Abstract
We demonstrate that subpopulations of adult human skeletal muscle-derived stem cells, myogenic endothelial cells (MECs), and perivascular stem cells (PSCs) can be simultaneously purified by fluorescence-activated cell sorting (FACS) from cryopreserved human primary skeletal muscle cell cultures (cryo-hPSMCs). For FACS isolation, we utilized a combination of cell lineage markers: the myogenic cell marker CD56, the endothelial cell marker UEA-1 receptor (UEA-1R), and the perivascular cell marker CD146. MECs expressing all three cell lineage markers (CD56+UEA-1R+CD146+/CD45−) and PSCs expressing only CD146 (CD146+/CD45−CD56−UEA-1R−) were isolated by FACS. To evaluate their myogenic capacities, the sorted cells, with and without expansion in culture, were transplanted into the cardiotoxin-injured skeletal muscles of immunodeficient mice. The purified MECs exhibited the highest regenerative capacity in the injured mouse muscles among all cell fractions tested, while PSCs remained superior to myoblasts and the unpurified primary skeletal muscle cells. Our findings show that both MECs and PSCs retain their high myogenic potentials after in vitro expansion, cryopreservation, and FACS sorting. The current study demonstrates that myogenic stem cells are prospectively isolatable from long-term cryopreserved primary skeletal muscle cell cultures. We emphasize the potential application of this new approach to extract therapeutic stem cells from human muscle cells cryogenically banked for clinical purposes.
Keywords: Myogenesis, Human skeletal muscle, Myogenic endothelial cells (MECs), Perivascular stem cells (PSCs), Cell therapy
INTRODUCTION
Mammalian skeletal muscle harbors multiple stem progenitor cell populations, which can repair and/or regenerate injured/defective tissues such as damaged/dystrophic skeletal muscles and ischemic hearts (1,2,5,7, 8,10,12–19). In particular, we previously reported the identification of two subpopulations of multipotent stem cells within human skeletal muscle [i.e., myogenic endothelial cells (MECs) (CD45−CD34+CD56+CD144+) and perivascular stem cells (PSCs) (CD34−CD45−CD56− CD146+)+, which exhibit multilineage mesodermal developmental potentials (6,20). These stem cell populations were specifically localized in situ within the walls of small blood vessels and can be prospectively purified by fluorescence-activated cell sorting (FACS) from fresh human skeletal muscle biopsies, through the use of a combination of positive and negative cell lineage markers (6,20). In vitro, purified MECs and PSCs displayed osteo-, chondro-, adipo-, and myogenic differentiation competence, and their high repair/regenerative capacities were not only demonstrated in injured mouse skeletal muscles but also in infarcted hearts (3,4,6, 11,20). However, it has never been documented whether these stem cell fractions could persist and retain their high myogenic capacities after the cryopreservation of human primary skeletal muscle cell cultures (cryo-hPSMCs). Furthermore, MECs and PSCs have been shown to be superior to myoblasts for muscle regeneration in previously performed studies; however, it has never been determined whether MECs isolated from cryopreserved, culture-expanded hPSMCs possessed the same superior regenerative capacity.
In order to identify and purify MECs and PSCs by FACS from in vitro expanded cryo-hPSMCs, we employed a collection of cell lineage markers reported in our previous studies, including the hematopoietic cell marker CD45, the myogenic cell marker CD56 (neural cell adhesion molecule; N-CAM), the perivascular cell marker CD146 (melanoma cell adhesion molecule; M-CAM/Mel-CAM/MUC18), and the endothelial cell marker UEA-1 receptor (Ulex europaeus agglutinin I receptor, UEA-1R) (6,20,21). UEA-1R was chosen as a substitute marker for CD34 and CD144 because these two endothelial cell markers are frequently lost during long-term culture whereas UEA-1 maintains consistent reactivity within endothelial cell lineage cultures (20,21). We hypothesized that MECs and PSCs (with and without culture expansion), purified from cryo-hPSMCs, retain their superior myogenic potential and exhibit a greater regeneration capacity of skeletal myofibers when compared to myoblasts.
MATERIALS AND METHODS
Human Muscle Biopsies and Animal Usage
In total, nine independent human skeletal muscle biopsies, from four female and five male donors (age range 4–75, mean 28), were used to obtain human primary skeletal muscle cells (hPSMCs). The procurement of human skeletal muscle biopsies from the National Disease Research Interchange (NDRI) was approved by the Institutional Review Board at the University of Pittsburgh Medical Center (UPMC). All the animal research experiments performed in this study were approved by the Animal Research and Care Committee at the Children’s Hospital of Pittsburgh of UPMC (Protocol #34-05) and the University of Pittsburgh (Protocol #0810310-B2).
Cell Isolation and Cryopreservation
The human skeletal muscle biopsies were placed in Hank’s Balanced Salt Solution (HBSS, Invitrogen) and transferred to the laboratory on ice. Briefly, tissues were finely minced and serially digested with 0.2% collagenase type XI, 0.25% dispase, and 0.1% trypsin, as previously described (20,21). Dispersed single cell suspensions were cultured in complete medium containing DMEM supplemented with 10% fetal bovine serum (FBS), 10% horse serum, 1% chicken embryo extract, and 1% penicillin/streptomycin (all from Invitrogen). After expansion, cells were cryopreserved at passages 2–8 in medium consisting of 50% complete culture medium and 50% freezing medium (80% FBS + 20% dimethyl sulfoxide) and stored in liquid nitrogen (21).
Flow Cytometry and Cell Sorting
To culture cryo-hPSMCs, cells were thawed and expanded for 2–6 passages. To perform flow cytometry analysis, cells were trypsinized, washed, and incubated with anti-human monoclonal antibodies/ligands: CD45-allophycocyanin-cyanine 7 (APC-Cy7), CD56-phycoerythrin-Cy7 (PE-Cy7), CD34-APC (all from Becton Dickinson), CD146-fluorescein isothiocyanate (FITC; Serotec), UEA-1-PE (Biomeda), von Willebrand factor (vWF)-FITC (US Biology), kinase insert domain receptor (also known as vascular endothelial growth factor receptor 2; VEGFR2) KDR-APC (R&D Systems), and CD144-PE (Beckman Coulter). Negative control samples received equivalent amounts of isotype-matched fluorophore-conjugated antibodies. For FACS purification, cells were incubated with CD45-APC-Cy7, CD56-PE-Cy7, CD146-FITC, UEA-1-PE, and with 7-amino-actinomycin D for dead cell exclusion. Sorted subpopulations were collected for immediate transplantation or transiently expanded in appropriate conditions as previously described (6,20).
Immunocytochemistry
For immunocytochemistry, cells were cytospun onto glass slides, fixed, and incubated with 10% serum. The following primary antibodies were used to detect cell lineage markers, including myogenic cell markers, CD56 (BD) and desmin (Sigma); perivascular cell markers, α-smooth muscle actin (Abcam) and CD146 (Cayman Chemical); endothelial cell markers/ligands, CD144 (Sigma), vWF (DAKO), CD34 (Novocastra), and biotinylated UEA-1 (Vector), followed by incubation with biotinylated secondary antibodies and/or Cy3-conjugated streptavidin (Sigma). Slides were observed and photographed on an epifluorescence microscope system (Nikon Eclipse E800).
Myogenesis In Vivo
To investigate whether the myogenic capacities of the cells were preserved, after cryopreservation, purified MECs, PSCs, myoblasts (Myos), endothelial cells (ECs), and unpurified muscle cells, without in vitro expansion, from six independent hPSMC samples were used for intramuscular injection. The newly sorted cells, on average, 11.8 ± 5.8 × 104 CD56+ Myo cells, 7.3 ± 4.4 × 104 CD146+ PSCs, 4.5 ± 2.6 × 104 UEA-1R+ ECs, and 2.9 ± 1.7 × 104 CD56+UEA-1R+CD146+ MECs as well as 30 × 104 corresponding unsorted cells, were resuspended in 20 μl of HBSS and used for transplantation.
To precisely measure the myogenic-regenerative capacity of each stem/progenitor cell subpopulation, newly sorted MECs, PSCs, and Myo cells were expanded in culture for 1–2 passages. Fifty thousand cells from each subpopulation as well as 5 × 104 corresponding unsorted cells were trypsinized, washed, and resuspended in 20 μl of phosphate-buffered saline (PBS). Four individual animal experiments were performed, with each using cell populations purified from a single FACS sort of one independent cryo-hPSMC culture.
Cells were injected into a single site of the gastrocnemius muscles of severe combined immunodeficient (SCID) mice that were injured 24 h before by injecting 1 μg of cardiotoxin in 20 μl of HBSS. The untreated group received sham injections of 20 μl of HBSS or PBS only. Treated muscles were collected 2 weeks post-injection for immunohistochemical analyses. Anti-human spectrin was used to identify human cell-derived skeletal myofibers in the mouse muscles. In order to quantitatively evaluate the myogenic regenerative capacity of each subpopulation, the number of human spectrin-positive skeletal myofibers was averaged from six randomly selected sections at the site of injection in each specimen and presented as the regenerative index (per section).
Statistical Analysis
Data were summarized as average ± SE. Statistical comparison between the groups (purified cells after expansion in vitro) was performed using one-way ANOVA with a 95% confidence interval. Bonferroni pairwise multiple comparison test was performed for ANOVA post hoc analysis. Statistical analyses were performed with SigmaStat software.
RESULTS
Heterogenous Cell Composition of Human Primary Skeletal Muscle Cell Cultures (hPSMCs) After Cryopreservation and Long-Term Expansion
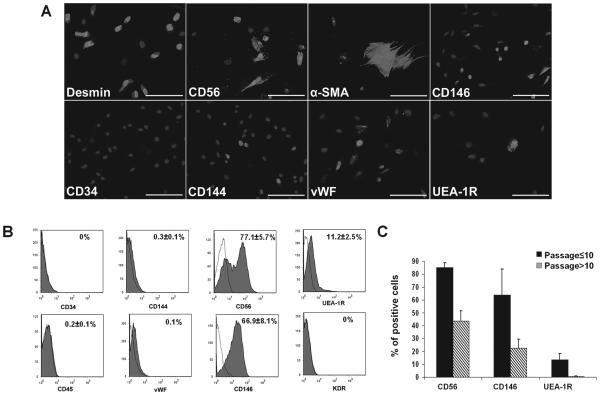
After expansion, the cryopreserved hPSMCs (cryo-hPSMCs) were examined by immunocytochemistry for cell surface marker expression. The majority of cryo-hPSMCs expressed desmin and CD56, and to a lesser extent, CD146 (Fig. 1). Only a fraction of cells expressed α-SMA, CD144, vWF, or UEA-1R. As predicted, the cultured human cryo-hPSMCs lacked CD34 expression. After excluding CD45+ hematopoietic cells (0.2 ± 0.1%), flow cytometry analysis quantitatively confirmed the presence of cells with diverse expressions of cell lineage makers by cryo-hPSMCs: 77.1 ± 5.7% CD56+, 66.9 ± 8.1% CD146+, 11.2 ± 2.5% UEA-1R+, 0.3 ± 0.1% CD144+, 0.1% vWF+, and null expression of CD34 and KDR (Fig. 1B). The number of cryo-hPSMCs positive for CD56, CD146, or UEA-1R decreased dramatically after passage 10 (Fig. 1C).
Figure 1.
Expression of cell lineage markers by cryopreserved human primary skeletal muscle cells (cryo-hPSMCs). (A) Immunocytochemistry revealed the expression of various cell lineage markers (light gray) by cryo-hPSMCs after expansion. Nuclei were stained with DAPI (dark gray). (B) Flow cytometry analysis quantitatively confirmed the diverse cell composition of cryo-hPSMCs. (C) The number of cryo-hPSMCs positive for CD56, CD146, or UEA-1R decreased when cells were cultured beyond passage 10. Scale bars: 100 μm.
Isolation of Myogenic Stem/Progenitor Cells From Cryo-hPSMCs
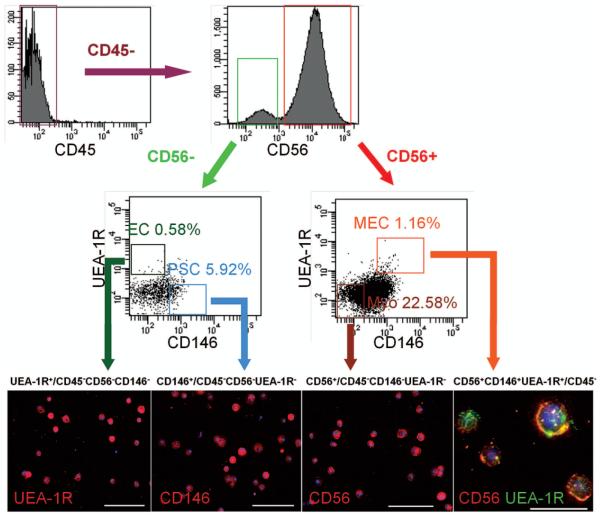
After revealing the heterogeneous nature of cryo-hPSMCs, we analyzed these cells for the existence of previously defined subpopulations by multicolor flow cytometry, based on their expression of hematopoietic (CD45), myogenic (CD56), endothelial (UEA-1R), and perivascular (CD146) cell lineage markers (6,20). After exclusion of CD45+ cells, four distinct cell fractions were identified, including myoblasts (Myo) (CD56+/CD45−CD146−UEA-1R−), endothelial cells (ECs) (UEA-1R+/CD45−CD56−CD146−), perivascular stem cells (PSCs) (CD146+/CD45−CD56−UEA-1R−), and myogenic endothelial cells (MECs), which expressed all three markers (CD56+UEA-1R+CD146+/CD45−). Long-term cultured cryo-hPSMCs included 22.58 ± 6.32% Myo, 0.58 ± 0.23% ECs, 5.92 ± 4.66% PSCs, and 1.16 ± 0.19% MECs (Fig. 2). These four cell subsets were subsequently fractionated by FACS, and on average we were able to recover the following number of each cell type: 25.61 ± 9.16 × 104 CD56+ Myo, 13.28 ± 7.37 × 104 UEA-1R+ ECs, 33.54 ± 20.53 × 104 CD146+ PSCs, and 3.84 ±0.96 × 104 CD56+UEA-1R+CD146+ MECs (Fig. 2).
Figure 2.
Identification and purification of myogenic stem cells within cryo-hPSMCs. After excluding CD45+ hematopoietic cells, CD45− cells were separated based on CD56 expression. CD56+ and CD56− populations were further gated on UEA-1R by CD146 to identify and/or sort four distinct cell populations: myogenic endothelial cells (MECs) (CD56+UEA-1R+CD146+/CD45−), myoblasts (Myos) (CD56+/CD45−CD146−UEA-1R−), perivascular stem cells (PSCs) (CD146+/CD45−CD56−UEA-1R−), and endothelial cells (ECs) (UEA-1R+/CD45−CD56−CD146−). The purities of the sorted populations were 90.73 ± 4.82%, 92.94 ± 1.23%, 93.86 ± 1.72, and 94.9 ± 0.64, respectively. Immunocytochemistry confirmed the expression of key cell lineage makers by freshly sorted cells: UEA-1R, CD146, and/or CD56. Nuclei were stained blue with DAPI. Scale bars: 100 μm; CD56/UEA-1R double staining: 20 μm.
Purified Myogenic Stem/Progenitor Cells Retain High Myogenic Potentials In Vivo
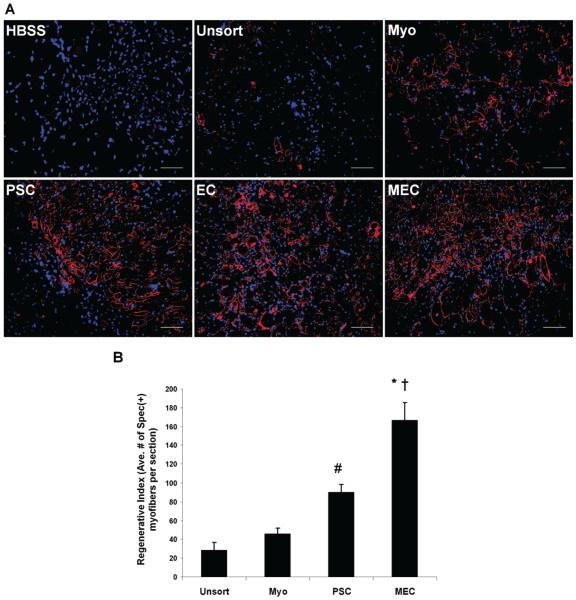
To evaluate whether the myogenic capacity was preserved after cryopreservation, all sorted cells were immediately transplanted (without culture expansion) into the cardiotoxin-injured gastrocnemius muscles of SCID mice (n = 6 per cell fraction). Unpurified muscle cells and HBSS injections were employed as treated and untreated controls, respectively. Mouse muscles were harvested 2 weeks postinjection, cryosectioned, and examined by immunohistochemistry to detect muscle fiber regeneration. An antibody against human spectrin, a myofiber cytoskeletal protein, was used to identify human cell-derived skeletal myofibers in the tissue sections. All of the newly purified cell fractions regenerated human spectrin-positive myofibers in the injured mouse skeletal muscles; however, the purified fractions appeared to regenerate a greater number of muscle fibers than the unpurified cryo-hPSMCs (Fig. 3A). As expected, a lack of spectrin-expressing muscle fibers was observed in the HBSS-injected muscles (Fig. 3A).
Figure 3.
Comparison of myogenic regenerative capacities in vivo. (A) Representative pictures of regenerating human spectrinpositive myofibers in cardiotoxin-injured mouse skeletal muscles transplanted with newly sorted cell fractions from a single donorderived cryo-hPSMC culture, including myogenic endothelial cells (MECs), perivascular stem cells (PSCs), endothelial cells (ECs), and myoblasts (Myos). Unsorted cryo-hPSMCs (Unsort) and HBSS were injected as treated and untreated controls respectively. Original magnification: 200×; scale bars: 50 μm. (B) Quantitative analyses of the myogenic regenerative capacities of sorted stem/ progenitor cell populations. Fifty thousand cells from each cell fraction that was minimally expanded in culture were injected. Quantitative analyses of spectrin-positive human skeletal myofibers on tissue sections revealed that MECs mediated the highest myogenic regeneration among all four cell fractions tested (*p < 0.001 vs. Myo and Unsort; †p = 0.004 vs. PSC). Injection of PSCs regenerated more human myofibers than injections of Myos (p > 0.05) and Unsort (#p = 0.017). Finally, Myos displayed a trend of higher myogenic capacity than Unsort, but no significant difference was observed (p > 0.05).
To quantitatively measure the myogenic regenerative capacity of each purified stem/progenitor cell population, newly sorted MECs, PSCs, and Myo cells were transiently expanded in culture for 1–2 passages. Fifty thousand cells from each of the subpopulations as well as 5 × 104 corresponding unsorted cells were transplanted into the same type of muscle injury model described above (n = 4 per cell fraction). Phosphate-buffered saline (PBS) injections were used as negative controls. ECs were not included in this experiment due to their unstable phenotype in culture. Quantitative analyses revealed that the myogenic regenerative index, indicated by the average number of human spectrin-positive skeletal myofibers per muscle section, was 166.3 ± 19.2 for the MECs, 90.1 ± 8.0 for the PSCs, 45.7 ± 6.2 for the myoblasts (Myo), and 28.7 ± 8.4 for the unsorted muscle cells (Unsort) (Fig. 3B). The MECs exhibited the highest regeneration index of all four cell fractions tested (p < 0.005) (Fig. 3B) and the PSCs regenerated more myofibers than the Myo (p > 0.05) and the Unsort (p = 0.017) groups (Fig. 3B). Although the purified myoblasts displayed a trend of higher myogenic capacity than the unsorted cells, no statistically significant difference was observed (p > 0.05) (Fig. 3B).
DISCUSSION
Skeletal muscle is known to possess multiple stem/progenitor cell populations that are associated with muscle development, maintenance, and regeneration (13,18). Upon purification, muscle stem/progenitor cells in general display more robust myogenic regenerative capacities than unpurified muscle cells in animal disease models, suggesting the advantage of isolating stem cells for therapeutic purposes (6,10,13,20). More recent data have shown that there is a functional heterogeneity in myogenesis even among the muscle precursor cell pool (2).
In the present study, we demonstrated that even after in vitro expansion and cryopreservation, primary human muscle cell cultures include various subpopulations, as indicated by expression of diverse cell lineage markers. Using a modified collection of cell lineage markers (CD45, CD56, CD146, and UEA-1R), we identified and purified to homogeneity four distinct cell populations from cryo-hPSMCs, including two stem cell subpopulations: PSCs (CD146+/CD45−CD56−UEA-1R−) and MECs (CD56+CD146+UEA-1R+/CD45−) (6,20,21). Newly sorted MECs, PSCs, ECs, and Myo cells were immediately transplanted into the cardiotoxin-injured skeletal muscles of SCID mice to examine the preservation of their myogenic potential after FACS, all of which regenerated human spectrin-positive myofibers in the injured mouse skeletal muscles. Quantitative analyses using sorted subpopulations that were minimally expanded in culture showed that the MECs displayed the highest muscle regenerative capacity among all cell subsets tested, and the PSCs were superior to myoblasts and unpurified cryo-hPSMCs.
These results were consistent with our previous observations from injections of cells isolated from fresh skeletal muscle biopsies (6,20). Taken together, our results suggest the presence of distinct subpopulations of highly myogenic stem/progenitor cells within culture-expanded, cryopreserved hPSMCs and support the feasibility of further purifying stem cell fractions from these unpurified cryopreserved human cells. Most importantly, these findings infer the practicability of prospective isolation of myogenic stem/progenitor cell populations from banked human skeletal muscle cells, highlighting a new technology to further enhance the availability and efficacy of cell-mediated therapies (9).
ACKNOWLEDGMENTS
This work was supported in part by grants to J.H from the National Institutes of Health (R01 DE013420-09) and the Department of Defense (W81XWH-09-1-0658) and from the William F. and Jean W. Donaldson Endowed Chair at the Children’s Hospital of Pittsburgh, the Henry J. Mankin Endowed Chair for Orthopaedic Research at University of Pittsburgh, and by a grant to B.P. from the National Institutes of Health (R21 HL083057-01A2). The authors also wish to thank Allison Logar for her excellent technical assistance on the flow cytometry and the cell sorting and James H. Cummins for his editorial assistance in the preparation of this manuscript. The following author contributions are recognized: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing (B.Z.); conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing (C.-W.C.); collection and/or assembly of data, data analysis, and interpretation (G.L.); collection and/or assembly of data, data analysis, and interpretation (S.D.T.); collection and/or assembly of data, data analysis and interpretation (M.P.); conception and design, financial support, data analysis and interpretation (B.P.); conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript (J.H.). Dr. Johnny Huard has received remuneration from Cook Myosite, Inc. for consulting services and for royalties received from technology licensing during the period that the above research was performed. All other authors declare no conflicts of interst.
REFERENCES
- 1.Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, Erickson J, Huard J, Chancellor MB. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008;19(6):881–883. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 2.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 134(1):37–47. 2008. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, Péault B. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20(5–6):429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Chen CW, Okada M, Tobita K, P?ault B, Huard J. Purified human muscle-derived pericytes support formation of vascular structures and promote angiogenesis after myocardial infarction. Circulation. 2009;120S1053(18 Suppl.) [Google Scholar]
- 5.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 25(4):885–894. 2007. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 6.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 3(3):301–313. 2008. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9(3):255–267. 2007. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 8.Drowley L, Okada M, Payne TR, Botta GP, Oshima H, Keller BB, Tobita K, Huard J. Sex of muscle stem cells does not influence potency for cardiac cell therapy. Cell Transplant. 18(10):1137–1146. 2009. doi: 10.3727/096368909X471305. [DOI] [PubMed] [Google Scholar]
- 9.Hirt-Burri N, de Buys Roessingh AS, Scaletta C, Gerber S, Pioletti DP, Applegate LA, Hohlfeld J. Human muscular fetal cells: A potential cell source for muscular therapies. Pediatr. Surg. Int. 24(1):37–47. 2008. doi: 10.1007/s00383-007-2040-5. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 9(10):642–647. 2002. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 11.Okada M, Payne TR, Zheng B, Oshima H, Momoi N, Tobita K, Keller BB, Phillippi JA, Péault B, Huard J. Myogenic endothelial cells purified from human skeletal muscle improve cardiac function after transplantation into infarcted myocardium. J. Am. Coll. Cardiol. 52(23):1869–1880. 2008. doi: 10.1016/j.jacc.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol. Ther. 12(6):1130–1141. 2005. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 13.Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 15(5):867–877. 2007. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 14.Pisani DF, Dechesne CA, Sacconi S, Delplace S, Belmonte N, Cochet O, Clement N, Wdziekonski B, Villageois AP, Butori C, Bagnis C, Di Santo JP, Kurzenne JY, Desnuelle C, Dani C. Isolation of a highly myogenic CD34-negative subset of human skeletal muscle cells free of adipogenic potential. Stem Cells. 28(4):753–764. 2010. doi: 10.1002/stem.317. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau J, Dumont N, Lebel C, Quenneville SP, Côté CH, Frenette J, Tremblay JP. Dystrophin expression following the transplantation of normal muscle precursor cells protects mdx muscle from contraction-induced damage. Cell Transplant. 19(5):589–596. 2010. doi: 10.3727/096368910X4863235. [DOI] [PubMed] [Google Scholar]
- 16.Seidel M, Borczyńska A, Rozwadowska N, Kurpisz M. Cell-based therapy for heart failure: Skeletal myoblasts. Cell Transplant. 18(7):695–707. 2009. doi: 10.3727/096368909X470810. [DOI] [PubMed] [Google Scholar]
- 17.Sherman W, He KL, Yi GH, Wang J, Harvey J, Lee MJ, Haimes H, Lee P, Miranda E, Kanwal S, Burkhoff D. Myoblast transfer in ischemic heart failure: Effects on rhythm stability. Cell Transplant. 2009;18(3):333–341. doi: 10.3727/096368909788534933. [DOI] [PubMed] [Google Scholar]
- 18.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: Functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 119(4):543–554. 2004. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Torrente Y, Belicchi M, Marchesi C, Dantona G, Cogiamanian F, Pisati F, Gavina M, Giordano R, Tonlorenzi R, Fagiolari G, Lamperti C, Porretti L, Lopa R, Sampaolesi M, Vicentini L, Grimoldi N, Tiberio F, Songa V, Baratta P, Prelle A, Forzenigo L, Guglieri M, Pansarasa O, Rinaldi C, Mouly V, Butler-Browne GS, Comi GP, Biondetti P, Moggio M, Gaini SM, Stocchetti N, Priori A, D’Angelo MG, Turconi A, Bottinelli R, Cossu G, Rebulla P, Bresolin N. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 16(6):563–577. 2007. doi: 10.3727/000000007783465064. [DOI] [PubMed] [Google Scholar]
- 20.Zheng B, Cao BH, Crisan M, Sun B, Li GH, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Péault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol. 2007;25(9):1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, Li GH, Logar A, Péault B, Huard J. The Orthopaedic Research Society (ORS) 54th Annual Meeting. San Francisco, CA: 2008. Identification of CD56+CD146+UEA-1+ cell population within cryopreserved human skeletal muscle cells which endowed with a high myogenic potential in vivo. [Google Scholar]