Abstract
The purpose of this study was to determine metal ion levels in central visual system structures of the DBA/2J mouse model of glaucoma. We used inductively coupled plasma mass spectrometry (ICP-MS) to measure levels of iron (Fe), copper (Cu), zinc (Zn), magnesium (Mg), manganese (Mn), and calcium (Ca) in the retina and retinal projection of 5-month (pre-glaucomatous) and 10-month (glaucomatous) old DBA/2J mice and age-matched C57BL/6J controls. We used microbeam X-ray fluorescence (μ-XRF) spectrometry to determine the spatial distribution of Fe, Zn, and Cu in the superior colliculus (SC), which is the major retinal target in rodents and one of the earliest sites of pathology in the DBA/2J mouse.
Our ICP-MS experiments showed that glaucomatous DBA/2J had lower retinal Fe concentrations than pre-glaucomatous DBA/2J and age-matched C57BL/6J mice. Pre-glaucomatous DBA/2J retina had greater Mg, Ca, and Zn concentrations than glaucomatous DBA/2J and greater Mg and Ca than age-matched controls. Retinal Mn levels were significantly deficient in glaucomatous DBA/2J mice compared to aged-matched C57BL/6J and pre-glaucomatous DBA/2J mice. Regardless of age, the SC of C57BL/6J mice contained greater Fe, Mg, Mn, and Zn concentrations than the SC of DBA/2J mice. Greater Fe concentrations were measured by μ-XRF in both the superficial and deep SC of C57BL/6J mice than in DBA/2J mice. For the first time, we show direct measurement of metal concentrations in central visual system structures affected in glaucoma and present evidence for strain-related differences in metal content that may be specific to glaucomatous pathology.
Glaucoma is the leading cause of irreversible blindness worldwide, affecting an estimated 80 million people by 2020 (Quigley and Broman 2006). The development of neuroprotective therapies for glaucoma has recently emerged as an essential strategy for preventing and treating this disabling disease (McKinnon et al. 2008). Despite greatly increased interest in this line of research, effective interventions remain elusive (Osborne 2009; Danesh-Meyer 2011), possibly because little is known about the causes, mechanisms, and progression of neurodegeneration in glaucoma. Insight into the underlying machinery behind glaucomatous neurodegeneration can come from research on other age-related neurodegenerative conditions, with which glaucoma shares similarities in epidemiology and proposed mechanisms (McKinnon 2003; Crish et al. 2010; Crish and Calkins 2011).
Trace metals are essential for normal cellular function. This is especially evident in the central nervous system; among other functions, metal ions play a large role at the synapse. Metal ions such as zinc (Zn), iron (Fe), manganese (Mn), and copper (Cu) are critical cofactors needed for neurotransmitter synthesis; calcium (Ca) is essential for neurotransmitter release and plasticity; and Zn and magnesium (Mg) modulate synaptic activity (Sourkes 1972; Paoletti et al. 2009; Corona et al. 2011; Südhof 2012). Given the importance of these molecules, concentrations in the nervous system are tightly regulated. One area of increasing interest as both a causative factor and target of intervention in neurodegenerative diseases is metal ion dyshomeostasis. Abnormal levels (both increases and decreases) of Cu, Zn, and Fe ions have been implicated in the pathogenesis and progression of a number of central nervous system neurodegenerative disorders, including Alzheimer’s disease (DeToma et al. 2012; Pithadia and Lim 2012; Kepp 2012; Savelieff et al. 2013), Parkinson’s disease (Dexter at el. 1992; Bisaglia et al. 2009), and Huntington’s disease (Dexter et al. 1992). Metals ions are important activators or cofactors for many transporters, transcription factors, and enzymes. Therefore, tight regulation of metal ion levels is essential for normal cellular function. Even minor alterations in these metal ions can have dramatic effects on cell physiology and survival (Sigel et al. 2006).
Given the overlap in pathological progression between glaucoma and other neurodegenerative diseases, it is surprising that only a handful of studies have investigated metal ions in glaucoma. In this sparse body of work, there is indirect evidence of metal ion involvement in glaucoma; upregulation of metal-regulating genes and proteins have been shown in human glaucomatous retinas (Farkas et al. 2004; Stasi et al. 2007), and in monkey (Farkas et al. 2004; Stasi et al. 2007; Miyahara et al. 2003) and mouse models of glaucoma (Stasi et al. 2007; Miyahara et al. 2003; Steele et al. 2006). The roles of the metals themselves in glaucomatous neurodegeneration is unknown; however, Cu and Zn ions have been evaluated in terms of their relationship with intraocular pressure (IOP) modulation (Akyol et al. 1990; Iqbal et al. 2002), the major modifiable risk factor in glaucoma. This relationship, however, was not investigated in the context of the neural changes that cause vision loss. A promising finding that was recently reported is that Mg supplementation was shown to improve visual field deficits in glaucoma (Aydin et al. 2010) – implying metal ion levels as an attractive target for intervention.
Using inductively coupled plasma mass spectrometry (ICP-MS), we directly examined the levels of six metals commonly implicated in age-related neurodegeneration: Fe, Cu, Zn, Mg, Mn, and Ca) in the retina and retinal projection of the DBA/2J mouse model of glaucoma. Furthermore, we used microbeam X-ray fluorescence (μ-XRF) spectrometry to determine the spatial distribution of Fe, Cu, and Zn in the superior colliculus (SC), the major retinal target in rodents and one of the earliest sites of pathology in the DBA/2J mouse (Crish et al. 2010).
Materials and Methods
Animals
DBA/2J and C57BL/6J mice were obtained from breeding colonies at the Northeast Ohio Medical University’s (NEOMED) Comparative Medicine Unit. Animals were originally obtained from Jackson Laboratories (Bar Harbor, ME). For ICP-MS, three animals from each strain were examined at two different: ages a) 5-months, an age that most DBA/2J mice would be considered “pre-glaucomatous” and b) 10-months, an age where we have found significant functional deficits but not large-scale neurodegeneration. For μ-XRF, two animals from each strain at 5 and 12 months of age were examined. All mice were housed in the same room under the same environmental and husbandry conditions. All experiments involving animals adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the NEOMED Institutional Animal Care and Use Committee.
Tissue Preparation
For ICP-MS, animals were anesthetized with 4% isoflurane prior to cervical dislocation and decapitation. Retinas, optic nerves, SC, and cerebellum were rapidly dissected using Teflon-coated instruments and frozen on dry ice. Cerebellum was collected as a control structure as it is unaffected in glaucoma. For μ-XRF, animals were anesthetized with ketamine/xylazine as described above and were transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde in 0.9% NaCl. Brains were removed and cryoprotected overnight in 20% sucrose/0.9% NaCl and a microtome was used to collect uniform 50 μm coronal sections through the midbrain containing SC.
Determination of metal concentrations
ICP-MS was performed at the Life Sciences Institute at the University of Michigan. Samples were treated with concentrated nitric acid for 2 h at 70 °C followed by dilution with the equal volume of water. Metals were determined using a Thermo Scientific Element2 Inductively Coupled Plasma-High Resolution Mass Spectrometer and referenced either to the weight of the sample or the protein concentration as determined by a BCA assay. Values are reported in μmol (μM) of metal/mg total wet weight or protein.
μ-XRF
Synchrotron microbeam X-ray fluorescence (μ-XRF) spectrometry was performed as described previously (Miller et al. 2006; Leskovjan et al. 2009; Leskovjan et all 2011). μ-XRF measurements were conducted at the Biophysics Collaborative Access Team (BioCAT) beam at the Advanced Photon Source (APS) at Argonne National Laboratory (Argonne, IL). Tissues from 16 colliculi were obtained (2 animals per age group from 5 month and 12 month C57BL/6J and DBA2/J mice). Each colliculus was treated as an independent sample. Tissues were placed in a high density polyethylene sample holder between two sheets of Ultralene (SPEX SamplePrep, Metuchen, NJ). Analyses of metal contents and distributions were conducted in non-overlapping regions of interest (ROI) of various sizes within the retinal ganglion cell layers in the retina and the SC region of the brain using μ-XRF at the Biophysics Collaborative Access Team (BioCAT) beamline 18 at the Advanced Photon Source (APS) at Argonne National Laboratory (Argonne, IL, USA). The synchrotron X-ray beam was tuned to 10 keV using a Si(111) double crystal monochromator. In the brain tissue, the SC is restricted to the upper 300 μm of the tissue that remains upon removal of the cortex; an ROI sufficiently large to encompass this area was scanned. The incident beam was focused to 10 μm × 10 μm or 20 μm × 20 μm at the sample using Kirkpatrick-Baez mirrors. Spectra were collected with a dwell time of 250 ms/pixel and 10 or 20 μm step size. Variations due to beam flux were normalized based on changes in I0.
Data were processed in MatLab using an adaptation of a program which produced a map of the distribution of metals. Metal concentrations were estimated by comparison with the intensity from thin metal foils (Fe, 0.1 μm, Cu, 0.1 μm, and Zn, 0.08 μm) measured under identical conditions. The approximate molar concentration was determined by accounting for the thickness of the tissue, assuming a density of 1 g/cm3 and dividing by the atomic weight of these elements.
Data Analysis
For the ICP-MS study, data were analyzed using 2 × 2 factorial analyses of variance (ANOVA) with strain (C57BL/6J vs. DBA/2J) and age (5 months vs. 10 months) serving as between subjects’ factors and metal concentrations serving as the dependent variables. Separate analyses were conducted for each metal in each of four targeted brain regions (i.e., retina, optic nerve, SC and cerebellum). Post-hoc comparisons using Tukey’s Honestly Significant Differences (HSD) tests were used to define relationships revealed by significant main effects and/or strain by age interactions.
For the μ-XRF study, our allotted beam time at the APS only allowed us to image sections from two 5 month and two 10 month C57BL/6J SC and one 5 month and two 12 month DBA/2J SC. Given the small sample size, we pooled data across ages from each strain. Our main comparisons were the superficial (retinorecipient) SC and deep SC between the strains. Data were analyzed with either one-way ANOVAs or nonparametric Kruskal-Wallis tests (in cases where datasets did not have normal distributions) to assess differences between DBA/2J mice and C57BL/6J controls in three different metal concentrations (Fe, Zn, and Cu) in either superficial or deep SC.
Results
ICP-MS
Retina
Main effects for age were shown in Fe (F1,23 = 6.40), Mg (F1,23 = 7.61), Ca (F1,23 = 11.67) and Zn (F1,23 = 10.43) concentrations (all p <0.05 for all); however, these results were qualified by significant strain by age interactions in metal concentration for Fe (F1,23 = 14.45, p <0.01), Mg (F1,23 = 4.92, p < 0.05), Ca (F1,23 = 8.08, p = 0.01), and Zn (F1,23 = 5.86, p <0.05). Additionally, a significant strain by age interaction was shown for retinal Mn level (F1,23 = 6.32, p <0.05). Analyses of the simple main effects for these interactions are described as follows and are illustrated in Figure 1. Mean metal concentrations in retina for each group analyzed are provided in Table 1.
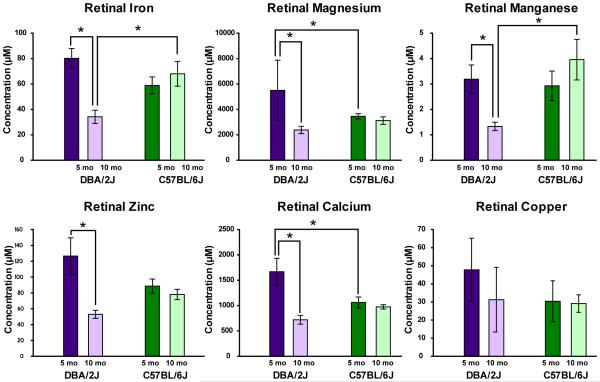
Figure 1.
Mean concentration (μM) of metals in the retina by mouse strain (DBA/2J; C57BL/6J) and age-group (5 and 10 months). Iron: Glaucomatous DBA/2J (10-month old) retina showed lower iron concentrations than 10-month old C57BL/6J control mice retina (p <0.01). Additionally, 10-month old DBA/2J retina had lower iron content than pre-glaucomatous (5-month old) DBA/2J retina (p <0.01). There were no differences in retinal iron content between strains at 5-months (p = ns). Manganese: Retinal manganese levels were significantly deficient in 10-month old DBA/2J mice compared to aged-matched C57BL/6J mice (p <0.01) and 5-month old DBA/2J mice (p <0.05). Retinal manganese levels did not differ between strains at 5-months (p = ns). Magnesium & calcium: 5-month old DBA/2J retina had greater magnesium and calcium concentrations than age-matched C57BL/6J mice (p < 0.05 for both Mg & Ca). 5-month old DBA/2J also had significantly greater retinal magnesium and calcium than 10-month old DBA/2J mice (p <0.01 for both Mg & Ca). By 10-months of age, both DBA/2J and C57BL/6J retina did not differ in magnesium or calcium content. Zinc: 5-month old DBA/2J retina mice showed over two-fold greater zinc concentrations than 10-month old DBA/2J retina (p <0.01). There were no other statistically significant differences between groups, but the data show a trend towards higher zinc concentrations in 5-month DBA/2J retina compared to all C57BL/6J retina. Statistically significant differences between groups are indicated by asterisks. n = 6/group
Table 1.
Mean metal concentration in retina by age and strain
| Mean concentration (μM)±SEM | ||||
|---|---|---|---|---|
| DBA/2J | C57BL/6J | |||
| Metal | 5-mo | 10-mo | 5-mo | 10-mo |
| Iron | 80.3±7.7 | 34.2±5.2 | 58.9±6.7 | 68.1±9.8 |
| Magnesium | 5500.6±1165.7 | 2381.5±288.0 | 3458.8±204.9 | 3120.5±295.2 |
| Manganese | 3.2±0.6 | 1.3±0.2 | 2.9±0.6 | 4.0±0.8 |
| Zinc | 126.6±23.0 | 53.0±5.1 | 88.6±8.9 | 78.1±6.5 |
| Calcium | 1668.0±265.8 | 720.7±86.0 | 1061.7±108.7 | 975.0±42.1 |
| Copper | 47.8±17.5 | 31.3±17.8 | 30.4±11.3 | 29.1±4.8 |
Iron
Tukey post-hoc analyses revealed that glaucomatous DBA/2J (10-month old) retina had lower Fe concentrations than retina from age-matched C57BL/6J control mice (p <0.01). Additionally, 10-month old DBA/2J retina had lower Fe than pre-glaucomatous (5-month old) DBA/2J retina (p < 0.01). Retina from 5-month old DBA/2J and age-matched C57BL/6J mice did not differ in Fe content (p = ns).
Manganese
Retinal Mn levels were significantly deficient in 10-month old DBA/2J mice compared to aged-matched C57BL/6J (p <0.01) and 5-month old DBA/2Js (p <0.05). Retinal Mn levels did not differ between strains at the 5-month age point (p = ns).
Magnesium
Pre-glaucomatous (5-month old) DBA/2J retina had greater Mg concentrations than their age-matched controls (p <0.05). The 5-month old DBA/2J also had significantly greater retinal Mg than 10-month old DBA/2J mice (p < 0.01). At 10-months of age, neither DBA/2J nor C57BL/6J retina differed in Mg content (p= ns).
Calcium
The same pattern of results shown for Mg concentration were obtained for retinal Ca levels. The 5-month DBA/2J retina had greater Ca concentrations than their age-matched C57BL/6J controls (p = 0.01) and 10-month DBA/2J mice samples (p <0.01). No differences in Ca content were seen between strains at 10-months (p = ns).
Zinc
The retina of pre-glaucomatous DBA/2J mice showed over two-fold greater Zn concentrations than 10-month old DBA/2J retina (p <0.01). There were no other statistically significant differences between groups, but the data show a trend towards higher Zn concentrations in 5-month DBA/2J retina compared to C57BL/6J (5-month and 10-month) retina as well (see Figure 1).
Copper
No differences in retinal Cu levels were shown between any of the groups of the study.
Optic nerve
A significant main effect for strain was shown in optic nerve Fe concentration, F1,23 = 6.277, p <0.05. Independent of age, DBA/2J mice exhibited lower concentrations of Fe (mean uM± SEM = 123.50 ± 28.77) in the optic nerve compared to C57BL/6J controls (mean uM± SE = 217.87 ± 21.88; Tukey test p <0.05). No other significant differences in metal concentration in the optic nerve were shown. Mean metal concentrations in ON for individual groups of the study are provided in Table 2.
Table 2.
Mean metal concentration in optic nerve by age and strain
| Mean concentration (μM) ± SEM | ||||
|---|---|---|---|---|
| DBA/2J | C57BL/6J | |||
| Metal | 5-mo | 10-mo | 5-mo | 10-mo |
|
| ||||
| Iron | 122.2±39.2 | 124.9±23.8 | 231.4±58.2 | 204.4±29.3 |
| Magnesium | 3203.7±784.5 | 3248.6±819.5 | 4332.3±1369.9 | 6242.6±238.2 |
| Manganese | 5.4±1.9 | 3.4±0.7 | 9.2±5.4 | 2.9±0.9 |
| Zinc | 95.2±25.6 | 124.5±55.1 | 117.4±36.1 | 116.3±21.8 |
| Calcium | 1205.4±265.3 | 922.6±235.8 | 1900.1±715.5 | 1521.0±226.4 |
| Copper | 56.3±18.9 | 37.4±7.5 | 81.2±18.8 | 261.2±138.2 |
Superior Colliculus
Factorial ANOVAs revealed main effects for strain in collicular Fe (F1,23 =22.14, p < 0.001), Mg (F1,23 = 12.43, p < 0.01), Mn (F1,23 = 27.06, p < 0.001), and Zn (F1,23 = 8.78, p <0.01) content. Regardless of age, the SC of C57BL/6J control mice contained greater metal concentrations than the SC of DBA/2J mice (see Table 3). While there are no significant age effects, it is notable that the data show trends towards lower metal concentrations in the glaucomatous (10-month old) DBA/2J SC in contrast to 5-month old DBA/2J and C57BL/6J controls. No differences in Ca and Cu levels were shown between any of the groups. The breakdown for individual group means for each metal concentration are shown in Table 4.
Table 3.
Mean metal concentration in DBA/2J and C57BL/6J superior colliculus collapsed across age groups
| Mean concentration (μM)±SEM | ||
|---|---|---|
| Metal | DBA/2J | C57BL/6J |
| Iron* | 193.46±12.47 | 304.24±21.84 |
| Magnesium* | 3233.03±224.52 | 4138.16±129.07 |
| Manganese* | 4.75±0.32 | 6.98±0.30 |
| Zinc* | 104.01±7.44 | 130.94±5.51 |
| Calcium | 937.75±157.22 | 1080.85±86.63 |
| Copper | 50.63±4.69 | 62.65±4.66 |
p<0.05
Table 4.
Individual group means for metal concentrations in the superior colliculus
| Mean concentration (μM)±SEM | ||||
|---|---|---|---|---|
| DBA/2J | C57BL/6J | |||
| Metal | 5-mo | 10-mo | 5-mo | 10-mo |
| Iron | 206.6±7.8 | 180.0±23.5 | 269.3±22.2 | 339.14±41.0 |
| Magnesium | 3509.5±126.6 | 2956.6±418.6 | 4186.2±186.1 | 4090.2±237.9 |
| Manganese | 5.1±0.2 | 4.4±0.6 | 7.4±0.4 | 6.6±0.5 |
| Zinc | 114.0±4.3 | 94.0±13.6 | 135.1±3.3 | 126.8±13.1 |
| Calcium | 890.0±76.1 | 985.5±320.2 | 1117.5±121.3 | 1044.2±163.2 |
| Copper | 51.0±5.2 | 50.25±8.3 | 71.9±6.2 | 53.4±5.8 |
Cerebellum
A significant main effect for strain was shown for cerebellar Zn content, F1,23 = 5.46, p <0.05. DBA/2J mice (mean uM ± SEM = 120.04 ± 4.59) had overall greater Zn concentrations in their cerebellum compared to C57BL/6J control mice (mean uM ± SEM= 106.6 ± 3.08; p <0.05). Cerebellar Fe content was shown to differ significantly as a function of animal age, F1,23 = 11.46, p <0.01. Older animals (10-months; mean uM ± SEM = 280.6 ± 13.97) showed more accumulation of iron in their cerebellum than their younger counterparts (5-months; mean uM ± SEM = 223.4 ± 9.23) regardless of strain. No differences in cerebellar Mg, Mn, Ca, or Cu were indicated between groups. Individual group means for metal concentration are shown in Table 5.
Table 5.
Mean metal concentration in cerebellum by age and strain
| Mean concentration (μM)±SEM | ||||
|---|---|---|---|---|
| DBA/2J | C57BL/6J | |||
| Metal | 5-mo | 10-mo | 5-mo | 10-mo |
| Iron | 231.0±10.1 | 267.5±24.6 | 215.8±15.8 | 293.7±13.7 |
| Magnesium | 3370.0±124.2 | 3455.5±122.7 | 3246.7±150.7 | 3276.7±103.3 |
| Manganese | 4.3±0.1 | 4.4±0.2 | 4.5±0.2 | 4.3±0.1 |
| Zinc | 117.5±4.9 | 122.6±8.2 | 106.4±5.0 | 106.9±4.1 |
| Calcium | 649.3±37.0 | 733.7±69.3 | 764.3±98.2 | 614.7±30.3 |
| Copper | 52.7±4.6 | 64.7±4.6 | 53.1±6.9 | 56.7±1.3 |
μ-XRF
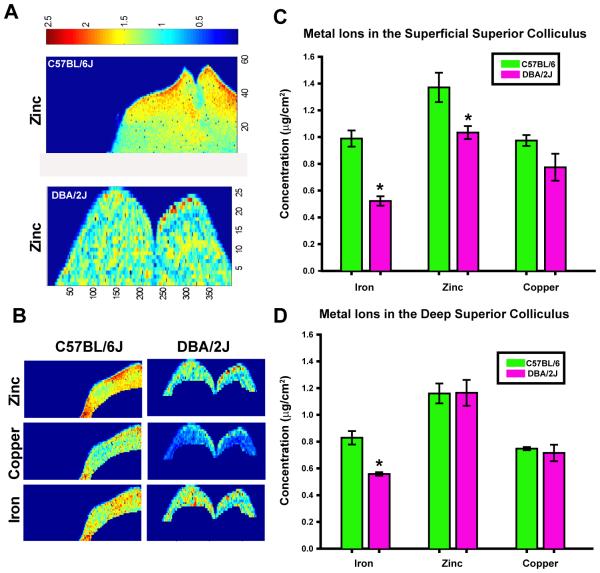
Due to small sample size, data were pooled across ages for each of the strains; therefore, no age-related effects were assessed. Initial qualitative analyses showed that the distribution of Fe was relatively uniform throughout the SC, while Cu and Zn were identifiable in more discrete regions of this tissue. The quantitative results of the μ-XRF study showed that Fe concentrations were significantly greater in C57BL/6J animals than DBA/2J mice in both the superficial (F1,7 = 44.58, p < 0.001) and deep SC (F1,7 = 19.92, p <0.01). Additionally, control animals had greater Zn concentrations in superficial SC than DBA/2J mice (F1,7 = 7.93, p <0.05). There was no difference in Zn in the deep SC. Consistent with the ICP-MS studies, no differences in Cu concentration were shown between strains in either area of the SC. Results of this experiment are shown in Figure 2.
Figure 2.
Data from μ-XRF spectrometry experiment. A) Spectrometry of slices through the midbrain showing zinc concentrations in C57BL/6J control and DBA/2J mice. Warmer colors indicate greater concentration of metal (ug/cm2). B) Examples of separation of the superficial superior colliculus for analysis of Zn, Cu and Fe in C57 and DBA/2J mice. C & D) Metal concentration by volume in superficial (panel C) and deep (panel D) superior colliculus (SC). Iron concentrations were increased in C57BL/6J control mice (n =4) compared to DBA/2J mice (n =3) in both the superficial and deep SC. C57BL/6J mice had greater Zn content in superficial SC than DBA/2J mice. There was no difference in Zn in the deep SC. No differences in Cu concentration were shown between strains in either area of the SC. Asterisks indicate statistically significant results. Due to small sample size, data were pooled across 5 & 12 month ages in both strains.
Discussion
Disruptions of metal levels can result in severe dysfunction and degeneration in the nervous system (Sigel et al. 2006). Abnormal metal metabolism has been postulated to play a role in the progression of pathology in glaucoma, yet few studies have quantified metal content in the tissues affected in this disease. Here, we examined metal concentration in the retinas and retinal projections of the DBA/2J mouse model of glaucoma and found significant decreases in a variety of metals.
The cerebellum was chosen as a control structure because it appears to be unrelated to the visual and auditory defects characteristic of the DBA/2J. It was easily accessed in our dissection and its globular structure allowed quick and reproducible selection of tissue. In assessing metal concentration, the cerebellum gave consistent results with low variability among experimental groups. Four of the metals tested did not show any difference between age or strain in this structure. Fe increased in similar magnitude with age in both strains, and only Zn showed a minor (~12%) increase in DBA/2J mice compared to C57BL/6J mice. Therefore, any differences in metal concentration shown in the central visual system structures likely are related to pathology associated with the visual system and not just a reflection of global changes in metal content of the brain.
Our retina results were striking as we found increased metal levels in young DBA/2J mice and reduced levels of metals in our aged DBA/2J animals. One explanation is that metal level elevations seen in other neurodegenerative diseases during overt degeneration (Sigel et al. 2006) may actually occur early in the DBA/2J retina. There is evidence that ceruloplasmin, a metal handling protein is elevated in 8 month DBA/2J mice (Steele et al. 2006). This raises the possibility that these elevations drive pathological progression through mechanisms reported in other disorders, such as increased oxidative stress and facilitation of protein aggregation (Gaeta and Hider 2005). At 5 months, we expect little if any RGC loss (Buckingham et al. 2008) in DBA/2J mice, yet significant dendritic pruning and synapse loss has been reported in other studies (Jakobs et al. 2005; Stevens et al. 2007). Given the importance of metals at synapses (Frederickson 1989; Tamano and Takeda 2011), it is possible that reductions in metal concentrations in 10-month old DBA/2J mouse visual structures are due to synaptic stripping and lack of resulting neuronal signaling.
Conversely, our colliculus data revealed decreased metal levels in the SC of DBA/2J mice regardless of age–although with some metals, there was evidence of trend towards lower concentrations in older animals. Early disruption of metal levels of the SC is consistent with our emerging understanding of early changes in glaucoma. The SC is the major target of the retina in rodents and an early site of the manifestation of pathology (Crish et al. 2010). Given that it is a location rich in retinal ganglion cell (RGC) synapses, it is not surprising that metal loss was observed. While RGC dendrites and synapses in the retina are also affected early in disease progression, we did not see significant metal decreases in the retina until older ages. There are few possibilities explaining this disparity. Due to the organization of the retina, RGC synapses from bipolar and other retinal cells that are affected disproportionately in glaucoma are relatively fewer in number than other synapses in this tissue. Contrasting with that, a larger percentage of the synapses in the superficial SC are synapses from RGCs (Valverde, 1973). Additionally, the retinocollicular synapses are classic glutamatergic presynaptic boutons and the synaptic compartment affected in the glaucomatous retina is postsynaptic. Much of the synaptic metals are stored presynaptically (Sourkes 1972; Paoletti et al. 2009; Corona et al. 2011) and it is unclear is how the presynaptic portion of these synapses are affected in early glaucoma. We might expect more changes in metal concentrations at presynaptic regions of RGCs (i.e. regions located in the brain).
Data obtained from the μ-XRF experiments supports results from our ICP-MS data that show reductions in iron content in the DBA/2J mouse SC. Our μ-XRF data also showed reduced zinc content in the superficial (retinorecipient) layers of the DBA/2J SC. While we were not able to test age-related differences due to small sample size, the μ-XRF technique shows promise for allowing us to evaluate sectorial changes in metal concentration in different portions of the SC. Sectorial changes in structure and function are pathological hallmarks of glaucoma (Soto et al. 2008; Crish et al. 2010). Assessing differences in metal concentration between adjacent tissue sections of the same structure based on pathology would be a powerful technique for determining subtle but important differences in metal concentration. Our data, while preliminary, provide proof of concept that this technique may have a great potential for yielding meaningful results going forward with larger sample sizes.
The Phenome Database at Jackson Laboratories (Yuan and Korstanje 2014) reported blood levels of Ca in DBA/2J and C57BL/6J mice vary little (1-7%) between the two strains at 6, 12, or 18 months of age (see Table 6). In mice that were similar to the age ranges we tested in the current study (6 & 12 months), blood levels of Fe presented were higher in DBA/2Js than C57BL/6J mice (Yuan and Korstanje 2014), opposite of our findings for Fe content in the SC and in the retina of 10-month old DBA/2J mice (recall Figure 1). By 18 months of age, older than we tested in the current study, blood levels of Fe between the strains were equivalent. Blood levels of Mg were similar between the two strains at 6 and 12 months of age (Table 6)–this was not the case with Mg content in the retina which was lower in C57BL/6J and 10-month old DBA/2J mice compared to 5-month old DBA/2J mice. Additionally, our SC data showed higher Mg concentrations overall in C57BL/6J mice. These differences in the reported blood metal concentration data and our visual system structure data suggest that any differences we are seeing between strains in the current study are unlikely to be nonspecific, but are associated with specific degeneration and dysfunction of the visual system. This assertion is also corroborated by our cerebellar control data that do not reflect strain-related differences in metal concentration.
Table 6.
Blood serum values for metals (mg/dL)*
| Age | 6-mo | 12-mo | 18-mo | |||
|---|---|---|---|---|---|---|
| DBA/2J | C57BL/6J | DBA/2J | C57BL/6J | DBA/2J | C57BL/6J | |
| Ca | 9.93 | 9.4 | 10.3 | 10.2 | 9.5 | 10.2 |
| Fe | 248.0 | 156.0 | 234.0 | 153.0 | 162.0 | 166.0 |
| Mg | 3.0 | 2.8 | 2.5 | 3.1 | 2.5 | 3.7 |
Table adapted from Yuan R. & Korstanje R. 2014. Aging study: Blood chemistry for 32 inbred strains of mice. MPD:Yuan3. Mouse Phenome Database web site, The Jackson Laboratory, Bar Harbor, Maine USA. http://phenome.jax.org.
We report here, for the first time, the direct measurement of metal concentrations in central visual system structures affected in glaucoma using ICP-MS and μ-XRF technology. While in their infancy, these explorations into the significance of metal concentrations in glaucoma, suggest this may be a fertile avenue for investigating ways to therapeutically manipulate metal levels in this disorder, as has been suggested in other neurodegenerative disorders (Corona et al. 2011).
Acknowledgements
The acknowledgment can be added as follows "The authors would like to acknowledge Dr. Raul Barrea of Sector 18 (BIOCAT beamline) beamline support, Andrew Crawford for help with MatLab programming, and Kevin O’Neill for help with processing XRF images. The authors also thank Dr. Ted Huston for assistance with the ICP-MS samples. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357.
This project was supported by grants (9 P41 GM103622-18) from the National Institute of General Medical Sciences of the National Institutes of Health.
This work was supported by the following funding sources:
The Ruth K. Broad Biomedical Foundation and the 2013 Research Fund (Project Number 1.130068.01) of UNIST (Ulsan National Institute of Science and Technology) (to M.H.L.).
EY022358 from the National Eye Institute (to S.D.C.)
NSF Graduate Research Fellowship (to A.S.D.).
References
- 1.Akyol N, Değer O, Keha EE, Kiliç S. Aqueous humour and serum zinc and copper concentrations of patients with glaucoma and cataract. Br J Ophthalmol. 1990;74(11):661–2. doi: 10.1136/bjo.74.11.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aydin B, Onol M, Hondur A, Kaya MG, Ozdemir H, Cengel A, Hasanreisoglu B. The effect of oral magnesium therapy on visual field and ocular blood flow in normotensive glaucoma. Eur J Ophthalmol. 2010;20(1):131–5. doi: 10.1177/112067211002000118. [DOI] [PubMed] [Google Scholar]
- 3.Bisaglia M, Tessari I, Mammi S, Bubacco L. Interaction between alpha-synuclein and metal ions, still looking for a role in the pathogenesis of Parkinson's disease. Neuromolecular Med. 2009;11(4):239–51. doi: 10.1007/s12017-009-8082-1. [DOI] [PubMed] [Google Scholar]
- 4.Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28(11):2735–44. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corona C, Pensalfini A, Frazzini V, Sensi SL. New therapeutic targets in Alzheimer's disease: brain deregulation of calcium and zinc. Cell Death Dis. 2011;2:e176. doi: 10.1038/cddis.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crish SD, Calkins DJ. Neurodegeneration in glaucoma: progression and calcium-dependent intracellular mechanisms. Neuroscience. 2011;176:1–11. doi: 10.1016/j.neuroscience.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107(11):5196–201. doi: 10.1073/pnas.0913141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011;22(2):78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- 9.DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Misfolded proteins in Alzheimer's disease and type II diabetes. Chem Soc Rev. 2012;41(2):608–21. doi: 10.1039/c1cs15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dexter DT, Jenner P, Schapira AH, Marsden CD. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. The Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol. 1992;32(Suppl):S94–100. doi: 10.1002/ana.410320716. [DOI] [PubMed] [Google Scholar]
- 11.Farkas RH, Chowers I, Hackam AS, Kageyama M, Nickells RW, Otteson DC, Duh EJ, Wang C, Valenta DF, Gunatilaka TL, Pease ME, Quigley HA, Zack DJ. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci. 2004;45(5):1410–7. doi: 10.1167/iovs.03-0872. [DOI] [PubMed] [Google Scholar]
- 12.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 13.Gaeta A, Hider RC. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol. 2005;146(8):1041–59. doi: 10.1038/sj.bjp.0706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal Z, Muhammad Z, Shah MT, Bashir S, Khan T, Khan MD. Relationship between the concentration of copper and iron in the aqueous humour and intraocular pressure in rabbits treated with topical steroids. Clin Experiment Ophthalmol. 2002;30(1):28–35. doi: 10.1046/j.1442-9071.2002.00480.x. [DOI] [PubMed] [Google Scholar]
- 15.Jakobs TC, Libby RT, Ben Y, John SW, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171(2):313–25. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kepp KP. Bioinorganic chemistry of Alzheimer's disease. Chem Rev. 2012;112(10):5193–239. doi: 10.1021/cr300009x. [DOI] [PubMed] [Google Scholar]
- 17.Leskovjan AC, Kretlow A, Lanzirotti A, Barrea R, Vogt S, Miller LM. Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer's Disease. NeuroImage. 2011;55:32–38. doi: 10.1016/j.neuroimage.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leskovjan AC, Lanzirotti A, Miller LM. Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer's Disease. NeuroImage. 2009;47:1215–20. doi: 10.1016/j.neuroimage.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinnon SJ. Glaucoma: ocular Alzheimer's disease? Front Biosci. 2003;8:s1140–56. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- 20.McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TJ. Current management of glaucoma and the need for complete therapy. Am J Manag Care. 2008;14(1 Suppl):S20–7. [PubMed] [Google Scholar]
- 21.Miller, LM, Wang Q, Telivala TP, Smith, RJ, Lanzirotti A, Miklossy J. Synchrotron-Based Infrared and X-ray Imaging Shows Focalized Accumulation of Cu and Zn Co-Localized with β-Amyloid Deposits in Alzheimer's Disease. Journal of Structural Biology. 2006;155:30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Miyahara T, Kikuchi T, Akimoto M, Kurokawa T, Shibuki H, Yoshimura N. Gene microarray analysis of experimental glaucomatous retina from cynomologous monkey. Invest Ophthalmol Vis Sci. 2003;44(10):4347–56. doi: 10.1167/iovs.02-1032. [DOI] [PubMed] [Google Scholar]
- 23.Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009;87(4):450–4. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 24.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158(1):126–36. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 25.Pithadia AS, Lim MH. Metal-associated amyloid-β species in Alzheimer's disease. Curr Opin Chem Biol. 2012;16(1-2):67–73. doi: 10.1016/j.cbpa.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savelieff MG, Lee S, Liu Y, Lim MH. Untangling amyloid-β, tau, and metals in Alzheimer's disease. ACS Chem Biol. 2013;8(5):856–65. doi: 10.1021/cb400080f. [DOI] [PubMed] [Google Scholar]
- 28.Schütz, A, Bergdahl IA, Ekholm A, Skerfving S. Measurement by ICP-MS of lead in plasma and whole blood of lead workers and controls. Occup Environ Med. 1996;53(11):736–740. doi: 10.1136/oem.53.11.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigel A, Sigel H, Sigel RKO. Neurodegenerative Diseases and Metal Ions: Metal Ions in the Life Sciences. West Sussex, England. 2006;1 [Google Scholar]
- 30.Soto I, Oglesby E, Buckingham BP, Son JL, Roberson ED, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28(2):548–561. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sourkes TL. Influence of specific nutrients on catecholamine synthesis and metabolism. Pharmacol Rev. 1972;24(2):349–59. [PubMed] [Google Scholar]
- 32.Stasi K, Nagel D, Yang X, Ren L, Mittag T, Danias J. Ceruloplasmin upregulation in retina of murine and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2007;48(2):727–32. doi: 10.1167/iovs.06-0497. [DOI] [PubMed] [Google Scholar]
- 33.Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):977–85. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 34.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4(1) doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamano H, Takeda A. Dynamic action of neurometals at the synapse. Metallomics. 2011;3(7):656–61. doi: 10.1039/c1mt00008j. [DOI] [PubMed] [Google Scholar]
- 37.Valverde F. The Neuropil in Superficial Layers of the Superior Colliculus of the Mouse. Z. Anat. Entwickl.-Gesch. 1973;142:117–147. doi: 10.1007/BF00519719. [DOI] [PubMed] [Google Scholar]
- 38.Yuan R, Korstanje R. Aging study: Blood chemistry for 32 inbred strains of mice. MPD:Yuan3. Mouse Phenome Database web site. The Jackson Laboratory, Bar Harbor, Maine USA. 2014 http://phenome.jax.org.