Sir,
Worldwide, most multiply antibiotic-resistant (MAR), and particularly carbapenem-resistant, Acinetobacter baumannii causing infections belong to global clone 1 (GC1) or global clone 2 (GC2). Though there have been incidences where a different clonal group was predominantly responsible for an epidemic outbreak, MAR isolates that are not members of these clones have been observed to cause infections in hospitals but are not as common.1
Isolate D46, collected at Royal North Shore Hospital, Sydney in 2010, was shown to be resistant to ampicillin, imipenem, meropenem, ticarcillin/clavulanate, ceftazidime, cefotaxime, streptomycin, spectinomycin, sulfamethoxazole, tetracycline, trimethoprim, chloramphenicol, florfenicol, kanamycin, neomycin, gentamicin, amikacin, tobramycin, nalidixic acid and ciprofloxacin, making it extensively antibiotic resistant. D46 was previously shown to be ST110 (Oxford MLST) and to harbour the aphA6 amikacin resistance gene within TnaphA6 and an aadB gene cassette (gentamicin, kanamycin and tobramycin resistance) in the small plasmid pRAY.2 An ISAba1 upstream of the chromosomal ampC confers resistance to third-generation cephalosporins, ceftazidime and cefotaxime.3
Here, we have further examined the causes of resistance. Using PCR as described previously,4 D46 was shown to contain the strA-strB, sul2 and tetA(B) genes, responsible for streptomycin, sulfamethoxazole and tetracycline resistance, respectively. These genes were in the same configuration as in AbGRI1-2 (Tn6167),4 but D46 does not have an island in comM. Carbapenem and ticarcillin/clavulanate resistance was due to the blaOXA-23 carbapenemase gene, which was within Tn2006.
To better understand how Tn2006 was acquired, conjugation was performed as described previously,5,6 using D46 as a donor and a rifampicin-resistant mutant of A. baumannii ATCC 17978 as a recipient. Transconjugants resistant to meropenem, imipenem and ticarcillin/clavulanate, indicative of blaOXA-23, and resistant to kanamycin, neomycin and amikacin, indicative of aphA6, were recovered. Hence, both Tn2006 and TnaphA6 were located on a conjugative plasmid.
The whole genome sequence of D46 was determined using Illumina HiSeq and assembled as described previously.5 The draft genome comprised 115 contigs with an average read depth of 88.7× coverage and D46 was determined to be ST25 according to the Pasteur MLST scheme. Three plasmids were detected. The sequence of the smallest plasmid, pD46-1 (6078 bp), contained aadB and was almost identical to pRAY* (a single base difference).2 pD46-2 is an 8731 bp cryptic plasmid that was identical to p1ABTCDC0715 (GenBank accession number CP002523) from a GC2 isolate.
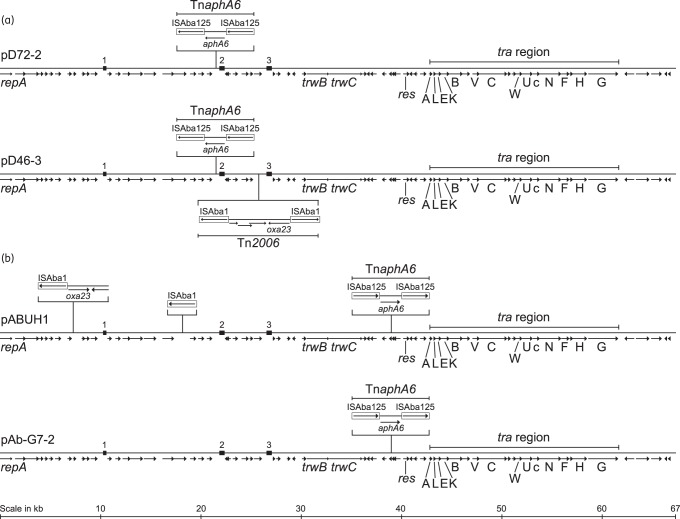
To determine whether D46 harboured Tn2006 together with TnaphA6 on a plasmid related to pAb-G7-25 and pD72-2,7 contigs that matched them were retrieved from the draft genome. Seven contigs were recovered and assembled using PCR.5,7 The amplicons were sequenced, finalizing the assembly. Plasmid pD46-3 has a 67 027 bp backbone sharing 99.99% identity (five single-base changes) with that of pD72-2 (Figure 1a). pD46-3 also harboured TnaphA6 in the same position as pD72-2 (Figure 1a). One end of two backbone contigs had inversely oriented fragments of ISAba1 and they were linked with blaOXA-23 in Tn2006 using PCR (Figure 1a). The complete 74 916 bp sequence of pD46-3 was deposited in GenBank under accession number KM977710. As the backbone of pD46-3 was almost identical to that of pD72-2 and pAb-G7-2, pD46-3 also contained the complete set of transfer genes (Figure 1). This is the first report of a completely sequenced A. baumannii plasmid that has been demonstrated to simultaneously transfer resistance to carbapenems and aminoglycosides.
Figure 1.
Comparison of repAci6 plasmids pD72-2, pD46-3 (a), pABUH1 and pAb-G7-2 (b). The plasmid backbones, linearized and opened at repA, are represented by horizontal lines. The extent of ORFs and genes are shown as arrows beneath the lines, with names given where a function is known. The transfer region is indicated above each line, the tra genes are indicated with capital letters and trbC is shown as c. The three repeat regions are indicated by the numbered boxes. The structures of TnaphA6, Tn2006 and Tn2008-like are shown above or below the backbone, with IS represented as open boxes containing an arrow showing their orientation.
A recent genomic study of a GC2 A. baumannii collection from a US hospital reported the sequence of pABUH1 (GenBank accession number AYOH01000010), which the authors suggested could be transferable.8 It was described as a plasmid related to pACICU2, harbouring ISAba1 upstream of aphA6 and having a copy of blaOXA-23 flanked by two copies of ISAba125 that is in the same position as ISAba125 in pACICU2.8 However, it was recently shown that pACICU2 is likely identical to pAb-G7-2 and in fact harbours TnaphA6 at this location.9 Our analysis of the sequence of pABUH1 revealed that it in fact carries TnaphA6 in this position (Figure 1b). Furthermore, ISAba1 is actually upstream of blaOXA-23, in a structure similar to Tn2008 (GenBank accession number GQ861438) that is flanked by a 9 bp direct repeat. However, in pABUH1 the sequence adjacent to the ISAba1 was 1613 bp whereas it is only 1351 bp in Tn2008.
The backbones of the repAci6 plasmids, pAb-G7-2, pD72-2, pD46-3, pABUH1 and pACICU2, are all very closely related, but they can be separated into two lineages based on the position of TnaphA6 (Figure 1). Representatives of the two lineages, pD46-3 and pABUH1, have each acquired the blaOXA-23 gene on separate occasions and a sixth repAci6 plasmid, pA85-3, has also gained blaOXA-23 within AbaR4.6 The acquisition of blaOXA-23 in different structures and in separate events indicates that this group of related plasmids plays a vital role in the dissemination of this carbapenemase gene and could be one of the factors responsible for making it a worldwide problem. Hence, surveillance of this group of A. baumannii plasmids will be vital in curtailing the spread of carbapenem resistance in A. baumannii.
Funding
This study was supported by grants from the School of Molecular Bioscience and the Wellcome Trust Sanger Institute. S. N. was supported by an Australian Postgraduate Award. K. E. H. was supported by NHMRC fellowship 628930.
Transparency declarations
None to declare.
References
- 1.Visca P, Seifert H, Towner KJ. Acinetobacter infection—an emerging threat to human health. IUBMB Life. 2011;63:1048–54. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- 2.Hamidian M, Nigro SJ, Hall RM. Variants of the gentamicin and tobramycin resistance plasmid pRAY are widely distributed in Acinetobacter. J Antimicrob Chemother. 2012;67:2833–6. doi: 10.1093/jac/dks318. [DOI] [PubMed] [Google Scholar]
- 3.Hamidian M, Hall RM. ISAba1 targets a specific position upstream of the intrinsic ampC gene of Acinetobacter baumannii leading to cephalosporin resistance. J Antimicrob Chemother. 2013;68:2682–3. doi: 10.1093/jac/dkt233. [DOI] [PubMed] [Google Scholar]
- 4.Nigro SJ, Hall RM. Tn6167, an antibiotic resistance island in an Australian carbapenem-resistant Acinetobacter baumannii GC2, ST92 isolate. J Antimicrob Chemother. 2012;67:1342–6. doi: 10.1093/jac/dks037. [DOI] [PubMed] [Google Scholar]
- 5.Hamidian M, Holt KE, Pickard D, et al. A GC1 Acinetobacter baumannii isolate carrying AbaR3 and the aminoglycoside resistance transposon TnaphA6 in a conjugative plasmid. J Antimicrob Chemother. 2014;69:955–8. doi: 10.1093/jac/dkt454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamidian M, Kenyon JJ, Holt KE, et al. A conjugative plasmid carrying the carbapenem resistance gene blaOXA-23 in AbaR4 in an extensively resistant GC1 Acinetobacter baumannii isolate. J Antimicrob Chemother. 2014;69:2625–8. doi: 10.1093/jac/dku188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigro SJ, Hall RM. Amikacin resistance plasmids in extensively antibiotic-resistant GC2 Acinetobacter baumannii from two Australian hospitals. J Antimicrob Chemother. 2014;69:3435–7. doi: 10.1093/jac/dku310. [DOI] [PubMed] [Google Scholar]
- 8.Wright MS, Haft DH, Harkins DM, et al. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. MBio. 2014;5:e00963–13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamidian M, Hall RM. pACICU2 is a conjugative plasmid of Acinetobacter carrying the aminoglycoside resistance transposon TnaphA6. J Antimicrob Chemother. 2014;69:1146–8. doi: 10.1093/jac/dkt488. [DOI] [PubMed] [Google Scholar]