Abstract
Rationale
Although selective 5-HT reuptake inhibitors (SSRIs) can reduce anxiety after chronic treatment, acute SSRI administration is associated with an increase in anxiety consistent with an acute increase in 5-HT neurotransmission. Exercise is anxiolytic in humans, and wheel running prevents anxiety-like behavioral consequences of uncontrollable stress in rats, but the effects of exercise on acute fluoxetine-induced anxiety-like behaviors are unknown.
Objectives
The current studies tested the hypothesis that acute administration of the SSRI fluoxetine would produce behaviors in rats resembling those produced by uncontrollable stress and that these behaviors would be blocked by prior wheel running.
Results
Adult, male Fisher 344 rats administered moderate (10 mg/kg) or high (20 mg/kg) doses of fluoxetine demonstrated exaggerated shock-elicited freezing and an interference with shuttle box escape compared to rats given either saline or low-dose fluoxetine (2.5 mg/kg). Fluoxetine-induced behaviors were similar to, but smaller in magnitude than, those produced by uncontrollable stress and were blocked by pretreatment with the 5-HT2C receptor antagonist SB 242084 (1 mg/kg). Rats allowed access to running wheels for 6 weeks were protected against the anxiety-like behaviors produced by a single injection of fluoxetine (10 mg/kg).
Conclusions
Behavioral effects of acute fluoxetine administration resemble those produced by uncontrollable stress. Results are consistent with the idea that exercise can produce resistance against the anxiogenic effects of acute increases in 5-HT and suggest that acute behavioral effects of antidepressants can depend on history of physical activity.
Keywords: Wheel running, Conditioned fear, Serotonin, 5-HT2C receptor, Depression, Anxiety, Learned helplessness, SSRI, Uncontrollable stress, Escape deficits
Introduction
Rats exposed to an acute uncontrollable stressor such as uncontrollable tail shock demonstrate a variety of behaviors that resemble anxiety, such as exaggerated freezing immediately following pairing of a shock-chamber-conditioned stimulus with a foot shock unconditioned stimulus (shock-elicited freezing), as well as deficits in shuttle box escape learning (Maier 1990). These behavioral consequences of uncontrollable stress have been called learned helplessness (Maier and Seligman 1976), have been argued to represent animal analogs of human anxiety (Maier and Watkins 1998), and can be both prevented and reversed by anxiolytic drugs (Drugan et al. 1984; Maier et al. 1994, 1990).
Growing evidence points to a critical role for hyper-activation of serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN) in mediating the behavioral consequences of uncontrollable stress (for recent reviews see, Greenwood and Fleshner 2008; Maier and Watkins 2005). Uncontrollable, relative to controllable, stress hyperactivates the DRN (Grahn et al. 1999) and produces exaggerated release of 5-HT in the DRN (Amat et al. 2006; Maswood et al. 1998). Exaggerated release of 5-HT within the DRN during uncontrollable stress could sensitize DRN 5-HT neurons by down-regulating somatodendritic 5-HT1A inhibitory autoreceptors in the DRN (Riad et al. 2001; Short et al. 2000), thus removing an important inhibitory influence over the firing of DRN 5-HT neurons. Indeed, hyper-activation of the DRN leads to sensitization of the DRN so that during behavioral testing 24 h after exposure to uncontrollable tail shock stress, there is exaggerated 5-HT release in DRN projection sites including the amygdala (Amat et al. 1998a,b; Bland et al. 2003). Importantly, manipulations that activate DRN 5-HT neurons (Maier et al. 1995a) or increase extracellular 5-HT in the DRN (Greenwood and Fleshner 2008) produce exaggerated shock-elicited freezing and interfere with shuttle box escape performance in the absence of stress, and the behavioral consequences of uncontrollable stressors can be prevented by DRN lesions (Maier et al. 1993) or manipulations that reduce activation of DRN 5-HT neurons (Maier et al. 1995b).
The role of 5-HT in the behavioral consequences of uncontrollable stress is consistent with the involvement of 5-HT in anxiety (Graeff et al. 1996; Lowry et al. 2005; Meloni et al. 2008). Anxiety-like behavioral effects of anxiogenic drugs such as the 5-HT2 receptor agonist m-chlorophenylpiperazine and the GABAA receptor partial inverse agonist N-methyl-beta-carboline-3-carboxamide correspond with activation of DRN 5-HT neurons (Abrams et al. 2005; Singewald and Sharp 2000) and can be blocked by 5-HT2C receptor antagonists (Bagdy et al. 2001; Hackler et al. 2007). Similarly, although selective-5-HT reuptake inhibitors (SSRIs) can reduce clinical symptoms in the spectrum of depression and anxiety disorders following several weeks of treatment (Feighner and Boyer 1991; Kent et al. 1998), SSRIs rapidly increase extracellular 5-HT in the DRN (Rutter et al. 1995), and symptom exacerbation, especially an increase in anxiety, is often reported during the onset of clinical treatment with SSRIs (Feighner and Boyer 1991; Goldstein and Goodnick 1998; Masand and Gupta 1999; Nutt and Glue 1989; Pohl et al. 1988). Consistent with the human data, acute administration of an SSRI can elicit anxiety-like behaviors in rodent models of anxiety (Bagdy et al. 2001; Belzung et al. 2001; Burghardt et al. 2007, 2004; To et al. 1999). Riad et al. (2004) reported that a single systemic injection of the SSRI fluoxetine can also rapidly internalize 5-HT1A inhibitory autoreceptors in the DRN. Given the similarities between uncontrollable tail shock stress and acute treatment with an SSRI (i.e. 5-HT release and 5-HT1A autoreceptor down-regulation), it might be expected that both uncontrollable stress and a single systemic injection of an SSRI would produce similar anxiety-like behaviors. The first aim of the current studies, therefore, was to test the hypothesis that a single injection of the SSRI fluoxetine would elicit behaviors similar to those produced by uncontrollable stress and that these behaviors would be dependent on fluoxetine dose and 5-HT2C receptor activation.
Prior studies investigating anxiety-like effects of acute SSRIs used sedentary animals only. Physical activity has anxiolytic and antidepressant effects in humans (Babyak et al. 2000; Blumenthal et al. 2007, 1999; Martinsen 1990) and can prevent (Binder et al. 2004; Bjornebekk et al. 2005; Dishman et al. 1997; Duman et al. 2008; Greenwood et al. 2003) and reverse (Greenwood et al. 2007a) depression-and anxiety-like behaviors in rodent models. Rats allowed 6 weeks of voluntary access to running wheels, for example, are protected against the DRN-hyperactivating and anxiety-like effects of uncontrollable stress, including exaggerated shock-elicited freezing and shuttle box escape deficits (Greenwood et al. 2005a, 2003). Exercise is thus a simple behavioral approach that could potentially be used to reduce the acute anxiogenic effects of increases in 5-HT, such as occurs during acute SSRI administration. The second goal of the current studies was, therefore, to determine if wheel running prior to a single systemic injection of the SSRI fluoxetine could prevent the typical behavioral consequences.
Materials and methods
Subjects
Adult, male Fischer 344 rats weighing 247.18±5.89 g at the time of behavioral testing were used in all experiments. Male Fischer 344 rats were used in these studies because our prior work with this strain has revealed that Fischer rats are stable runners that display minimal individual variability in running behavior. All rats were individually housed in Nalgene Plexiglas cages (45×25.2×14.7 cm) in a temperature- (22°C) and humidity-controlled environment. Lights were maintained on a 12:12-h light/dark cycle (lights on 0600–1800). Animals were allowed to acclimate to these housing conditions for 1–2 weeks prior to any experimental manipulation. Care was taken to minimize animal discomfort during all procedures. Rats had ad libitum access to food and water, were weighed weekly, and were handled daily during the week prior to experimental manipulation. All experimental protocols were approved by the University of Colorado Animal Care and Use Committee.
Drug injections
The SSRI fluoxetine hydrochloride and the selective 5-HT2C receptor antagonist SB 242084 were purchased from Sigma (St. Louis, MO, USA). Fluoxetine was dissolved in sterile saline at concentrations of 1.25, 5, and 10 mg/ml and administered i.p. at a volume of 2 ml/kg body weight. SB 242084 was dissolved in sterile saline at a concentration of 1 mg/ml and was administered i.p. at a volume of 1 ml/kg. This dose of SB 242084 was chosen based on prior work reporting anxiolytic effects of 1 mg/kg SB 242084 in the absence of changes in spontaneous activity (Jones et al. 2002; Kennett et al. 1997; Millan et al. 2001).
Wheel running
All rats in the wheel running group were randomly assigned to be housed with in-cage running wheels (Mini Mitter, Bend, OR, USA). Other than the presence or absence of the running wheel, housing conditions of sedentary and physically active rats were identical. Daily wheel revolutions were recorded digitally sing Mini Mitter (Bend, OR, USA) Vital View Data Acquisition software, and weekly running distance was calculated by multiplying wheel circumference (1.081 m) by the number of wheel revolutions. Prior work has demonstrated that several relevant exercise-induced adaptations, including the protective effect of exercise against the behavioral consequences of uncontrollable stress, take between 3 and 6 weeks to develop (Greenwood et al. 2005a,b). Animals in the current study were thus allowed voluntary access to running wheels for 6 weeks.
Uncontrollable stress
The uncontrollable tail shock procedure followed protocols previously used in our laboratory that are known to produce exaggerated shock-elicited freezing and shuttle box escape deficits (Greenwood et al. 2003, 2007b). Rats were randomly assigned to either be left in their home cages (No Stress) or to be exposed to the uncontrollable stress procedure (Stress). Stressed rats were restrained in Plexi-glas tubes (23.4 cm in length and 7.0 cm in diameter) that allowed protrusion of the tail from the back of the tube. Electrodes attached to the tail delivered 100 5-s tail shocks (1.5 mA) on a 1-min variable-interval schedule (Coulbourn Instruments, Allentown, PA, USA). This number and intensity of tail shocks were chosen based on prior work using this same stressor procedure (Greenwood et al. 2003, 2007b; Maier 1990) and a titration of the number of tail shocks required to produce escape deficits (Takase et al. 2005). Following termination of shock, rats were returned to their home cages. The entire stress procedure lasted 2 h and occurred from 08:00 to 10:00 h, 24 h prior to behavioral testing.
Behavioral testing
Behavioral testing procedures were similar to protocols previously used in our laboratory (Greenwood et al. 2003, 2007b). Fear conditioning and shuttle box escape behavior took place sequentially in shuttle boxes (20″W×10″D×12″H, Coulbourn Instruments, Whitehall, PA, USA) consisting of a grid floor and Plexiglas walls. Shuttle boxes were contained within custom built sound-attenuating chambers and were illuminated by bright overhead lights mounted on both sides of the shuttle box ceiling. At the beginning of a testing session, rats were placed one at a time into shuttle boxes and allowed to explore both sides of the box for 5 min. During this 5-min exploration period, each rat was scored every 10 s as either freezing or not freezing (pre-shock freezing). In order to be scored as freezing, there had to be an absence of all movement except for that required for respiration. Rats then received three 0.6-mA foot shocks that could be terminated by crossing to the opposite side of the shuttle box in a fixed-ratio 1 (FR-1) schedule. During escape trials, the grid floor on both sides of the shuttle box delivered foot shocks. Only after rats had fully crossed through the shuttle box door was the shock terminated. During each of these FR-1 trials, the latency to cross to the opposite side of the shuttle box was recorded (FR-1 escape latencies). Immediately following the third FR-1 trial, rats were observed for 20 min and again scored for freezing (shock-elicited freezing). This shock-elicited freezing is a measure of fear conditioned to cues present in the shuttle box (Fanselow and Lester 1988). The post-foot shock observation period was followed by 25 fixed-ratio 2 (FR-2) escape trials. During FR-2 trials, rats were required to cross to the other side of the shuttle box and then back to terminate the foot shock. Each shock was terminated after 30 s if an escape response had not occurred. In these cases, an escape latency of 30 s was assigned. Shocks occurred with an average inter-trial interval of 60 s, and a single test session lasted approximately 1 h. All behavioral tests occurred between 0900 and 1200 by an experimenter blind to treatment condition of the animals.
Corticosterone measurement
Trunk blood was collected 1 h following administration of fluoxetine. Plasma corticosterone levels were assessed using the Corticosterone Enzyme Immunoassay Kit (Assay Designs; Ann Arbor, MI, USA) following the manufacturer’s instructions. Samples were diluted 1:50 in Steroid Displacement Reagent made by adding 5.0 μl of the concentrated Steroid Displacement Reagent to 10.0 ml of Assay Buffer 15.
Procedures
To compare the behavioral effects of acute fluoxetine to those produced by uncontrollable tail shock stress, rats (n= 10/group) were placed into shuttle boxes, and freezing and escape behaviors were recorded 1 h following injections of either saline or fluoxetine (10.0 mg/kg). The 1-h time point was chosen based on prior work reporting (1) 5-HT release in the DRN (Rutter et al. 1995), (2) internalization of 5-HT1A autoreceptors in the DRN (Riad et al. 2004), and (3) anxiety-like behaviors (Bagdy et al. 2001; Burghardt et al. 2007, 2004), all occurring within 1 h after systemic fluoxetine administration. Another group of rats (n=6) did not receive an injection but were instead exposed to uncontrollable tail shock stress. Behavioral effects of uncontrollable stress are difficult to interpret soon after the tail shock procedure due to lingering effects of tail shock on motor activity. For this reason, uncontrollable tail shock occurred 24 h prior to behavioral testing, as is customary for learned helplessness procedures (Greenwood and Fleshner 2008; Maier and Watkins 2005).
A second experiment investigated the dose–response relationship between fluoxetine and shock-elicited freezing and escape behaviors. Rats received a single injection of either saline (n=10), 2.5 (n=8), 10 (n=8), or 20 (n=8) mg/kg fluoxetine and were tested for freezing and escape behaviors in shuttle boxes 1 h later.
Prior work indicates that anxiogenic effects of acute SSRI administration can be prevented by pretreatment with the 5-HT2C receptor antagonist SB 242084 (Bagdy et al. 2001; Burghardt et al. 2007). To determine if acute fluoxetine-induced exaggerated shock-elicited freezing and escape deficits are similarly dependent on 5-HT2C receptor activation, saline or SB 242084 (1 mg/kg) was administered 15 min prior to saline or fluoxetine (10.0 mg/kg), and behavioral testing occurred 1 h later (n=8/group).
A final experiment investigated the effects of prior wheel running on anxiety-like behaviors produced by acute fluoxetine. Following 6 weeks of the wheel running or sedentary conditions, rats received either saline or 10 mg/kg fluoxetine (n=10/group). Behavioral testing occurred in shuttle boxes 1 h later. The effect of wheel running on corticosterone elevations elicited by acute fluoxetine was also determined in a separate experiment. Acute fluoxetine has been reported to increase circulating levels of the stress hormone corticosterone (Duncan et al. 1998; Serra et al. 2001). Wheel running can attenuate hypothalamic–pituitary–adrenal axis responses to mild stressors (Day et al. 2006; Droste et al. 2007, 2006), thus, it is of interest to determine if wheel running affects increases in circulating corticosterone elicited by acute fluoxetine. Sedentary and physically active (6 weeks of wheel running) rats were sacrificed 1 h following an injection of either saline (n=8/ group) or fluoxetine (10 mg/kg; n=7/group), and trunk blood was collected for analysis of corticosterone. No behavioral testing was performed in the animals used for corticosterone analysis.
Data analysis
Pre-shock freezing scores were collapsed into 1 pre-shock score and analyzed with one-way ANOVA. Shock-elicited freezing was collapsed into ten 2-min blocks and analyzed with repeated measures ANOVA. Shock-elicited freezing for the entire 20-min freezing period was also averaged and analyzed with ANOVA. Escape latencies during the initial three FR-1 escape trials were averaged for each rat and analyzed with ANOVA. FR-2 escape latencies were collapsed into five blocks of five trials each and analyzed with repeated measures ANOVA. FR-2 escape latencies were also averaged and analyzed with ANOVA. Corticosterone levels were analyzed with two-way ANOVA. Repeated measures ANOVA was used to determine group differences in body weight. All ANOVAs were followed by Fisher’s protected least significant difference post hoc analysis when required. All analysis performed were considered significant when p≤0.05.
Results
Acute fluoxetine produces behaviors similar to those produced by uncontrollable stress
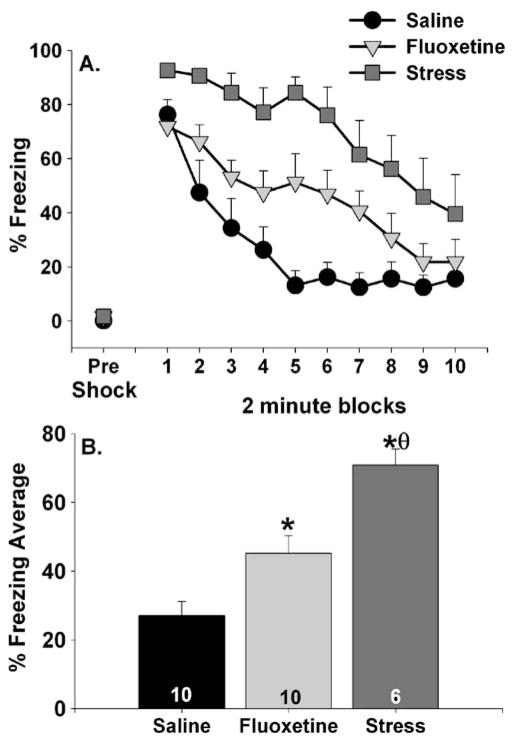
The behavioral effects of acute fluoxetine (10.0 mg/kg) were compared to those produced by uncontrollable tail shock stress. Figure 1a shows pre-shock freezing behavior and shock-elicited freezing across 2-min blocks over the course of the 20-min observation period. Prior to receiving foot shocks in the shuttle box, freezing behavior in all groups was very low. Neither fluoxetine nor stress treatment affected freezing behavior during the first 5 min prior to administration of foot shock (Fig. 1a; pre-shock). Freezing increased in all groups following the three FR-1 trials. In contrast to saline-treated rats whose freezing behavior returned to very low levels by 10 min, freezing behavior remained elevated in both stressed and fluoxetine-treated rats. Shock-elicited freezing was particularly exaggerated in the stressed group whose level of freezing remained elevated even after 20 min. Repeated measures ANOVA revealed a significant main effect of group (F (2, 23) = 18.1; p<0.0001) and of time (F (9, 207)=17.64; p< 0.0001) on 2-min blocks of freezing. The average freezing scores for the duration of the observation period are shown in Fig. 1b. The uncontrollable stress and fluoxetine groups both differed from the saline group. Uncontrollable stress also produced a greater exaggeration of shock-elicited freezing than did fluoxetine treatment.
Fig. 1.
Effects of fluoxetine (10 mg/kg) and uncontrollable tail shock stress on freezing behavior immediately before (pre-shock) and immediately following (representing fear conditioned to contextual or discrete cues in the shuttle box) three foot shocks in a shuttle box. Fluoxetine was administered 1 h and tail shock stress 24 h prior to behavioral testing. Data are presented as a 2-min blocks of freezing and b the mean percent shock-elicited freezing for the entire 20-min observation period. Data represent means ± SEM. Asterisks p<0.05 relative to saline-treated rats; theta p<0.05 relative to fluoxetine-treated rats. The number in each bar represents the number of animals included in that group
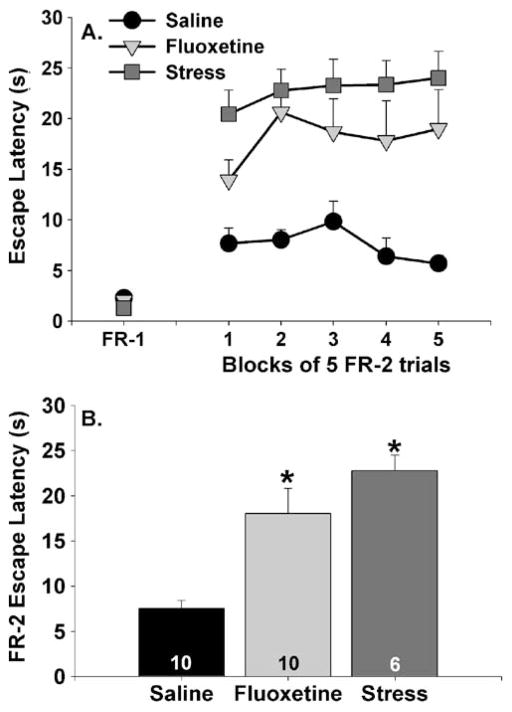
The effects of stress and fluoxetine treatment on FR-1 and FR-2 shuttle box escape latencies are shown in Fig. 2a. Rats in all groups were capable of escaping from FR-1 trials within 5 s (Fig. 2a, FR-1 time point). Neither stress nor fluoxetine treatment affected FR-1 escape latencies. Inspection of FR-2 escape latencies revealed the typical deficit in shuttle box escape learning produced by uncontrollable tail shock stress. Additionally, fluoxetine produced a significant disruption in shuttle box escape learning. As expected, rats given saline were able to learn to escape from foot shocks and escaped from all FR-2 trials in less than 10 s. Repeated measures ANOVA revealed a significant main effect of group (F (2, 23)=13.32; p<0.0001) on escape latencies during blocks of five escape trials. Neither the main effect of time nor the interaction between group and time reached significance. The average escape latencies across all 25 FR-2 trials are shown in Fig. 2b. The stress and fluoxetine groups differed from the saline group but did not differ from each other.
Fig. 2.
Effects of fluoxetine (10 mg/kg) and uncontrollable tail shock stress on fixed-ratio 1 (FR-1) and fixed-ratio 2 (FR-2) escape performance. Fluoxetine was administered 1 h and tail shock 24 h prior to behavioral testing. Data are presented as a blocks of five escape trials and b the mean escape latency for all 25 FR-2 escape trials. Data represent means ± SEM. Asterisks p<0.05 relative to the saline group. The number in each bar represents the number of animals included in that group
Behavioral effects of acute fluoxetine are dose dependent
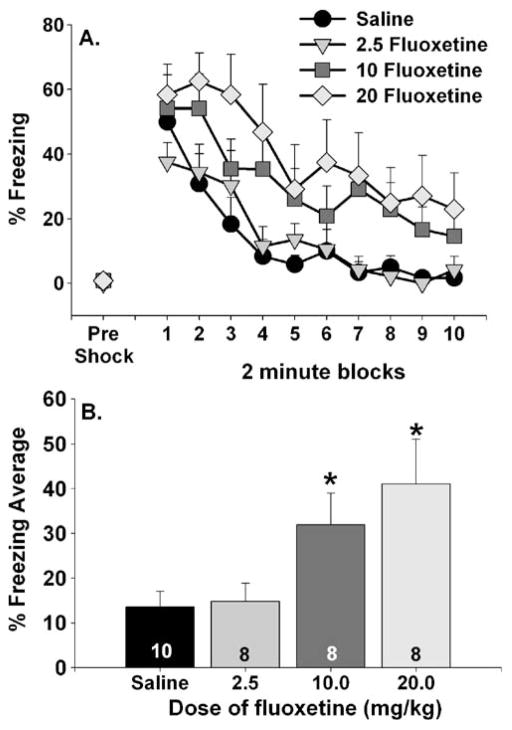
Fluoxetine increased shock-elicited freezing and FR-2 escape latencies in a dose-dependent manner. None of the doses of fluoxetine used affected freezing behavior prior to the three FR-1 trials (Fig. 3a, pre-shock). Following the FR-1 trials, however, both 10 and 20 mg/kg of fluoxetine exaggerated freezing relative to saline and 2.5 mg/kg fluoxetine. This was verified by repeated measures ANOVA, which revealed significant effects of group (F (3, 30)=4.03; p<0.05) and time (F (9, 270)=24.37; p<0.0001) on 2-min freezing blocks. The interaction between group and time was not significant. Analysis of the average freezing scores (Fig. 3b) revealed that shock-elicited freezing exhibited by the 20 mg/kg fluoxetine group was increased relative to both the saline and 2.5 mg/kg fluoxetine groups, which were not different from each other. The 10 mg/kg fluoxetine group only differed from the saline group.
Fig. 3.
Effects of dose of fluoxetine on freezing behavior immediately before (pre-shock) and immediately following (representing fear conditioned to contextual or discrete cues in the shuttle box) three foot shocks in a shuttle box. Drugs were administered 1 h prior to behavioral testing. Data are presented as a 2-min blocks of freezing and b the mean percent freezing for the entire 20-min observation period. Data are means ± SEM. Asterisks p<0.05 relative to saline and 2.5 mg/kg fluoxetine groups. Group sizes are shown within the bars
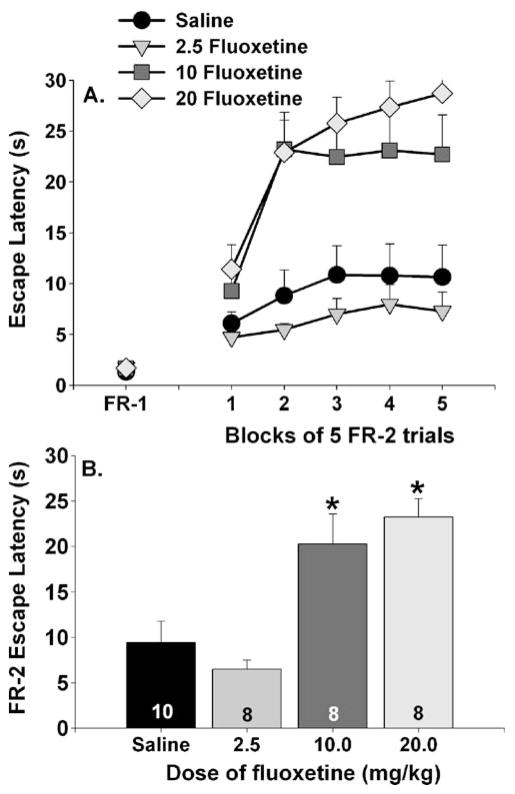
Figure 4 shows the effects fluoxetine dose on FR-1 and FR-2 escape. FR-1 escape latencies did not differ between groups; however, both 10 and 20 mg/kg of fluoxetine interfered with FR-2 escape compared with saline and 2.5 mg/kg fluoxetine (Fig. 4a). There were significant main effects of group (3, 30)=11.7; p<0.0001), trial block (4, 120)=29.9; p<0.0001), and a reliable interaction between group and trial block (F (12, 120)=4.24; p<0.0001) on FR-2 escape latencies. Both the 10 and 20 mg/kg fluoxetine groups differed from the saline and 2.5 mg/kg fluoxetine groups during all five trial blocks except the first, during which the 10 mg/kg group did not differ from the saline group. Neither the 10 and 20 mg/kg fluoxetine groups nor the saline and 2.5 mg/kg fluoxetine groups differed from each other. Average FR-2 escape latencies appear in Fig. 4b. Both the 10 and 20 mg/kg fluoxetine groups differed from the saline and 2.5 mg/kg fluoxetine groups. No other group differences were significant. That the two highest doses of fluoxetine tested produced similar behavioral consequences suggests that both doses were above a certain threshold required to produce the observed effects.
Fig. 4.
Effects of dose of fluoxetine on fixed-ratio 1 (FR-1) and fixed-ratio 2 (FR-2) escape performance. Drugs were administered 1 h prior to behavioral testing. Data are presented as a blocks of five escape trials and b the mean escape latency for all 25 FR-2 escape trials. Data represent means ± SEM. Asterisks p<0.05 relative to saline and 2.5 mg/kg fluoxetine groups. The number in each bar represents the number of animals included in that group
Behavioral effects of acute fluoxetine are dependent on 5-HT2C receptor activation
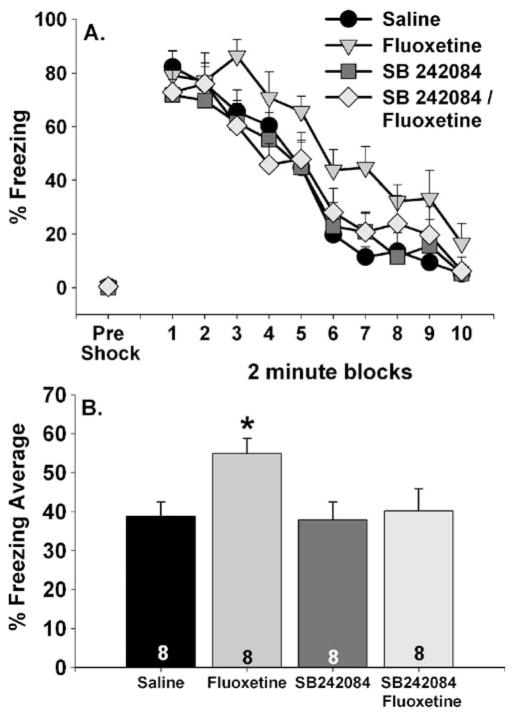
To determine if acute fluoxetine-induced behaviors are dependent on 5-HT2C receptor activation, saline or SB 242084 (1 mg/kg) was administered 15 min prior to saline or fluoxetine (10 mg/kg) administration. SB 242084 treatment prior to fluoxetine blocked the behavioral effects of fluoxetine. Freezing behavior is shown in Fig. 5. All groups displayed similar freezing behavior prior to the FR-1 trials (Fig. 5a, pre-shock). Freezing behavior increased following the three FR-1 trials in all groups (Fig. 5a); however, freezing increased more in the fluoxetine group relative to all other groups. Repeated measures ANOVA revealed significant main effects of group (F (3, 28)=3.1; p<0.05) and time (F (9, 252)=55.02; p<0.0001) on 2-min freezing blocks. The average shock-elicited freezing displayed by the fluoxetine group was significantly higher than all the other groups, which were not different from each other (Fig. 5b).
Fig. 5.
Effects of the 5-HT2C antagonist SB 242084 on fluoxetine-induced exaggerated shock-elicited freezing. SB 242084 (1 mg/kg) was administered 15 min prior to saline or fluoxetine (10 mg/kg). Freezing behavior immediately before (pre-shock) and immediately following (representing fear conditioned to contextual or discrete cues in the shuttle box) three foot shocks was assessed in a shuttle box 1 h later. Data are presented as a 2-min blocks of freezing and b the mean percent shock-elicited freezing for the entire 20-min observation period. Data are means ± SEM. Asterisk p<0.05 relative to saline, SB 242084, and SB 242084/fluoxetine groups. The number in each bar represents the number of animals included in that group
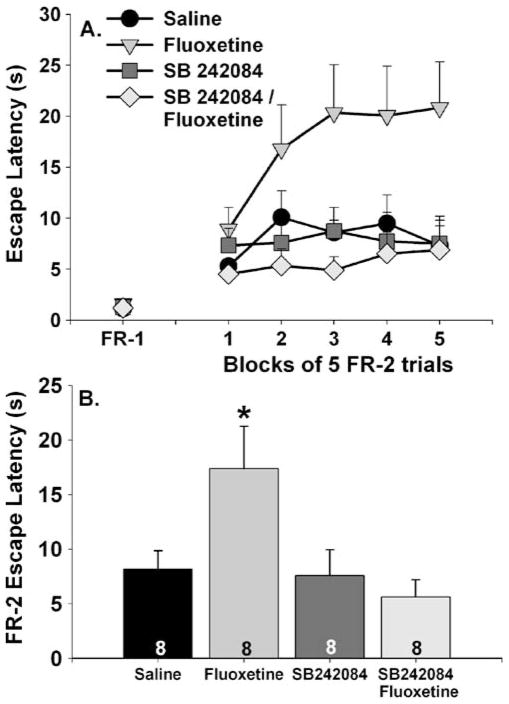
The effects of fluoxetine and SB 242084 on FR-1 and FR-2 escape latencies are shown in Fig. 6a. FR-1 escape latencies were similar between groups. Fluoxetine again interfered with FR-2 escape, and pretreatment with SB 242084 blocked this effect. The main effects of group (F (3, 28)=4.2; p<0.05) and time (F (4, 112)=6.9; p<0.0001) and the interaction between group and time (F (12, 112)=2.9; p<0.05) were all significant. The fluoxetine group had longer FR-2 latencies compared to all other groups during all of the trial blocks except the first. Fluoxetine increased average FR-2 escape latency compared to all other groups, which did not differ from each other (Fig. 6b).
Fig. 6.
Effects of the 5-HT2C antagonist SB 242084 on fluoxetine-induced escape deficit. SB 242084 (1 mg/kg) was administered 15 min prior to saline or fluoxetine (10 mg/kg). Rats were placed into shuttle boxes for assessment of fixed-ratio 1 (FR-1) and fixed-ratio 2 (FR-2) escape performance 1 h later. Data are presented as a blocks of five escape trials and b the mean escape latency for all 25 FR-2 escape trials. Data represent means ± SEM. Asterisk p<0.05 relative to saline, SB 242084, and SB 242084/fluoxetine groups. Group sizes are shown within the bars
Wheel running reduces the behavioral effects of acute fluoxetine
Body weights of sedentary and exercised rats increased steadily over the course of the 6-week experiment, and wheel running did not affect body weight. Weights of sedentary (156.7±4.1 g) and exercised (160.8±2.7 g) rats did not differ prior to the onset of running nor at any point thereafter. Repeated measures ANOVA revealed a reliable main effect of time on body weight (F (5, 190)=692.5; p< 0.0001), but neither the main effect of exercise nor the time by exercise interaction was significant (data not shown). Running behavior of rats allowed access to running wheels was similar to what we have previously observed in Fischer 344 rats (Greenwood et al. 2005a; Greenwood et al. 2005b). Rats ran an average of 6.9±0.98 km during the first week of wheel access. Weekly running distance increased rapidly during the first 3 weeks of wheel access to a maximum of 21.2±3.24 km during the third week of running. Running distance remained relatively constant just below this level for the remainder of the study (data not shown).
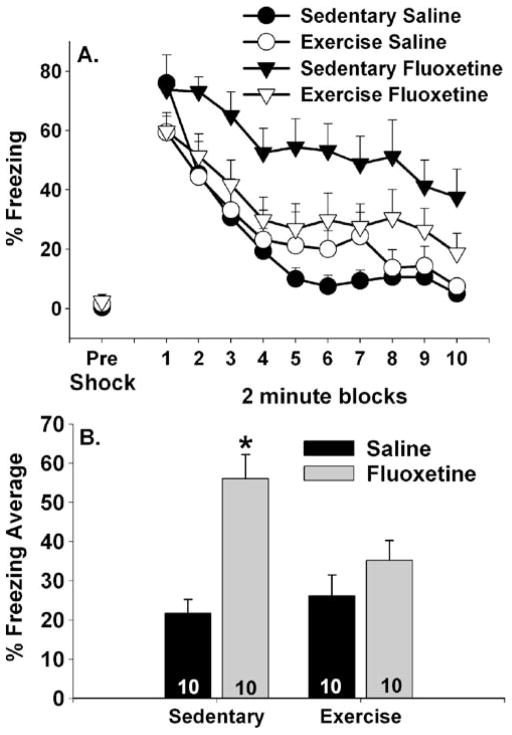
Six weeks of wheel running significantly reduced the acute behavioral effects of fluoxetine measured in the shuttle box following fluoxetine (10 mg/kg) administration. Pre-shock freezing and shock-elicited freezing behavior during 2-min blocks are shown in Fig. 7a. Again, during the first 5 min in the shuttle boxes, animals spent very little time freezing regardless of prior activity or drug treatment (Fig. 7a; pre-shock). Freezing scores increased in all groups following the three FR-1 trials. Freezing displayed by both sedentary and exercise groups treated with saline returned to low levels by the end of the 20-min freezing period. As before, sedentary rats treated with fluoxetine displayed exaggerated shock-elicited freezing that remained elevated even after 20 min. Wheel running prevented the effect of fluoxetine on freezing. This was confirmed with repeated measures ANOVA that revealed significant main effects of drug (F (1, 35)=18.07; p=0.0002) and time (F (9, 315)= 22.06; p<0.0001) but not exercise on shock-elicited freezing. There were reliable interactions between drug and exercise (F (1, 35)=6.12; p<0.05). No other interactions were significant. The Sedentary/Fluoxetine group differed from both the Sedentary/Saline and the Exercise/ Saline groups at all time points except the first 2-min block of freezing. The Sedentary/Saline and Exercise/Saline groups never differed from each other. The Exercise/ Fluoxetine group never differed from the Exercise/Saline group and only reliably differed from the Sedentary/Saline group during the sixth 2-min freezing block. The Exercise/ Fluoxetine group reliably differed from the Sedentary/ Fluoxetine group during all 2-min blocks except for the first and seventh through tenth freezing block. Similar results were obtained from analysis of the average freezing scores (Fig. 7b). The Sedentary/Fluoxetine group differed from all other groups. No other group differences were significant.
Fig. 7.
Effects of exercise on fluoxetine-induced exaggerated shock-elicited freezing. Rats remained sedentary or were allowed 6 weeks of voluntary access to running wheels prior to receiving a single injection of either saline or fluoxetine (10 mg/kg). Freezing behavior immediately before (pre-shock) and immediately following (representing fear conditioned to contextual or discrete cues in the shuttle box) three foot shocks was assessed in a shuttle box 1 h after fluoxetine injection. Data are presented as a 2-min blocks of freezing and b the mean percent shock-elicited freezing for the entire 20-min observation period. Data are means ± SEM. Asterisk p<0.05 relative to sedentary/ saline, exercised/saline, and exercised/fluoxetine groups. Group sizes are shown within the bars
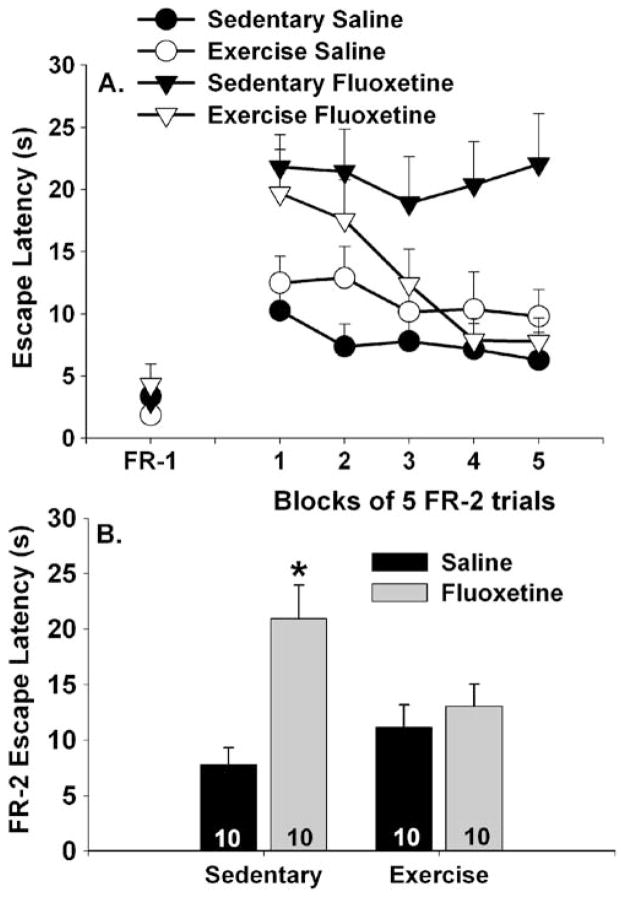
Wheel running significantly reduced the effect of fluoxetine on shuttle box escape latency. FR-1 and FR-2 escape latencies over blocks of five trials are depicted in Fig. 8a. Neither drug treatment nor exercise altered the latency to cross the box once during the three FR-1 trials. Rats in all groups displayed average FR-1 latencies of less than 5.0 s. Both sedentary and exercised rats treated with saline were able to learn to escape from foot shock during the 25 FR-2 trials. Rats treated with saline were able to escape in less than 12 s regardless of prior wheel running. Fluoxetine again produced a deficit in shuttle box escape. However, the effect of fluoxetine on escape latencies depended upon physical activity status. Fluoxetine treatment produced robust escape deficits in sedentary rats, whereas fluoxetine has less of an effect in physically active rats. This was confirmed with repeated measures ANOVA that indicated reliable main effects of drug (F (1,35)=11.35; p=0.001) and time (F (4,140)=5.48; p=0.0004) and significant interactions between drug and exercise (F (1,35)=6.3; p<0.05), time and exercise (F (4,140)=2.35; p=0.05), and time, drug, and exercise (F (4,140)=2.33; p= 0.05) during the five blocks of five FR-2 trials. Neither the main effect of exercise nor the interaction between time and drug was significant. Post hoc analysis revealed that although fluoxetine treatment had a similar effect on sedentary and exercised rats during the first three trial blocks, the escape latency of the exercise/fluoxetine group improved by the final two trial blocks. Sedentary/Fluoxetine group differed from the Sedentary/Saline and Exercise/Saline groups at each of the five blocks of FR-2 trials. The Sedentary/Saline and Exercise/Saline groups were never different from each other. The Exercise/Fluoxetine group never differed from the Exercise/Saline group and only differed from the Sedentary/Saline group during the first and second blocks of FR-2 trials. Figure 8b shows the average escape latency across all 25 FR-2 escape trials. The Sedentary/Fluoxetine group differed from all other groups, which did not differ from each other.
Fig. 8.
Effects of exercise on fluoxetine-induced escape deficit. Rats remained sedentary or were allowed 6 weeks of voluntary access to running wheels prior to receiving a single injection of either saline or fluoxetine (10 mg/kg). Rats were placed into shuttle boxes for assessment of fixed-ratio 1 (FR-1) and fixed-ratio 2 (FR-2) escape performance 1 h later. Data are presented as a blocks of five escape trials and b the mean escape latency for all 25 FR-2 escape trials. Data represent means ± SEM. Asterisk p<0.05 relative to sedentary/saline, exercised/saline, and exercised/fluoxetine groups. Numbers in each bar represent the number of rats included in that group
Fluoxetine (10 mg/kg) elevated circulating corticosterone similarly in sedentary and exercised rats (Fig. 9). There was a main effect of drug (F (1, 26)=7.1; p<0.05), but neither the main effect of exercise nor the drug-by-exercise interaction was significant.
Fig. 9.
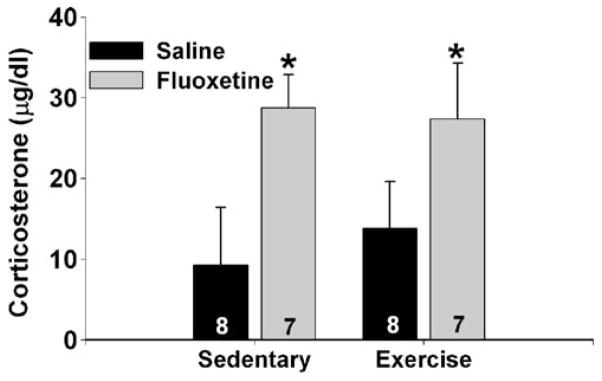
Effects of exercise and fluoxetine on circulating corticosterone. Rats remained sedentary or were allowed 6 weeks of voluntary access to running wheels prior to receiving a single injection of either saline or fluoxetine (10 mg/kg). One hour after fluoxetine administration, rats were sacrificed, and trunk blood was collected for analysis of plasma corticosterone. Data represent means ± SEM. Asterisks p<0.05 relative to saline-treated groups. Numbers in each bar represent the number of rats included in that group
Discussion
Here, we report the novel findings that a single injection of the SSRI fluoxetine produced behaviors similar to, but of a smaller magnitude, than those produced by uncontrollable tail shock stress, namely an exaggeration of shock-elicited freezing and a deficit in shuttle box escape behavior. Similar to other reports demonstrating anxiolytic effects of 5-HT2C receptor blockade (Bagdy et al. 2001; Burghardt et al. 2007; Harada et al. 2006, 2008; Jones et al. 2002; Kennett et al. 1997; Millan et al. 2001), the anxiogenic effects of acute fluoxetine were blocked by pretreatment with the 5-HT2C receptor antagonist SB 242084. Additionally, 6 weeks of wheel running prior to fluoxetine administration reduced the acute behavioral effects of fluoxetine. These results are consistent with the suggestion (Greenwood and Fleshner 2008; Greenwood et al. 2003) that exercise can provide protection against anxiogenic effects of rapid increases in 5-HT. Prior exercise may be effective in reducing the anxiety that can be exacerbated during the onset of pharmacotherapy in clinical patients.
Fluoxetine-induced behaviors
The current results are consistent with prior work indicating that an acute increase in 5-HT is associated with anxiety (Abrams et al. 2005; Graeff et al. 1996; Hackler et al. 2007; Lowry et al. 2005) and can interfere with escape behaviors (Brown et al. 1982). Additionally, anxiety-like behaviors have been reported following a single administration of an SSRI in several other animal models of anxiety including social interaction (Bagdy et al. 2001; To et al. 1999), the elevated plus maze (Kurt et al. 2000), and the free-exploration test (Belzung et al. 2001). Most relevant to the current studies is the work by Burghardt and colleagues showing that a single injection of an SSRI can increase freezing during both the acquisition phase (Burghardt et al. 2004 #1321) and the retention phase of auditory fear conditioning (Burghardt et al. 2004 #1321; Burghardt et al. 2007 #1571). Similar to the 5-HT2C-dependent behavioral effects of fluoxetine reported here, the effect of acute SSRI administration on auditory fear conditioning can also be prevented by 5-HT2C receptor blockade (Burghardt et al. 2007), as can acute SSRI-induced reductions in social interaction (Bagdy et al. 2001).
Neither fluoxetine nor uncontrollable stress affected freezing during 5 min of habituation to the shuttle box prior to receiving foot shocks. Thus, the exaggerated shock-elicited freezing produced by fluoxetine and stress in these experiments likely represents exaggerated fear conditioned to contextual or discrete cues present in the shuttle box and not a non-specific effect on freezing. The deficit in FR-2 escape similarly occurred in the absence of an FR-1 escape deficit, suggesting that neither fluoxetine nor stress impair general motor function at the time points tested. Instead, these treatments likely interfere with the more complex instrumental processing required for successful FR-2 escape learning.
It is of interest to consider specific mechanisms by which 5-HT could contribute to the observed behavioral effects of acute fluoxetine. Similar to the mechanism proposed for the induction of learned helplessness by uncontrollable stressors (Greenwood and Fleshner 2008; Maier and Watkins 2005), fluoxetine-induced internalization of 5-HT1A autoreceptors in the DRN (Riad et al. 2004) could sensitize DRN 5-HT neurons, leading to exaggerated 5-HT responses during behavioral testing. Whether caused indirectly by 5-HT1A autoreceptor internalization in the DRN or by direct 5-HT transporter blockade, excessive 5-HT in the amygdala could potentially increase freezing (Graeff et al. 1996), whereas excessive 5-HT in the periaqueductal gray could interfere with fight or flight responding required for a successful FR-2 escape response (Graeff et al. 1993, 1997). 5-HT in the striatum has also recently been associated with deficits in instrumental learning, although this effect may be mediated by the 5-HT6 receptor (Mitchell et al. 2007). Severe stress has recently been shown to increase 5-HT2C gene expression in the amygdala (Harada et al. 2008), and 5-HT2C receptors are expressed in the amygdala, periaqueductal gray, and striatum (Huang et al. 2007; Pompeiano et al. 1994), further implicating the involvement of these regions in the observed anxiety-like effects of acute fluoxetine. It is also possible that the exaggerated freezing state produced by acute fluoxetine in response to the FR-1 shocks could, itself, interfere with the ability to perform the FR-2 escape response. Indeed, although the FR-2 escape deficit produced by uncontrollable tail shock stress is independent of freezing (Maier 1990), the presence of conditioned freezing is sufficient to interfere with shuttle box escape responding (Greenwood et al. 2006). Regardless of the mechanisms involved, the current data illustrate an anxiogenic effect of acute SSRI administration that is similar to what is observed in human clinical populations and several other animal models.
The observation that a single injection of an SSRI can increase shock-elicited freezing deserves special discussion considering prior work on the effects of acute SSRIs on fear conditioning. As mentioned, the current results are consistent with Burghardt et al. (2004) who report that a single systemic injection of the SSRI citalopram (10 mg/kg) administered 1 h prior to auditory fear conditioning enhances conditioned freezing both during training (similar to the shock-elicited freezing measured in the current studies) and during testing 24 h later. In contrast, however, administration of the same dose of fluoxetine prior to either training (Inoue et al. 1996) or testing (Hashimoto et al. 1996) can reduce freezing to a context paired with foot shocks 24 h earlier. The effects of acute SSRI administration on shock-elicited freezing were not reported in these latter studies.
Burghardt et al.( 2007) have suggested that the contrasting effects of acute SSRIs on fear conditioning could be due to the fact that different brain circuits support auditory vs. contextually conditioned fear. Both the hippocampus and amygdala are critical for the complex contextual processing required for contextual fear conditioning, whereas the amygdala, but not the hippocampus, supports simple unimodal associations and is thus important for auditory fear conditioning (Phillips and LeDoux 1992). The divergent effects of acute SSRIs on auditory and contextual fear conditioning might reflect, therefore, differences in the effects of the SSRI on these brain circuits. In other words, acute SSRI administration might inhibit hippocampal-dependent fear learning and memory while enhancing fear that is not dependent on the hippocampus. The current studies investigated the effects of acute SSRI administration on shock-elicited freezing, that is, freezing expressed immediately following presentation of shocks during contextual fear training. Freezing expressed immediately after shock presentation in this paradigm is supported by the amygdala but not the hippocampus (Kim et al. 1993). Thus, the current observations that SSRI administration increased shock-elicited freezing is consistent with the idea that acute SSRIs can enhance hippocampal-independent fear processes. This interpretation also supports the proposed role of 5-HT1A autoreceptor internalization in mediating the behavioral effects of acute fluoxetine because 5-HT1A autoreceptors are more potent at inhibiting 5-HT neurons in the DRN, which provide the majority of 5-HT afferents to the amygdala, then they are at inhibiting 5-HT neurons in the median raphe nucleus, which more heavily innervate the hippocampus (Lowry 2002; Sinton and Fallon 1988).
Effects of wheel running on fluoxetine-induced behaviors
Rats allowed 6 weeks of voluntary access to running wheels were protected against the exaggerated shock-elicited freezing and escape deficits produced by acute fluoxetine, just as 6 weeks of wheel running protects against similar behavioral consequences of uncontrollable tail shock stress (Greenwood et al. 2003). Interestingly, wheel running reduced the behavioral effects of acute fluoxetine but had no effect on fluoxetine-induced corticosterone. Exercise, therefore, does not globally attenuate the acute effects of fluoxetine. Instead, there seems to be some selectivity to the effects of exercise on the consequences of acute fluoxetine administration.
There are many factors that could contribute to the anxiolytic effects of exercise reported here. Changes in the expression or function of the 5-HT transporter could account for the observed effects of exercise. Wheel running reduces levels of 5-HT transporter mRNA in the raphe nuclei (Greenwood et al. 2005b), but it is unknown if the change in mRNA levels results in a functional change in the 5-HT transporter and, if so, whether this change is restricted to the raphe nuclei or occurs in terminal regions. Another possibility is exercise-induced desensitization of post-synaptic 5-HT receptors important in mediating the effects of fluoxetine such as the 5-HT2C receptor. Indeed, there is some evidence that exercise decreases sensitivity of 5-HT2 receptors in the brains of both humans (Broocks et al. 1999, 2001) and laboratory animals (Dwyer and Browning 2000), although the limited work on the effects of exercise on other post-synaptic 5-HT receptors has been met with mixed results (Chaouloff 1994; Chennaoui et al. 2001; Dey 1994). The effects of exercise on the 5-HT system could depend on whether forced or voluntary exercise was employed. Finally, voluntary wheel running increases mRNA for the 5-HT1A autoreceptor in the rat DRN and the median raphe nucleus (Greenwood et al. 2005b, 2003). This allows for the possibility that exercise could prevent the anxiety-like effects of fluoxetine by increasing 5-HT1A-mediated autoinhibition of 5-HT neurons, thus, reducing 5-HT release in response to foot shocks during behavioral testing in the shuttle box.
Results presented here support a role for 5-HT2C receptors in the anxiogenic effects of acute SSRI administration and suggest that the anxiety that can occur during the onset of clinical treatment with SSRIs could depend on history of physical activity. The effectiveness of exercise participation as an adjunct therapy during the onset of SSRI treatment to reduce potential deleterious behavioral effects associated with acute SSRI administration, including anxiety and non-compliance, warrants further investigation.
Acknowledgments
Supported by NIMH068283 (MF), National Alliance for Research on Schizophrenia and Depression (MF), and American Foundation for Suicide Prevention (BG)
References
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998a;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998b;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12:151–162. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol. 2005;8:357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Bland ST, Twining C, Watkins LR, Maier SF. Stressor controllability modulates stress-induced serotonin but not dopamine efflux in the nucleus accumbens shell. Synapse. 2003;49:206–208. doi: 10.1002/syn.10229. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broocks A, Meyer T, George A, Hillmer-Vogel U, Meyer D, Bandelow B, Hajak G, Bartmann U, Gleiter CH, Ruther E. Decreased neuroendocrine responses to meta-chlorophenylpiperazine (m-CPP) but normal responses to ipsapirone in marathon runners. Neuropsychopharmacology. 1999;20:150–161. doi: 10.1016/S0893-133X(98)00056-6. [DOI] [PubMed] [Google Scholar]
- Broocks A, Meyer T, Gleiter CH, Hillmer-Vogel U, George A, Bartmann U, Bandelow B. Effect of aerobic exercise on behavioral and neuroendocrine responses to meta-chlorophenyl-piperazine and to ipsapirone in untrained healthy subjects. Psychopharmacology (Berl) 2001;155:234–241. doi: 10.1007/s002130100706. [DOI] [PubMed] [Google Scholar]
- Brown L, Rosellini RA, Samuels OB, Riley EP. Evidence for a serotonergic mechanism of the learned helplessness phenomenon. Pharmacol Biochem Behav. 1982;17:877–883. doi: 10.1016/0091-3057(82)90465-8. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F. Influence of physical exercise on 5-HT1A receptor-and anxiety-related behaviours. Neurosci Lett. 1994;176:226–230. doi: 10.1016/0304-3940(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Drogou C, Gomez-Merino D, Grimaldi B, Fillion G, Guezennec CY. Endurance training effects on 5-HT(1B) receptors mRNA expression in cerebellum, striatum, frontal cortex and hippocampus of rats. Neurosci Lett. 2001;307:33–36. doi: 10.1016/s0304-3940(01)01901-2. [DOI] [PubMed] [Google Scholar]
- Day HE, Wolf EM, Herlihy L, Campeau S. The effect of voluntary exercise on the acute HPA axis response to mild stress in rats. Neuroscience meeting planner. Society for Neuroscience Online; Atlanta, GA. 2006. Program No. 563.20. [Google Scholar]
- Dey S. Physical exercise as a novel antidepressant agent: possible role of serotonin receptor subtypes. Physiol Behav. 1994;55:323–329. doi: 10.1016/0031-9384(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain mono-amine levels after footshock. Brain Res Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic–pituitary–adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol. 2006;18:915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic–pituitary–adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Ryan SM, Minor TR, Maier SF. Librium prevents the analgesia and shuttlebox escape deficit typically observed following inescapable shock. Pharmacol Biochem Behav. 1984;21:749–754. doi: 10.1016/s0091-3057(84)80014-3. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Carson SW, Breese GR. Differential effects of chronic antidepressant treatment on swim stress- and fluoxetine-induced secretion of corticosterone and progesterone. J Pharmacol Exp Ther. 1998;285:579–587. [PMC free article] [PubMed] [Google Scholar]
- Dwyer D, Browning J. Endurance training in Wistar rats decreases receptor sensitivity to a serotonin agonist. Acta Physiol Scand. 2000;170:211–216. doi: 10.1046/j.1365-201x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- Fanselow M, Lester L. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and learning. Erlbaum; Hillsdale, NJ: 1988. pp. 185–211. [Google Scholar]
- Feighner JP, Boyer WF. Selective serotonin re-uptake inhibitors: the clinical use of citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. Wiley; New York: 1991. [Google Scholar]
- Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders—III. Tolerability, safety and pharmacoeconomics. J Psychopharmacol. 1998;12:S55–S87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RM. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res. 1993;58:123–131. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–799. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Research. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008 doi: 10.1007/s12017-008-8029-y. in press. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005a;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005b;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Fleshner M. Conditioned helplessness: a modified version of learned helplessness that is resistant to the protective effects of exercise and is dependent upon fear conditioning. Neuroscience meeting planner. Society for Neuroscience Online; Atlanta, GA. 2006. Program No. 564.1/ HH15. [Google Scholar]
- Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci. 2007a;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007b;144:1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, Gore JC, Sanders-Bush E. 5-Hydroxytryptamine2C receptor contribution to m-chlorophenylpiperazine and N-methyl-beta-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J Pharmacol Exp Ther. 2007;320:1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- Harada K, Aota M, Inoue T, Matsuda R, Mihara T, Yamaji T, Ishibashi K, Matsuoka N. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol. 2006;553:171–184. doi: 10.1016/j.ejphar.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Harada K, Yamaji T, Matsuoka N. Activation of the serotonin 5-HT2C receptor is involved in the enhanced anxiety in rats after single-prolonged stress. Pharmacol Biochem Behav. 2008;89:11–16. doi: 10.1016/j.pbb.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Inoue T, Koyama T. Serotonin reuptake inhibitors reduce conditioned fear stress-induced freezing behavior in rats. Psychopharmacology (Berl) 1996;123:182–186. doi: 10.1007/BF02246175. [DOI] [PubMed] [Google Scholar]
- Huang XF, Tan YY, Huang X, Wang Q. Effect of chronic treatment with clozapine and haloperidol on 5-HT(2A and 2C) receptor mRNA expression in the rat brain. Neurosci Res. 2007;59:314–321. doi: 10.1016/j.neures.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hashimoto S, Tsuchiya K, Izumi T, Ohmori T, Koyama T. Effect of citalopram, a selective serotonin reuptake inhibitor, on the acquisition of conditioned freezing. Eur J Pharmacol. 1996;311:1–6. doi: 10.1016/0014-2999(96)00391-3. [DOI] [PubMed] [Google Scholar]
- Jones N, Duxon MS, King SM. 5-HT2C receptor mediation of unconditioned escape behaviour in the unstable elevated exposed plus maze. Psychopharmacology (Berl) 2002;164:214–220. doi: 10.1007/s00213-002-1197-9. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Kent JM, Coplan JD, Gorman JM. Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry. 1998;44:812–824. doi: 10.1016/s0006-3223(98)00210-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kurt M, Arik AC, Celik S. The effects of sertraline and fluoxetine on anxiety in the elevated plus-maze test in mice. J Basic Clin Physiol Pharmacol. 2000;11:173–180. doi: 10.1515/jbcpp.2000.11.2.173. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic–pituitary–adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability, anxiety, and serotonin. Cognit Ther Res. 1998;22:595–613. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: theory and evidence. JEP: Gen. 1976;105:3–46. [Google Scholar]
- Maier SF, Silbert LH, Woodmansee WW, Desan PH. Adinazolam both prevents and reverses the long-term reduction of daily activity produced by inescapable shock. Pharmacol Biochem Behav. 1990;36:767–773. doi: 10.1016/0091-3057(90)90075-s. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Kalman BA, Grahn RE. Chlordiazepoxide micro-injected into the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behav Neurosci. 1994;108:121–130. doi: 10.1037//0735-7044.108.1.121. [DOI] [PubMed] [Google Scholar]
- Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995a;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995b;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Martinsen EW. Physical fitness, anxiety and depression. Br J Hosp Med. 1990;43:194, 196, 199. [PubMed] [Google Scholar]
- Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69–84. [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Reedy CL, Cohen BM, Carlezon WA., Jr Activation of raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol Psychiatry. 2008;63:832–839. doi: 10.1016/j.biopsych.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Dorey G, Casara P, Dekeyne A. Anxiolytic properties of the selective, non-peptidergic CRF(1) antagonists, CP154,526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology. 2001;25:585–600. doi: 10.1016/S0893-133X(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–30. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Glue P. Clinical pharmacology of anxiolytics and antidepressants: a psychopharmacological perspective. Pharmacol Ther. 1989;44:309–34. doi: 10.1016/0163-7258(89)90006-5. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pohl R, Yeragani VK, Balon R, Lycaki H. The jitteriness syndrome in panic disorder patients treated with antidepressants. J Clin Psychiatry. 1988;49:100–104. [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter JJ, Gundlah C, Auerbach SB. Systemic uptake inhibition decreases serotonin release via somatodendritic autoreceptor activation. Synapse. 1995;20:225–233. doi: 10.1002/syn.890200306. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Muggironi M, Parodo V, Papi G, Sari R, Dazzi L, Spiga F, Purdy RH, Biggio G. Opposite effects of short-versus long-term administration of fluoxetine on the concentrations of neuroactive steroids in rat plasma and brain. Psychopharmacology (Berl) 2001;158:48–54. doi: 10.1007/s002130100853. [DOI] [PubMed] [Google Scholar]
- Short KR, Patel MR, Lee SH, Tolarino CA. Uncontrollable stress induced both anxiety and downregulation of dorsal raphe 5-HT1a receptors in rats: both follow the same time course. Soc Neurosc Abstr. 2000;26:2267. [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Fallon SL. Electrophysiological evidence for a functional differentiation between subtypes of the 5-HT1 receptor. Eur J Pharmacol. 1988;157:173–181. doi: 10.1016/0014-2999(88)90380-9. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- To CT, Anheuer ZE, Bagdy G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10:553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]