Abstract
Objectives
Phenotypic drug susceptibility testing (DST) for Mycobacterium tuberculosis takes several weeks to complete and second-line DST is often poorly reproducible, potentially leading to compromised clinical decisions. Following a fatal case of XDR TB, we investigated the potential benefit of using whole-genome sequencing to generate an in silico drug susceptibility profile.
Methods
The clinical course of the patient was reviewed, assessing the times at which phenotypic DST data became available and changes made to the therapeutic regimen. Whole-genome sequencing was performed on the earliest available isolate and variants associated with drug resistance were identified.
Results
The final DST report, including second-line drugs, was issued 10 weeks after patient presentation and 8 weeks after initial growth of M. tuberculosis. In the interim, the patient may have received a compromised regimen that had the potential to select for further drug resistance. The in silico susceptibility profile, extrapolated from evolving evidence in the literature, provided comparable or superior data to the DST results for second-line drugs and could be generated in a much shorter timeframe.
Conclusions
We propose routine whole-genome sequencing of all MDR M. tuberculosis isolates in adequately resourced settings. This will improve individual patient care, monitor for transmission events and advance our understanding of resistance-associated mutations.
Keywords: extensively drug resistant, multidrug resistant, XDR, MDR
Introduction
The global TB epidemic is increasingly being driven by the emergence and spread of drug-resistant strains.1 The WHO estimates that nearly half a million (450 000; range: 300 000–600 000) people developed MDR TB (resistant to isoniazid and rifampicin) in 2012, of whom <20% received appropriate treatment.2 The diagnosis and treatment of MDR TB present major challenges. Dependence on traditional drug susceptibility testing (DST) incurs significant delays in diagnosis, which force clinicians to initiate empirical treatment that may be suboptimal, leading to the multiplication of drug resistance and adverse patient outcomes.
Initiating treatment with an optimal regimen is especially important in patients with XDR TB (MDR with additional resistance to fluoroquinolones and at least one second-line injectable drug), since therapeutic options remain limited. Approximately 10% of MDR cases are thought to be XDR2 and its global rise is driven by treatment that is poorly aligned with local resistance patterns,3 as well as by efficient transmission of some XDR strains.4 Given the poor treatment outcomes of XDR TB patients,5 every effort should be made to reduce delays in case detection and characterization of susceptibility profiles.
Conventional phenotypic DST requires a minimum of 2 weeks: one for initial detection of microbial growth and another to assess critical concentrations of first-line anti-TB drugs.6 In reality, full characterization of an XDR strain may take months, since expanded DST is usually performed sequentially and often some tests need to be repeated. In addition, drug resistance breakpoints for most second-line drugs are poorly standardized. Genotypic tests such as the commercial GeneXpert MTB/RIF® and GenoType MTBDRplus® (Hain Lifescience) assays offer rapid assessment of drug resistance mutations against key first-line anti-TB drugs, with testing of second-line agents using the GenoType MTBDRsl® (Hain Lifescience) assay if required. However, culture-based DST remains the reference standard, given the limited number of mutations evaluated by current genotypic tests and our incomplete understanding of the mechanisms of resistance.
Whole-genome sequencing has offered novel insight into the evolution and spread of Mycobacterium tuberculosis, but its application in routine patient care has been limited by the perceived lack of clinical relevance, high cost, complexity of interpretation and slow turn-around times. Recent technological advances have made routine whole-genome sequencing of select M. tuberculosis strains in reference laboratories in a clinically relevant timeframe technically and economically feasible. We present a case of XDR TB diagnosed in New South Wales, Australia, and illustrate the potential clinical value of routine whole-genome sequencing of highly resistant M. tuberculosis strains.
Patient and methods
A man in his thirties presented to hospital with cough, night sweats and weight loss for more than 3 months. He was of East African ancestry and had spent much of his adult life as a refugee in the Horn of Africa. He first received treatment for TB in Ethiopia in early adolescence; the basis for the diagnosis was uncertain and he discontinued treatment after 3 months due to war. A decade later in Djibouti, he was treated for sputum smear-positive TB. He received 6 months of standard first-line therapy, comprising isoniazid, rifampicin, pyrazinamide and ethambutol during the 2 month intensive phase, with isoniazid/rifampicin during the 4 month continuation phase. He was documented to be sputum smear negative after completion and declared cured.
Three years later he was diagnosed with a third episode of TB. His third treatment course (also received in Djibouti) comprised 2 months of isoniazid/rifampicin/pyrazinamide/ethambutol and streptomycin, followed by 1 month of isoniazid/rifampicin/pyrazinamide/ethambutol and a further 5 months of isoniazid/rifampicin/ethambutol. He was reported to be culture negative at the end of treatment. He subsequently received approval to settle in Australia, on condition that he commit to regular TB screening. He had stable chest radiograph findings and culture-negative sputum for 2 years after migration (Figure 1a), but was subsequently lost to follow-up until his symptomatic presentation, 5 years after migration.
Figure 1.
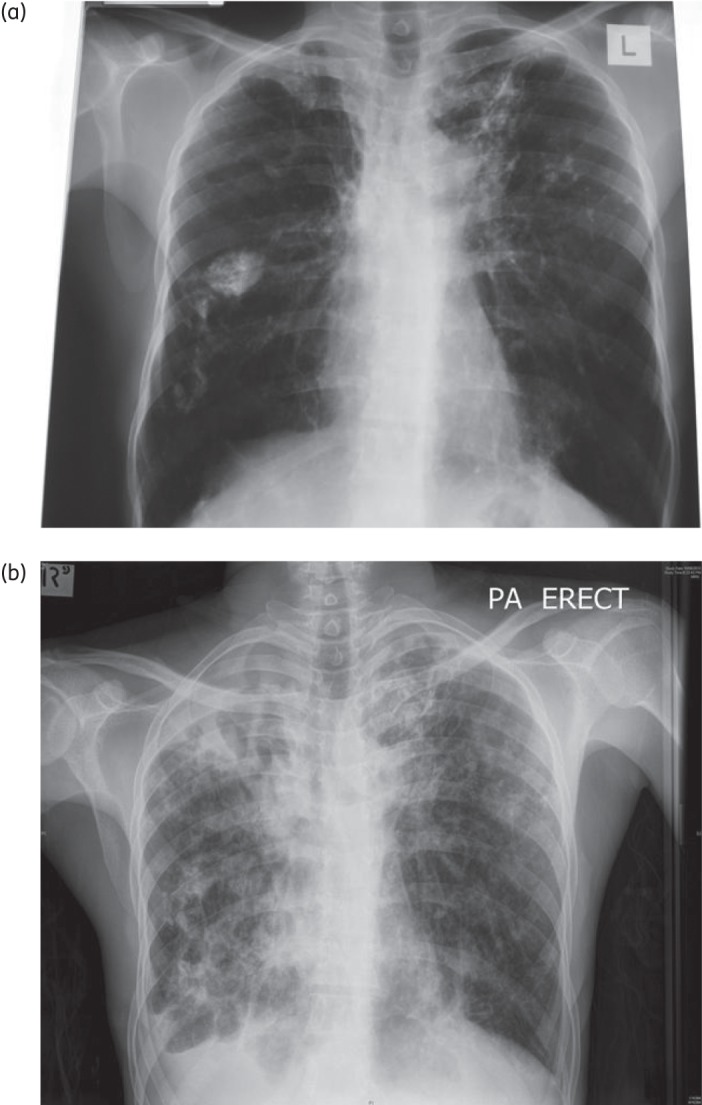
(a) Chest radiograph when asymptomatic; 3 years before presentation. (b) Chest radiograph with symptomatic presentation.
Physical examination on presentation revealed cachexia (38 kg) and finger clubbing. The chest radiograph showed bilateral cavities with extensive fibrocalcific changes (Figure 1b). Sputum microscopy was 3+ for acid-fast bacilli and M. tuberculosis was confirmed on culture. HIV serology was negative. Given his long disease-free interval and poor clinical condition, the patient was commenced immediately on regular first-line therapy with daily isoniazid/rifampicin/pyrazinamide/ethambutol, pending the outcome of genotypic and phenotypic DST. GeneXpert MTB/RIF® testing was not performed, but GenoType MTBDRplus® (Hain Lifescience) assay results suggested MDR TB. When this information became available at week 3, amikacin and moxifloxacin were added to his regimen.
Five weeks after presentation, phenotypic DST results showed resistance to isoniazid (MIC >0.4 mg/L), rifampicin and pyrazinamide, with ethambutol susceptibility. Additional results suggested resistance to streptomycin, amikacin, capreomycin, ciprofloxacin and ethionamide, fulfilling the WHO definition of XDR TB. All medications were temporarily ceased until more complete second-line susceptibilities became available. The expanded DST report, issued 10 weeks after initial diagnosis, listed susceptibility to para-aminosalicylic acid, cycloserine and clofazimine. He was restarted on moxifloxacin, high-dose ethambutol (25 mg/kg) and pyrazinamide with the addition of para-aminosalicylic acid, cycloserine, clofazimine and amoxicillin/clavulanate. On this regimen his clinical situation improved, with no further fevers, reduction in cough, 17 kg of weight gain and some radiological improvement. Unfortunately the patient developed major depression and cycloserine had to be discontinued; it was replaced by trimethoprim/sulfamethoxazole and clarithromycin. Susceptibility testing on an isolate collected 9 months after presentation reported intermediate-level resistance to moxifloxacin (MIC between 1.0 and 2.0 mg/L) and susceptibility to linezolid.
The patient remained in hospital in strict isolation in a negative-pressure room for 2 years, since his sputum remained culture positive for M. tuberculosis despite smear conversion. Given his stable clinical condition, suitable accommodation was ultimately found where the infectious risk could be minimized and directly observed therapy provided 7 days a week. However, within 4 months of discharge he was readmitted to hospital with extensive ascites, he was culture positive for M. tuberculosis and he died following a major pulmonary haemorrhage.
Whole-genome sequencing
After the patient's death, an early M. tuberculosis isolate was submitted for whole-genome sequencing using the Illumina HiSeq 2000 platform, producing 100 base paired-end reads with >500-fold average depth of coverage after mapping onto the M. tuberculosis H37Rv reference genome (NCBI GenBank NC_000962).
Several genetic variants in different genes associated with drug resistance in M. tuberculosis were identified by whole-genome sequencing, providing a plausible genetic basis for the observed phenotypic resistance to isoniazid, rifampicin and pyrazinamide, as well as all fluoroquinolones and injectables (capreomycin and aminoglycosides).7–11 These are shown in Table 1. Although this patient's strain was initially reported to be susceptible to ethambutol using phenotypic methods, the sequenced isolate possessed multiple mutations associated with ethambutol resistance.
Table 1.
Genetic variantsa identified with potential to contribute to drug resistance
| Drug | Gene | H37Rv identifier | Variant | Likelihood of resistanceb | Clinical relevance |
|---|---|---|---|---|---|
| Isoniazid7,11 | katG | Rv1908c | S315T | high | no value in treatment |
| fabD | Rv2243 | S275N | high | ||
| iniA | Rv0342 | H481Q | low | ||
| Rv1592c | Rv1592c | I322V | low | ||
| proA | Rv2427c | V140L | low | ||
| Rifampicin7,11 | rpoB | Rv0667 | S450Wc | high | no value in treatment |
| Pyrazinamide7,11 | pncA | Rv2043c | D158EAGd | high | no value in treatment |
| T160M | high | ||||
| T167A | low | ||||
| Ethambutol7,11 | embC | Rv3793 | T270I | possible | no value in treatment |
| N394D | possible | ||||
| embA | Rv3794 | V206M | possible | ||
| P913S | possible | ||||
| embB | Rv3795 | D328G | possible | ||
| E378A | possible | ||||
| G406S | high | ||||
| rmlD | Rv3266c | S257P | possible | ||
| embR | Rv1267c | D107N | possible | ||
| C110Y | possible | ||||
| iniA | Rv0342 | H481Q | possible | ||
| Fluoroquinolones7–9,11 | gyrA | Rv0006 | E21Q | low | limited value, should not be counted as an ‘active drug’ |
| A90Ve | high | ||||
| D94Ye | high | ||||
| A384V | very low | ||||
| G668D | very low26 | ||||
| gyrB | Rv0005 | M330I | very low | ||
| E540D | low27 | ||||
| Aminoglycosides, streptomycin and capreomycin7,10 | rrs | Rvnr01 | A1401G | high | no value in treatment |
| rpsL | Rv0682 | K43R | high | ||
| gidB | Rv3919c | S100F | possible | ||
| Ethionamide7 | ethA | Rv3854c | C403W | possible | limited value, should not be counted as an ‘active drug’ |
aCompared with H37Rv as the reference strain.
bHigh—strong evidence for a causal association with a drug-resistant phenotype; low—conflicting evidence or evidence suggesting a weak association with a drug-resistant phenotype; possible—theoretical possibility of causal association with a drug-resistant phenotype, but insufficient evidence.
cS531W with Escherichia coli numbering.
dFrameshift mutation.
eHeteroresistance—mutations detected in 63% (A90V) and 16% (D94Y) of reads.
Discussion
In retrospect, this patient's treatment might have been optimized to provide him with the best possible chance of cure if whole-genome sequencing data had been available at the time. More importantly, in future the growing body of knowledge on molecular markers of drug resistance and the availability of new antimycobacterial agents should provide better guidance and more treatment options to clinicians. In our case, the sequenced isolate had genetic evidence of resistance to ethambutol, pyrazinamide and moxifloxacin, which were components of both his expanded first-line regimen and his initial XDR regimen. When XDR TB treatment was ultimately commenced, the only drugs in the regimen with likely activity were para-aminosalicylic acid and cycloserine, with possible contributions from clofazimine and amoxicillin/clavulanate.
The regimen was suboptimal due to delayed DST results and inadequate laboratory guidance on likely ethambutol resistance. The difficulties of establishing ethambutol breakpoints and performing reliable phenotypic DST have been documented before.12 In addition, DST results arrived in stages over many weeks, hampering rational treatment decision-making. This is of particular importance in TB, where three to four active drugs are required to prevent amplification of drug resistance, and the addition of a single drug to a failing regimen is irresponsible. The therapeutic regimen was compromised when cycloserine had to be ceased and was replaced with trimethoprim/sulfamethoxazole, which act on the same metabolic pathway as para-aminosalicylic acid, and clarithromycin, which has little activity against M. tuberculosis.13,14 With the availability of genomic information, the objective of including at least four active drugs15 might have been achieved if the addition of a carbapenem16 or linezolid17 was considered early in the treatment course. Linezolid was not included in the regimen because of its significant side effect profile, minimal efficacy data and the belief that four active agents were available (ethambutol, PAS, cycloserine and clofazimine). The patient did show clinical improvement on this regimen. Neither delamanid18 nor bedaquiline19 was available and there was little evidence at that time to suggest that carbapenems were sufficiently effective to warrant inclusion.
This case study emphasizes the heavy clinical reliance on phenotypic DST, which is too slow and insufficiently validated for drugs other than isoniazid, rifampicin, quinolones and the injectables to guide individual patient management. Our patient's treatment failed despite the anticipation of MDR TB and the prompt addition of two extra drugs (moxifloxacin and amikacin) to his regimen following preliminary genetic testing for MDR. Given the urgency to optimize treatment before additional drug resistance develops, every effort should be made to optimize the regimen from the very beginning.
We propose that all isolates found to be likely MDR on initial genetic testing (GeneXpert MTB/RIF® or GenoType MTBDRplus®) should undergo expedited whole-genome sequencing. A realistic turn-around time for whole-genome sequencing is 1–2 weeks from nucleic acid extraction and costs of US$70–250 per isolate pale in comparison with patient management costs. The estimated total cost of this patient's care was equivalent to US$1 million, with average XDR TB treatment costs in developed countries estimated at US$483 000.20 The price and turn-around time of whole-genome sequencing is rapidly decreasing with ongoing technological advances. Routine whole-genome sequencing would also facilitate detailed transmission analysis, to guide targeted public health interventions and monitor infection control practices.21–25 Phenotypic testing would still be required to validate findings, comply with international recommendations and assist the elucidation of unknown resistance mechanisms, but this may change in future with growing confidence in the genetic markers of resistance. An additional safety advantage of whole-genome sequencing for laboratory personnel can be obtained if there are fewer manipulations of live cultures.
Routine whole-genome sequencing of all MDR M. tuberculosis isolates will assist patient management and guide public health responses, but is currently feasible only in well-resourced settings. It is important to drive innovation and refine the clinical application of this new technology, since widespread use is anticipated in the near future and TB patients globally stand to benefit.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
References
- 1.Abubakar I, Zignol M, Falzon D, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13:529–39. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2013. Geneva, Geneva: WHO; 2013. [Google Scholar]
- 3.Chihota VN, Müller B, Mlambo CK, et al. Population structure of multi- and extensively drug-resistant Mycobacterium tuberculosis strains in South Africa. J Clin Microbiol. 2012;50:995–1002. doi: 10.1128/JCM.05832-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klopper M, Warren RM, Hayes C, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2013;19:449–55. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383:1230–9. doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- 6.Goloubeva V, Lecocq M, Lassowsky P, et al. Evaluation of Mycobacteria Growth Indicator Tube for direct and indirect drug susceptibility testing of Mycobacterium tuberculosis from respiratory specimens in a Siberian prison hospital. J Clin Microbiol. 2001;39:1501–5. doi: 10.1128/JCM.39.4.1501-1505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandgren A, Strong M, Muthukrishnan P, et al. Tuberculosis drug resistance mutation database. PLoS Med. 2009;6:e1000002. doi: 10.1371/journal.pmed.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik S, Willby M, Sikes D, et al. New insights into fluoroquinolone resistance in Mycobacterium tuberculosis: functional genetic analysis of gyrA and gyrB mutations. PLoS One. 2012;7:e39754. doi: 10.1371/journal.pone.0039754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruri F, Sterling TR, Kaiga AW, et al. A systematic review of gyrase mutations associated with fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed gyrase numbering system. J Antimicrob Chemother. 2012;67:819–31. doi: 10.1093/jac/dkr566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georghiou SB, Magana M, Garfein RS, et al. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One. 2012;7:e33275. doi: 10.1371/journal.pone.0033275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebenzahl-Guimaraes H, Jacobson KR, et al. Systematic review of allelic exchange experiments aimed at identifying mutations that confer drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2014;69:331–42. doi: 10.1093/jac/dkt358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schön T, Juréen P, Giske CG, et al. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother. 2009;64:786–93. doi: 10.1093/jac/dkp262. [DOI] [PubMed] [Google Scholar]
- 13.Köser CU, Summers DK, Archer JAC. Role of the dihydrofolate reductase DfrA (Rv2763c) in trimethoprim-sulfamethoxazole (co-trimoxazole) resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2010;54:4951–2. doi: 10.1128/AAC.00876-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fivian-Hughes AS, Houghton J, Davis EO. Mycobacterium tuberculosis thymidylate synthase gene thyX is essential and potentially bifunctional, while thyA deletion confers resistance to p-aminosalicylic acid. Microbiology. 2012;158:308–18. doi: 10.1099/mic.0.053983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caminero JA, Sotgiu G, Zumla A, et al. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–9. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzo SD, Alffenaar JW, Sotgiu G, et al. Efficacy and safety of meropenem–clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J. 2013;41:1386–92. doi: 10.1183/09031936.00124312. [DOI] [PubMed] [Google Scholar]
- 17.Sotgiu G, Centis R, D'Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40:1430–42. doi: 10.1183/09031936.00022912. [DOI] [PubMed] [Google Scholar]
- 18.Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41:1393–400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diacon AH, Donald PR, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56:3271–6. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LoBue P, Sizemore C, Castro KG. Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. Morb Mortal Wkly Rep. 2009;58:1–43. [PubMed] [Google Scholar]
- 21.Schürch AC, Kremer K, Kiers A, et al. The tempo and mode of molecular evolution of Mycobacterium tuberculosis at patient-to-patient scale. Infect Genet Evol. 2010;10:108–14. doi: 10.1016/j.meegid.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. New Engl J Med. 2011;364:730–9. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 23.Roetzer A, Diel R, Kohl TA, et al. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med. 2013;10:e1001387. doi: 10.1371/journal.pmed.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker TM, Ip CL, Harrell RH, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13:137–46. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Lago L, Comas I, Navarro Y, et al. Whole genome sequencing analysis of intrapatient microevolution in Mycobacterium tuberculosis: potential impact on the inference of tuberculosis transmission. J Infect Dis. 2014;209:98–108. doi: 10.1093/infdis/jit439. [DOI] [PubMed] [Google Scholar]
- 26.Lau RWT, Ho P-L, Kao RYT, et al. Molecular characterization of fluoroquinolone resistance in Mycobacterium tuberculosis: functional analysis of gyrA mutation at position 74. Antimicrob Agents Chemother. 2011;55:608–14. doi: 10.1128/AAC.00920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Nakajima C, Yokoyama K, et al. Impact of the E540V amino acid substitution in GyrB of Mycobacterium tuberculosis on quinolone resistance. Antimicrob Agents Chemother. 2011;55:3661–7. doi: 10.1128/AAC.00042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


