Abstract
Repeated administration of psychostimulant drugs or stress can elicit a sensitized response to the stimulating and reinforcing properties of the drug. Here we explore the mechanisms in the nucleus accumbens (NAc) whereby an acute restraint stress augments the acute locomotor response to cocaine. This was accomplished by a combination of behavioral pharmacology, microdialysis measures of extracellular dopamine and glutamate, and Western blotting for GluR1 subunit of the AMPA glutamate receptor (AMPAR). A single exposure to restraint stress 3 weeks before testing revealed that enduring locomotor sensitization to cocaine was paralleled by an increase in extracellular dopamine in the core, but not the shell subcompartment of the NAc. Wistar rats pre-exposed to acute stress showed increased basal levels of glutamate in the core but the increase in glutamate by acute cocaine was blunted. The alterations in extracellular glutamate seem to be relevant, since blocking AMPAR by CNQX microinjection into the core prevented both the behavioral cross-sensitization and the augmented increase in cocaine-induced extracellular dopamine. Further implicating glutamate, the locomotor response to AMPAR stimulation in the core was potentiated, but not in the shell of pre-stressed animals, and this was accompanied by an increase in NAc GluR1 surface expression. This study provides evidence that the long-term expression of restraint stress-induced behavioral cross-sensitization to cocaine recapitulates some mechanisms thought to underpin the sensitization induced by daily cocaine administration, and shows that long-term neurobiological changes induced in the NAc by acute stress are consequential in the expression of cross-sensitization to cocaine.
Keywords: psychostimulant and stress, microdialysis, nucleus accumbens core and shell, locomotor activity, AMPA receptor surface expression
1. Introduction
A facilitatory influence of stress is often attributed to the abuse of addictive drugs (O'Doherty, 1991). In support of this clinical observation, animal studies employing various models of drug addiction show that acute or repeated stress augments drug-induced mesocorticolimbic dopamine transmission, as well as the locomotor activating ( Lu et al., 2003; Yap & Miczek, 2008) and rewarding (Lett et al, 1989; Capriles & Cancela, 1999; Vezina et al., 2002; Capriles et al., 2003) effects of cocaine and other addictive drugs. The stress-induced neuroadaptations in mesocorticolimbic dopamine are thought to be initiated by the fact that both addictive drugs and stress increase the release of corticotropin-releasing factor (CRF) into the ventral tegmental area (VTA), which augments the response of dopamine neurons to glutamatergic inputs (Saal et al., 2003; Ungless et al., 2003; Wang et al., 2005).
These adaptations in VTA glutamate transmission by stress are thought to mediate enduring changes in both dopamine and glutamate transmission in the nucleus accumbens (NAc) (Kalivas & Stewart, 1991; Mameli et al., 2009). The NAc is a heterogeneous structure that can be separated histologically into core and shell subdivisions (Pennartz, 1994). Dopamine release in the shell and core is differentially sensitive to drugs of abuse (Di Chiara, 2002) and a role for glutamate within the core subcompartment has been shown in the expression of cocaine-induced behavioral sensitization (Pierce et al, 1996). Regarding cross-sensitization with stress, only the core exhibits sensitized dopamine release in response to d-amphetamine when the interval between restraint stress and d-amphetamine is one week, and blocking NMDA glutamate receptors inhibits restraint stress-induced augmentation in d-amphetamine-mediated dopamine release and locomotor activity (Pacchioni et al., 2007). In parallel with the sensitization studies, cue-, stress- or drug-induced reinstatement of cocaine- or heroin-seeking is associated with increased glutamate and dopamine release into the core (McFarland et al., 2003; McFarland et al., 2004; LaLumiere & Kalivas, 2008). Also, increases in NAc AMPA glutamate receptors (AMPAR) are thought to contribute to the expression of cocaine-induced behavioral sensitization and drug-seeking (Churchill et al., 1999; Boudreau & Wolf, 2005; Conrad et al., 2008; Ghasemzadeh et al., 2009; Schumann & Yaka, 2009). Consistently, the increase in locomotor activity and reinstatement of drug-seeking induced by AMPAR stimulation is augmented after chronic cocaine administration (Pierce et al, 1996; Suto et al., 2004).
Although a role for glutamate transmission in the NAc is well-established in some of the behavioral effects of chronic cocaine administration, the involvement of glutamate in the enduring augmentation of cocaine-induced locomotor activity produced by a single exposure to a stressful life event has not been characterized. The main hypothesis inspiring this study is that sensitization of the motor stimulant properties of cocaine measured 21 days after a single restraint stress is regulated by enhanced glutamate transmission in the core subcompartment of the NAc. To examine this hypothesis, stress-induced long-term changes in dopamine and glutamate release were measured with microdialysis, and a role for AMPAR was determined by behavioral pharmacology and immunoblotting for the AMPAR subunit GluR1 in the NAc core.
2. Materials and Methods
Animals
Adult male Wistar rats (250–350 g) were bred and housed in the Facultad de Ciencias Químicas vivarium. They were maintained at 22°C under a 12 h light/dark cycle, with free access to food and water. At least a week before the beginning of the treatment, rats were separated from the colony group and housed in groups of four in cages of 12 cm x 30 cm x 50 cm. The animals were approximately two months old (+/− one week). All the rats were tested during the light cycle, between 9.00 and 16.00 hs. All procedures were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals as approved by the Animal Care and Use Committee of the Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Argentina.
Stress
The acute stress group was restrained for two hours (anytime between 10:00 a.m. and 2:00 p.m.) in restraining devices while control animals were left undisturbed in their home cages. The Plexiglass cylinders were designed so that the rats’ tails emerged from the rear. The animals appeared healthy as evidenced by their coat texture and slight changes in body weight. Twenty-one days after this single stress episode, all animals were assigned to the behavioral, neurochemical or Western blot surface AMPAR studies.
Surgery
Rats weighing 250–350 g were anesthetized with ketamine and xilazine (55 mg/kg and 11 mg/Kg, respectively) and mounted into a Stoelting stereotaxic instrument with the incisor bar at −3.3 cm above the interaural line. Afterwards, cannulae were implanted according to the coordinates from Paxinos & Watson, (2007). For the microinjection experiments, cannulae (14 mm, 27 gauge stainless steel) were implanted bilaterally in the NAc core (AP: +1.4; ML: +/− 1.5, DV: −4.3) or shell (AP: +1.4; ML: +/−0.8, DV: −4.3). The cannulae were secured in place with two stainless steel screws tapped into the skull, and dental cement. All animals were allowed to recover for 5 or 6 days. For the microdialysis experiments cannulae (20 mm, 27 gauge stainless steel) were implanted unilaterally in the NAc core (AP: +1.4; ML: +/− 1.5, DV: −7.8) or shell (AP: +1.4; ML: +/−0.8, DV: −7.8). The cannulae were secured in place with one stainless steel screw tapped into the skull, and dental cement. All animals were allowed to recover for 18–22 h.
Locomotor Activity
The testing apparatus consisted of eight rectangular cages (30.5 ×19.5 ×46.5 cm) equipped with two parallel infrared photocell beams located 3 cm above the floor. Interruption of either beam resulted in a photocell count. All rats were tested once or twice between 09.00 am and 4.00 pm under white light in a quiet room. They were placed individually in each cage with motor activity counts monitored at 10 min intervals.
Microinjection Procedure
On the challenge day, after one-hour adaptation to the photocell apparatus, the obturators were removed from the microinjection guide cannulae and replaced with an injection needle (30 gauge stainless steel) that extended 2.5 mm from below the tip of the guide cannulae into the core or shell. Bilateral infusions were made over 1 min at a volume of 0.3 µl/side (experiment 5) or 0.5 µl/side (experiment 6). Twenty seconds later, the injector was removed and the rat was returned to the photocell cage.
Microdialysis Probe Construction, Implantation and Collection of Dialysates
A vertical concentric dialysis probe was prepared with AN69 fibers (Hospal, Bologna Italy) according to the method of Di Chiara et al., (1993) with minor modifications. The length of the active dialyzing area for the NAc core and shell was 2.0 mm. The probe was fastened to the skull with glass polyalkenoate (ionomer) cement (Meron; Voco GmbH, Cuxhaven, Germany). After surgery was performed as described above, all rats were placed in individual acrylic bowls and allowed to recover for at least 18 h. The day following surgery, the dialysis membrane was perfused with Ringer’s solution (NaCl 145 nM, KCl 4.0 nM, CaCl2 2.2 nM) at a constant flow rate of 1µl/min. Samples of the dialysate were automatically collected every 30 min in vials kept at 4°C in a refrigerated fraction collector.
HPLC System for Dopamine Quantification
The perfusate was assayed for dopamine content by reverse-phase HPLC coupled with electrochemical detection (ESA Coulochem II). The mobile phase was composed of 50 mM NaH2PO4; 5 mM Na2HPO4; 0.1 mM EDTA-Na; 0.5 mM n-octyl-sodium sulphate; and 12% methanol; pH was adjusted to 5.5. The mobile phase was delivered by a pump (Model 582, solvent delivery model; ESA, Chelmsford, MA) at a flow of 1 ml/min through a RP 18 column (C18, 125-4.6 mm, 5 mm). Samples were injected via a 20 µl injection loop. Dopamine was detected using a coulometric detector consisting of three electrodes: a guardcell (+350 mV), an oxidation analytical electrode (+175 mV), and a reduction analytical electrode (-175 mV). Peaks were recorded, and height measured by a computer using an ESA Chromatography Data System. The obtained values were compared with an external standard curve.
HPLC System for Glutamate Quantification
The perfusate was assayed for glutamate content by reverse-phase HPLC coupled with electrochemical detection (ESA Coulochem III). The mobile phase was composed of 100 mM Na2HPO4, 1.75% acetonitrile and 15 % methanol; pH was adjusted to 6.67 with phosphoric acid. The mobile phase was delivered by a pump (Model 582, solvent delivery model; ESA, Chelmsford, MA) at a flow of 0.5 ml/min through a Waters Xterra MS (15 cm × 4.6 mm; 3.0 µm). The released glutamate was measured by derivatization with OPA/OME, as described by Donzanti & Yamamoto, (1988). In brief, 15 µl of derivatizing reagent (OPA/OME dissolved in tetraborate buffer) were mixed with 20 µl of the microdialysis eluate. After 2 min, it was injected into the HPLC. OPA/OME: o-phthalaldehyde and o-βmercapto ethanol. Samples were injected via a 20 µl injection loop. Glutamate was detected using a coulometric detector consisting of three electrodes: a guardcell (+650 mV), an oxidation analytical electrode (+150 mV), and a reduction analytical electrode (+550 mV). Peaks were recorded, and the height measured by a computer using an ESA Chromatography Data System. The obtained values were compared with an external standard curve.
Determination of Basal Levels of Extracellular Glutamate
The basal concentration of extracellular glutamate was determined by adding glutamate to the dialysis perfusate at concentrations above and below the expected extracellular concentration to generate a series of points that were interpolated to measure the concentration at which no-net-flux of glutamate occurred across the dialysis membrane. Perfusion of dialysis buffer was begun in the morning and 2.30 hs later, 0, 2.5, 5.0 and 10.0 µM glutamate was advanced through the probe. Four 30-minute dialysis samples were obtained at each concentration of glutamate, and the last three samples were averaged for a determination of the net flux of glutamate.
AMPA Receptor Expression
Surface Biotinylation
Animals were sacrificed at 45 min after a cocaine or saline challenge injection because our previous study found that cocaine elicited an increase in GluR1 at this time in rats that were pretreated with chronic stress (Esparza et al., 2012). The dissected pieces of tissue contained both core and shell subregions of the NAc, although there was relatively more core tissue. NAc tissue was pooled from two animals to obtain enough material to quantify GluR1 surface expression (n=6/group). The dissected area was transferred to ice-cold sulfo-NHS-LC-biotin (Pierce) in PBS (0.3 mg/ml) and incubated for 1 h, then rinsed in cold Tris-glycine to quench free biotin (5 min) followed by washes with ice-cold TBS (5 min, 3×). Microdissected NAc were homogenized in 200 µl of RIPA buffer (150 mM NaCl, 10 mM NaH2PO4, 2 mM EDTA, 50 mM NaF, 10 mM Na-pyrophosphate, 10 mM Na-iodoacetamide, 1 mM Na-orthovanadate, 1% Triton X-100, 0.5% SDS, and 0.5% deoxycholate). An additional 100 µl of homogenization buffer was added to obtain 300 µl of total slice homogenate. Homogenates were centrifuged at 13,000 × g for 30 min to pellet the insoluble fraction. For the total fraction of GluR1 (surface plus internal), 50 µl of the supernatant was mixed and heated with 12.5 µl of 4xSDS sample buffer. Biotinylated surface proteins in the remaining supernatant (200 µl) were immunoprecipitated with 50 µl of 50% avidin-agarose beads (ImmunoPure Immobilized Avidin; Pierce) for 2 h at 4°C. The beads were pelleted, and 150 µl of the supernatant (internal fraction) was mixed and heated with 37.5 µl of 4×SDS sample buffer. The beads were then rinsed three times with ice-cold TBS and heated in 50 µl of 2×SDS sample buffer (surface fraction). The surface fraction was subjected to quantitative immunoblotting for AMPAR using anti-GluR1 (AB 1504, Millipore). In the AMPAR plasma membrane expression study, plasma membrane-associated AMPAR was defined as the surface fraction of SDS sample buffer eluent collected from avidin beads, normalized to the total fraction of AMPAR (surface plus internal, which represents total GluR1 present prior to avidin bead addition) in each sample.
Drug and Antibodies
Cocaine was purchased from Verardo Laboratories (Buenos Aires, Argentina). A rabbit monoclonal anti-GluR1 was kindly provided by Dr Peter Kalivas (1:400; Millipore). The secondary antibody was a peroxidase conjugated anti-rabbit (1:2000; Jackson Laboratories, Baltimore Pike, PA).
Experimental Procedure
Experiment 1: response to cocaine
Locomotor activity was registered in response to saline or cocaine (5; 10; or 15 mg/kg i.p.) in both non-stressed and stressed rats for 2 h, after a 1 h habituation period. The number of rats in each group (non-stress and stress) was 8.
Experiments 2 and 3: dopamine and glutamate dialysis, respectively
After collecting the dialysate for 90 min to determine baseline data (four consecutive samples differing by no more than 10%), both non-stressed and stressed rats were injected with saline and samples were collected for 120 min. This was followed by a cocaine injection (15 mg/kg i.p.) and samples were collected for another 180 min. This microdialysis protocol was applied to both core and shell. The number of rats in each group (non-stress and stress) was 6–10.
Experiment 4: no-net-flux in core and shell
After collecting the dialysate for 120 min to determine the baseline data (five consecutive samples differing by no more than 10%), both non-stressed and stressed rats were perfused in the dialysis buffer with three escalating concentrations of glutamate (2.5; 5.0 and 10.0 µM). These glutamate perfusions were administered sequentially in ascending order through the probe into the core and shell during 120 min at each concentration. The number of rats in each group (non-stress and stress) was 6–10.
Experiment 5: locomotor response to AMPA
Non-stressed and stressed rats were habituated to the photocell apparatus for 60 min. Thereafter, locomotor activity was recorded for 120 min in response to a saline or AMPA (0.03 or 0.10 µg/side) microinjection in either the core or the shell. The number of rats in each group (non-stress/vehicle, non-stress/AMPA, stress/vehicle, stress/AMPA) was 5–7.
Experiment 6: CNQX pretreatment
Non-stressed and stressed rats were habituated to the photocell apparatus for 60 min and microinjected in the core with DMSO (0.1; 1.0; 10.0%) or CNQX (0.01; 0.1; 1.0 nmol/side). Five minutes later, they received a saline or cocaine (15 mg/kg i.p.) injection and were returned to the photocell apparatus where motor activity was monitored for an additional 120 min. Using a minimum of three days intervals, each rat received a maximum of two combinations of DMSO or CNQX, and saline or cocaine injections. The drug treatments were made in random order using a cross-over design, including: 1) DMSO microinjection in the core plus systemic saline, followed two days later by a DMSO microinjection in the core and systemic cocaine (15 mg/kg i.p.), or 2) CNQX microinjection in the core plus systemic saline, followed two days later by a CNQX microinjection in the core and systemic cocaine (15 mg/kg i.p.). The number of rats in each group (non-stress/vehicle, non-stress/CNQX, stress/vehicle, stress/CNQX) was 5–8.
Experiment 7: dopamine dialysis in the core after CNQX
After the collection of baseline data for 120 min, all rats were perfused in the dialysis buffer with DMSO (10%) or CNQX (1.0 nM). The animals were then injected with saline and 120 min later challenged with cocaine (15 mg/kg i.p.). Samples were collected for 180 min. The number of rats in each group (non-stress/vehicle, non-stress/CNQX, stress/vehicle, stress/CNQX) was 7–8.
Experiment 8: AMPAR surface expression
The animals were sacrificed 45 min after an acute injection of saline or cocaine (15mg/kg i.p.) and the NAc, including both core and shell, dissected to examine the expression of AMPAR. The number of rats in each group (non-stress/saline, non-stress/cocaine, stress/saline, stress/cocaine) was 6.
Histology
At the end of the microdialysis and microinjection experiments, and in order to verify the probe and cannulae placements, animals were anesthetized with choral hydrate, brain dissected and then fixed with a formalin solution (10%). All brains were sectioned on a freezing microtome in serial coronal slices (60 µm). The location of the probes and cannulae was reconstructed, and positioned referring to the Paxinos & Watson, (2007) atlas. All animals whose probe traces were found located outside the target area were discarded.
Data Analysis
The locomotor and microdialysis data were analyzed by two-way ANOVA with repeated measures over time (Fig. 1B, C, D and E; 2; 4C, D, E and F; 5B, C, D and E; 7), and total photocell counts data were analyzed by two-way ANOVA with repeated measures over dose (Fig. 1A; Fig. 4A and B; 5A). The western blot data were evaluated using two-way ANOVA (Fig. 8). All ANOVAs were followed by a Bonferroni test for post-hoc comparisons. The no-net-flux data were statistically evaluated using an unpaired two-tailed t-test (Fig. 3A and B).
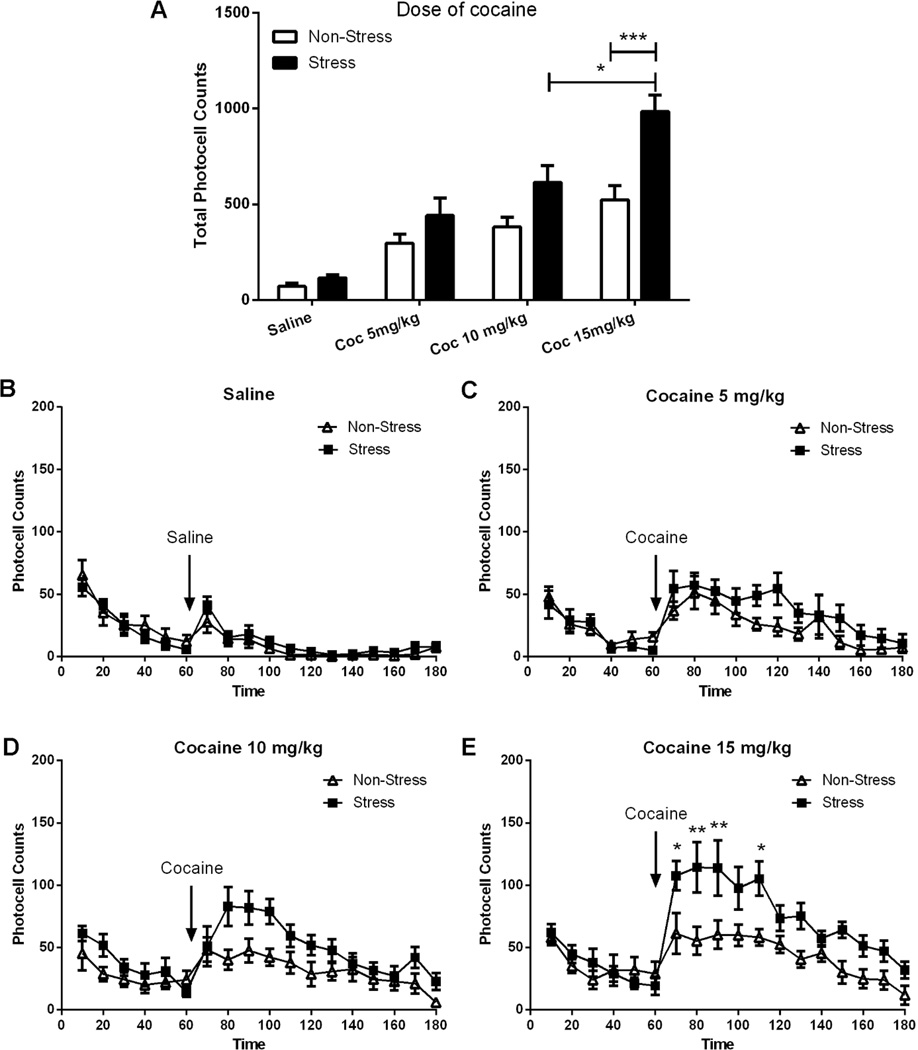
Figure 1. Locomotor activity in response to cocaine (0–15 mg/kg i.p.) in non-stressed and stressed rats.
The number of rats in each group (non-stress and stress) were 8. Horizontal photocell counts are represented as the mean +/− SEM. A Total horizontal photocell counts 120 min after the injection of saline or cocaine (5, 10 or 15 mg/kg), the data were analyzed by a two-way ANOVA (non-stressed/stressed x dose) interaction, F(3,55)=3.83, p<0.05; treatment, F(1,55)=23.31, p<0.001; dose, F(3,55)=36.87, p<0.001; Bonferroni post-hoc test revealed a significant difference comparing stress to control at cocaine 15 mg/kg, **p<0.001. T-test(7)=2.65, p<0.05, showed significant difference at cocaine 15 mg/kg in stress group. B Time course of the horizontal photocell counts for saline-injected animals. Time-frame from 10 to 60 min corresponds to the acclimatation period before saline administration at 70 min. C Time-course for cocaine-injected rats (5 mg/kg). D Time-course corresponding to cocaine-injected rats (10 mg/kg). E Time-course for cocaine-injected rats (15 mg/kg). Locomotor data were analyzed by a two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Interaction, F(17,238)=2.47, p<0.01; treatment, F(1,238)=11.88, p<0.01; time, F(17,238)=12.00, p<0.001; **p<0.01 and *p<0.05 comparing stress to control using a Bonferroni post-hoc test.
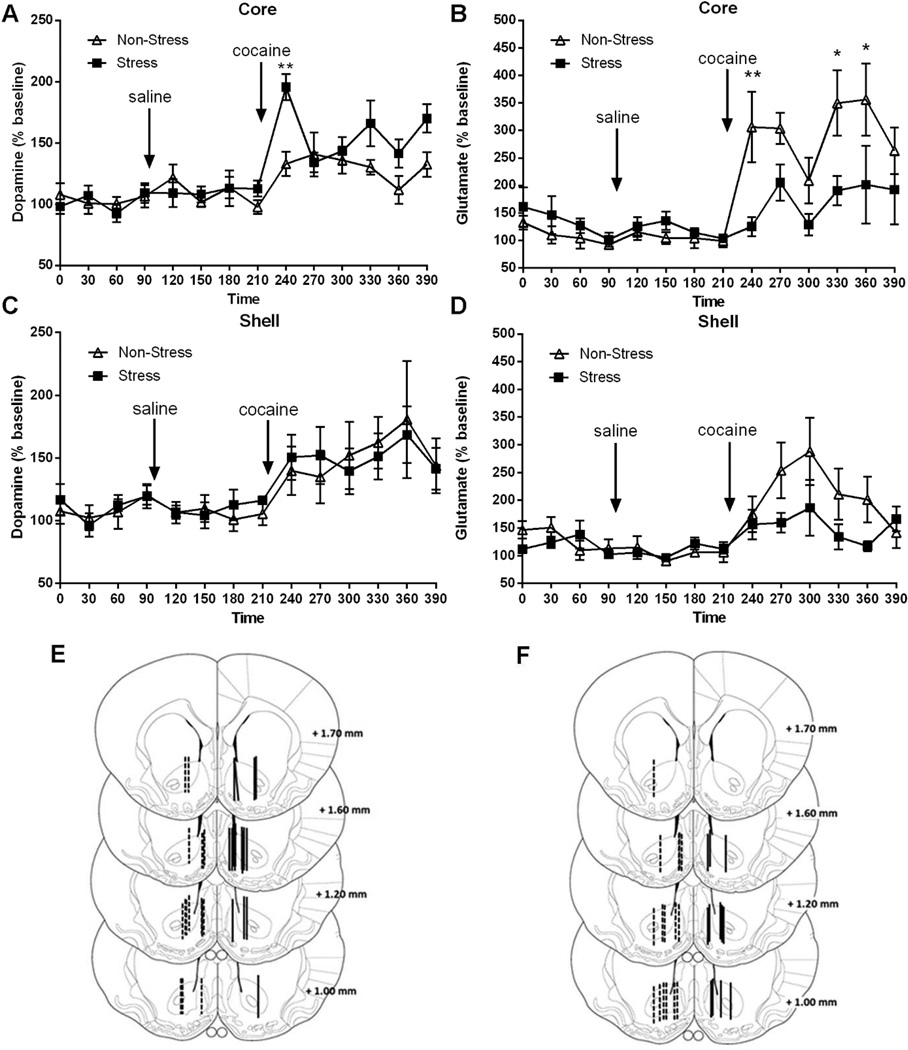
Figure 2. Extracellular dopamine and glutamate levels in Core and Shell measured by microdialysis in response to saline or cocaine (15 mg/kg).
The number of rats in each group (non-stress and stress) was 6–10. Data are expressed as percent of change from baseline. A-B Dopamine and glutamate levels in Core. Baseline: measurements were made from 0 to 90 min. Saline were administered at 90 min, while cocaine were injected at 210 min. Samples were collected every 30 min at 1.0 µl/min. The data were analyzed by a two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Dopamine: interaction, F(13,208) =2.49, p<0.001; treatment, F(1,208)=5.08, p<0.05; time, F(13,208)=9.77, p<0.001; ***p<0.001, comparing stress to control using a Bonferroni post-hoc test. Glutamate: interaction F(13,169)=2.79, p<0.01; treatment, F(1,169)=6.10, p<0.05; time, F(13,169)=7.94, p<0.001; *p<0.05, **p<0.01 comparing pre-stress to control using a Bonferroni post-hoc test. C-D Dopamine and glutamate levels in Shell. There were no significant differences between treatments. E-F Summary illustration of the location of the active membrane of the dialysis probe in the Core and Shell for dopamine and glutamate, respectively, according to Paxinos & Watson, 2007. The dashed lines represent cannula placements in non-stress group and solid lines depict placements in stress group. Basal dopamine levels were equivalent between pre-stress and control animals (Core: non-stress baseline=26.44 ± 2.23 fmol/sample; stress baseline=29.43 ± 1.73 fmol/sample. Shell: non-stress baseline=25.70 ± 1.00 fmol/sample; stress baseline=30.72 ± 1.92 fmol/sample). Basal glutamate levels were significantly different in Core, but not Shell, between pre-stress and control animals (Core: non-stress baseline=8.13 ± 0.84 pmol/sample; stress baseline=15.46 ± 2.30 pmol/sample, t(59)=3.18, p<0.005. Shell: non-stress baseline=11.72 ± 2.35 pmol/sample; stress baseline=15.32 ± 3.15 pmol/sample).
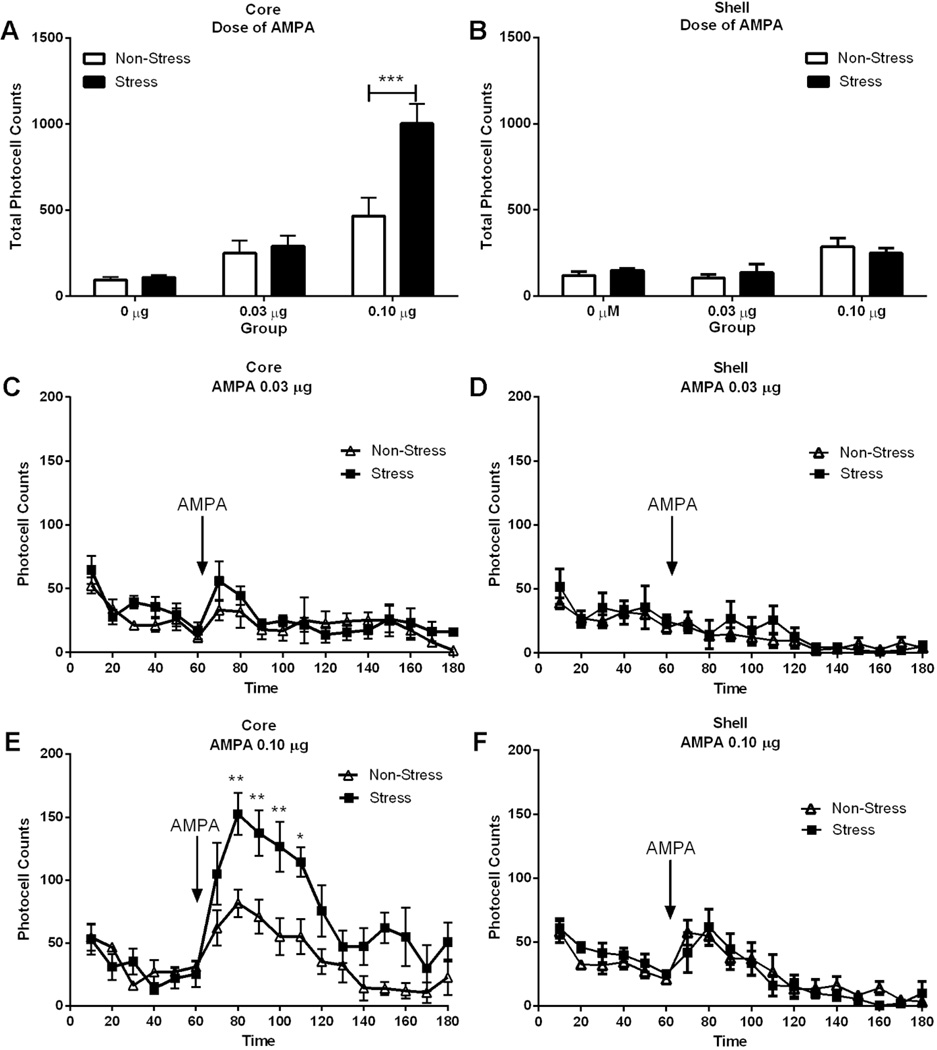
Figure 4. AMPA-induced locomotor activity is augmented in pre-stress rats.
The number of rats in each group (non-stress/vehicle, non-stress/AMPA, stress/vehicle, stress/AMPA) were 5–7. Data are represented as mean +/− SEM horizontal photocell counts. A-B Total horizontal photocell counts 120 min after the AMPA microinjection. Data were analyzed by a two-way ANOVA (non-stressed/stressed x dose) with repeated measure over dose. Core: interaction, F(2,39)=12,47; p<0.001; treatment F(1,39)=18,12, p<0.001; dose, F(2,39)=68.29, p<0.001; ***p<0.001, comparing pre-stress with control using a Bonferroni post-hoc. Shell: dose, F(2,32)=12,17; p<0.001. C-D Time-course of locomotor activity shown in the top panel corresponding to 0.03 µg AMPA in Core and Shell. There were no significant differences between treatments. Time-frame: 10 to 60 min acclimation before AMPA administration at 70 min. E-F Time-course of the locomotor activity shown in the top panel corresponding to 0.10 µg AMPA in Core and Shell. Data were analyzed by a two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Core: interaction, F(17,136)=2.74, p<0.001; treatment, F(1,136)=14.20, p<0.01; time, F(17,136)=12.42, p<0.001; *p<0.05, **p<0.001, comparing pre-stress with control using a Bonferroni post-hoc. Shell: the data analyzed did not reveal significant differences between both stress and non-stress groups.
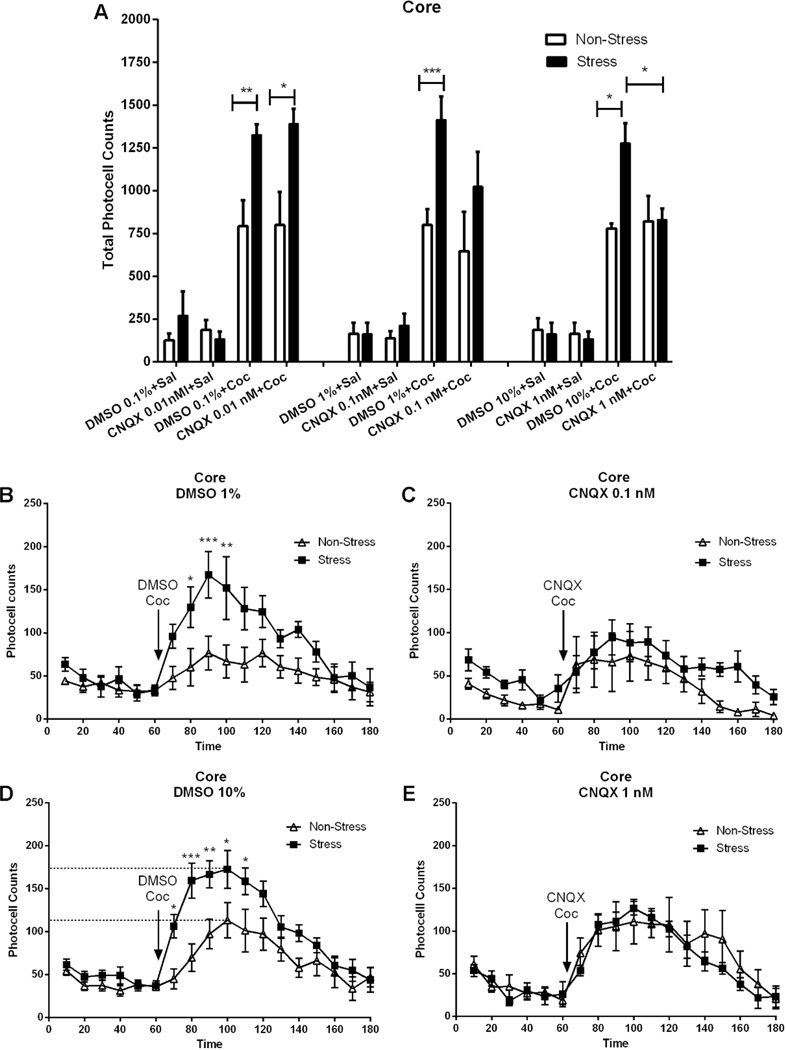
Figure 5. Blockade of AMPA receptors prevents the augmented locomotor response to cocaine in pre-stress animals.
The number of rats in each group (non-stress/vehicle, non-stress/CNQX, stress/vehicle, stress/CNQX) is 5–8. Data are shown as the mean +/− SEM horizontal photocell counts. A Total horizontal photocell counts 120 min after DMSO microinjection (0.1, 1.0, 10 %) or CNQX (0.01, 0.1, 1.0 nmol) associated with a saline or cocaine (15 mg/kg i.p.) injection. Data were analyzed by a two-way ANOVA (non-stressed/stressed x dose) with repeated measures over dose. Interaction, F(11,91)=2.69, p<0.01; treatment, F(1,91)=20.80, p<0.0001; dose, F(11,91)=26.87, p<0.0001; *p<0.05, **p<0.01, ***p<0.001, comparing stress to control using a Bonferroni post-hoc test. T-test(5)=3.48, p<0.05, showed significant difference between the stress group at DMSO (10 %) and CNQX (1 nmol). B Time-course of the horizontal photocell counts shown in the top panel corresponding to DMSO 1 % plus cocaine. Time-frame 10 to 60 min corresponds to the acclimation period before DMSO plus cocaine administration at 70 min. Data were analyzed by a two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Interaction, F(17,170)=2.33, p<0.01; treatment, F(1,170)=10.13, p<0.01; time, F(17,170)=8.49, p<0.001; *p<0.05, **p<0.01, ***p<0.001 comparing stress to control using a Bonferroni post-hoc test. C Time course of the horizontal photocell counts shown in the top panel corresponding to CNQX 0.1 nmol plus cocaine. Time-course: 10 to 60 min corresponds to the 60 min corresponds to the acclimatation period before CNQX plus cocaine administration at 70 min. D Time-course of the horizontal photocell counts shown in the top panel corresponding to DMSO 10 % plus cocaine. Data were analyzed by a two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Interaction, F(17,221)=2.58, p<0.001; treatment, F(1,221)=11.49, p<0.01; time, F(17,221)=18.42, p<0.001; *p<0.05, **p<0.01, ***p<0.001 comparing stress to control using a Bonferroni post-hoc test. E Time-course of the horizontal photocell counts shown in the top panel corresponding to CNQX 1 nmol plus cocaine.
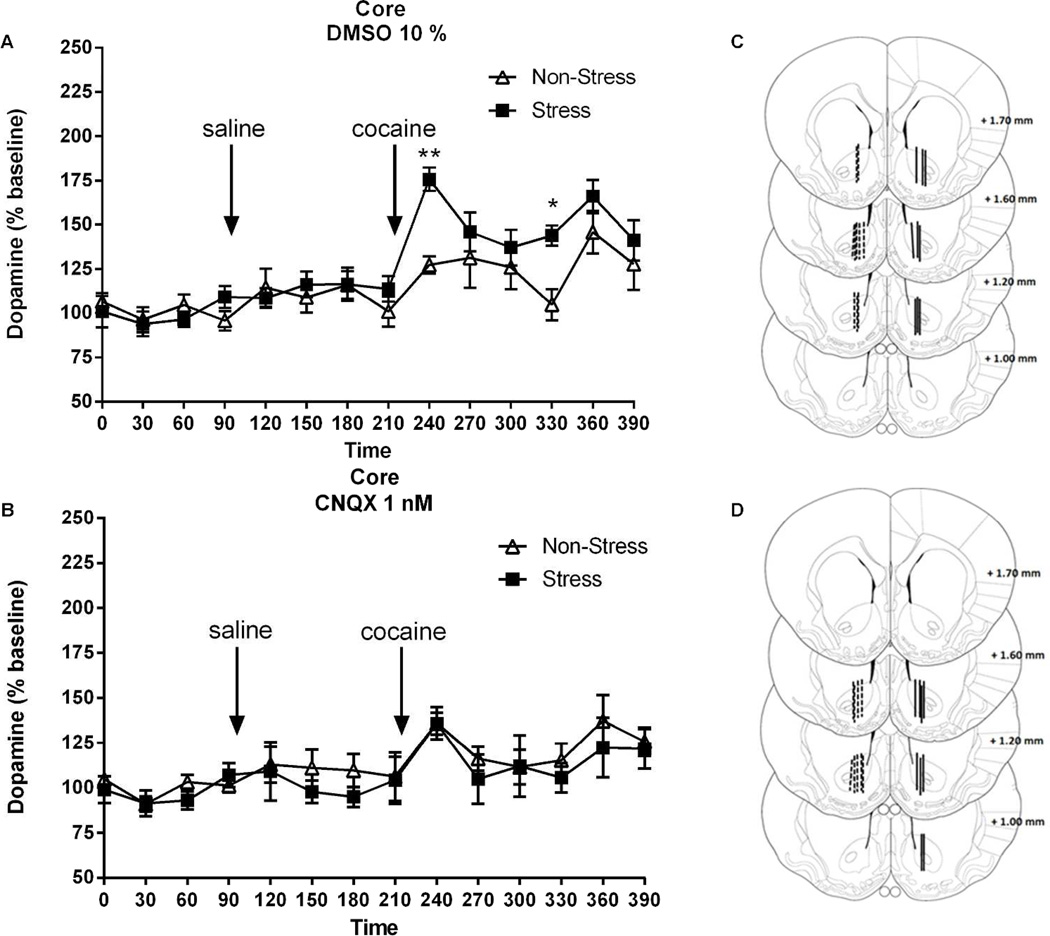
Figure 7. Blockade of AMPA receptors in the core prevents the augmented rise in extracellular dopamine elicited by cocaine.
The number of rats in each group (non-stress/vehicle, non-stress/CNQX, stress/vehicle, stress/CNQX) were 7–8. Data were normalized to percent change from baseline. A Core: time-frame 0 to 90 min corresponds to the acclimation period before DMSO plus saline administration at 120 min. The arrow pointing at 240 min denotes the time of DMSO plus cocaine (15 mg/kg) administration. Data were analyzed by two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Interaction, F(13,195)=1.85, p<0.05; treatment, F(1,195)=4.99, p<0.05; time, F(13,195)=10.28, p<0.001; *p<0.05, **p<0.01, comparing pre-stress with control using a Bonferroni post-hocB Core: time-frame 0 to 90 min corresponds to the adaptation before the CNQX plus saline administration at 120 min. The 240 min correspond to the CNQX (1 nM) plus cocaine (15 mg/kg) administration. Data were analyzed by two-way ANOVA (non-stressed/stressed x time) with repeated measures over time. Time, F(13,221)=3.76, p<0.0001. C-D Summary example of the location of the active membrane of the dialysis probe in the core for dopamine according to Paxinos & Watson, 2007 after DMSO or CNQX respectively. The dashed lines represent cannula placements in non-stress group and solid lines depict placements in stress group.
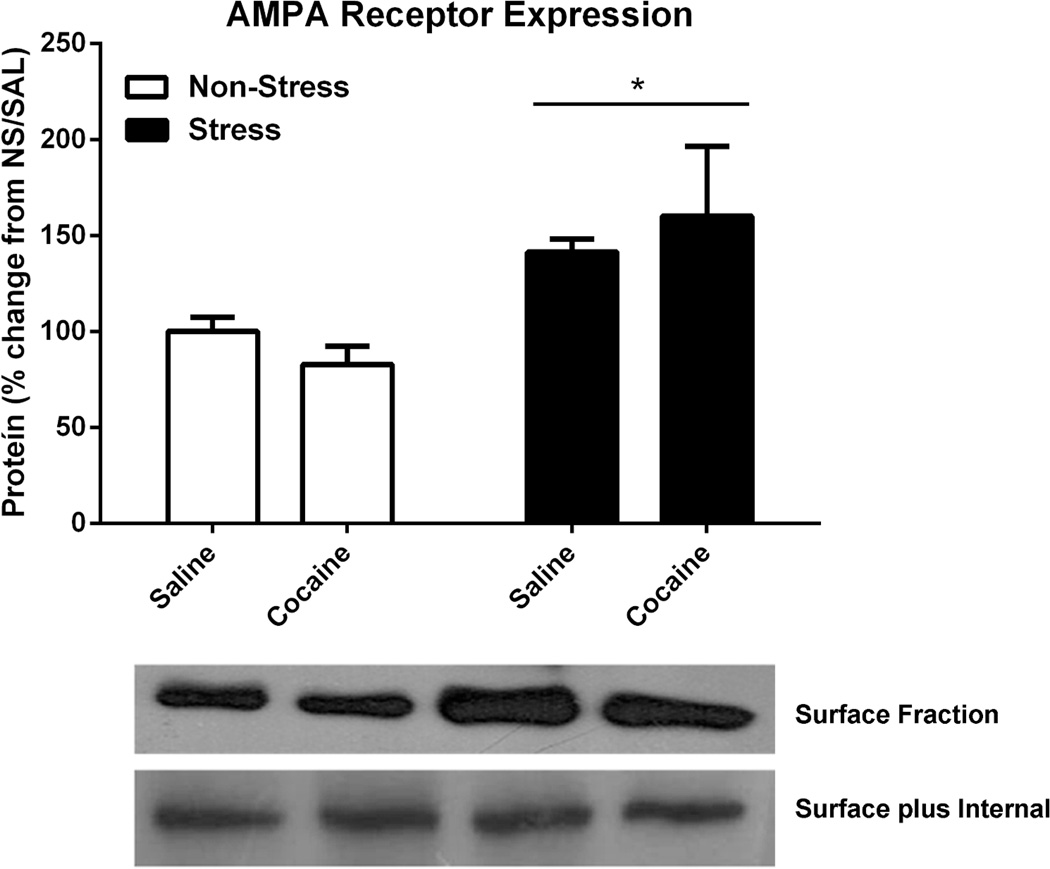
Figure 8. AMPA receptor expression in nucleus accumbens.
Three weeks after acute stress, rats were injected with saline or cocaine (15 mg/kg, i.p.), sacrificed 45 min later and the expression of AMPA surface expression were examined. The number of rats in each group (non-stress/saline, non-stress/cocaine, stress/saline, stress/cocaine) were 6. The results were analysed by a two-way ANOVA and a significant main effect of stress vs no stress were measured F(1,16)=9,254, p<0.01. *p<0.05 comparing pre-stress and non-stress groups.
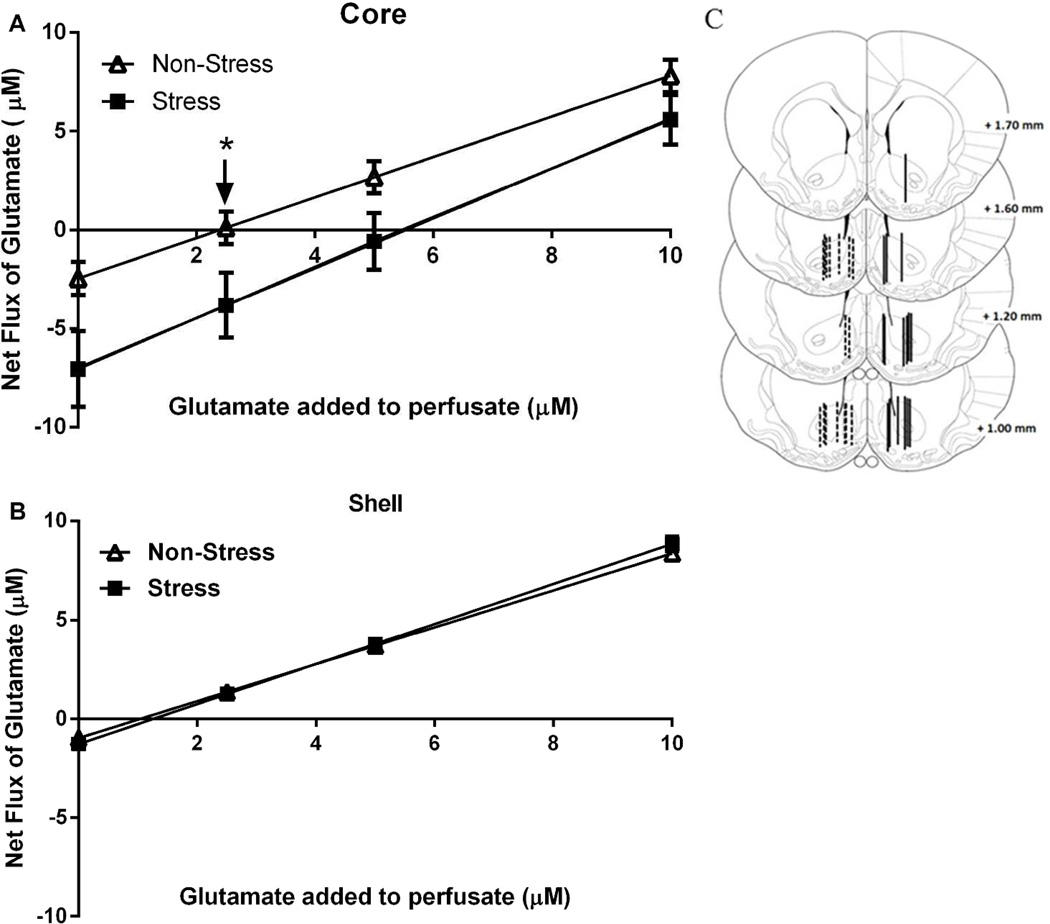
Figure 3. No-Net-Flux microdialysis in the Core and Shell reveals that acute stress augmented the basal levels of extracellular glutamate.
Plots from the no-net-flux in vivo microdialysis experiment. Net flux (y axis) is the difference between the glutamate concentration dialyzed into and recovered from the brain. The number of rats in each group (non-stress and stress) were 6–10 A Core: the x-intercept corresponding to the extracellular glutamate concentration were extrapolated from fitted regression lines and differed between treatment groups: non-stress: 2.09 ± 0.63 µM and stress 5.33 ± 1.24 µM. Data were analyzed by a t-test(16)=2.148, *p<0.05. The slopes of the regression line were not different between the two groups: non-stress: 1.03 ± 0.10 and stress: 1.26 ± 0.21. B Shell: neither the basal concentrations (non-stress: 1.04 ± 0.11 µM and stress: 1.23 ± 0.19 µM) nor the slope of the regression line (non-stress: 0.94 ± 0.04 and stress: 1.02 ± 0.03) differed between the two groups. C Illustration of the location of the active membrane of the dialysis probe in the Core and Shell according to Paxinos & Watson, 2007. The dashed and solid lines represent cannula placements in non-stress group and stress group, respectively.
3. Results
Long Term Stress-Induced Sensitization to Cocaine is Dose Dependent
Figure 1A–E shows a dose-response analysis of the locomotor stimulating properties of cocaine (5, 10, or 15 mg/kg i.p.) in rats receiving acute immobilization stress 21 days earlier, compared with sham non-stressed animals. The motor response to cocaine was augmented in the pre-stressed animals in a dose-dependent manner, while no difference was observed in the response to saline between the stress and non-stress groups, as revealed by analysis of total photocell counts over 120 min after injection (Fig. 1A, interaction F(3,51)=3.23, p<0.05; treatment, F(1,51)=19.67, p<0.001; dose, F(3,51)=23.84, p<0.001), and after time-course analysis of the response to 0, 5, 10 or 15 mg/kg in the stress and non-stress groups (Dose 10 mg/ kg: Fig. 1D, interaction, F(17,187)=1.88, p<0.05; treatment, F(1,187)=5.93, p<0.05; time, F(17,187)=5.53, p<0.001; Dose 15 mg/ kg: Fig. 1E, interaction, F(17,221)=3.16, p<0.001; treatment, F(1,221)=12.57, p<0.01; time, F(17,221)=9.42, p<0.001). Bonferroni post-hoc comparisons applied to the total photocell counts indicated that a dose of 10 mg/kg significantly enhanced the motor stimulant effect, and that the response to 15 mg/kg was further augmented in pre-stressed compared with control subjects. In contrast, there was no difference in the response to saline or to 5 mg/kg in pre-stressed and non-stressed animals (Fig. 1A). Bonferroni post-hoc comparisons applied to the time-course data indicated that, after 10 mg/kg, a significant difference between the stress and non-stress groups (Fig. 1D) was observed at 100 min, whereas after 15 mg/kg the significant differences were observed at 80, 90 and 110 min (Fig. 1E).
Cocaine-Induced Dopamine but not Glutamate is Augmented in the Core by Acute Stress
Extracellular dopamine and glutamate were measured in the core and shell in order to determine if acute stress induces cross-sensitization in the capacity of cocaine injection to increase dopamine. All animals were injected with saline and 90 min later with cocaine (15 mg/kg, i.p.), and dialysis samples collected for an additional 180 min. Figure 2A shows that the increase in dopamine levels in the core was higher in pre-stressed animals compared with controls during the first 30 min after cocaine administration (interaction, F(13,208)=2.49, p<0.001; treatment, F(1,208)=5.08, p<0.05; time, F(13,208)=9.77, p<0.001). Bonferroni post-hoc comparisons showed a significant increase in the percentage of basal dopamine output at 30 min in stressed rats following a cocaine injection, compared to baseline levels and to values in the non-stress group. Interestingly, the increase in glutamate levels by cocaine was blunted in the core of pre-stress versus control subjects (Fig. 2B, interaction F(13,169)=2.79, p<0.01; treatment, F(1,169)=6.10, p<0.05; time, F(13,169)=7.94, p<0.001). Bonferroni post-hoc comparisons indicated a significant difference at 30, 120 and 150 min after cocaine between pre-stressed and non-stressed animals. In contrast with the core, figure 2C shows that dopamine levels in the shell increased after cocaine, but there was no difference between pre-stressed and non-stressed animals. Pre-stressed animals also showed a trend towards lower levels of cocaine-induced glutamate in the shell (Fig. 2D). A saline injection did not modify either dopamine or glutamate release in non-stressed or in stressed animals in any of the brain areas studied. Figure 2E and F show the location of dialysis probes in the core and shell, respectively. The basal dopamine levels were equivalent between pre-stress and control animals (Core: non-stress baseline=26.44 ± 2.23 fmol/sample; stress baseline=29.43 ± 1.73 fmol/sample. Shell: non-stress baseline=25.70 ± 1.00 fmol/sample; stress baseline=30.72 ± 1.92 fmol/sample). Basal glutamate levels were significantly different in Core, but not Shell, between pre-stress and control animals (Core: non-stress baseline=8.13 ± 0.84 pmol/sample; stress baseline=15.46 ± 2.30 pmol/sample, t(59)=3.18, p<0.005. Shell: non-stress baseline=11.72 ± 2.35 pmol/sample; stress baseline=15.32 ± 3.15 pmol/sample).
Acute Stress Pretreatment Increased Basal Glutamate in Core but not Shell
No-net-flux microdialysis revealed that acute stress administered 21 days earlier produced an enduring increase in basal extracellular glutamate levels in the core (t-test(16)=2.148, *p<0.05), while no effect was measured in the shell (see X-intercepts in Fig. 3A–B). The slopes of the regression lines were equivalent between treatment groups, indicating equivalent probe recovery.
Pre-stress Potentiated the Motor Response to AMPAR Activation in the Core but not the Shell
Figure 4 demonstrates that AMPA administered in the core, but not the shell, 21 days after acute stress exposure, induced a greater increase in locomotor activity compared with the non-stress group. A significant augmentation in locomotor activity was observed only after the highest dose of AMPA (0.10 µg) into the core, and is revealed by an analysis of the total photocell counts over 120 min after injection (Fig. 4A, interaction, F(2,39)=12,47; p<0.001; treatment F(1,39)=18,12, p<0.001; dose, F(2,39)=68.29, p<0.001; Bonferroni post-hoc test indicated that the stress group shows significantly higher total photocell counts at the highest dose of AMPA compared to the non-stress group) and after time-course analysis (Fig. 4E, interaction, F(17,136)=2.74, p<0.001; treatment, F(1,136)=14.20, p<0.01; time, F(17,136)=12.42, p<0.001; Bonferroni post-hoc test indicated that the pre-stressed animals shows significantly higher photocell counts at 80, 90, 100 and 110 min compared to the non-stress group).
Blocking AMPAR in the Core Prevents the Capacity of Pre-stress to Augment Cocaine-induced Motor Activity Increases in Extracellular Dopamine
Since the motor response to both cocaine and intra-core AMPA was augmented by pre-stress, the possibility that blocking AMPAR prevents the augmentation of cocaine-induced locomotion and extracellular dopamine was examined. Figure 5A shows the total horizontal photocell counts over 120 min after DMSO (0.1, 1.0, 10%) or CNQX (0.01, 0.1, 1.0 nmol) microinjection into the core associated with a saline or cocaine (15 mg/kg i.p.) injection. While cocaine increased motor activity in all treatment groups, the highest dose of CNQX prevented the augmented response in pre-stressed animals (interaction, F(11,91)=2.69, p<0.01; treatment, F(1,91)=20.80, p<0.001; dose, F(11,91)=26.87, p<0.001). A Bonferroni post-hoc test indicated that the pre-stressed animals show significantly higher total photocell counts at all doses of DMSO as well as at the lower dose of CNQX, compared to the non-stress group. A t-test revealed that the highest dose of CNQX abrogated the enhanced response in pre-stressed animals compared to that observed after DMSO in the stress group (t-test(5)=3.48, p<0.05). The time course data in figure 5C and E reveal that both 0.1 and 1.0 nmol CNQX significantly inhibited the sensitized motor response to cocaine in pre-stressed animals, without significantly reducing the acute response to cocaine in control animals. On the other hand, when 1 and 10% of DMSO were microinjected in the core of pre-stressed animals, an increase of the motor response to cocaine was observed in contrast to that of sham non-stressed animals (Fig. 5B, interaction, F(17,170)=2.33, p<0.01; treatment, F(1,170)=10.13, p<0.01; time, F(17,170)=8.49, p<0.001; Fig. 5D, interaction, F(17,221)=2.58, p<0.001; treatment, F(1,221)=11.49, p<0.01; time, F(17,221)=18.42, p<0.001). A Bonferroni post-hoc test revealed that the stress group shows similar significant increases of photocell counts at different times following cocaine injection, after either a 1% or 10% DMSO dose compared to the non-stressed group.
Akin to cocaine-induced motor activity, microdialysis performed in the core revealed that the sensitized increase in extracellular dopamine levels elicited by cocaine in pre-stressed animals was inhibited by CNQX (1 nM) but not DMSO 10% (Fig. 7 A-B, interaction, F(13,195)=1.85, p<0.05; treatment, F(1,195)=4.99, p<0.05; time, F(13,195)=10.28, p<0.001). Bonferroni post-hoc comparisons showed a significant increase in the percentage of basal DA output, 30 min and 120 min after cocaine challenge in the pre-stressed animals, compared to control animals.
Acute Pre-stress Increased NAc Surface Expression of GluR1
Surface expression of the AMPAR subunit GluR1 was higher in pre-stressed animals than in the non-stress groups (Fig. 8, treatment, F1,16)=9,254, p<0.01). However, there was no effect of acute cocaine administration in either group on AMPAR.
4. Discussion
These data show that an acute stressful experience causes an enduring augmentation in cocaine-induced locomotor activity and increases in extracellular dopamine. Moreover, these augmented responses depended on AMPAR stimulation in the core, but not the shell of the NAc. Accordingly, the motor response to direct AMPAR stimulation in the core was also augmented in pre-stress animals, and there was an enduring elevation in the surface expression of the GluR1 AMPAR subunit in the NAc by pre-exposure to acute stress. All of these adaptations to acute stress recapitulate the behavioral and neurochemical augmentation produced by daily exposure to cocaine, and is indicative of cocaine sensitization. Thus, acute stress causes an enduring change in pharmacology and physiology of the core that potentiates the effects of acute cocaine, and may contribute to the addiction liability of cocaine.
Although the majority of effects by acute stress recapitulate the enduring neuroadaptations elicited in the core by daily cocaine, a marked distinction was found in the basal and cocaine stimulated levels of extracellular glutamate. Thus, in contrast to measurements made after discontinuing daily cocaine (Pierce et al, 1996; Baker et al., 2003), the basal levels of extracellular glutamate were elevated following acute stress. The mechanisms underpinning this following daily cocaine arise from reduced cystine-glutamate exchange (Baker et al., 2003). However, daily cocaine also produces an enduring reduction in glial glutamate transport via down-regulating glutamate transporter-1 (GLT-1) (Knackstedt et al., 2010). Thus, it is possible that acute stress may down-regulate only GLT-1 and the reduction in uptake would be expected to elevate basal glutamate levels. Although this possibility is not indicated by the parallel slopes of the regression curves in the no-net-flux experiment, since enhanced transmitter elimination can change probe recovery slope (Bungay et al., 2003) the linkage between transmitter uptake and slope has been challenged especially for measures of extracellular glutamate (Bungay et al., 2003; Chen et al, 2006; Pendyam et al., 2009).
Behavioral and Dopamine Cross-Sensitization Between Acute Stress and Cocaine is Manifested in the Core but not Shell
In all instances where the responsiveness of the core and shell were compared, the enduring alterations by an acute stress exposure were manifested more in the core than the shell. Previous findings from our lab show that enhanced dopamine overflow in response to amphetamine is also observed only in the core of animals previously exposed to acute stress (Pacchioni et al., 2007). Augmented psychostimulant effects induced by pre-exposure to stressors on motor activity and dopamine levels in the NAc has been observed by several laboratories (Camp & Robinson, 1988; Kalivas & Stewart, 1991; Deroche et al., 1995; Rouge-Pont et al., 1995; Lu et al., 2003 ). The prepotent involvement of the core over the shell regarding enduring neurochemical adaptations that may contribute to behavioral sensitization is consistent with a portion, but not all of the literature. For example, sensitization to morphine, amphetamine, and cocaine produces an increase in dopamine release in the core, but not in the shell upon challenge injection after 10–14 days withdrawal (Cadoni & Di Chiara, 1999; Cadoni et al., 2000). In addition, food restriction sensitizes to the motor stimulant effects of cocaine and amphetamine and this change is associated with reciprocal drug-induced changes in dopamine responsiveness in the core as compared with the shell, where dopamine release was either unchanged or reduced (Cadoni et al., 2000). However, amphetamine injected directly into the shell produces a greater increase in local dopamine release after chronic cocaine at either early or late withdrawal compared to saline-treated rats and, in the core, amphetamine elicited less dopamine efflux in cocaine-treated rats at early withdrawal, while there was no difference between saline and cocaine-treated rats in amphetamine-induced dopamine efflux at late withdrawal (Pierce et al, 1995). Related to this, Chen et al., (1996) found that a cocaine challenge enhanced NAc dopamine release after 7 days withdrawal from chronic cocaine compared to drug naive controls, but that this enhancement occurred only in response to systemically administered cocaine, not when cocaine was administered by reverse dialysis into the NAc. When differential sampling of NAc subregions was performed during self-administration, a greater dopamine elevation was observed in shell than in core (Lecca et al., 2007; Suto et al., 2010). However, Lecca et al., (2007) showed that, after 3 weeks of daily drug availability, passive exposure to cocaine resulted in a progressive loss of the preferential response of shell dopamine, with actual reversal into a preferential core response.
Taken together, these findings suggest that enhanced dopamine release in the core is a general feature of the long-term expression of drug- and stress-induced behavioral sensitization to cocaine. Interestingly, cues associated with cocaine self-administration presented non-contingently also selectively increased dopamine release in the core, with no effect in the shell (Ito et al., 2000). Since dopaminergic mechanisms within core and shell contribute differentially to reward-related behaviors (Ito et al., 2000; Ito et al., 2004; Ikemoto et al, 2007; Ambroggi et al., 2008; Nicola et al, 2010) future studies should investigate the relevance of the differential contribution of stress on cocaine-induced changes of dopamine from core vs shell on reward.
AMPA Mechanisms Underlying Restraint Stress-Induced Cross-Sensitization to Cocaine
The present studies yielded three findings supporting involvement of AMPAR in the enduring sensitized locomotor and dopaminergic responses to cocaine by an acute stress. First, the sensitized cocaine-induced locomotor response and increase in extracellular dopamine was prevented by pretreatment with the AMPA antagonist CNQX in the core. Similarly, Pierce et al, (1996) showed that the expression of behavioral sensitization produced by daily cocaine is also blocked by AMPAR antagonists in the core, although they did not study the influence of CNQX on cocaine-induced dopamine sensitization. Many studies support an idea that adaptations in excitatory underlie the cross-sensitization between drugs and stress. For example, both acute administration of drugs of abuse (amphetamine, cocaine, alcohol, nicotine, morphine) and stress (cold water swimming) can enhance synaptic strength in NAc medium spiny neurons (MSNs) or midbrain dopamine neurons via a glucocorticoid-dependent mechanism (Campioni et al, 2009; Saal et al, 2003). Moreover, electrophysiological recordings have shown that CRF regulates the excitability of VTA dopamine neurons (Korotkova et al., 2006; Korotkova et al., 2007; Wanat et al., 2008) and glutamatergic synaptic inputs into these neurons (Ungless et al., 2003). The inhibitory effect of intra-core CNQX on the expression of dopamine sensitization to cocaine following stress may result for affecting a polysynaptic pathway from the NAc to the VTA, and thereby bring about changes in the dopamine terminals in the core. Supporting this speculation, Choi et al., (2005) showed that blocking AMPAR within the nucleus accumbens coincident with stimulating D2/3 dopamine receptors decreased neuronal activity in the VTA. Interestingly, a CRF-glutamate interaction in the VTA not only regulates the initiation of addiction-related behaviors, but may also play a role in the expression of these behaviors (Wang et al., 2005; Harris et al., 2006; Wang et al., 2007).
The second finding supporting glutamatergic involvement in cross-sensitization between stress and cocaine is that the motor stimulant actions of an AMPA agonist were potentiated following microinjection into the core, but not the shell of pre-stressed animals. A similar behavioral augmentation to AMPA has been observed after repeated cocaine or amphetamine administration (Pierce et al, 1996; Suto et al., 2004). Thirdly, there was an enduring increase in the surface expression of GluR1 in the NAc of pre-stressed animals. Although the entire NAc was analyzed in this experiment to provide sufficient tissue to conduct the biotinylation assay, given the apparent selectivity for the core in the other neuroadaptations induced by pre-stress, it seems probable that this elevation may rely more on changes in the core than in the shell. Although this possibility requires empirical validation, it is supported by the fact that elevating GluR1 in the shell inhibits rather than promotes the expression of cocaine sensitization (Bachtell et al., 2008). Overall, the increase in GluR1 is consistent with an enduring increase in membrane insertion of AMPAR in cocaine-withdrawn animals (Boudreau & Wolf, 2005; Ghasemzadeh et al., 2009; Schumann & Yaka, 2009) and the marked increase in AMPA currents in NAc spiny cells after withdrawal from cocaine (Kourrich et al., 2007; Moussawi et al., 2011).
The pioneering studies showing that corticosterone secretion is one of the mechanisms underlying stress-induced cross-sensitization to amphetamine, morphine and cocaine, may also point to a potential mechanism whereby stress can elicit changes in AMPAR (Deroche et al., 1992; Deroche et al.; 1993; Deroche et al., 1995; Rouge-Pont et al., 1995; Rouge-Pont et al., 1998; Prasad et al., 1998). It has been suggested that through action on mesolimbic dopamine neurons an increase in glucocorticoid hormones underlies the increased vulnerability to drug abuse (Marinelli & Piazza, 2002; de Jong & de Kloet, 2004). Also, selective deletion of Nr3c1 (the glucocorticoid receptor gene) in mouse of spiny neurons expressing D1 dopamine receptors decreased the motivation of mice to self-administer cocaine (Ambroggi et al., 2009). Recent studies also reveal that corticosterone increases AMPAR surface mobility and synaptic surface content in the hippocampus (Martin et al., 2009; Conboy & Sandi, 2010, Groc et al., 2008).
In addition to corticosterone, several lines of evidence indicate a critical role for CRF action on the dopamine system in stress-related behaviors. It has been shown that CRF antagonists inhibit stress-induced reinstatement of cocaine-seeking (Wang et al., 2005; Wang et al., 2007) as well as cross-sensitization to psychostimulants (Cole et al., 1990; Boyson et al., 2011). Taken together, these data point to CRF and corticosterone secretion by stress as important potential mechanisms in the stress-induced increase in AMPAR that we find facilitates the motor stimulant effects of psychostimulants.
Conclusions
This study identifies mechanisms in common among the enduring changes in the brain produced by acute restraint stress and daily cocaine administration. Notably, certain enduring neuroadaptations in dopamine and glutamate transmission and/or pharmacology seen in the core after withdrawal from daily cocaine were also elicited following only a single exposure to stress. In particular, we identified a role for AMPAR in the nucleus accumbens akin to what has been shown previously using animal models of cocaine relapse (Cornish & Kalivas, 2000; Suto et al., 2004; Conrad et al., 2008; Famous et al., 2008). This indicates that an acute life stressor can produce long-lasting facilitation of processes that have been strongly linked to cocaine addiction in animal models. Taking all the experiments together, this study helps mechanistically buttress the clinical observation that life stress may be a vulnerability factor in the development of cocaine addiction.
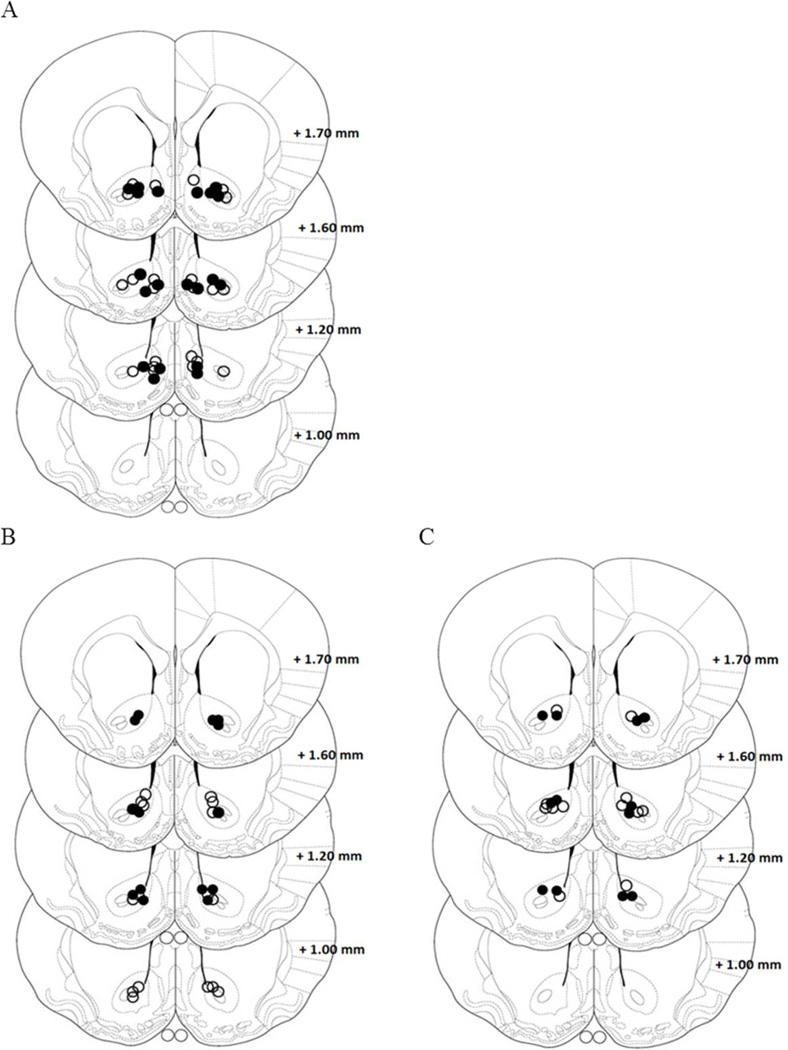
Figure 6. Location of the microinjection guide cannula in the nucleus accumbens from experiments 5 and 6.
A. Illustration showing the location of AMPA microinjection cannula tips in the Core. The open circles represent cannula placements in non-stress group and open filled circles depict placements in stress group. B-C Illustration showing the location of DMSO and CNQX microinjection cannula tips in the Core. The open circles represent cannula placements in non-stress group and open filled circles depict placements in stress group.
Acknowledgements
This work was supported by grants from FONCyT, CONICET, SECyT and MinCyT (Argentina). The authors are grateful to Estela Salde for her laboratory technical assistance and Joss Heywood for English technical assistance.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DMSO
dimethyl sulfoxide
- GLT-1
glutamate transporter-1
- NAc
nucleus accumbens
- NMDA
n-methyl-d-aspartic acid
- OPA/OME
o-phthalaldehyde and o-βmercapto ethanol
- Pfc
prefrontal cortex
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Reciprocal changes in dopamine responsiveness in the nucleus accumbens shell and core and in the dorsal caudate-putamen in rats sensitized to morphine. Neuroscience. 1999;90:447–455. doi: 10.1016/s0306-4522(98)00466-7. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stress. Behav Brain Res. 1988;30:69–88. doi: 10.1016/0166-4328(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Campioni MR, Xu M, Mc Gehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;6:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Cancela LM. Effect of acute and chronic stress restraint on amphetamine-associated place preference: involvement of dopamine D(1) and D(2) receptors. Eur J Pharmacol. 1999;386:127–134. doi: 10.1016/s0014-2999(99)00746-3. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M. Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res. 1990;512:343–346. doi: 10.1016/0006-8993(90)90646-S. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-15-j0006.2000. RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Marmur R, Paredes W, Pulles A, Gardner EL. Systemic cocaine challenge after chronic cocaine treatment reveals sensitization of extracellular dopamine levels in nucleus accumbens but direct cocaine perfusion into nucleus accumbens does not: implications for the neural locus of cocaine sensitization. Life Sci. 1996;58:PL139–PL146. doi: 10.1016/s0024-3205(96)80014-2. [DOI] [PubMed] [Google Scholar]
- Chen KC. Effects of tissue trauma on the characteristics of microdialysis zeronet-flux method sampling neurotransmitters. J Theor Biol. 2006;238:863–881. doi: 10.1016/j.jtbi.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Choi KH, Clements RL, Greenshaw AJ. Simultaneous AMPA/kainate receptor blockade and dopamine D(2/3) receptor stimulation in the nucleus accumbens decreases brain stimulation reward in rats. Behav Brain Res. 2005;158:79–88. doi: 10.1016/j.bbr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- de Jong IE, de Kloet ER. Glucocorticoids and vulnerability to psychostimulant drugs: toward substrate and mechanism. Ann N Y Acad Sci. 2004;1018:192–198. doi: 10.1196/annals.1296.022. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Maccari S, Le Moal M, Simon H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992;598:343–348. doi: 10.1016/0006-8993(92)90205-n. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Casolini P, Le Moal M, Simon H. Sensitization to the psychomotor effects of amphetamine and morphine induced by food restriction depends on corticosterone secretion. Brain Res. 1993;611:352–356. doi: 10.1016/0006-8993(93)90526-s. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Frau R, Carboni E. On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology (Berl) 1993;112:398–402. doi: 10.1007/BF02244939. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- Esparza MA, Bollati F, Garcia-Keller C, Virgolini M, Lopez LM, Brusco A, Shen HW, Kalivas P, Cancela LM. Stress-induced sensitization to cocaine: actin cytoskeleton remodeling within mesocorticolimbic nuclei. Eur J Neurosci. 2012;36(8):3103–3117. doi: 10.1111/j.1460-9568.2012.08239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav. 2006;49:615–625. doi: 10.1016/j.yhbeh.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Klyuch BP, Ponomarenko AA, Lin JS, Haas HL, Sergeeva OA. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology. 2007;52:626–633. doi: 10.1016/j.neuropharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Martin S, Henley JM, Holman D, Zhou M, Wiegert O, van Spronsen M, Joels M, Hoogenraad CC, Krugers HJ. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4:e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty F. Is drug use a response to stress? Drug Alcohol Depend. 1991;29:97–106. doi: 10.1016/0376-8716(91)90026-u. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. San Diego: Academic Press; 2007. [Google Scholar]
- Pendyam S, Mohan A, Kalivas PW, Nair SS. Computational model of extracellular glutamate in the nucleus accumbens incorporates neuroadaptations by chronic cocaine. Neuroscience. 2009;158:1266–1276. doi: 10.1016/j.neuroscience.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMG, H. J. Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pierce RCK, PW Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–1029. [PubMed] [Google Scholar]
- Pierce RCB, Duffy K, Kalivas P, PW Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Sorg BA. Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal. Psychopharmacology (Berl) 1998;136:24–33. doi: 10.1007/s002130050535. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology (Berl) 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Stress and Rodent Models of Drug Addiction: Role of VTA-Accumbens-PFC-Amygdala Circuit. Drug Discov Today Dis Models. 2008;5:259–270. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]