Abstract
Objectives
There is a paucity of evidence regarding the optimal dosing of anti-TB drugs in children. The aim of this study was to identify the pharmacokinetic parameters of first-line anti-TB drugs and the concentrations achieved after implementation of the 2010 WHO-recommended paediatric dosages.
Methods
We conducted a prospective, observational pharmacokinetic study in children 10 years old or younger who were on isoniazid, rifampicin, pyrazinamide and ethambutol therapy in Durban, KwaZulu-Natal, South Africa. Blood was collected at six timepoints over a 24 h period, chosen using optimal sampling theory. The drug concentrations were simultaneously modelled to identify the compartmental pharmacokinetics of each drug in each child, using the ADAPT program.
Results
The best six sampling timepoints in children were identified as 0 (pre-dose) and 0.42, 1.76, 3.37, 10.31 and 24 h post-dose. Thirty-one children were recruited and blood was drawn at these timepoints. Rifampicin, ethambutol and pyrazinamide were best described using a one-compartment model, while isoniazid was best described with a two-compartment model. Only 2/31 (6%), 20/31 (65%), 17/31 (55%) and 2/13 (15%) of children attained the WHO 2 h target therapeutic concentrations of rifampicin, isoniazid, pyrazinamide and ethambutol, respectively. Moreover, only 24/31 (77%), 6/31 (19%) and 8/31 (26%) achieved the AUCs associated with an optimal clinical response to rifampicin, pyrazinamide and isoniazid, respectively. No single risk factor was significantly associated with below-normal drug levels.
Conclusions
The drug concentrations of all first-line anti-TB drugs were markedly below the target therapeutic concentrations in most South African children who received the revised WHO-recommended paediatric weight-based dosages.
Keywords: paediatric tuberculosis, anti-tuberculosis drug pharmacokinetics, paediatric pharmacokinetics
Introduction
TB continues to be a major global public health threat in which children bear a significant proportion of the disease mortality and morbidity. In South Africa, childhood TB accounts for 15%–20% of the burden. To compound this, the additional problem of MDR, XDR and totally drug-resistant strains has emerged.1,2 Despite these ominous threats, the first-line treatment regimen for TB, comprising isoniazid, rifampicin, pyrazinamide and often ethambutol, has remained stagnant for several decades. With the failure to ensure an adequate control of the childhood TB burden, an evaluation of drug concentrations associated with standard dosing with the existing drugs is paramount since inadequate drug levels may contribute to treatment failure and the problem of MDR TB.2
The design of paediatric pharmacokinetic (PK) studies needs to be optimized. Paediatric PK studies have often relied on a convenience sampling strategy and a desire to incorporate the 2 h timepoint. However, this ‘random’ and arbitrary sampling strategy leads to imprecision in PK estimation and is a common source of error.3–5 The duration of sampling is most accurate when the sampling time encompasses at least three elimination t1/2 values for all drugs.4 An approach that takes these concerns into account is the application of optimal sampling theory, based on Fisher information.3,5 Blood draws are performed at particular ‘information-rich’ timepoints, allowing for the identification of unbiased estimates of PK parameters. Here, we applied optimal sampling theory to the sampling strategy design so that more accurate PK parameter estimates could be identified in children.
The importance of accurately identifying PK parameter estimates in children is to ensure that optimal dosing strategies can be designed. An optimal dose is that which achieves a target concentration that is known to be associated with optimal microbial and clinical outcomes. The reference concentrations most commonly utilized by the WHO have been 2 h drug concentrations.6 In order to achieve these target 2 h drug concentrations, the WHO recently recommended new treatment doses for children.7 These 2 h concentrations are often confused with ‘peak’ (Cmax) concentrations; however, McIlleron et al.8 have shown that they differ. Moreover, 2 h concentrations have been found not to be predictive of clinical outcomes in several studies in adults.9–11 Furthermore, these 2 h concentrations were not designed to address the question of acquired drug resistance (ADR); ADR is unquestionably driven by low drug concentrations, which initiate a series of molecular events termed ‘the antibiotic resistance arrow of time’.11–22 On the other hand, studies in the hollow fibre model (HFM) and in murine TB, and our reanalysis of older guinea pig studies, have identified that it is instead the AUC0–24/MIC and Cmax/MIC of these first-line drugs that drive efficacy and suppress ADR.15–18,22–24 The HFM studies and computer-aided clinical trial simulations predicted that PK variability was the main driver of therapy failure and ADR in South Africa.11 This was confirmed in three clinical studies in adults.12,13,25 In one clinical study of 142 adult South Africans, >90% of therapy failure (death, relapse and microbial failure) and 100% of ADR was explained by having low AUC0–24 and Cmax values for pyrazinamide, rifampicin and isoniazid.12 These findings have since been validated in a separate prospective clinical study.25 Moreover, these concentration thresholds predictive of outcome in adult TB were virtually the same as those identified in HFM and in mice.15–18,22 Here, we investigated how often South African children treated with the new WHO-recommended doses achieved the older reference concentrations used by the WHO as well as how often they achieved the AUC0–24 thresholds that predicted clinical outcomes in adults.
Methods
Regulatory compliance
The Institutional Review Boards of the University of KwaZulu-Natal and Johns Hopkins University approved this study.
Study population and setting
Pharmacokinetics of Anti-Tuberculosis Medications in South African Children (PHATISA) is a prospective, single-centre, observational PK study that was conducted at the King Edward VIII Hospital in Durban, South Africa, from May 2012 to March 2013. Children 10 years of age or younger with the diagnosis of TB were enrolled. The diagnosis of TB was based on clinical symptoms, radiological findings, tuberculin skin testing, a history of household contact and microbiological confirmation. Children were excluded from the study if they had a haemoglobin level <60 g/L, an ALT level more than three times the normal value for their age, evidence of coagulopathy based on an abnormal prothrombin time/partial thromboplastin time, a probable diagnosis of abdominal TB based on clinical findings or any history of intolerance or allergy to the first-line anti-TB drugs. Children who were enrolled in another study were also excluded.
Baseline clinical data were obtained for each participant, including age, nutritional status (weight, height, mid upper-arm circumference), ALT, creatinine, blood urea nitrogen and chest radiography. HIV testing was performed by antibody testing for children older than 18 months of age and by HIV DNA PCR for those younger than 18 months of age. Dietary information and concomitant medications were recorded for the 24 h of the blood sampling.
Definitions
The diagnosis of definite TB was made if there was microbiological evidence (by positivity on sputum or tissue culture of Mycobacterium tuberculosis) or probable TB (by a positive acid-fast bacilli smear and/or a classic radiological finding); the children who did not meet these criteria but showed symptoms of TB (possible TB) and were started on treatment were also included in the study.26 The children were started on the standard first-line anti-TB agents, rifampicin, isoniazid and pyrazinamide with addition of ethambutol for severe forms of TB in accordance with the new WHO guidelines. These guidelines recommend that children receive 10–15 mg/kg of isoniazid, 10–15 mg/kg of rifampicin, 30–40 mg/kg of pyrazinamide and 15–25 mg/kg of ethambutol.7 Informed consent was always obtained from the parents or guardians prior to enrolment, regardless of the age of the child.
Drug treatments
The drugs were provided by the hospital pharmacy and were obtained from Aspen Pharmacare and Sanofi-Aventis South Africa (Pty) Ltd. They were ground and given as food emulsions for children too young to swallow tablets. The drugs were given as fixed-dose combinations. The doses were rounded to the nearest value using the available tablet sizes—combined rifampicin, isoniazid and pyrazinamide 60, 30 and 150 mg tablets, combined rifampicin and isoniazid 60 and 30 mg or 60 and 60 mg tablets, pyrazinamide 150 and 500 mg tablets, and ethambutol 100 and 400 mg tablets—according to weight-based charts.
Study and sampling procedures
Blood samples were obtained from each participant between the 4th and 12th day after the initiation of anti-TB therapy. In order to avoid biased estimations of PK parameters, optimal sampling theory was utilized to identify information-rich timepoints for each of the four drugs with the use of ADAPT II software.3–5,27 Six timepoints were identified. Peripheral intravenous catheters were used for sample collections. At each timepoint, 2 mL of blood was collected in EDTA-coated tubes. Each specimen was immediately placed on ice until processing. Blood samples were centrifuged at 2000 g for 10 min. The plasma layer was separated within 30 min after sampling and stored in a cryovial at −80°C until the time of analysis.
Drug concentration measurement assays
The measurement of drug concentrations was carried out by a previously published three-drug assay using LC coupled to tandem MS (AB-Sciex Qtrap® 4500 LC/MS/MS system).28 The internal standard was 6-amino nicotinic acid. Calibration and quality control standards, along with a blank plasma aliquot and an internal standard aliquot, were used for all runs. Dilutions of standard drug solutions were used to cover the range of concentrations expected for each drug. Three quality control solutions were used to span the range of serum drug concentrations: lowest (QL), intermediate (QM) and highest (QH). The interday and intraday coefficients of variation were below 10%. Selected multiple reaction monitoring transitions were run in the positive ion mode of [M+H]+. Precursor ions to product ions were isoniazid [mass-to-charge ratio (m/z) 138.1→51.9], rifampicin (m/z, 823.1→791.2), pyrazinamide (m/z, 124.1→52.1) and 6-amino nicotinic acid (m/z, 138.7→58.9). Analyst® 1.5 software version 1.5.1 was used. All the calibration curves were linear up to the maximum values stipulated and we did not use a gradient for separation. Analyses were done isocratically at 50% acetonitrile/water, both with 0.1% formic acid. Based on a signal-to-noise ratio of 10 for the limit of quantification, the signal-to-noise ratios from standards were 64–7500 ng mL for isoniazid, 570–75 000 ng mL for pyrazinamide and 14–25 000 ng mL for rifampicin.
Compartmental PK analyses
All concentrations of rifampicin, isoniazid, pyrazinamide and ethambutol were modelled using the ADAPT 5 software program.29 First, we utilized the standard two-stage estimation method to generate initial estimates of PK parameters for each drug for a one-, two- or three-compartment model, with first-order input and elimination. The compartmental parameter estimates were then used in the POPINIT subroutine of ADAPT. Next, each drug was modelled to identify PK parameter estimates for each child using the maximum-likelihood solution via the expectation-maximization algorithm. The choice of best compartmental model was then made based on the lowest Akaike information criterion (AIC) score and Bayesian information criteria (BIC). While they should ideally agree, the BIC, which penalizes for complexity, was used for the final decision. However, we also applied the rule of parsimony, based on Occam's razor, which was that if AIC and BIC chose the more complex model, but that model did not significantly improve the parameter estimates compared with the less complex model, then the simpler model offered the best explanation.
Statistical analysis
STATA version 12 was used for the statistical analysis. The baseline characteristics and secondary PK parameters such as Cmax, Tmax and AUC0–24 were summarized as means ± SD. The 2 h concentration values were considered to be binary data: above the reference target concentration or below. The 2 h reference concentrations were 8 mg/L for rifampicin, 3 mg/L for isoniazid, 20 mg/L for pyrazinamide and 2 mg/L for ethambutol based on previous studies that utilized these concentrations to design new doses.30 These were used simply to identify whether the intended target concentrations for the new WHO doses had been attained. Next, we wanted to identify the proportion of children with these WHO-recommended doses who achieved or exceeded the AUC0–24 values shown to be associated with clinical outcome in adult patients with pulmonary TB.12 These were a pyrazinamide AUC0–24 ≤363 mg·h/L, a rifampicin AUC0–24 ≤13 mg·h/L and an isoniazid AUC0–24 ≤52 mg·h/L. For this latter analysis, the concentrations were not dose- or weight-normalized.
For each of the four drugs, the Cmax and AUC were dose-normalized, depicted as Cmax/D and AUC0–24/D, respectively. Graphical exploratory data analysis showed that the Cmax and AUC0–24 values were not normally distributed; hence, non-parametric tests were used to investigate the differences in Cmax and AUC among the covariates. The Wilcoxon rank sum test was used to delineate the differences in Cmax/D and AUC0–24/D between younger and older children (≤2 years versus >2 years), sex (male versus female), HIV serostatus (positive versus negative) and nutritional status (malnourished versus non-malnourished).
Results
The application of optimal sampling theory revealed that the best six sampling timepoints in children were 0 (pre-dose) and 0.42, 1.76, 3.37, 10.31 and 24 h post-dose (the 0 and 24 h timepoints were prespecified). These sampling times were then used to time the blood draws. A total of 36 children, treated between May 2012 and March 2013, were eligible for the study. Three parents declined informed consent. One patient improved with antibiotic therapy for bacterial pneumonia and the clinical diagnosis of probable TB was removed, with discontinuation of anti-TB therapy. Another patient was diagnosed with probable gastrointestinal TB after imaging and was disqualified from the study. The baseline anthropometric and clinical data of the 31 remaining children are summarized in Table 1. A total of 81% children presented with pulmonary TB; 19% had disseminated disease. The percentage of drug concentrations measured that were below the limits of quantification in any of the 31 children at any of the timepoints identified by D-optimality was 0% for rifampicin, pyrazinamide and ethambutol (a total of 558 measurements). For isoniazid, 2% of the 186 measurements were below the limit of detection, all of which were at the 0 h timepoint. The results indicate that the optimal sampling strategy derived from sampling theory was likely to be optimal.
Table 1.
Baseline demographics and clinical characteristics
| Clinical factor | |
|---|---|
| Demographics | |
| age (years), median (range) | 2.29 (0.25–10.5) |
| ≤2 years, n | 16 |
| >2 years, n | 15 |
| weight (kg), median (range) | 11.5 (6.1–19) |
| height (cm), median (range) | 84 (66–114) |
| malnourished, n (%) | 20 (65) |
| HIV positive, n (%) | 7 (23) |
| female, n (%) | 13 (42) |
| Laboratory data | |
| haemoglobin (g/L), median (range) | 92 (70–112) |
| ALT (IU/L), median (range) | 16 (9–71) |
| creatinine (μmol/L), median (range) | 28.5 (20–62) |
| Microbiological data | |
| smear positive, n/n | 5/23 |
| culture positive, n/n | 7/20 |
| Hain positive, n/n | 7/8 |
| GeneXpert, n/n | 1/13 |
| Diagnostic data | |
| positive tuberculin skin test, n/n | 13/17 |
| chest X-ray findings, n/n | 22/31 |
| cavitary lesion, n/n | 4/22 |
| parenchymal consolidation, n/n | 19/22 |
| perihilar adenopathy, n/n | 14/22 |
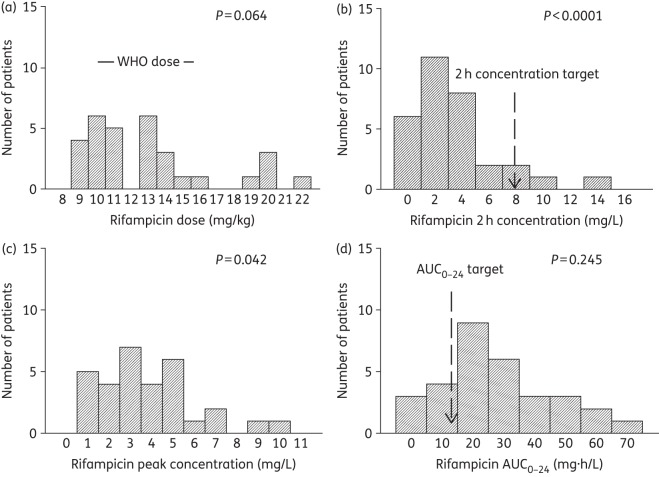
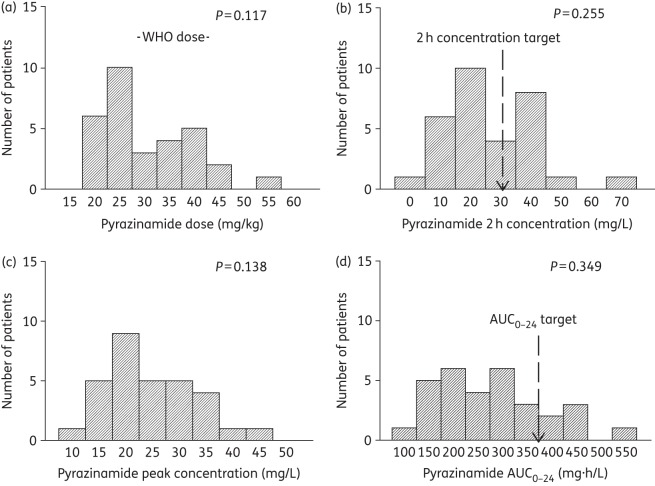
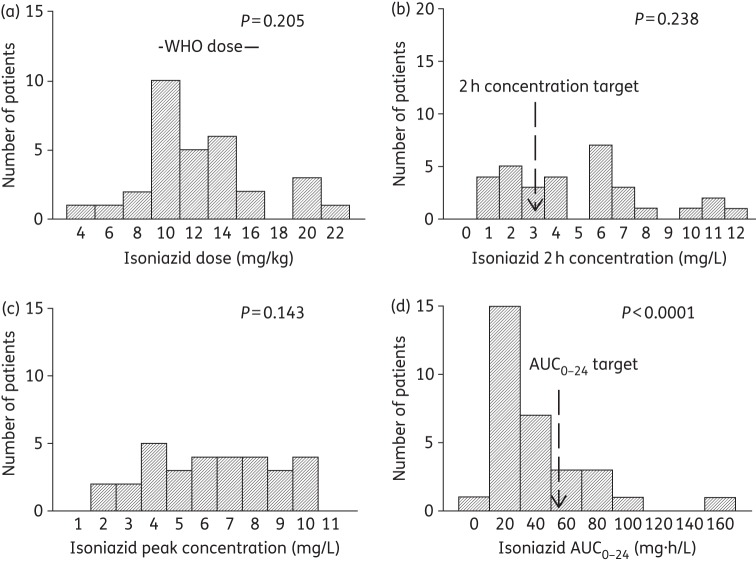
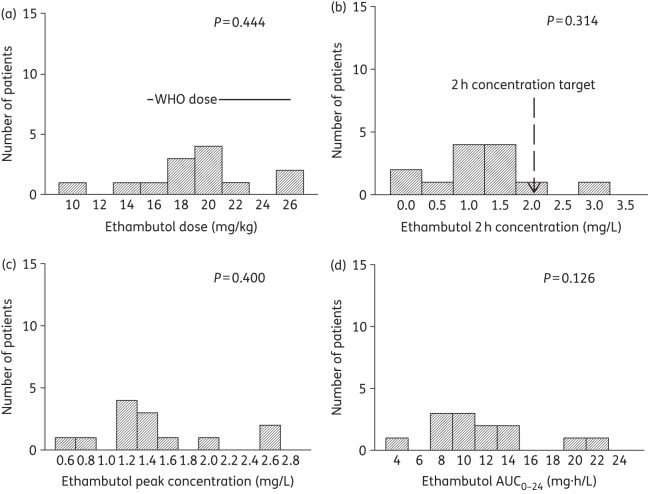
All the children were inpatients and the doses were received under the supervision of nurses; however, 11 children received their doses as outpatients. The dosages received by participants for each of the four drugs were ascertained by checking the drug bottles as well as the prescribed dosages on the participants' charts [Figures 1–4, panel (a)]. Overall, 90% (28/31), 84% (26/31), 42% (13/31) and 85% (11/13) of the participants received dosages within the revised WHO recommendations for rifampicin, isoniazid, pyrazinamide and ethambutol, respectively [Figures 1–4, panel (a)].
Figure 2.
Doses and concentrations of rifampicin achieved in 31 South African children. A P value ≥ 0.05 is significant for a normal distribution. (a) The ratio of the lowest-to-highest rifampicin dose was 2.5-fold, and doses fell mostly either into the WHO-recommended doses or even higher. (b) The 2 h rifampicin concentration had a lowest-to-highest ratio of 308.3, more than 100-fold higher than the ratio for the dose. (c) The peak rifampicin concentration had a lowest-to-highest ratio of 18.2, which was thus lower than the variability of the rifampicin 2 h concentration. (d) The rifampicin AUC0–24 that was achieved varied 37.8-fold between the lowest and highest values. More than 10-fold of this was due to the dose.
Figure 3.
Doses and concentrations of pyrazinamide achieved in 31 South African children. A P value ≥0.05 is significant for a normal distribution. (a) The ratio of the lowest-to-highest pyrazinamide dose was 2.8-fold, with a large proportion of doses below the WHO-recommended doses. (b) The 2 h pyrazinamide concentration had a lowest-to-highest ratio of 28.3, severalfold higher than the ratio for the dose. (c) The peak pyrazinamide concentration had a lowest-to-highest ratio of 4.2, which was thus lower than the variability of the pyrazinamide 2 h concentration. (d) The pyrazinamide AUC0–24 that was achieved varied 4.8-fold between the lowest and highest concentrations.
Figure 1.
Doses and concentrations of isoniazid achieved in 31 South African children. The P values are for the D'Agostino and Pearson omnibus normality test, whereby a P value ≥0.05 is significant for a normal distribution, while lower P vales indicate a non-normal distribution. (a) Isoniazid doses administered are compared with the WHO-recommended doses. The majority of patients were dosed according to the WHO-recommended doses. The ratio between the highest and lowest dose administered was 4.7. (b) The 2 h isoniazid concentration had a lowest-to-highest ratio of 14.7, severalfold higher than that imposed by the dose. The true Tmax had a median and range of 1.75 (0.33–3.67) h, indicating a wide variability in Tmax, and that the 2 h concentration rarely coincided with peak concentration of isoniazid in the children. (c) The peak isoniazid concentration differed from the 2 h concentration in terms of distribution. The ratio between the lowest and highest peak was 5.6, closely tracking the doses administered. (d) The isoniazid AUC0–24 that was achieved varied 22.5-fold between the lowest and highest values, much higher than the ratio for the doses.
Figure 4.
Doses and concentrations of ethambutol achieved in 13 South African children. A P value ≥0.05 is significant for a normal distribution. (a) The ratio of the lowest-to-highest ethambutol dose was 2.5-fold, with a large proportion below the WHO-recommended doses. (b) The ethambutol 2 h concentration had a lowest-to-highest ratio of 139.1, >100-fold higher than the ratio for the dose. (c) The ethambutol peak concentration had a lowest-to-highest ratio of 4.3, which was thus lower than the variability of the ethambutol 2 h concentration. (d) The ethambutol AUC0–24 that was achieved varied 4.9-fold between the lowest and highest concentrations.
The Spearman's rank correlation coefficient was used to determine whether correlations existed between the underdosing of one drug and another. This evaluation revealed a strong statistically significant correlation between the underdosing of isoniazid and rifampicin (ρ = 0.746, P = 0.001), and isoniazid and pyrazinamide (ρ = 0.373, P = 0.039), but not between rifampicin and pyrazinamide (ρ = 0.278, P = 0.13). This correlation reflects the fact that fixed-dose combinations were used, an effect compounded by weight-banding.
Rifampicin, pyrazinamide and ethambutol were best described using a one-compartment model, while isoniazid was best described using a two-compartment model. The mean population PK parameter estimates for all four drugs are shown in Table 2. Secondary PK estimates, such as Cmax, 2 h concentrations and AUC0–24, are shown in Figures 1–4, which highlight the wide interpatient variability for each of the drugs. Table 3 shows how poor the 2 h concentration and Cmax were as predictors of AUC0–24. The r2 values were mediocre, except for pyrazinamide, which had a moderate r2. The duration of therapy until blood draws for measurement of drug concentration was a median of 7.0 (range 4–12) days. The relationship between the duration of therapy and the drug concentration is shown in Table 4, which shows that none of the slopes significantly differed from 0. Thus, the duration of therapy until blood draws for the PK study was not associated with a low or high serum drug concentration, even for rifampicin, which undergoes autoinduction.
Table 2.
PK parameter estimates for first-line anti-TB drugs in South African children
| Isoniazid | Rifampicin | Pyrazinamide | Ethambutol | |
|---|---|---|---|---|
| Total clearance (L·h−1) | 12.2 (7.58) | 12.7 (9.9) | 2.7 (0.9) | 20.6 (6.1) |
| Volume of central compartment (L) | 56.4 (7.5) | 85.1 (42.7) | 24.0 (2.3) | 135 (21.2) |
| Absorption constant (h−1) | 10.0 (4.4) | 14.8 (7.4) | 0.9 (0.3) | 1.7 (2.1) |
| Intercompartmental clearance (L·h−1) | 10.2 (2.8) | NA | NA | |
| Volume of peripheral compartment (L) | 1.0 (0.3) | NA | NA |
NA, not applicable for a one-compartment model.
Values are shown as mean (±SD).
Table 3.
Relationship between different concentrations of first-line anti-TB agents
| Correlation of concentration measures (r2) |
|||
|---|---|---|---|
| Cmax versus AUC | 2 h versus Cmax | 2 h versus AUC | |
| Isoniazid | 0.464 | 0.644 | 0.209 |
| Rifampicin | 0.440 | 0.767 | 0.297 |
| Ethambutol | 0.772 | 0.002 | 0.008 |
| Pyrazinamide | 0.819 | 0.798 | 0.725 |
Table 4.
Coefficient of determination for the relationship between drug concentration and time to PK sampling
| Isoniazid | Rifampicin | Pyrazinamide | Ethambutol | |
|---|---|---|---|---|
| 2 h concentration | <0.01 | 0.04 | 0.11 | 0.05 |
| PK model-derived peak | <0.01 | <0.01 | 0.10 | 0.02 |
| PK model-derived AUC0–24 | 0.02 | 0.05 | 0.09 | <0.01 |
Table 5 shows a summary of the proportion of children who achieved the reference 2 h drug concentrations that have been used for dose design in children. For all four drugs, a substantial proportion of the children achieved 2 h concentrations below the reference targets, especially rifampicin and pyrazinamide. This means that for children in KwaZulu-Natal, the new WHO-recommended doses still fail to achieve their intended target concentration. Table 4 also shows that the median value and range of 2 h concentration differed from those for Cmax for all drugs, except for the pyrazinamide median (but not the range).
Table 5.
Concentrations achieved in South African children treated with WHO-recommended dosing
| Pyrazinamide (n = 31) | Rifampicin (n = 31) | Isoniazid (n = 31) | Ethambutol (n = 13) | |
|---|---|---|---|---|
| Observed 1.92 h concentration | ||||
| median (range); mg/L | 22.55 (2.35–66.35) | 2.87 (0.05–14.18) | 4.50 (0.82–11.80) | 1.10 (0.02–3.07) |
| children with concentration below reference, n (%) | 14 (45) | 29 (94) | 11 (35) | 11 (85) |
| PK model-derived peak | ||||
| median (range); mg/L | 22.51 (11.18–47.17) | 3.47 (0.56–10.20) | 6.05 (1.83–10.28) | 1.44 (0.62–6.28) |
| PK model-derived AUC0–24 | ||||
| median (range); mg·h/L | 233.9 (110.10–525.7) | 21.2 (1.8–67.3) | 28.7 (6.8–153.0) | 10.8 (4.7–22.7) |
| children with AUC0–24 below optimal, n (%) | 25 (81) | 7 (23) | 23 (74) | — |
Table 5 also shows that, for isoniazid and pyrazinamide, most children did not achieve the AUC0–24 values that have been shown to be associated with optimal long-term responses such as cure in adults. In the case of rifampicin, a recent clinical study in adults identified a rifampicin AUC of 35 mg·h/L as being predictive of the speed of the sterilizing effect and 2 month sputum conversion rates.25 Twenty-two (71%) of the 31 children had a rifampicin AUC ≤35 mg·h/L, which suggests that they would have low sterilizing effect rates and delayed cure. Thus, overall, rifampicin, isoniazid and pyrazinamide exposures were below the optimal AUC0–24 in a majority of the children.
Next, we performed a univariate analysis for failure to achieve target 2 h drug levels for each of the four study drugs against clinical and demographic factors; only HIV positivity was significantly associated with a low 2 h/D for isoniazid (P = 0.04, Wilcoxon rank sum comparison). There was a trend towards significance for a low AUC0–24/D for isoniazid and pyrazinamide (P = 0.07 for both drugs).
Discussion
There are several findings from our study. First and foremost was the surprisingly high proportion of children with plasma concentrations below normal of all four drugs, even in those receiving the recommended revised WHO dosages. Our findings suggest a need to increase the doses of these drugs in children to above what is currently recommended by the WHO. On the other hand, the target concentrations used to make this recommendation assume that the 2 h and AUC concentrations needed for an optimal effect in adults are the same as in children. This is currently unknown and should be investigated, given possible differences in bacterial burden between adults and children with TB.31
Second, the PK parameter estimates in the children we studied differed from those in other parts of sub-Saharan Africa and from India.32,33 A study from Cape Town, South Africa, reported different AUCs and Cmax concentrations in children who received the revised WHO-recommended doses. As a result, the proportion of children who achieved drug concentrations below normal was lower than in our current study. Both studies are correct and the differences simply illustrate the large between-patient PK variability in children between different regions of the same country, perhaps due to genetic, demographic and nutritional factors as well as co-morbities.32,33 Indeed, in a study of ofloxacin use in adults from the same two places, PK parameter estimates differed between them for MDR TB patients.34 Similarly, PK parameters and their variability are expected to differ in children in different countries. This variability strongly emphasizes the crucial need to establish population PK parameter estimates in children in each different locale where there is a large paediatric TB burden: children in Mumbai differ from those in KwaZulu-Natal, who differ from those in Cape Town. Our findings should be used to allow a more targeted local adjustment of doses by clinicians.
In summary, despite implementation of the 2010 WHO dosing guidelines, a considerable proportion of children still achieve anti-TB drug concentrations that are below normal. Since the metabolism of each of the drugs arises from different xenobiotic metabolism enzymes encoded by unlinked alleles, the below-normal concentrations observed across all four drugs suggest that dosing practices (in this case in pursuance of WHO guidelines) rather than pharmacogenetic factors are one of the major reasons for the low drug concentrations. The current practice of prescribing first-line TB drugs is weight based; hence, a 2-month-old infant will receive the same 10 mg/kg dose of isoniazid as a 10-year-old child. This approach ignores the significant physiological differences that exist between infants and children based on considerations of allometric and fractal geometry, in addition to the weight difference.35–38 Thus, further well-powered studies are needed to elucidate optimal age-based and weight-based dose schedules in the paediatric population. In addition, studies that also compare the clinical responses in children with normal or below-normal levels are needed to further inform and strengthen the underlying concern that a failure to achieve the therapeutic targets in the anti-TB pharmacotherapy for children may be a driver of poor outcomes and ADR.
Funding
This study was funded by HHMI and NIH grants AI 079590 and AI 097138.
Transparency declarations
None to declare.
Acknowledgements
We would like to thank Gary Maartens and colleagues at the University of Cape Town for generously providing quality controls for our LCMS protocols. We would also like to thank Dr Jotam Pasipanodya at UT Southwestern for help with the design of the PHATISA study. The financial support of HHMI and NIH is gratefully acknowledged.
References
- 1.WHO. WHO Report 2011: Global Tuberculosis Control. 2011. http://www.who.int/tb/country/en/
- 2.Dheda K, Gumbo T, Gandhi NR, et al. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med. 2014;2:321–38. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drusano GL, Forrest A, Yuen G, et al. Optimal sampling theory: effect of error in a nominal parameter value on bias and precision of parameter estimation. J Clin Pharmacol. 1994;34:967–74. doi: 10.1002/j.1552-4604.1994.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 4.Reed MD. Optimal sampling theory: an overview of its application to pharmacokinetic studies in infants and children. Pediatrics. 1999;104:627–32. [PubMed] [Google Scholar]
- 5.Tam VH, Preston SL, Drusano GL. Optimal sampling schedule design for populations of patients. Antimicrob Agents Chemother. 2003;47:2888–91. doi: 10.1128/AAC.47.9.2888-2891.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–83. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 7.WHO. 2010. Rapid Advice: Treatment of Tuberculosis in Childrenhttp://whqlibdoc.who.int/publications/2010/9789241500449_eng.pdf?ua=1 .
- 8.McIlleron H, Wash P, Burger A, et al. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother. 2006;50:1170–7. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narita M, Hisada M, Thimmappa B, et al. Tuberculosis recurrence: multivariate analysis of serum levels of tuberculosis drugs, human immunodeficiency virus status, and other risk factors. Clin Infect Dis. 2001;32:515–7. doi: 10.1086/318490. [DOI] [PubMed] [Google Scholar]
- 10.Burhan E, Ruesen C, Ruslami R, et al. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob Agents Chemother. 2013;57:3614–9. doi: 10.1128/AAC.02468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava S, Pasipanodya JG, Meek C, et al. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–9. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasipanodya JG, McIlleron H, Burger A, et al. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208:1464–73. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55:169–77. doi: 10.1093/cid/cis353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumbo T, Louie A, Deziel MR, et al. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis. 2004;190:1642–51. doi: 10.1086/424849. [DOI] [PubMed] [Google Scholar]
- 15.Gumbo T, Louie A, Deziel MR, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51:3781–8. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007;51:2329–36. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumbo T, Siyambalapitiyage Dona CS, Meek C, et al. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother. 2009;53:3197–204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava S, Musuka S, Sherman C, et al. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis. 2010;201:1225–31. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasipanodya JG, Gumbo T. A new evolutionary and pharmacokinetic-pharmacodynamic scenario for rapid emergence of resistance to single and multiple anti-tuberculosis drugs. Curr Opin Pharmacol. 2011;11:457–63. doi: 10.1016/j.coph.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmalstieg AM, Srivastava S, Belkaya S, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother. 2012;56:4806–15. doi: 10.1128/AAC.05546-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered versus directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57:21–31. doi: 10.1093/cid/cit167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother. 2011;55:24–34. doi: 10.1128/AAC.00749-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaram R, Gaonkar S, Kaur P, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47:2118–24. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaram R, Shandil RK, Gaonkar S, et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2004;48:2951–7. doi: 10.1128/AAC.48.8.2951-2957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chigutsa E, Pasipanodya JG, Sirgel FA, et al. The impact of non-linear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother. 2014 doi: 10.1128/AAC.03931-14. doi:10.1128/AAC.03931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cundall DB. The diagnosis of pulmonary tuberculosis in malnourished Kenyan children. Ann Trop Paediatr. 1986;6:249–55. doi: 10.1080/02724936.1986.11748450. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Endrenyi L. A computationally efficient approach for the design of population pharmacokinetic studies. J Pharmacokinet Biopharm. 1992;20:279–94. doi: 10.1007/BF01062528. [DOI] [PubMed] [Google Scholar]
- 28.Song SH, Jun SH, Park KU, et al. Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1331–8. doi: 10.1002/rcm.2961. [DOI] [PubMed] [Google Scholar]
- 29.D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles, CA: Biomedical Simulations Resource; 2009. [Google Scholar]
- 30.McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48:1547–53. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 31.Newton SM, Brent AJ, Anderson S, et al. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thee S, Seddon JA, Donald PR, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55:5560–7. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramachandran G, Hemanth Kumar AK, Bhavani PK, et al. Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis. 2013;17:800–6. doi: 10.5588/ijtld.12.0628. [DOI] [PubMed] [Google Scholar]
- 34.Chigutsa E, Meredith S, Wiesner L, et al. Population pharmacokinetics and pharmacodynamics of ofloxacin in South African patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2012;56:3857–63. doi: 10.1128/AAC.00048-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–6. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- 36.West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–9. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 37.West GB, Savage VM, Gillooly J, et al. Physiology: why does metabolic rate scale with body size? Nature. 2003;421:713. doi: 10.1038/421713a. [DOI] [PubMed] [Google Scholar]
- 38.Hope WW, Seibel NL, Schwartz CL, et al. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob Agents Chemother. 2007;51:3714–9. doi: 10.1128/AAC.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]