Abstract
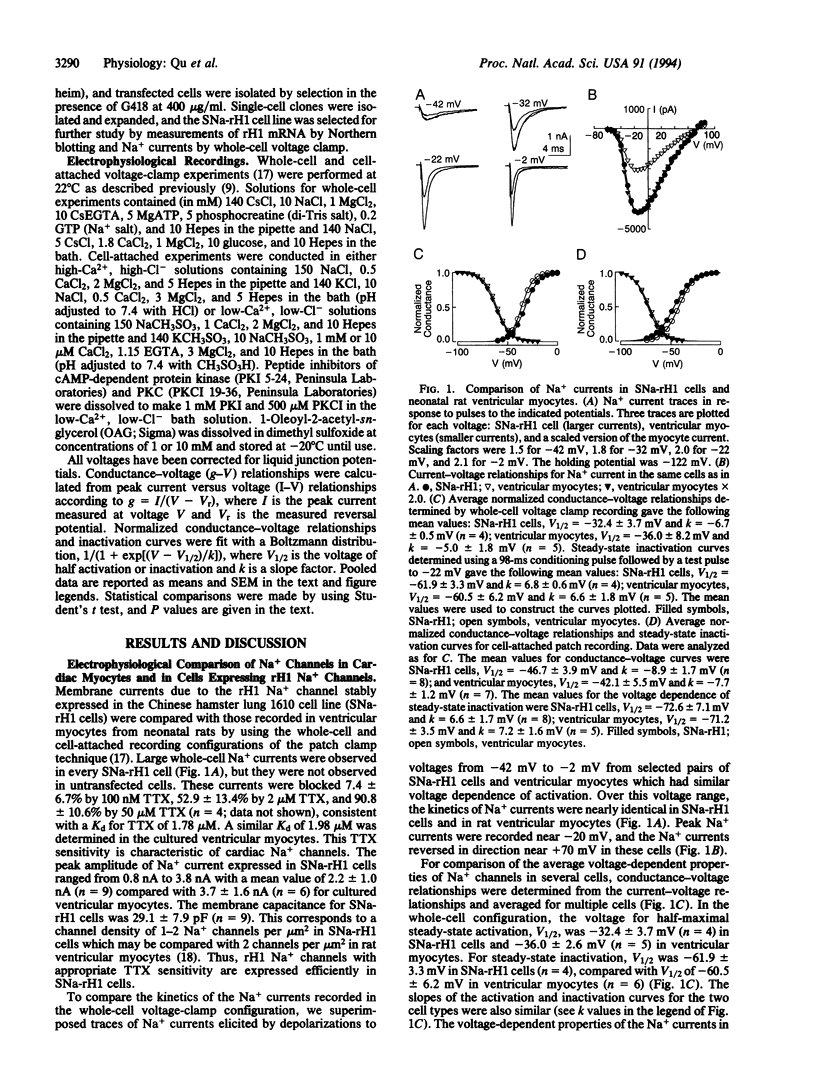
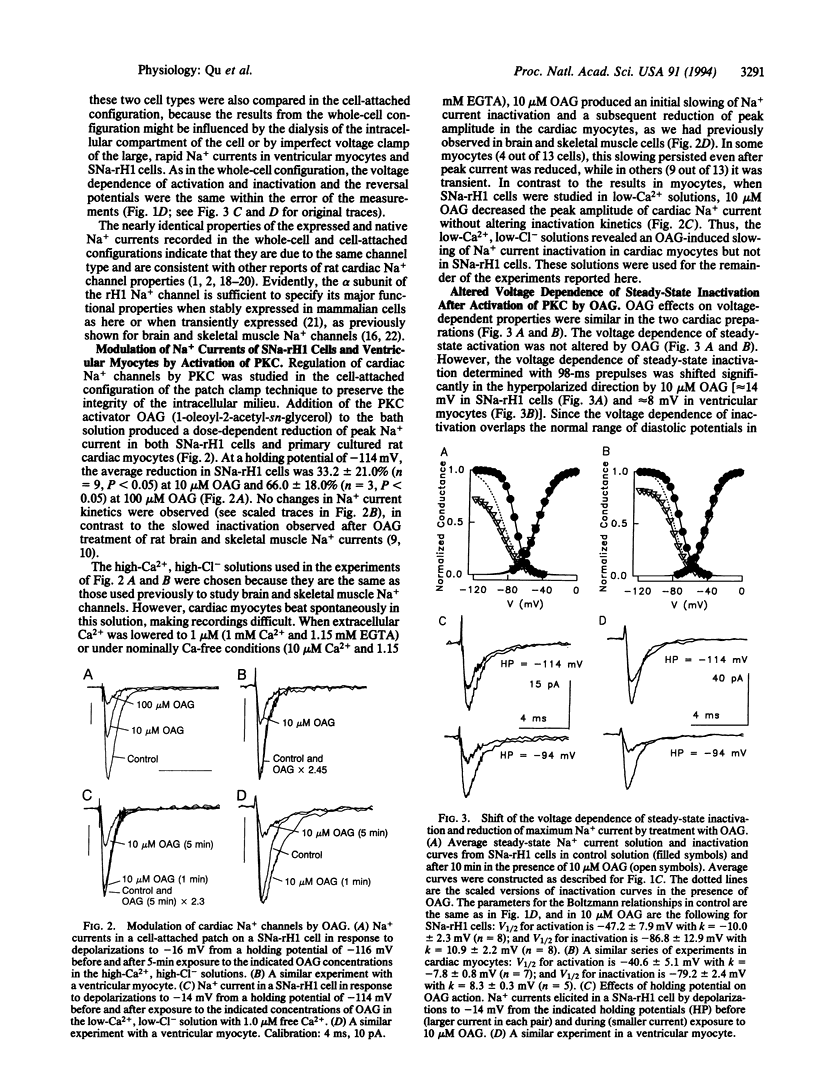
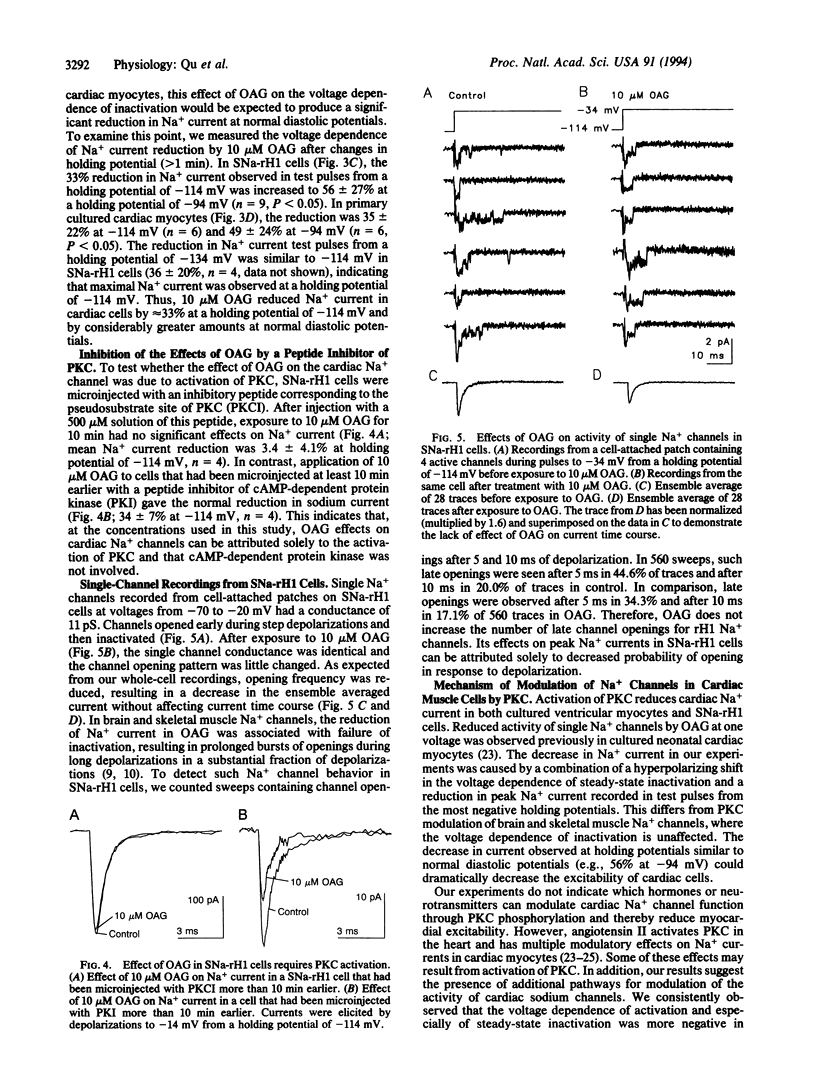
Cardiac rH1 Na+ channel alpha subunits were expressed in cells of the Chinese hamster lung 1610 cell line by transfection, and a stable cell line expressing cardiac Na+ channels (SNa-rH1) was isolated. Mean Na+ currents of 2.2 +/- 1.0 nA were recorded, which corresponds to a cell surface density of approximately 1-2 channels active at the peak of the Na+ current per micron2. The expressed cardiac Na+ current was tetrodotoxin resistant (Kd = 1.8 microM) and had voltage-dependent properties similar to those of the Na+ current in neonatal ventricular myocytes. Activation of protein kinase C by 1-oleoyl-2-acetyl-sn-glycerol (OAG) (10 microM) decreased this current approximately 33% at a holding potential of -114 mV and 56% at -94 mV. This reduction in peak current was caused in part by an 8- to 14-mV shift of steady-state inactivation in the hyperpolarized direction. Na+ channel activation was unchanged. Effects of OAG in SNa-rH1 cells and in neonatal rat cardiac myocytes were similar, except that the time course of inactivation was slowed either transiently or persistently when protein kinase C was activated in myocytes bathed in low-Ca2+ (1 microM) or Ca(2+)-free solution but was unaffected in SNa-rH1 cells. The effects of OAG on cardiac Na+ current were blocked in cells that had been previously microinjected with a peptide inhibitor of protein kinase C but not with a peptide inhibitor of cAMP-dependent protein kinase, indicating that protein kinase C is responsible for the effect of OAG. Single-channel recordings from SNa-rH1 cells showed that the probability of channel opening was reduced by OAG, but the conductance was unaffected. OAG did not induce the late Na+ channel openings observed with PKC modulation of neuronal and skeletal muscle Na+ channels. Thus, the substantial reduction in Na+ current at normal diastolic depolarizations with 10 microM OAG is due to failure of channel opening in response to depolarization. Such Na+ current reductions may have profound effects on cardiac cell excitability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer M., Best P. M., Reuter H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature. 1976 Sep 23;263(5575):344–345. doi: 10.1038/263344a0. [DOI] [PubMed] [Google Scholar]
- Benz I., Herzig J. W., Kohlhardt M. Opposite effects of angiotensin II and the protein kinase C activator OAG on cardiac Na+ channels. J Membr Biol. 1992 Nov;130(2):183–190. doi: 10.1007/BF00231895. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981 Sep;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs L. L., Satin J., Fozzard H. A., Rogart R. B. Functional expression of the rat heart I Na+ channel isoform. Demonstration of properties characteristic of native cardiac Na+ channels. FEBS Lett. 1990 Nov 26;275(1-2):195–200. doi: 10.1016/0014-5793(90)81470-9. [DOI] [PubMed] [Google Scholar]
- Dascal N., Lotan I. Activation of protein kinase C alters voltage dependence of a Na+ channel. Neuron. 1991 Jan;6(1):165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Drugge E. D., Rosen M. R., Robinson R. B. Neuronal regulation of the development of the alpha-adrenergic chronotropic response in the rat heart. Circ Res. 1985 Sep;57(3):415–423. doi: 10.1161/01.res.57.3.415. [DOI] [PubMed] [Google Scholar]
- Gellens M. E., George A. L., Jr, Chen L. Q., Chahine M., Horn R., Barchi R. L., Kallen R. G. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kallen R. G., Sheng Z. H., Yang J., Chen L. Q., Rogart R. B., Barchi R. L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990 Feb;4(2):233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., Lacerda A. E., Brown A. M. Angiotensin II modulates cardiac Na+ channels in neonatal rat. Circ Res. 1989 Dec;65(6):1804–1809. doi: 10.1161/01.res.65.6.1804. [DOI] [PubMed] [Google Scholar]
- Nilius B., Tytgat J., Albitz R. Modulation of cardiac Na channels by angiotensin II. Biochim Biophys Acta. 1989 Dec 14;1014(3):259–262. doi: 10.1016/0167-4889(89)90221-8. [DOI] [PubMed] [Google Scholar]
- Numann R., Catterall W. A., Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991 Oct 4;254(5028):115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A., Hanck D. A. Mechanism of cAMP-dependent modulation of cardiac sodium channel current kinetics. Circ Res. 1993 Apr;72(4):807–815. doi: 10.1161/01.res.72.4.807. [DOI] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer W., Dascal N., Lotan I., Wallner M., Weigl L. Molecular mechanism of protein kinase C modulation of sodium channel alpha-subunits expressed in Xenopus oocytes. FEBS Lett. 1991 Oct 21;291(2):341–344. doi: 10.1016/0014-5793(91)81316-z. [DOI] [PubMed] [Google Scholar]
- Ukomadu C., Zhou J., Sigworth F. J., Agnew W. S. muI Na+ channels expressed transiently in human embryonic kidney cells: biochemical and biophysical properties. Neuron. 1992 Apr;8(4):663–676. doi: 10.1016/0896-6273(92)90088-u. [DOI] [PubMed] [Google Scholar]
- West J. W., Numann R., Murphy B. J., Scheuer T., Catterall W. A. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991 Nov 8;254(5033):866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- West J. W., Scheuer T., Maechler L., Catterall W. A. Efficient expression of rat brain type IIA Na+ channel alpha subunits in a somatic cell line. Neuron. 1992 Jan;8(1):59–70. doi: 10.1016/0896-6273(92)90108-p. [DOI] [PubMed] [Google Scholar]
- White M. M., Chen L. Q., Kleinfield R., Kallen R. G., Barchi R. L. SkM2, a Na+ channel cDNA clone from denervated skeletal muscle, encodes a tetrodotoxin-insensitive Na+ channel. Mol Pharmacol. 1991 May;39(5):604–608. [PubMed] [Google Scholar]
- Zhang J. F., Robinson R. B., Siegelbaum S. A. Sympathetic neurons mediate developmental change in cardiac sodium channel gating through long-term neurotransmitter action. Neuron. 1992 Jul;9(1):97–103. doi: 10.1016/0896-6273(92)90224-2. [DOI] [PubMed] [Google Scholar]



