Abstract
Liver cirrhosis involves chronic wound healing and fibrotic processes. Mesenchymal stromal cells (MSCs) are multipotent adult progenitor cells that are used as vehicles of therapeutic genes. Insulin growth factor like-I (IGF-I) was shown to counteract liver fibrosis. We aimed at analyzing the effect of applying IGF-I overexpressing mouse bone marrow-derived MSCs on hepatic fibrosis. Fibrosis was induced by chronic thioacetamide application or bile duct ligation. MSCs engineered to produce green fluorescent protein (GFP) (AdGFP-MSCs) or IGF-I (AdIGF-I-MSCs) were applied systemically, and changes in collagen deposition and in the expression of key pro-fibrogenic and pro-regenerative genes/proteins were assessed. In addition, immunogenicity of transduced cells was analyzed. Liver fibrosis was further ameliorated after a single-dose application of AdIGF-I-MSCs when compared with AdGFP-MSCs and/or recombinant IGF-I treatments. Interestingly, an early and transitory upregulation in IGF-I and hepatocyte growth factor (HGF) mRNA expression was found in the liver of MSC-treated animals, which was more pronounced in AdIGF-I-MSCs condition. A reduction in hepatic stellate cell activation status was found after incubation with MSCs conditioned media. In addition, the AdIGF-I-MSCs cell-free supernatant induced the expression of IGF-I and HGF in primary cultured hepatocytes. From day 1 after transplantation, the proliferation marker proliferating cell nuclear antigen was upregulated in the liver of AdIGF-I-MSCs group, mainly in hepatocytes. MSCs were in vivo traced till day 14 after injection. In addition, multiple doses of Ad-IGF-I-MSCs likely suppressed antiviral immune response and it further reduced collagen deposition. Our results uncover early events that are likely involved in the anti-fibrogenic effect of genetically modified MSCs and overall would support the use of AdIGF-I-MSCs in treatment of liver fibrosis.
Introduction
Liver cirrhosis is characterized by an excessive accumulation of collagen and other extracellular matrix proteins, which lead to the impairment of the hepatic function [1]. It is the first indication for liver transplantation [2] but due to the scarcity of donors, new therapeutic approaches are urgently needed.
Mesenchymal stromal cells (also known as mesenchymal stem cells; MSCs) are regarded as multipotent progenitors [3], and they are able to modulate inflammatory responses [4] and to migrate to injury sites [5]. Systemic administration of MSCs in different animal models was found to ameliorate liver fibrosis [6], although mechanisms therein involved are largely unknown. It has been hypothesized that MSCs would exert their effect by diverse mechanisms [6–8]; nevertheless, they have been therein evaluated several days after MSCs application and might, in turn, depend on earlier as yet unexplored events.
It has been proposed that the use of MSCs as vehicles of therapeutic genes might result in an enhanced amelioration of liver fibrosis [6]. In the same context, it has been described that the application of multiple doses of MSCs in athymic mice might result in a better outcome when compared with single doses [9]. However, little is known regarding whether or not applications of genetically modified MSCs into immunocompetent mice might induce immunogenicity against such cells or antigens derived from the adenovirus (Ad) being used to express therapeutic genes.
Insulin growth factor like-I (IGF-I) is an anabolic hormone with an important role in metabolism that is mainly synthesized by the liver. Its expression levels are known to be reduced in the cirrhotic liver [10]. The systemic application of IGF-I as recombinant protein or incorporated in a viral vector construct was found to improve liver function in the context of liver cirrhosis in animal models and in patients [11–13]. IGF-I might likely exert its antifibrotic effect [14] and/or improve liver function partly by modulating transforming growth factor-beta 1 (TGF-β1) expression/signaling events [15] and/or by inducing hepatocyte proliferation [16] or survival [17].
Here, we show for first time that the application of genetically modified MSCs to express IGF-I (AdIGF-I-MSCs) further ameliorates liver fibrosis. We observed that this effect is associated with reduced hepatic stellate cells (HSCs) activation. Early events after MSC transplantation include increased expression of IGF-I, hepatocyte growth factor (HGF), proliferating cell nuclear antigen (PCNA), and/or TWEAK depending on the type of treatment, suggesting the involvement of regenerative mechanisms. AdIGF-I-MSCs supernatant was found to induce the expression of IGF-I and HGF in cultured hepatocytes. Multiple applications of AdIGF-I-MSCs result in further reduction in collagen deposition when compared with single-dose treatment. Finally, we show that the administration of genetically modified MSCs would not likely be associated with an increase in immune responses against MSCs or adenoviral proteins.
Materials and Methods
Isolation and characterization of mouse bone marrow MSCs
Male BALB/c mice (6- to 8-week old) were sacrificed by cervical dislocation, and bone marrow (BM) cells were flushed out from tibia and femur. Mononuclear cells were isolated using Ficoll–PaqueTM Plus density gradient (1.077 g/mL; GE Healthcare). Cells were plated at 4,000 cells per cm2 and incubated in Dulbecco's modified Eagle's medium low glucose (DMEM lg; Invitrogen/Life Technologies) supplemented with 10% fetal bovine serum (FBS; Gibco/Invitrogen). Medium was replaced 3 days later, and cells were expanded till passage 4–8.
Adenoviral vector preparation and MSCs transduction
MSCs were seeded at 70% of confluence in complete medium. Medium was then removed, and cells were infected at a multiplicity of infection (MOI) of 30 in DMEM lg and 2% FBS in half of total volume for 2 h. After that, medium was completed with 10% FBS in DMEM lg. Expression of IGF-I or green fluorescent protein (GFP) was evaluated at 3 days after transduction by radio-immunoassay (Supplementary Data; Supplementary Data are available online at www.liebertpub.com/scd) or by direct observation under fluorescence microscopy. Otherwise stated, in different experimental protocols, cells were also used at 3 days after adenoviral infection.
In vivo experiments
Six- to 8-week-old male BALB/c mice were purchased from CNEA (Comisión Nacional de Energía Atómica, Ezeiza, Buenos Aires, Argentina). Animals were maintained at our Animal Resources Facility (Faculty of Biomedical Sciences, Austral University) in accordance with the experimental ethical committee and the NIH guidelines on the ethical use of animals. Fibrosis was induced by intraperitoneal administration of 0.2 mg/g bodyweight of thioacetamide (TAA) (Sigma-Aldrich Co.), thrice per week, during 6 weeks, and maintained for 2 additional weeks by applying the same compound and dose twice a week. During week 6, animals were intravenously injected (tail) with saline, AdGFP-MSCs, or AdIGF-I-MSCs. Animals (n=5/condition) were sacrificed at 1, 3, or 14 days after cellular/saline treatment, and liver samples were dissected out for subsequent studies. Other experimental groups consisted of animals treated with/without AdGFP-MSCs plus recombinant IGF-I (rIGF-I) (kindly provided by Dr. P. Fortes): 1 μg/100 g body weight, in two divided doses. The latter was subcutaneously applied daily from day 1 to 14.
In vivo tracking of MSCs was performed as previously described [18]. In brief, MSCs were stained with CMDil for histological analysis and DiR (Molecular Probes, Invitrogen) for fluorescence imaging (FI). MSCs (5×105) were intravenously injected into mice previously treated with TAA for 6 weeks. FI was performed using the Xenogen In Vivo Imaging System (IVIS; Caliper Life Sciences). Mice injected with CMDiI-DiR-labeled MSCs were analyzed at 1–14 days after MSC injection. Images represent the radiant efficiency and were analyzed with IVIS Living Image (Caliper Life Sciences) software. Regions of interest were manually drawn around the isolated organs to assess the fluorescence signal emitted. Results were expressed as total radiant efficiency in units of photons/second within the region of interest [p/s]/[μW/cm2]. In addition, cells were traced within tissue by mean of GFP immunohistochemistry.
To test the effects of multiple MSCs doses on collagen deposition using similar experimental settings, in addition to single MSCs or saline dose conditions, AdIGF-I-MSCs or AdGFP-MSCs were applied at weeks 6, 8, and 10 after TAA treatment onset, and all animals were sacrificed at week 12 to obtain liver samples and the sirius red-stained area on tissue sections. After week 6, all animals received two doses of TAA per week. See bile duct ligation and immunostaining protocols in Supplementary Data.
Reverse transcription–polymerase chain reaction
Liver tissue was homogenized, and total RNA was extracted by using Trizol Reagent (Sigma-Aldrich Co.). Total RNA (4 μg) was reverse transcribed with 200 U of SuperScript II Reverse Transcriptase (Invitrogen) using 500 ng of Oligo (dT) primers. cDNAs were subjected to a real-time polymerase chain reaction (qPCR) (Stratagene Mx3005p; Stratagene). The mRNA levels were quantified by SYBR® Green (Invitrogen). See further details in Supplementary Data.
Statistical analysis
Data are expressed as mean±SEM. Statistical analysis was performed using Student's t-test or Mann–Whitney test, according to data distribution. Differences were considered significant when P<0.05. All experiments were performed at least thrice in triplicate.
Results
Adenoviral transduction of IGF-I does not induce immunogenicity in MSCs
The phenotype and differentiation potential of BALB/C BM-derived MSC cultures was confirmed (Table 1 and not shown). MSCs were transduced using specific adenoviruses carrying GFP and IGF-I full length at an MOI of 30. The efficiency of cell transduction was analyzed by direct observation of fluorescent green signal (AdGFP-MSCs; efficiency: 82.25%±0.79%; not shown) or by enzyme-linked immunosorbent assay (ELISA) (AdIGF-I-MSCs). A significant increase in IGF-I levels was found in the supernatant of AdIGF-I-MSCs when compared with AdGFP-MSCs (Fig. 1A) or naïve MSCs (not shown). The genetic modification of MSCs did not significantly affect their surface marker expression pattern and differentiation potential (Table 1 and not shown). As expected, mixed lymphocyte reaction studies showed that genetically modified MSCs did not induce further proliferation of C57Bl/6-derived splenocytes when compared with naïve MSCs (1,480±68.13 vs. 1,354±143.3 vs. 1,014±219.9; MSCs vs. AdGFP-MSCs vs. AdIGF-I-MSCs; P>0.05). In order to analyze whether or not genetically modified MSCs would be immunogenic, multiple doses of cells were applied to immunocompetent mice and peripheral blood was withdrawn at day 0 to 14. No neutralizing antibodies against AdGFP were elicited in tested animals at any time point analyzed (Table 2).
Table 1.
Phenotypic Characterization of MSCs and AdIGF-I-MSCs
| Marker | MSCs (%) | AdIGF-I-MSCs (%) |
|---|---|---|
| Sca-1 | 97.6 | 97 |
| CD105 | 88.2 | 91 |
| CD44 | 96.9 | 96.7 |
| CD45 | 1.5 | 0.6 |
| CD45R | 9.8 | 11.2 |
| GR1 | 9.8 | 5.8 |
| CD11b | 0.3 | 0.3 |
| CD3 | 1.1 | 0.6 |
| MHC-II | 0.2 | 0.2 |
| CD80 | 24 | 31 |
| CD86 | 0.7 | 0.4 |
| Sca-1 | 97.6 | 97 |
| CD105 | 88.2 | 91 |
| CD44 | 96.9 | 96.7 |
| CD45 | 1.5 | 0.6 |
MSC, mesenchymal stromal cell.
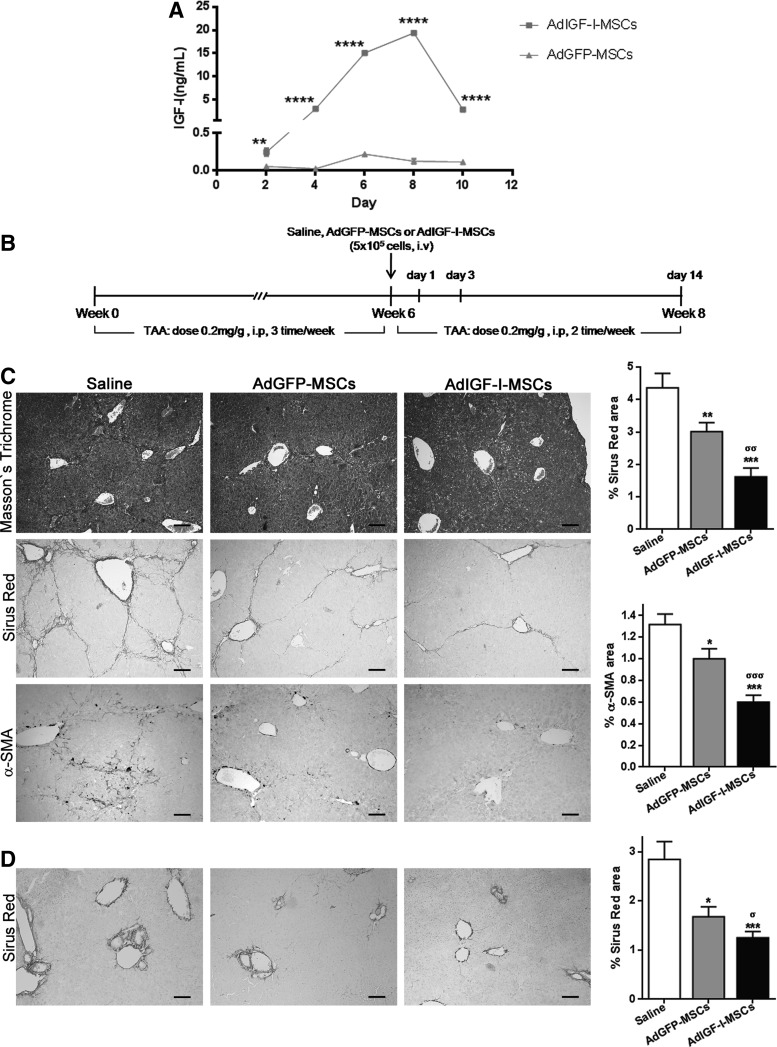
FIG. 1.
AdIGF-I-MSCs treatment further ameliorates liver fibrosis. (A) Dosage of insulin growth factor like-I (IGF-I) in culture's supernatant of AdIGF-I-MSCs and AdGFP-MSCs at day 2, 4, 6, 8, and 10 postinfection. **P<0.01; ****P<0.0001; * versus AdGFP-MSCs. (B) Experimental in vivo model (see Materials and Methods section). (C) Representative microphotographies of Masson's trichrome and sirius red-stained and alpha-smooth muscle actin (α-SMA) immunostained liver sections (scale bars: 100 μm). Quantification of collagen deposits based on sirius red-stained sections and of α-SMA-positive cells, by morphometric analysis [thioacetamide (TAA) model]. (D) Representative microphotographies of sirius red-stained liver sections from mice subjected to bile duct ligation and treated with mesenchymal stromal cells (MSCs) (BDL model). Quantification of collagen deposits based on sirius red stainings. *σP<0.05; **σσP<0.01; ***σσσP<0.001. * versus saline; σ versus AdGFP-MSCs.
Table 2.
Adenovirus-Neutralizing Antibody Titers in Serum of MSCs or AdIGF-I-MSC-Treated Mice After One or Several Cell Applications
| Titer (Dil−1) | ||
|---|---|---|
| Sample | MSCs | AdGFP-MSCs |
| Basal | n.d. | n.d. |
| Day 3 | n.d. | n.d. |
| Day 14 | n.d. | n.d. |
| Control+ | 3,200 | |
| Control− | n.d. | |
n.d., nondetected.
Application of AdIGF-I-MSCs further ameliorates liver fibrosis in mice through reduction of HSC activation
In order to analyze whether AdIGF-I-MSCs might be able to further ameliorate liver fibrosis in mice when compared with appropriate controls, TAA was chronically applied to 6- to 8-week-old BALB/c mice, thricee times a week, during 8 weeks. At the sixth week, 5×105 MSCs were intravenously injected. At the eighth week, animals were sacrificed and peripheral blood and liver samples were collected (Fig. 1B). As shown in Figure 1C, a reduction in the amount of collagen deposition was observed in sections from AdGFP-MSCs-treated animals when compared with saline-treated ones. Interestingly, the application of AdIGF-I-MSCs resulted in a higher amelioration of fibrosis degree when compared with AdGFP-MSCs (Fig. 1C). Consistently, a decrease in alpha-smooth muscle actin (α-SMA) (a well-known marker of activated stellate cells) stained area was found in sections from AdGFP-MSCs-treated mice when compared with saline, and it was further significantly reduced when animals were treated with AdIGF-I-MSCs (Fig. 1). As expected, a significant decrease in the total Knodell score was observed in samples from MSCs-treated animals when compared with vehicle; furthermore, a significant decrease in the fibrosis score resulted when AdIGF-I-MSCs were compared against AdGFP-MSCs or saline (Table 3). Similar results regarding collagen deposition in the liver, as seen after picrosirius red staining, were obtained using a bile duct ligation model (Fig. 1D).
Table 3.
Histopathological Assessment of Liver Samples After MSC Treatments
| Saline | AdGFP-MSCs | AdIGF-I-MSCs | |
|---|---|---|---|
| Periportal or periseptal hepatitis | 3.0±0.00 | 2.2±0.20 | 2.2±0.20 |
| Confluent necrosis | 3.4±0.40 | 2.6±0.40 | 2.2±0.2* |
| Focal lytic necrosis, apoptosis and focal inflammation | 2.6±0.24 | 2.2±0.20 | 2.2±0.45 |
| Portal inflammation | 3.4±0.24 | 2.4±0.24 | 2.6±0.24 |
| Fibrosis | 4.6±0.24 | 4.0±0.32 | 2.8±0.2**σ |
| Knodell score | 17.0±0.45 | 12.5±0.64* | 11.5±0.29** |
Knodell scores (mean±SD) were calculated by an expert pathologist. *σP<0.05; **P<0.01; * versus saline; σ versus AdGFP-MSCs.
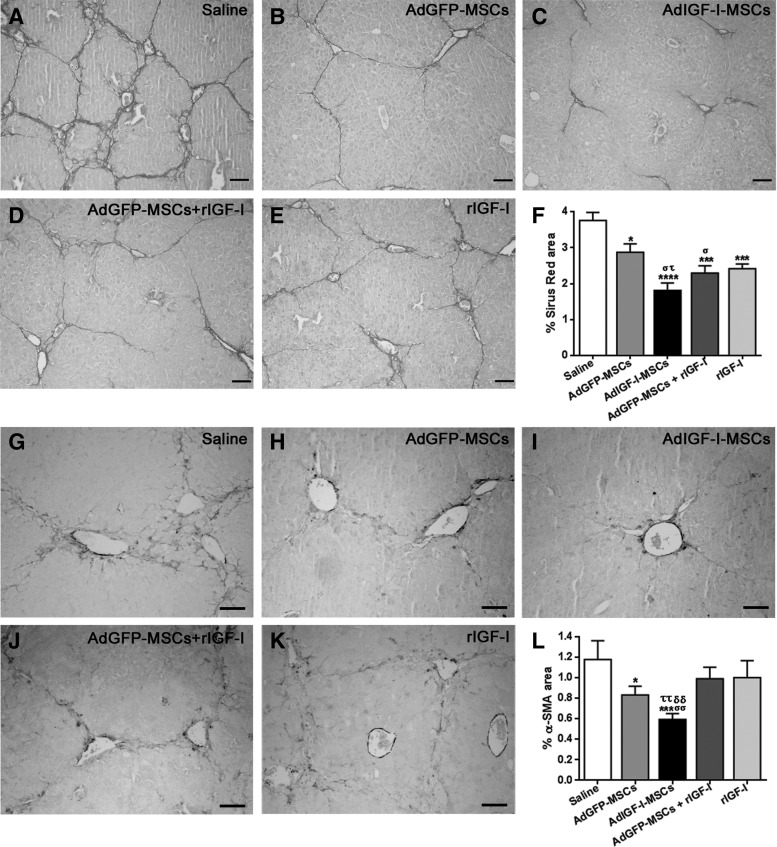
We then asked whether the anti-fibrotic effect of a single application of AdIGF-I-MSCs might be similar or better than applying AdGFP-MSCs in combination with rIGF-I or rIGF-I alone. Interestingly, both collagen deposition (as measured by picrosirius red staining; Fig. 2A–F) and HSCs activation (as determined by immunohistochemistry against α-SMA; Fig. 2G–L) were significantly reduced in the liver of AdIGF-I-MSC-treated animals when compared with AdGFP-MSCs+rIGF-I or rIGF conditions. Considering these results, rIGF-I conditions are not considered in next experiments due to their comparatively nonimproving antifibrotic effects, their high costs, and animal discomfort of daily subcutaneous applications of the protein.
FIG. 2.
AdIGF-I-MSCs antifibrotic effects are, at least, comparable to AdGFP-MSCs treatment. (A–E) Representative pictures showing picrosirius red staining on liver sections derived from saline (A), AdGFP-MSCs (B), AdIGF-I-MSCs (C), AdGFP-MSCs+rIGF-I (D), or recombinant IGF-I (rIGF-I)-treated animals (E). (F) Quantification of collagen deposits based on sirius red staining. (G–K) Representative pictures showing α-SMA immunostained area on liver sections derived from saline (G), AdGFP-MSCs (H), AdIGF-I-MSCs (I), AdGFP-MSCs+rIGF-I (J), or rIGF-I-treated mice (K). (L) Quantification of α-SMA immunostained area. Scale bars: 50 μm. *στP<0.05; σσττδδP<0.01; ***P<0.001; ****P<0.0001; * versus saline; σ versus AdGFP-MSCs; τ versus AdGFP-MSCs+rIGF-I; δ versus rIGF-I.
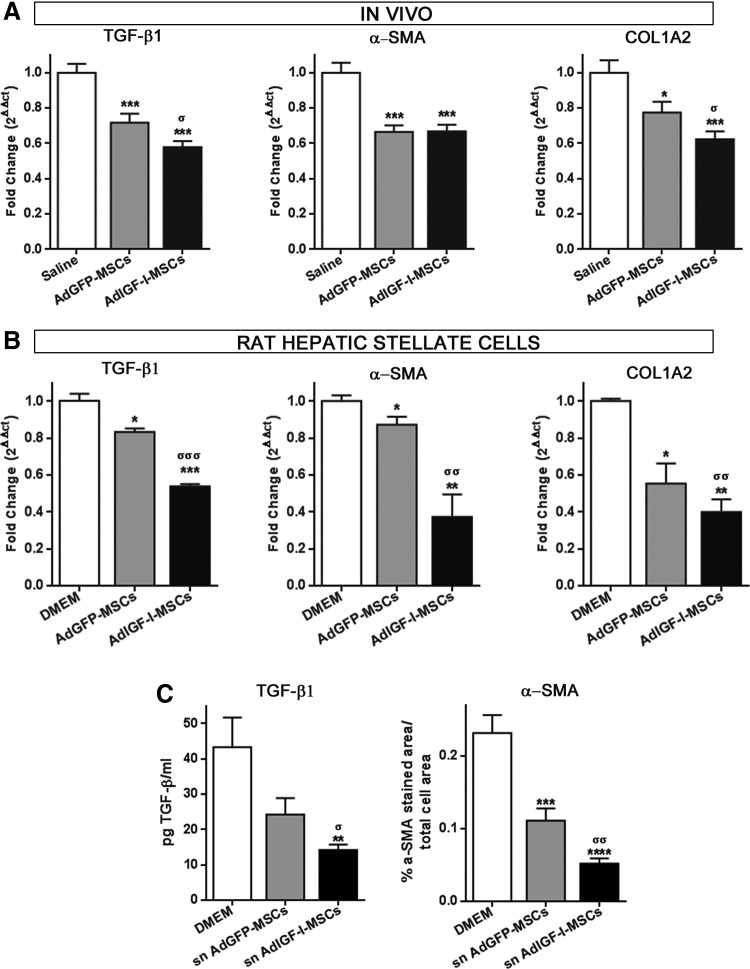
In order to confirm the effect of different MSCs treatments in liver fibrosis process, mRNA expression levels of the master pro-fibrogenic molecule TGF-β1 and of α-SMA and collagen 1A2 (COL1A2) were analyzed by qPCR. As expected, expression levels of all these genes were reduced after MSC treatments when compared with saline (Fig. 3A). Moreover and consistent with previous results, TGF-β1 and COL1A2 mRNA levels were also downregulated in samples obtained from AdIGF-I-MSCs-treated animals when compared with those treated with AdGFP-MSCs (Fig. 3A).
FIG. 3.
AdIGF-I-MSCs treatment reduces the activation of hepatic stellate cells (HSCs). (A) Analysis of mRNA levels of transforming growth factor-beta 1 (TGF-β1), α-SMA, and collagen 1A2 (COL1A2) at 14 days after MSCs application. (B) Quantitative graphs showing mRNA expression levels of TGF-β1, α-SMA, and COL1A2 in HSCs after 40 h of incubation with Dulbecco's modified Eagle's medium (DMEM) (control) and supernatants of AdIGF-I-MSCs or AdGFP-MSCs. (C) Quantitative graphs showing active TGF-β1 and α-SMA protein expression levels in HSCs after 40 h of incubation with DMEM (control) and supernatants of AdIGF-I-MSCs or AdGFP-MSCs. TGF-β1 was measured at 48 h after incubation *σP<0.05; **σσP<0.01; ***σσσP<0.001; * versus saline; σ versus AdGFP-MSCs; ****P<0.0001 versus DMEM. sn, supernatant.
To further demonstrate an MSC-mediated reduction in the activation of HSCs, CFSC-2G cells were incubated overnight (18 h) with supernatant obtained from AdGFP-MSCs and AdIGF-I-MSCs cultures. Interestingly, TGF-β1 was found to be downregulated in AdIGF-I-MSCs condition when compared with controls, while α-SMA and COL1A2 remained unchanged (not shown). Considering that TGF-β1 might be responsible for the induction of α-SMA expression, HSCs were incubated for 1 additional day (40 h in total) and this resulted in a reduction in α-SMA mRNA expression levels in cultures treated with AdGFP-MSCs when compared with DMEM (Fig. 3B). Moreover, α-SMA mRNA expression levels were further reduced in HSCs when they were treated with AdIGF-I-MSC supernatant (Fig. 3B). As expected, a similar pattern of changes in mRNA expression levels was found for TGF-β1 and COL1A2 genes (Fig. 3B). These results were confirmed at the protein level for active TGF-β1 and α-SMA (Fig. 3C). A similar pattern was observed when TGF-β1 protein expression levels were analyzed in serum by ELISA (not shown; 33.75±2.28 vs. 25.14±3.29 vs. 20.21±1.25; saline vs. AdGFP-MSCs vs. AdIGF-I-MSCs; P=0.04, saline vs. AdIGF-I-MSCs). These data suggest that AdIGF-I-MSCs treatment further reduces the activation of HSCs when compared with AdGFP-MSCs condition, thus resulting in the amelioration of liver fibrosis.
In vivo tracking of MSCs within the fibrotic liver
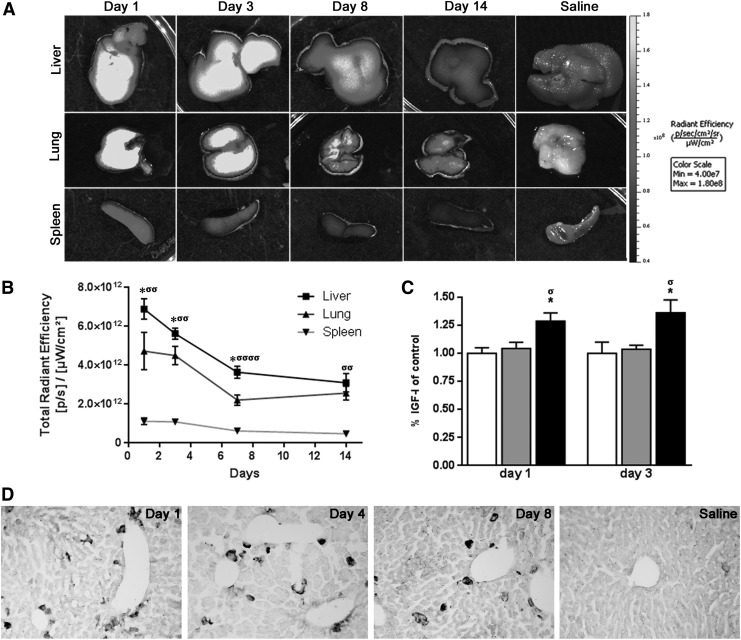
In order to address the survival and biodistribution of MSCs after their application in vivo, AdIGF-I-MSCs were stained with an infrared dye (DiR) and systemically applied into 6 week TAA-treated animals. The highest levels of DiR signal were observed in the liver at first day after MSCs application (Fig. 4A, B). In addition, MSCs-derived signals were found, although at lower levels in the lung and in the spleen (Fig. 4A, B). In all cases, a decrease in DiR levels was observed in these three tissues with time after cellular administration (Fig. 4A, B). No DiR levels were detected in TAA-treated mice receiving saline (vehicle). Consistent with these results, when IGF-I protein were measured in liver samples by ELISA at 1 and 3 days after MSCs/vehicle administrations a significant increase in their expression levels was observed after AdIGF-I-MSCs treatment when compared with controls (Fig. 4C).
FIG. 4.
In vivo survival and biodistribution of MSCs. (A) DiR-labeled AdIGF-I-MSCs were systemically administered and monitored from 1 to 14 days later by fluorescence imaging (FI). At different survival times, tissues were removed and lungs, liver, and spleen were exposed to FI. Images represent the radiant efficiency. (B) Regions of interest calculated for the isolated liver, spleen, and lung and results were expressed as total radiant efficiency ([p/s]/[μW/cm2]). Note that MSC-derived signals were more abundantly found in the liver and peak at 1 day after cellular application and decrease thereafter. Animals with liver fibrosis receiving vehicle instead of MSCs showed no significant signals at 4 days after this treatment. *P<0.05; σσP<0.01; σσσσP<0.0001; * versus lung; σ versus spleen. (C) IGF-I protein levels in liver samples as measured by enzyme-linked immunosorbent assay (D) Representative images from green fluorescent protein (GFP)-immunostained sections at 1, 4, and 8 days after AdGFP-MSCs application or at 1 day after vehicle administration, as control. *σP<0.05; **σσP<0.01; ***σσσP<0.001; * versus saline; σ versus AdGFP-MSCs. Scale bars: 50 μm.
In order to confirm previous results, livers from AdGFP-MSCs-treated mice sacrificed at 1, 4, and 8 days after cellular application were sectioned and immunostained for GFP expression. Consistent with FI analyses, significant numbers of GFP+ cells were found at all survival times analyzed although diminishing over time (Fig. 4D). No GFP+ signals were observed in sections from animals receiving vehicle instead of cells or when primary antibody incubation was omitted (Fig. 4D and not shown).
Early events after MSCs transplantation in the fibrotic liver
Several mechanisms were proposed to explain the beneficial effects of MSCs when they are used to counteract liver fibrogenesis. However, in previous reports, liver samples were analyzed several days after cell infusion and earlier events were not investigated. Considering that the peak in MSCs numbers within the liver was found at 1 day after their application, we then asked whether some relevant changes in the liver expression profile might take place early after MSC transplantation.
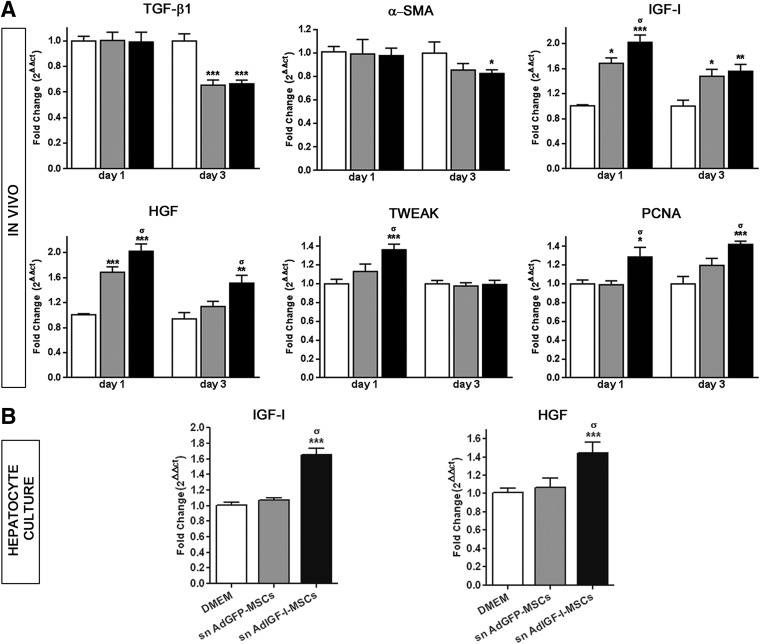
As shown in Figure 5A, mRNA expression of TGF-β1 and α-SMA remained unchanged at 1 day after experimental treatment. Interestingly, TGF-β1 was downregulated in all MSCs-treated animals at 3 days after cell therapy when compared with saline (Fig. 5A); however, a minor but significant reduction in hepatic α-SMA expression levels was only found in AdIGF-I-MSC-treated mice (Fig. 5A), suggesting that a downregulation in TGF-β1 expression levels might precede that of α-SMA. We then decided to explore which molecular changes would take place in the fibrotic liver before TGF-β1 and α-SMA downregulation. At day 1 after cellular treatments, an increase in IGF-I and HGF mRNA expression levels was observed in livers from AdGFP-MSC-treated mice when compared with saline (Fig. 5A). Importantly, the mRNA abundance of these molecules was even higher in liver samples obtained from AdIGF-I-MSC-treated mice (Fig. 5A). In addition, at 1 day after AdIGF-I-MSCs administration, a significant increase in TWEAK (TNF-like weak inducer of apoptosis; a marker related to oval cells) was observed when compared with the other experimental groups (Fig. 5A).
FIG. 5.
Early changes in the expression of profibrogenic and antifibrogenic/regenerative factors after MSC treatments. Induction of IGF-I and hepatocyte growth factor (HGF) expression in hepatocytes. (A) Quantitative graphs showing mRNA expression levels of TGF-β1, α-SMA, IGF-I, HGF, TWEAK, and proliferating cell nuclear antigen (PCNA) at 1 and 3 days after vehicle (white bars), AdGFP-MSCs (gray bars) and AdIGF-I-MSCs (black bars) treatments. (B) Quantitative graphs showing mRNA expression levels of IGF-I or HGF by hepatocytes incubated overnight with DMEM, or supernatants (sn) from AdGFP-MSCs or AdIGF-I-MSCs cultures. σP<0.05 versus AdGFP-MSCs (A) or sn AdGFP-MSCs (B); *P<0.05 versus saline (A); **P<0.01 versus saline (A); ***P<0.001 versus DMEM.
At 3 days after treatment, IGF-I remained upregulated in the liver of MSC-treated animals when compared with saline, although at lower levels (Fig. 5A); a similar pattern was found for HGF, which remained upregulated only in the AdIGF-I-MSCs group (Fig. 5A). No differences in hepatic IGF-I or HGF mRNA values were found between experimental groups at 14 days after MSCs or saline administration (not shown). Considering that IGF and HGF upregulation might influence liver regeneration and hepatocyte proliferation, we decided to analyze the expression levels of the proliferation marker PCNA. Notably, PCNA was found to be significantly induced at 1 and 3 days after application of AdIGF-I-MSCs when compared with AdGFP-MSC or saline (Fig. 5A).
From previous data, we can conclude that AdIGF-I-MSC application in the context of liver fibrosis likely results in early liver upregulation of IGF-I and HGF, increased liver cell proliferation (at 1 day after transplantation), and a subsequent reduction in HSCs activation (at 3 days after treatment), events which are likely involved in the observed amelioration of liver fibrosis.
AdIGF-I-MSCs conditioned media treatment induces the upregulation of IGF-I and HGF mRNA expression levels in hepatocytes
We previously showed that MSCs results in the induction of IGF-I and HGF mRNA expression levels in the liver at 1 day after their application in vivo. In order to analyze whether MSCs treatment might induce the expression of IGF-I and/or HGF in hepatocytes, liver hepatic cells were incubated overnight with supernatant from AdGFP-MSCs, AdIGF-I-MSCs, or DMEM. Interestingly, an upregulation in IGF-I and HGF mRNA expression levels was observed in hepatocytes treated with AdIGF-I-MSCs supernatant when compared with controls (Fig. 5B). In addition, a 50% increase in PCNA mRNA expression was found in hepatocytes treated with AdIGF-I-MSCs supernatant when compared with saline (1.51±0.08 vs. 1±0.01; P<0.01). From these results, we can conclude that factors released by AdIGF-I-MSCs induce the expression of IGF-I and HGF in hepatocytes.
In order to analyze whether AdIGF-I transduction might modify the expression profile of MSCs, qPCR analyses were performed. Interestingly, IGF-I and HGF mRNA expression was found to be induced in AdIGF-I tranduced cells when compared with controls, whereas TWEAK remained unchanged in all treatments (Supplementary Fig. S1).
Changes in liver cell proliferation after administration of MSCs and effects of multiple doses of MSCs application on collagen deposition
We have previously shown that PCNA mRNA values were increased at 1 day after AdIGF-I-MSC infusion and were maintained significantly higher than in other experimental conditions after 2 additional days. Moreover, the pattern of PCNA mRNA expression at 1 or 3 days after AdGFP-MSCs application suggests a trend to increase proliferation rates when compared with saline treatment (Fig. 5A).
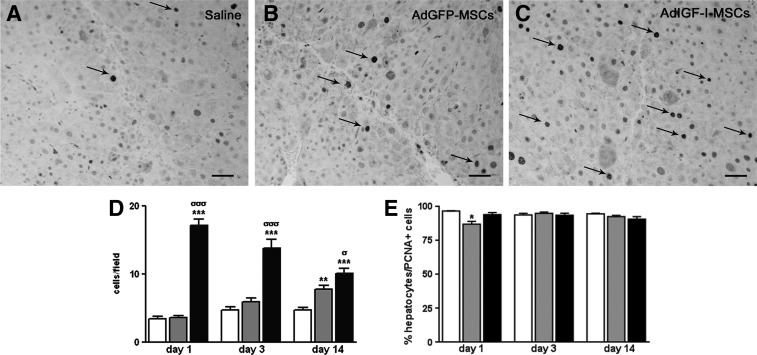
We then asked whether liver cell proliferation might be enhanced in samples obtained from MSC-treated animals at later time points (14 days after cell transplantation). Immunohistochemistry against PCNA showed a specific labeling in the nuclei of parenchymal cells, mostly hepatocytes (Fig. 6E). A significant increase in protein expression levels in liver samples from AdIGF-I-MSC-treated mice was observed when compared with other conditions (Fig. 6A–D). In addition, the number of PCNA+ was also increased in AdGFP-MSC-treated mice at day 14 when compared with saline (Fig. 6A, B, D). Finally, results from PCNA immunohistochemistry analyses at different time points (Fig. 6D) enable us to conclude that the number of PCNA-positive cells increases after AdGFP-MSCs administration and becomes significant at day 14; conversely, PCNA expression peaks at day 1 after AdIGF-I-MSCs infusion and declines afterward, although it remains higher at all time points analyzed when compared with AdGFP-MSCs and vehicle-treated groups.
FIG. 6.
MSCs treatments induce the proliferation of liver parenchymal cells. (A–C) Representative microphotographies of PCNA immunostained liver sections from saline (A), AdGFP-MSCs (B), and AdIGF-I-MSC-(C) treated mice (scale bars: 50 μm). (D) Quantification of liver PCNA immunostained cells at 1, 3, and 14 days after saline (white bars), AdGFP-MSCs (gray bars) or AdIGF-I-MSCs (black bars) administration. (E) Quantification of percentage of hepatocytes expressing PCNA on total population of cells expressing this marker. σP<0.05; **P<0.01; ***σσσP<0.001; * versus saline; σ versus AdGFP-MSCs.
Considering that multiple doses of genetically modified MSCs, using recombinant adenovirus, seem not to induce immunogenicity on these cells, the effect of multiple applications of MSCs over collagen deposition was analyzed by quantifying sirius red-stained area on liver sections obtained from animals subjected to different treatments. All conditions using MSCs ameliorated liver condition when compared with saline (sirius red-stained area: 4.36±0.44; arbitrary units) treatment (not shown). Interestingly, a reduction in collagen accumulation was observed in animals receiving multiple doses of AdGFP-MSCs when compared with those subjected to only one dose of similar treatment (1.77±0.27 vs. 3.025±0.22; P=0.0007; Mann–Whitney test). Moreover, the application of multiple doses of AdIGF-I-MSCs was found to result in a further reduction in collagen deposition when compared with a single dose of AdIGF-I-MSCs (0.65±0.05 vs. 1.719±0.17; P<0.0001; t-test) or with multiple doses of AdGFP-MSCs (P=0.0002, Mann–Whitney test) (not shown).
Discussion
In this study, we show that systemic application of BM-derived AdIGF-I-MSCs results in the amelioration of liver fibrosis. In addition, we observed that three subsequent doses of AdIGF-I-MSCs, separated by 2 weeks in between, further reduced collagen deposition in comparison with a single dose of similar experimental condition. Furthermore, the application of naïve or adenoviral-transduced MSCs into immunocompetent mice was found not to induce the proliferation of splenocytes obtained from a different mouse strain or immunogenicity against adenoviral antigens. These data might have important consequences for future therapeutic use of MSCs, as repeated applications of genetically modified MSCs to overexpress IGF-I using adenovirus might likely result in significantly higher benefits for the long-term treatment of established liver fibrosis with minor adverse effects.
One mechanism partly explaining our results after single-dose MSCs treatment is the reduction in the activation of HSCs, as shown by the downregulation in α-SMA expression levels in livers from AdGFP-MSC-treated animals when compared with saline, and in CFSC-2G rat HSCs, a feature that was further significant after AdIGF-I-MSCs treatment in both experimental settings. These findings are consistent with changes in TGF-β1, a key molecule involved in HSCs activation and fibrogenesis [1], and in COL1A2 mRNA expression patterns. Interestingly, a downregulation in TGF-β1 mRNA expression in CFSC-2G cells was observed when they were cultured overnight in AdIGF-I-MSC supernatant when compared with control conditions, which was lately followed by a downregulation in α-SMA and in COL1A2 mRNA expression levels. Consistent dynamic changes in the pattern of TGF-β1 and α-SMA mRNA expression in the fibrotic liver were also found when MSCs were applied in vivo. Since liver TGF-β1 mRNA levels were decreased only at 3 days after MSCs transplantation, we conclude that this mechanism could be secondary to earlier MSCs-induced local events.
The trophic factors IGF-I and HGF were previously reported to influence TGF-β1 signaling and liver fibrosis [15,19] and were, therefore, considered good candidates for triggering early events likely involved in subsequent TGF-β1 downregulation. Indeed, when AdGFP-MSCs were administered, both IGF-I and HGF mRNA expression levels were upregulated at the first day after MSC treatment, when compared with saline group. Interestingly, treatment with AdIGF-I-MSCs resulted in a further upregulation of both factors at the first day after cell infusion and IGF-I mRNA levels were still significantly higher at day 3 in this group than in vehicle-treated controls. Moreover, at day 3, hepatic HGF expression was higher in AdIGF-I-MSC-treated mice compared with the other two groups. Interestingly, AdIGF-I-MSCs treatment elicited a peak of TWEAK expression at first day after treatment, suggesting the possible involvement of oval stem cell expansion [20], an issue that requires further elucidation. Consistent with these results and with previous reports showing involvement of IGF-I and HGF in the induction of hepatocyte proliferation [16,21], our data suggest that parenchymal cells are stimulated to proliferate in MSC-treated animals. Significantly higher levels of PCNA expression were found in AdIGF-I-MSCs when compared with other experimental conditions at all time points analyzed. In addition, while PCNA protein expression in the AdIGF-I-MSCs treatment peaks at first day after cell infusion and it is downregulated thereafter, it seems gradually upregulated after AdGFP-MSC administration, becoming significant at 14 days when compared with saline treatment. Whether or not the increase in cell proliferation is related to a reduced activation of HSCs and/or to the attenuation of liver fibrosis remains to be elucidated.
It is worth mentioning according to evidence here shown using primary culture settings that factors released by AdIGF-I-MSCs seem to be responsible for an induction in the expression of IGF-I and HGF by hepatocytes. This feature might, at least, partially explain the early increase in IGF-I and HGF observed in the liver after MSC transplantation. Our results suggest that very early events taking place at the first day after MSCs transplantation and likely involving upregulation of HGF and IGF-I expression might be critical in the reduction of liver fibrosis degree observed after these cellular treatments. Indeed, we have been able to detect GFP-labeled transplanted cells in the injured liver tissue. This issue is not trivial, as many reports aimed at tracking MSCs in vivo have not been able to distinguish specific signals from autofluorescence, a feature frequently observed in a fibrotic liver [22,23]. In addition, for cell tracking purposes, other researchers have labeled human MSCs in vitro with Hoechst. Nevertheless, very often instead of showing a typical human staining pattern, Hoechst+ cells within the liver look similar to mouse ones [24]. Our data showing a peak in MSC numbers in the fibrotic liver at 1 day after their administration and a progressive decline in the amount of cells as well as in the expression levels of IGF-I, HGF, TWEAK, and PCNA (in the latter case, for Ad-IGF-I-MSCs condition) thereafter might suggest that early events triggered by MSCs administration might be critical for achieving their therapeutic benefits.
In summary, we have shown a number of molecular events within the fibrotic liver being triggered shortly after MSCs application, resulting in a reduced activation of HSCs, the increase in IGF-I and HGF expression by hepatocytes, the reduction of fibrogenesis, and/or the stimulation of hepatocellular proliferation. Moreover, we found that all these therapeutic effects could be enhanced by engineering MSCs to secrete IGF-I, a condition which is, at least, as beneficial as injecting the cells and applying in parallel rIGF. Finally, our data indicate that a combination of cell and gene therapy and the application of multiple doses of genetically modified MSCs to overexpress IGF-I could represent a promising novel approach to treat liver cirrhosis.
Supplementary Material
Acknowledgments
The authors thank Rodolfo Goya (UNLP, CONICET) for kindly providing them with Ad-IGF-I and Ad-GFP. They also thank Mariano Marrodan and Marcela Giovenco for experimental help, and Soledad Arregui, Vanina Ferreira, and Guillermo Gaston for technical assistance. This work was supported by grants from Austral University (for J.B. I04-12; for M.G.G. T13-12; for J.B.A. 17-09; and for G.M. T13-11) and from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT; PICT-2007/00082, PICT 2008/00123, PICTO 2008/00122, PICTO 2008/00115, and PICT 2010/2818).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Friedman SL. (2008). Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R. and Brenner DA. (2005). Liver fibrosis. J Clin Invest 115:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ. and Fibbe WE. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood 110:3499–3506 [DOI] [PubMed] [Google Scholar]
- 5.di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, et al. (2008). Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 57:223–231 [DOI] [PubMed] [Google Scholar]
- 6.Aquino JB, Bolontrade MF, Garcia MG, Podhajcer OL. and Mazzolini G. (2010). Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther 17:692–708 [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Xu R, Zhang Z, Chen L, Shi M. and Wang FS. (2011). Implications of the immunoregulatory functions of mesenchymal stem cells in the treatment of human liver diseases. Cell Mol Immunol 8:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z. and Baharvand H. (2010). Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int 34:601–605 [DOI] [PubMed] [Google Scholar]
- 9.Miryounesi M, Piryaei A, Pournasr B, Aghdami N. and Baharvand H. (2013). Repeated versus single transplantation of mesenchymal stem cells in carbon tetrachloride-induced liver injury in mice. Cell Biol Int. DOI: 10.1002/cbin.10048 [DOI] [PubMed] [Google Scholar]
- 10.Bonefeld K. and Moller S. (2011). Insulin-like growth factor-I and the liver. Liver Int 31:911–919 [DOI] [PubMed] [Google Scholar]
- 11.Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S. and Prieto J. (1997). Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology 113:1682–1691 [DOI] [PubMed] [Google Scholar]
- 12.Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, Castilla-Cortazar I, Frystyk J, Flyvbjerg A, et al. (2005). Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol 43:630–636 [DOI] [PubMed] [Google Scholar]
- 13.Sobrevals L, Rodriguez C, Romero-Trevejo JL, Gondi G, Monreal I, Paneda A, Juanarena N, Arcelus S, Razquin N, et al. (2010). Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology 51:912–921 [DOI] [PubMed] [Google Scholar]
- 14.Blaas L, Kornfeld JW, Schramek D, Musteanu M, Zollner G, Gumhold J, van Zijl F, Schneller D, Esterbauer H, et al. (2010). Disruption of the growth hormone—signal transducer and activator of transcription 5—insulinlike growth factor 1 axis severely aggravates liver fibrosis in a mouse model of cholestasis. Hepatology 51:1319–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song K, Cornelius SC, Reiss M. and Danielpour D. (2003). Insulin-like growth factor-I inhibits transcriptional responses of transforming growth factor-beta by phosphatidylinositol 3-kinase/Akt-dependent suppression of the activation of Smad3 but not Smad2. J Biol Chem 278:38342–38351 [DOI] [PubMed] [Google Scholar]
- 16.Koch KS, Shapiro P, Skelly H. and Leffert HL. (1982). Rat hepatocyte proliferation is stimulated by insulin-like peptides in defined medium. Biochem Biophys Res Commun 109:1054–1060 [DOI] [PubMed] [Google Scholar]
- 17.Kundu AK, Nagaoka M, Chowdhury EH, Hirose S, Sasagawa T. and Akaike T. (2003). IGF-1 induces growth, survival and morphological change of primary hepatocytes on a galactose-bared polymer through both MAPK and beta-catenin pathways. Cell Struct Funct 28:255–263 [DOI] [PubMed] [Google Scholar]
- 18.Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, Aquino JB, Fiore E, Rizzo MM, Rodriguez A, Lorenti A, Andriani O, Podhajcer O, and Mazzolin G. (2011). Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm 8:1538–1548 [DOI] [PubMed] [Google Scholar]
- 19.Inagaki Y, Higashi K, Kushida M, Hong YY, Nakao S, Higashiyama R, Moro T, Itoh J, Mikami T, et al. (2008). Hepatocyte growth factor suppresses profibrogenic signal transduction via nuclear export of Smad3 with galectin-7. Gastroenterology 134:1180–1190 [DOI] [PubMed] [Google Scholar]
- 20.Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP. and Forbes SJ. (2011). Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology 53:2003–2015 [DOI] [PubMed] [Google Scholar]
- 21.Burr AW, Toole K, Chapman C, Hines JE. and Burt AD. (1998). Anti-hepatocyte growth factor antibody inhibits hepatocyte proliferation during liver regeneration. J Pathol 185:298–302 [DOI] [PubMed] [Google Scholar]
- 22.Ren H, Zhao Q, Cheng T, Lu S, Chen Z, Meng L, Zhu X, Yang S, Xing W, et al. (2010). No contribution of umbilical cord mesenchymal stromal cells to capillarization and venularization of hepatic sinusoids accompanied by hepatic differentiation in carbon tetrachloride-induced mouse liver fibrosis. Cytotherapy 12:371–383 [DOI] [PubMed] [Google Scholar]
- 23.Salguero Palacios R, Roderfeld M, Hemmann S, Rath T, Atanasova S, Tschuschner A, Gressner OA, Weiskirchen R, Graf J. and Roeb E. (2008). Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest 88:1192–1203 [DOI] [PubMed] [Google Scholar]
- 24.Moser FG, Dorman BP. and Ruddle FH. (1975). Mouse-human heterokaryon analysis with a 33258 Hoechst-Giemsa technique. J Cell Biol 66:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.