Carol Prospero, Julie Simons, Gina Baffa; University of Texas Southwestern Medical Center: Ilana Zeltzer (PI), Tia Tortoriello, Deborah McElroy, Deborah Town.
Angiography core laboratory--Duke University: John Rhodes and J. Curt Fudge.
Echocardiography core laboratories--Children’s Hospital of Wisconsin: Peter Frommelt; Children’s Hospital Boston: Gerald Marx.
Genetics Core Laboratory--Children’s Hospital of Philadelphia: Catherine Stolle.
Protocol Review Committee--Michael Artman (Chair), Erle Austin, Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne.
Data and Safety Monitoring Board--John Kugler (Chair), Rae-Ellen Kavey (Executive Secretary), David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb.
Footnotes
Registered with ClinicalTrials.gov: <>
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabbutt S, Nord AS, Jarvik GP, Bernbaum J, Wernovsky G, Gerdes M, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121:476–483. doi: 10.1542/peds.2007-1282. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105:1082–89. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, et al. Neurodevelopmental outcome of patients after the Fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137:646–52. doi: 10.1067/mpd.2000.108952. [DOI] [PubMed] [Google Scholar]
- 4.Fuller S, Nord AS, Gerdes M, Wernovsky G, Jarvik GP, Bernbaum J, et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36(1):40–48. doi: 10.1016/j.ejcts.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: The Single Ventricle Reconstruction Trial. Circulation. 2012;125:2081–91. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freed DH, Robertson CM, Sauve RS, Joffe AR, Rebeyka IM, Ross DB, et al. Intermediate-term outcomes of the arterial switch operation for transposition of great arteries in neonates; alive but well? J Thorac Cardiovasc Surg. 2006;132 (4):845–52. doi: 10.1016/j.jtcvs.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127(9):971–9. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, et al. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143(1):67–73. doi: 10.1016/S0022-3476(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 9.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1397–403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 10.Von Rhein M, Dimitropoulos A, Valsangiacomo Buechel ER, Landolt MA, Latal B. Risk factors for neurodevelopmental impairments in school-age children after cardiac surgery with full-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;144 (3):577–83. doi: 10.1016/j.jtcvs.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Creighton DE, Robertson CMT, Sauve RS, Moddemann DM, Alton GY, Nettel–Aguire A, et al. Neurocognitive functional and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120(3):e478–e486. doi: 10.1542/peds.2006-3250. [DOI] [PubMed] [Google Scholar]
- 12.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136(4):968–75. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. JTCVS. 2012;144 (4):896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlichting JA, Soliman AS, Schairer C, Schottenfeld D, Merajver SD. Inflammatory and non-inflammatory breast cancer survival by socioeconomic position in the Surveillance, Epidemiology and End results database, 1990–2008. Breast Cancer Res Treat. 2012;134:1257–68. doi: 10.1007/s10549-012-2133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 17.Squires J, Potter L, Bricker D, editors. Ages and Stages Questionnaire. 2. Brookes Publishing Company; 1999. [Google Scholar]
- 18.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. 2. Minneapolis, MN: Pearson; [Google Scholar]
- 19.Varni JW, Seid M, Kurtin PS. The Peds QL4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4. 0 Generic Core Scales in healthy and patient populations. Med Care. 2001;39(8):800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Uzark K, Jones K, Burwinkle TM, Varni JW. The Pediatric Quality of Life Inventory in Children with Heart Disease. Prog Pediatr Cardiol. 2003;18(2):243–50. [Google Scholar]
- 21.Stein REK, Jessop DJ. Functional status II® A measure of child health status. Med Care. 1990;28:1041–55. doi: 10.1097/00005650-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Atallah J, Dinu IA, Joffee AR, Robertson CM, Sauve RS, Dyck JD, et al. Two-year survival and mental and psychomotor outcomes after the Norwood analysis of the modified Blalock Taussig shunt and right ventricle-to-pulmonary artery shunt surgical eras. Circulation. 2008;118(14):1410–8. doi: 10.1161/CIRCULATIONAHA.107.741579. [DOI] [PubMed] [Google Scholar]
- 23.Hack M, Taylor HG, Drotar G, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–41. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 24.Woodward BJ, Papile LA, Lowe JR, Laadt VL, Shaffer ML, Montman R, et al. Uses of the Ages and Stages Questionnaire and Bayley Scales of Infant Development-II in neurodevelopmental follow-up of extremely low birth weight infants. J Perinatol. 2011;31(10):641–6. doi: 10.1038/jp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahle WT, Lu M, Ohye RG, Gaynor JW, Goldberg CS, Sleeper LA, et al. A predictive model for neurodevelopmental outcome after the Norwood procedure. Pediatr Cardiol. 2012;34 (2):327–33. doi: 10.1007/s00246-012-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visconti KJ, Rimmer D, Gauvreau, del Nido P, Mayer JE, Jr, Hagino I, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one year neurodevelopmental outcome. Ann Thorac Surg. 2006;82(6):2207–11. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 27.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131(1):190–7. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg CS, Bove EL, Devaney EJ, Mollen E, Schwartz E, Tindall S, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133(4):880–7. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Algra SO, Jansen N, van der Tweel I, Schouten A, Groenendaal F, Toet MC, et al. Neurological injury after neonatal cardiac surgery: a randomized controlled trial of two perfusion techniques. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003312. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–72. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.