Abstract
IL-13 has been implicated in the pathogenesis of allergic diseases, including atopic dermatitis (AD). However, a direct role of IL-13 in AD has not been established. We aimed to develop an inducible transgenic model in which IL-13 can be expressed in the skin and to define the resulting dermal phenotype and mechanisms involved. The keratin 5 promoter was used with a tetracycline-inducible system to target IL-13 to the skin. The clinical manifestations, dermal histology, cytokine gene regulation, and systemic immune responses in the transgenic mice were assessed. IL-13 was produced exclusively in the skin and caused a chronic inflammatory phenotype characterized by xerosis and pruritic eczematous lesions; dermal infiltration of CD4 + T cells, mast cells, eosinophils, macrophages, and Langerhans cells; upregulation of chemokine and cytokine genes, including thymic stromal lymphopoietin; and skin remodeling with fibrosis and increased vasculature. The dermal phenotype was accompanied by elevated serum total IgE and IgG1 and increased production of IL-4 and IL-13 by CD4 + cells from lymphoid tissues and peripheral blood mononuclear cells. IL-13 is a potent stimulator of dermal inflammation and remodeling and this transgenic model of AD is a good tool for investigating the underlying mechanisms in the pathogenesis of AD.
INTRODUCTION
Atopic dermatitis (AD) is a pruritic, chronic inflammatory skin disease that affects 15–20% of children and 1–3% of adults. The prevalence of AD has been steadily increasing during the past three decades (Leung et al., 2004). Th2-dominated immune responses are believed to contribute to the pathogenesis of AD, particularly in the early stage. Analysis of biopsies of affected skin of AD patients revealed increased numbers of Th2 cells expressing IL-4 and IL-13 mRNA (Hamid et al., 1994; Novak et al., 2003). IL-4 and IL-13 are important Th2 cytokines.
IL-13 has some effects in Th2 inflammation that are key features in both asthma and AD. The effects include the ability to induce IgE production (Emson et al., 1998), CD23 expression (Zurawski and de Vries, 1994), endothelial P-selectin and vascular cell adhesion molecule-1 expression (Bochner et al., 1995; Woltmann et al., 2000), and inhibition of eosinophil apoptosis (Horie et al., 1997). Overexpression of IL-13 in the murine lung causes eosinophil, lymphocyte and macrophage-rich inflammation, mucus metaplasia, and airway hyperresponsiveness on methacholine challenge (Zhu et al., 1999). In AD, memory T cells expressing the skin homing receptor, cutaneous lymphocyte-associated antigen, produced increased levels of IL-13 (Akdis et al., 1997). IL-13-stimulated keratinocytes preferentially attract CD4 + CCR4 + T lymphocytes (Purwar et al., 2006). IL-13 also downregulates antimicrobial peptide expression in eczematous skin, which may account for their propensity toward recurrent skin infections (Ong et al., 2002; Nomura et al., 2003).
These studies have established a close relationship between IL-13 and AD. However, neither the direct effects of IL-13 on dermal tissue nor the effector functions of IL-13 in the skin have been addressed. We hypothesized that IL-13 alone is sufficient to initiate inflammatory responses in the skin that mimic AD. In this study we developed an externally regulatable overexpression transgenic model system in which IL-13 is selectively expressed in murine skin. Analyses show that expression of IL-13 in the skin resulted in a chronic pruritic inflammatory phenotype with many characteristics closely resembling those of human AD. Many proinflammatory cytokines, including thymic stromal lymphopoietin (TSLP), were highly upregulated in the skin and the dermal phenotype was accompanied by a systemic Th2-prone environment.
RESULTS
Generation of transgenic mice
To obtain mice that express IL-13 specifically in the skin we first generated mice carrying the transgene TRE-Tight-IL-13 (Figure 1a) and crossbred these mice with the K5-tTA mouse line (Diamond et al., 2000). The offspring that carried both transgenes were designated as K5-tTA-IL-13 mice (or Tg(+) mice) and were used in experiments in comparison with Tg(−) littermate control mice.
Figure 1. Targeting IL-13 to the skin.
(a) Schematic construct of TRE-Tight-IL-13. (b) Tissue specificity of IL-13 expression in Tg(+) mice as determined by RT–PCR. The experiments were started by withdrawing Dox and total RNA from different tissues was analyzed for IL-13 mRNA expression. (c) IL-13 mRNA expression in the skin of Tg(+) mice in comparison with Tg(−) mice 6 weeks off Dox. (d) IL-13 protein in the skin extract 8 weeks after Dox withdrawal (n = 36, each group).
Inducible expression of IL-13 in the skin
We then tested if expression of IL-13 could be induced by doxycycline (Dox) and was restricted to the skin. The keratin 5 (K5) promoter controlled tTA is inactive in the presence of Dox and active in the absence of Dox. To express IL-13 only in the adult mice, we gave Dox (1 mg ml−1 with 4% sucrose) in the drinking water of the K5-tTA-IL-13 mice and their Tg(−) littermates until they were at least 6 weeks of age. In the beginning of each experiment, Dox was withdrawn and the induction of the IL-13 transgene was initiated.
IL-13 was not detected at either the mRNA or protein level in any tissues of Tg(−) mice or in Tg(+) mice with Dox suppression (data not shown). However, after Dox withdrawal for 6 weeks IL-13 mRNA was readily detected in the skin but not in other tissues of Tg(+) mice (Figures 1b and c). Similarly, IL-13 protein was readily detected in the skin extracts of Tg(+) mice after induction (Figure 1d). All subsequent experiments were performed to compare Tg(+) mice and Tg(−) littermate controls without Dox starting from 6 weeks of age. Immunohistochemistry (IHC) showed that IL-13 was expressed in the keratinocytes, with less staining in the dermal layer, indicating that IL-13 was produced by keratinocytes (data not shown). These results demonstrated that using the K5-tTA system, we targeted the IL-13 transgene to the skin in an inducible fashion.
IL-13-induced inflammation in the skin
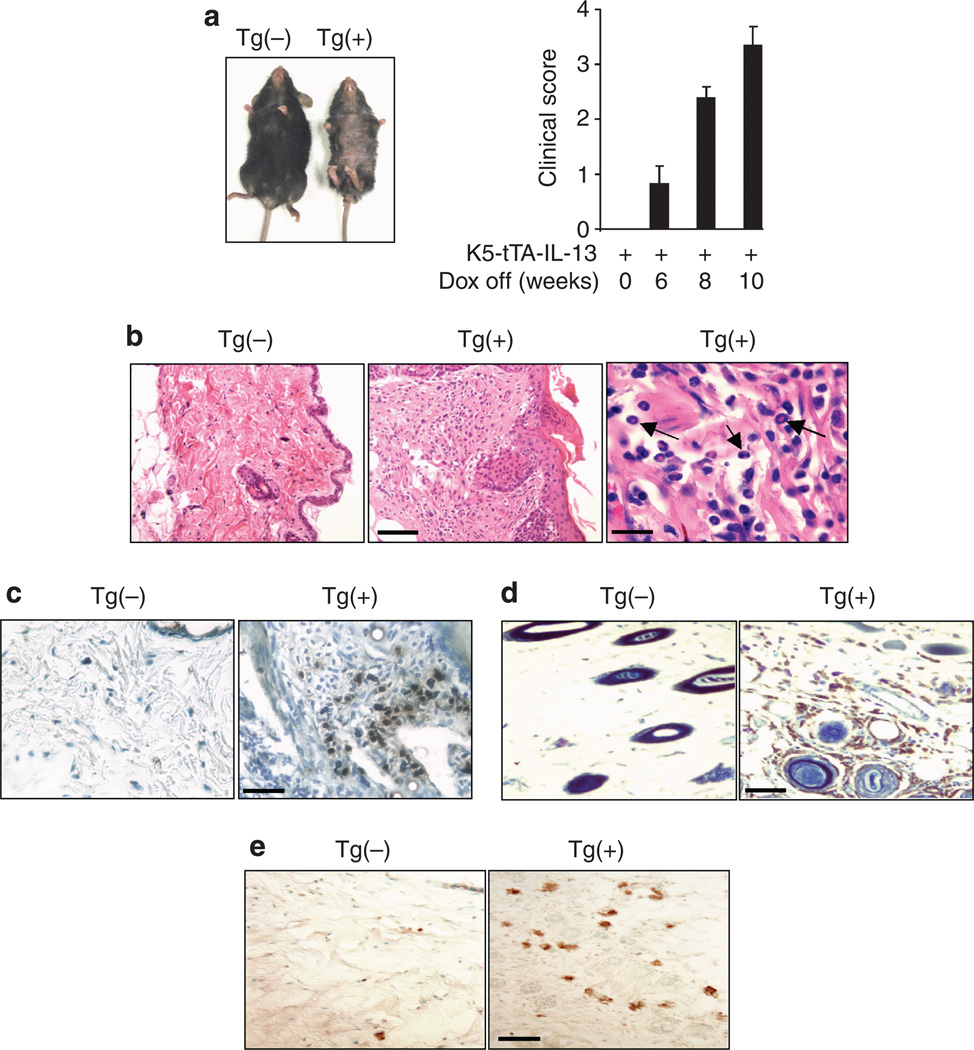
Gross and histological examination showed no abnormalities in the skin of Tg(−) mice with or without Dox, indicating that Dox had no effect on the skin. Similarly, Tg(+) mice given Dox did not show any sign of inflammation because no IL-13 was expressed under this condition (Figure 2a). In contrast, 6–8 weeks after Dox withdrawal, Tg(+) mice developed pruritus (indicated by constant and intensive scratching of the affected areas), loss of hair, erythema, crusting, excoriation, bacterial pyoderma, and erosions in the skin, mostly involving the back and abdominal areas. With continued IL-13 induction, the lesions progressed and became more extensive, including dry lichenified skin lesions (Figure 2a). Quantitative evaluations of the mice using the criteria described in Materials and Methods showed that Tg(+) mice with the IL-13 transgene turned on had increased clinical scores over time (Figure 2a). Histologically, in comparison to the Tg(−) littermates, the lesional skin from Tg(+) mice showed thickening of the epidermal and dermal layers, spongiosis, hyperkeratosis, and marked cellular infiltration (Figure 2b). The inflammatory infiltration was characterized by increased numbers of mononuclear cells and eosinophils in the subepidermal, intradermal, and perivascular spaces whereas neutrophils were rarely seen (Figure 2b). To further define the cell types, skin samples were stained using antibodies to specific markers for eosinophils, CD4 + cells, and F4/80 + cells, including activated macrophages and Langerhans cells, which are important cell types that contribute to the inflammatory response in human AD (Leung et al., 2004). Significantly increased accumulation of major basic protein + eosinophils (Figure 2c) and F4/80 + cells (Figure 2d) was seen in the dermal layer of the skin of Tg(+) mice as compared to the Tg(−) controls. Similarly, in the lesional skin of Tg(+) animals increased numbers of CD4 + cells were found (Figure 2e). Quantitative evaluation of these cell types is shown in Table 1. These findings demonstrated that expression of IL-13 in the skin resulted in a chronic pruritic inflammatory skin phenotype with many characteristic changes closely resembling eczematous inflammation observed in human AD.
Figure 2. IL-13-induced AD.
(a) Gross comparison of a Tg(+) mouse and a Tg(−) mouse off Dox for 8 weeks. Tg(+) mice developed inflammatory skin lesions in the back and abdomen areas with hair loss, dry skin, excoriation, crusting, and bacterial pyoderma. Clinical scores of dermatitis in a representative group of Tg(+) mice (n = 7) after induction of IL-13 transgene for varying time. Tg(−) mice and Tg(+) mice without induction did not show any clinical signs of dermatitis. (b) Skin sections (H&E) showing thickening of epidermal and dermal layers, spongiosis, and infiltration of eosinophils (arrows) and mononuclear cells in Tg(+) mice. Scale bars: left and middle panels = 100 µm, right = 20 µm. IHC analyses of infiltrating cells (c) MBP + eosinophils (dark brown). Bar = 50 µm; (d) F4/80 + cells (brown). Bar = 50 µm; and (e) CD4 + cells (brown). Bar = 50 µm.
Table 1.
Cellularity in the skin
| Stain | Tg(−) (n=4) | Tg(+) (n=5) | P-values | |
|---|---|---|---|---|
| CD4+ cells | IHC | 2.1 ± 0.13 | 8.1 ± 1.011 | 0.016 |
| Eosinophils | HE | 0.31 ± 0.18 | 7.4 ± 0.941 | 0.031 |
| F4/80+ cells | IHC | 4.5 ± 0.34 | 14.66 ± 3.151 | 0.011 |
| Mast cells | TB | 14.41 ± 1.23 | 52.42 ± 4.611 | 0.02 |
Skin sections were stained with H&E (HE), toluidine blue (TB), or immunohistochemistry (IHC) and the unit is number of cells per high power field. Values are mean ± SEM.
Difference is statistically significant.
IL-13-induced increased mast cells and mediators
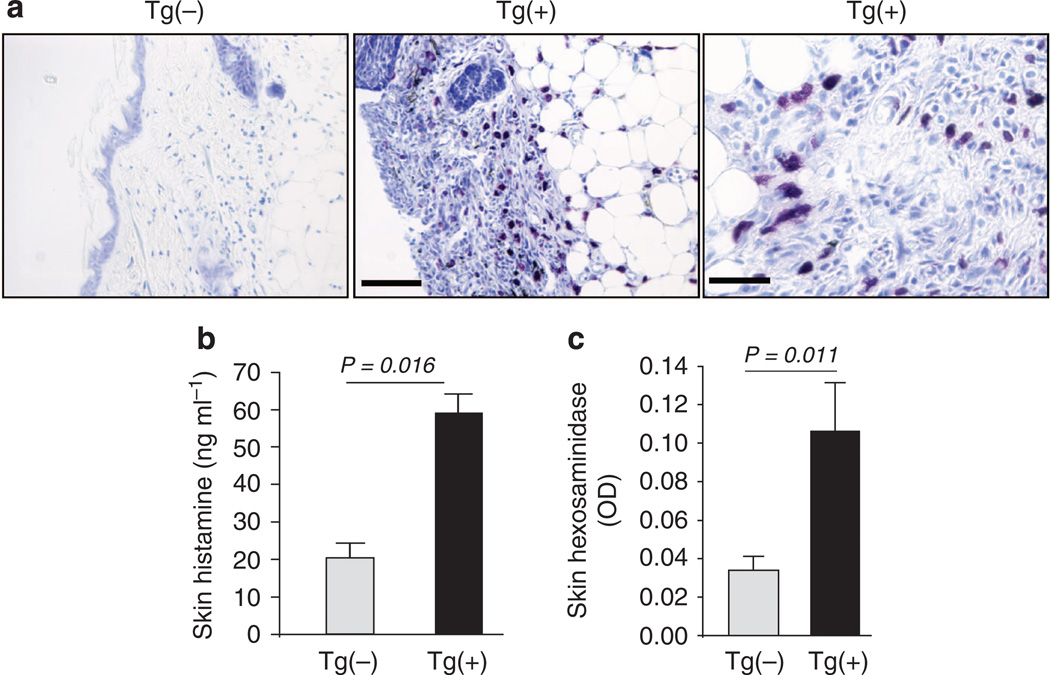
Mast cells and their mediators contribute to the pathogenesis of human AD. Increased numbers of mast cells were found in the skin of AD patients. Studies were undertaken using toluidine blue staining to detect mast cells in the skin of K5-tTA-IL-13 mice and their littermate controls. Some mast cells were present in the skin of Tg(−) mice. However, IL-13 caused a fourfold increase in the numbers of mast cells in the lesional and nonlesional skin of Tg(+) animals (Figures 3a and b). This was associated with increased tissue histamine and β-hexosaminidase in the skin of Tg(+) mice as compared with those in Tg(−) mice (Figures 3b and c).
Figure 3. Mast cells and mast-cell mediators in the skin.
(a) Toluidine blue staining of skin samples from Tg(−) and Tg(+) mice that were off Dox for 8 weeks. Scale bars: left and middle panels = 100 µm, right = 20 µm. (b) Total tissue histamine and (n = 7 per group). (c) β-hexosaminidase content in the skin extracts (n = 12 per group).
IL-13-induced upregulation of chemokine and cytokine expression in the skin
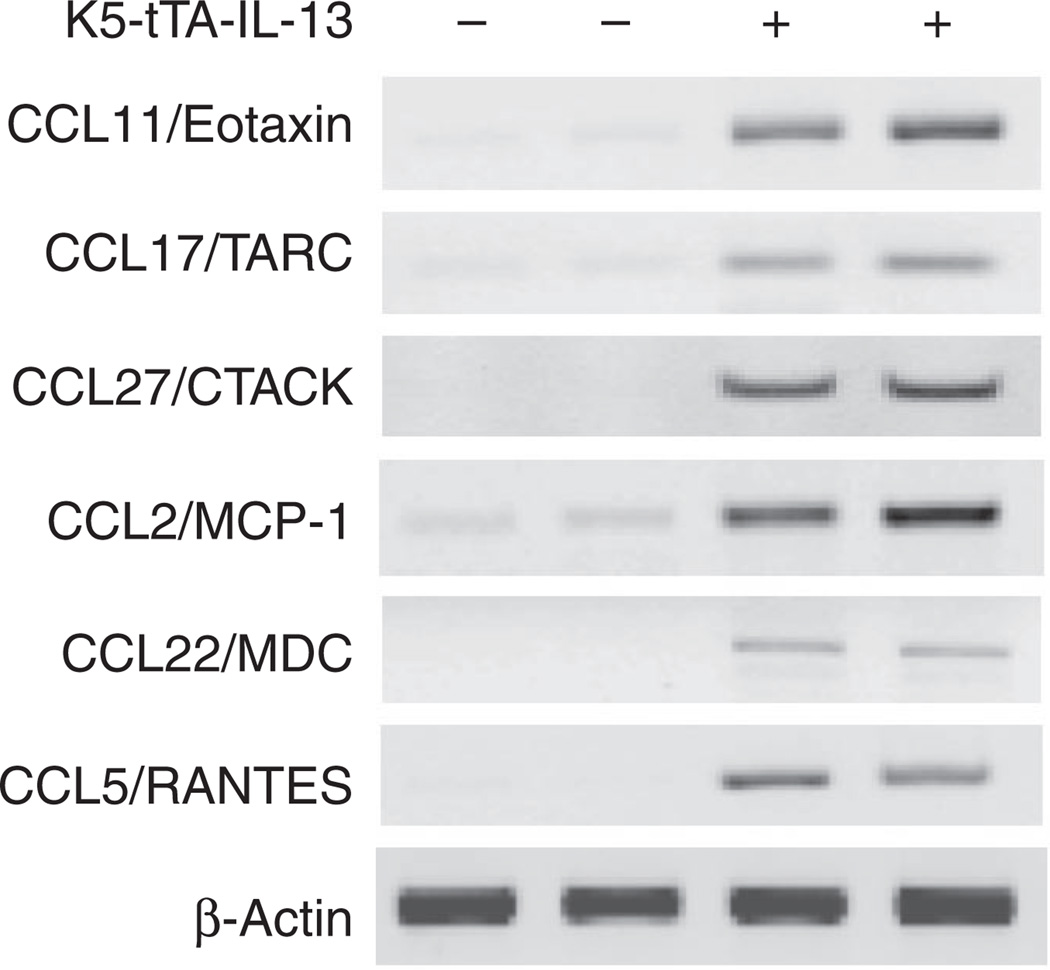
To understand the mechanisms by which IL-13 induced skin inflammation, we examined the expression of selected chemokines in Tg(+) and Tg(−) mice. In the skin of Tg(+) mice, the levels of mRNA encoding CCL11/Eotaxin, CCL17/TARC, CCL27/CTACK, CCL2/MCP-1, CCL22/MDC, and CCL5/RANTES were highly increased compared with those in the skin of Tg(−) mice (Figure 4), indicating that IL-13 is a potent stimulator of proinflammatory chemokines in the skin.
Figure 4. Chemokine mRNA expression in the skin.
Total RNA purified from skin samples from K5-tTA-IL-13 Tg(+) mice and Tg(−) controls was analyzed using gene-specific primers. β-Actin gene was used as an internal control. At least two independent experiments were performed for each of the genes.
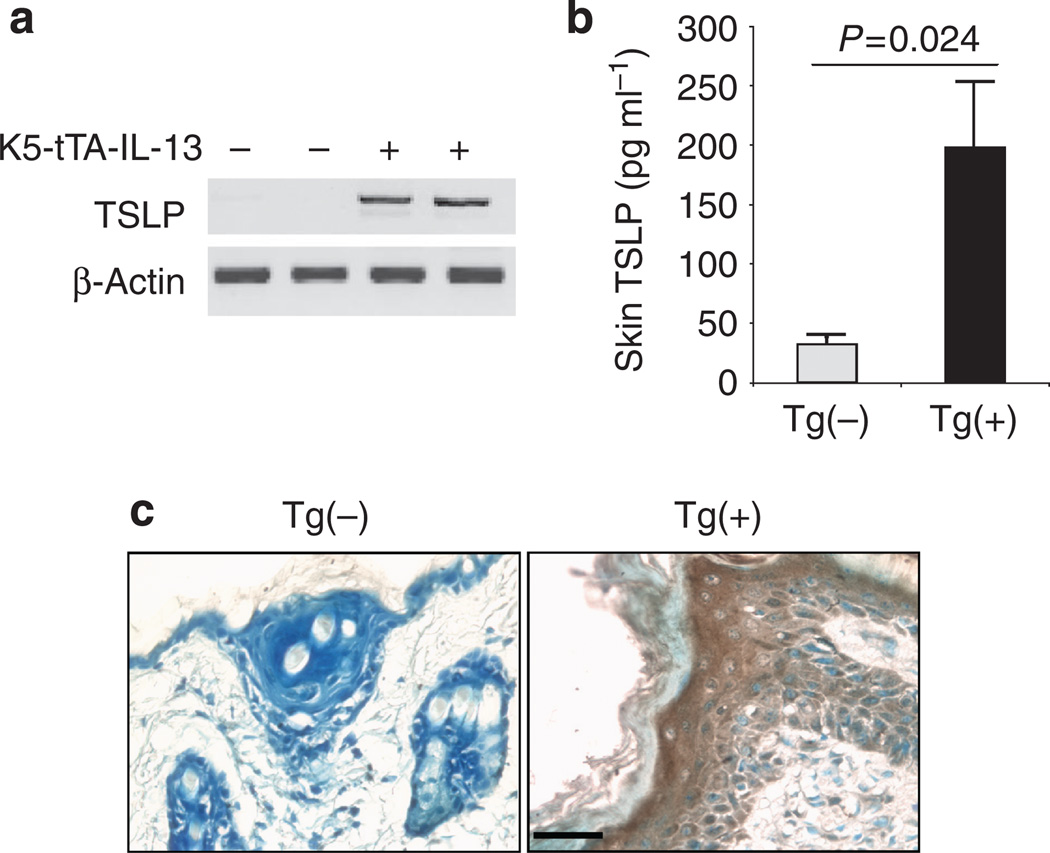
We further investigated whether other cytokines were involved in IL-13-induced inflammation in the skin. TSLP is a critical cytokine in mediating dendritic cell-mediated Th2 inflammatory responses (Liu, 2006). Although no TSLP mRNA could be detected by reverse transcription–PCR, some basal level of TSLP protein was detected in the skin of Tg(−) mice. However, significantly increased levels of TSLP mRNA and protein were found in the skin of Tg(+) mice (Figures 5a and b). IHC with anti-TSLP revealed that few cells were stained positive in the skin of Tg(−) mice. In contrast, numerous cells, particularly keratinocytes were stained positive by anti-TSLP in the skin of Tg(+) mice (Figure 5c). These results indicate that IL-13 is a potent stimulator of TSLP expression in the skin and keratinocytes are major cells that produce TSLP in response to IL-13.
Figure 5. Upregulation of TSLP expression in the skin.
(a) RT–PCR for TSLP mRNA in the skin. (b) TSLP protein in the skin extracts (n = 6 for each group). (c) IHC identification of cell types expressing TSLP. Skin sections from Tg(+) and Tg(−) mice were stained using anti-TSLP. Bar = 50 µm. Shown are representative slides of three pairs of stained samples.
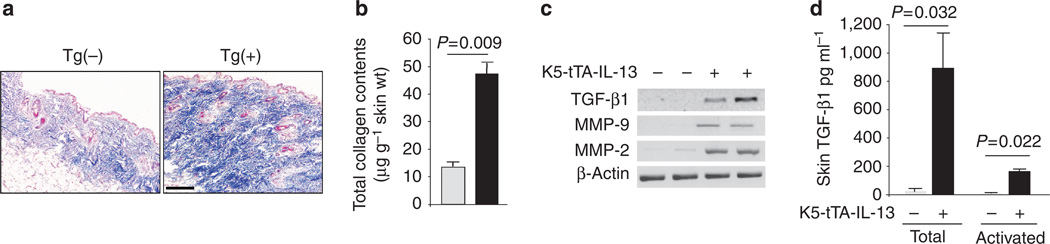
Effect of IL-13 on collagen accumulation in the skin
To investigate whether IL-13 could cause fibrosis in the skin, we investigated the collagen content in the skin of Tg(+) and Tg(−) mice. Trichrome staining and soluble collagen assay showed that IL-13 caused significantly increased collagen deposition in the skin of Tg(+) mice (Figures 6a and b). To further understand the mechanisms of IL-13-induced fibrosis in the skin, we determined the expression of transforming growth factor-β1 (TGF-β1) and related TGF-β1 activating factors matrix metalloproteinase-9 (MMP-9) and µmP-2. Comparing to Tg(−) mice, the amount of mRNA coding for TGF-β1, µmP-9, and µmP-2 was significantly increased in the skin of Tg(+) mice (Figure 6c). Furthermore, both total and spontaneously activated TGF-β1 were significantly increased in the skin of Tg(+) mice, comparing to Tg(−) mice (Figure 6d). These findings suggest that IL-13 stimulates dermal fibrosis, possibly through upregulation of TGF-β1, µmP-9, and µmP-2.
Figure 6. IL-13-induced fibrosis and upregulation of TGF-β1.
(a) Trichrome staining of skin sections from Tg(+) and Tg(−) mice after Dox off for 8 weeks. Bar = 100 µm. (b) Quantitative analysis (Sircol Assay) of collagen content in the skin (n = 6, each group). (c) TGF-β1 mRNA and (d) TGF-β1 protein (n = 6, each group).
Effect of IL-13 on vasculature of the skin
IL-13 is a potent inducer of angiogenesis and a stimulator of vascular endothelial growth factor (VEGF) in the murine lung (Lee et al., 2004). We examined the potential effects of IL-13 on vasculature in the skin and the expression of VEGF. Compared to Tg(−) mice, there were increased blood vessels in the subcutaneous area of the skin of Tg(+) mice (Figure 7a). ELISA assays showed basal level expression of VEGF in the skin of Tg(−) mice. However, the levels of VEGF in the skin of Tg(+) mice were significantly higher (Figure 7b). These data suggest that IL-13 stimulates VEGF in the sites of skin inflammation and VEGF in turn may contribute to the vascular remodeling and enhanced Th2 inflammation in the skin.
Figure 7. IL-13-induced increased vasculature and upregulation of VEGF.
(a) Increased numbers of blood vessels were present in the skin of Tg(+) mice as compared with that of Tg(−) mice (H&E). Bars: upper panels = 200 µm and lower panels = 50 µm. (b) ELISA analysis of VEGF protein in skin extracts (n = 6 each group).
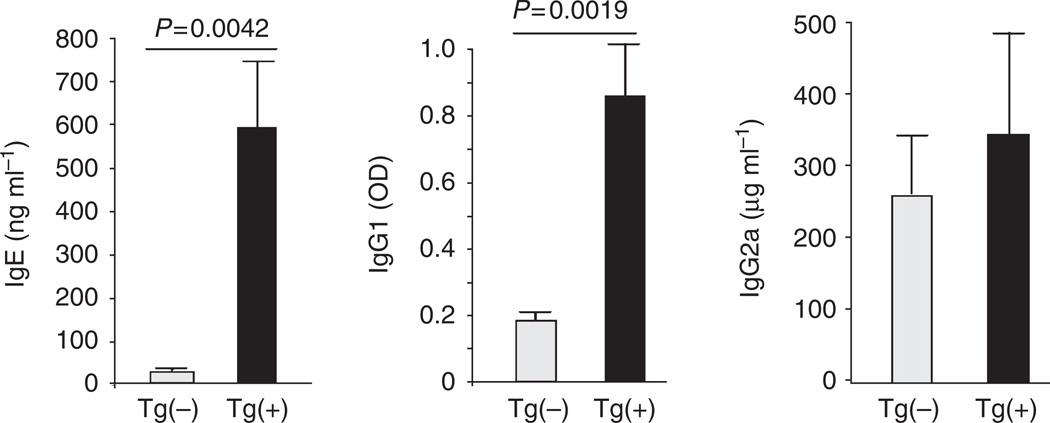
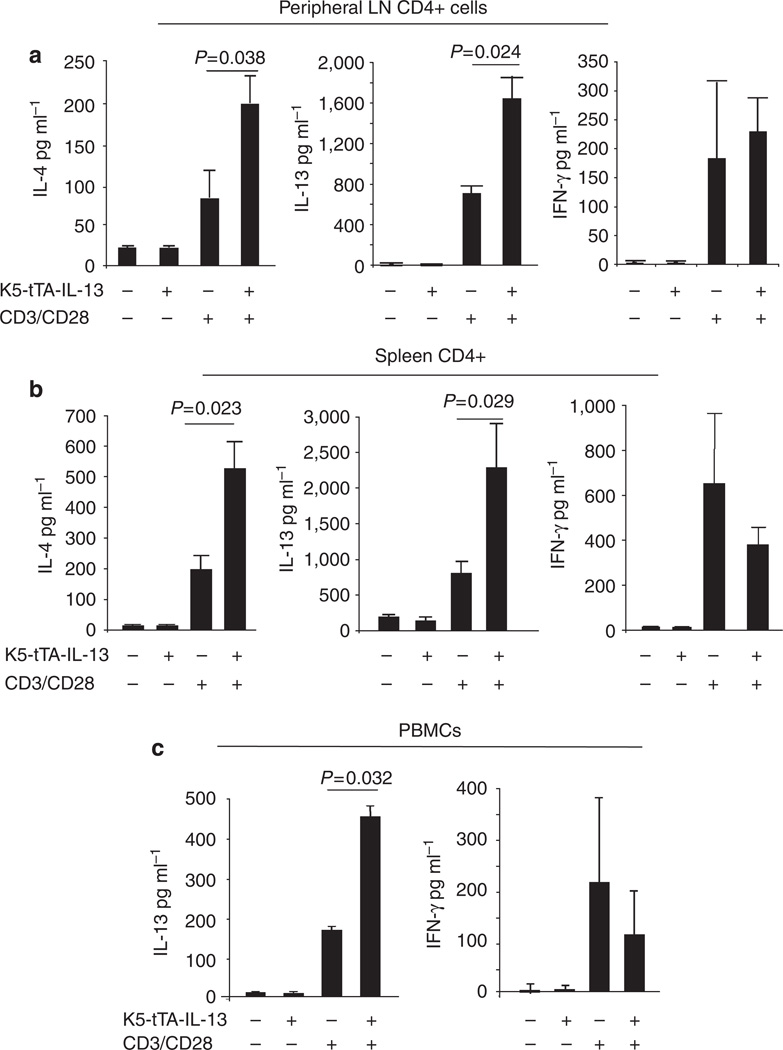
IL-13-induced dermatitis and associated systemic immune responses
To determine if skin inflammation induced by IL-13 was associated with systemic immune responses, we compared the levels of serum immunoglobulins and cytokine production by lymphocytes and peripheral blood mononuclear cell (PBMC) in Tg(+) and Tg(−) mice 8 weeks after the induction of the transgene. The serum levels of total IgE and IgG1, but not IgG2a, were significantly increased in Tg(+) animals (Figure 8). Experiments were undertaken to measure cytokine production by activated lymphocytes from the cutaneous draining lymph nodes (inguinal, cervical, and axillary) and spleen of Tg(−) and Tg(+) animals after stimulation with anti-CD3/CD28 for 72 hours. Lymphocytes from Tg(+) animals produced significantly higher levels of IL-4 and IL-13 than those from Tg(−) animals, whereas the levels of IFN-γ were similar (Figures 9a and b). Similarly, after stimulation with anti-CD3/CD28, PBMC from Tg(+) mice produced significantly higher levels of IL-13 than those from Tg(−) controls, whereas the levels of IFN-γ showed no difference (Figure 9c). These studies suggest that IL-13-induced AD predisposes to a systemic Th2-prone immune environment.
Figure 8. Dermal expression of IL-13 resulted in altered serum immunoglobulins.
Serum samples from Tg(+) and Tg(−) mice off Dox for 8 weeks were collected and analyzed by ELISA. Shown are serum total IgE, IgG1, and IgG2a (n = 25 each group).
Figure 9. Cytokine production by PBMC and lymphocytes.
PBMC and lymphocytes from cutaneous draining lymph nodes were isolated from Tg(+) and Tg(−) mice 8 weeks after Dox withdrawal and stimulated with anti-CD3/CD28 for 3 days in culture. (a) IL-13 and IFN-γ production by peripheral LN CD4 + cells (n = 14 per group). (b) IL-13 and IFN-γ production by PBMC (n = 5 per group).
DISCUSSION
Th2 inflammation is an important component in AD, particularly in the early phase (Leung et al., 2004; Boguniewicz and Leung, 2006; McGirt and Beck, 2006). IL-13 is an important cytokine in Th2 related diseases. Increased expression of IL-13 has been found in the skin tissue of AD patients. For these reasons it is believed that IL-13 is an important player in the pathogenesis of AD. However, to date, no study has been performed to show the direct tissue effects of IL-13 in the skin. Furthermore, the downstream molecular events induced by IL-13 in the skin have not been defined. In this study we established an inducible transgenic mouse model in which IL-13 can be selectively targeted to the skin. The results demonstrate that transgenic expression of IL-13 leads to a skin phenotype that mirrors human AD in many aspects with a chronic pruritic eczematous clinical presentation, infiltration of CD4 + T cells, eosinophils, F4/80 + macrophages and Langerhans cells and mast cells in the epidermis and dermis layers, and skin remodeling, including dermal fibrosis and increased vasculature.
These changes are accompanied by upregulation of a variety of chemokines. Several studies showed that in patients with AD serum levels of CCL11, CCL17, CCL22, CCL26, and CCL27 correlated with disease activity (Yamada et al., 1996; Kaburagi et al., 2001; Homey et al., 2002; Horikawa et al., 2002; Hijnen et al., 2004; Shimada et al., 2004; Jahnz-Rozyk et al., 2005; Park et al., 2005). We found that in the skin of Tg(+) mice IL-13 is a potent stimulator of the expression of CCL11/eotaxin, CCL2/MCP-1, CCL22/MDC, CCL27/CTACK, and CCL5/RANTES, which are chemotactic for T cells, eosinophils, mast cells, and mononuclear cells. As IL-13 itself is not a chemokine, upregulation of these chemokines and others by IL-13 would be an important process leading to the accumulation of different inflammatory cells and subsequent pathologies in the skin of Tg(+) mice. It is also possible that IL-13-induced inflammation resulted in accumulation of growth factors that can facilitate local proliferation of cell types, such as mast cells. These studies demonstrated that IL-13 is crucial in generating and sustaining Th2-dominant skin inflammation in AD.
The IL-13-induced phenotype is not limited to the skin as evidenced by activation and increased Th2 cytokine production by PBMC, lymphocytes from draining lymph nodes and spleen, and increased serum IgE and IgG1. These features are commonly seen in AD patients and indicate a systemic environment favoring Th2 responses. The activation of T cells by IL-13 is most likely an indirect effect because T cells do not express IL-13 receptor α1, which is required for IL-13 signaling. Another implication is that these could be precursors for further development of other allergic diseases such as allergic rhinitis and asthma, the so-called “atopic march”. Our model is uniquely suitable to address these questions. Whether there will be enhanced inflammatory response in the lung of these mice to allergen stimulation is under investigation, as is the question whether the skin and immune phenotype persists after the transgene is turned off.
One of the cytokines that were upregulated in the skin of IL-13 transgenic mice is TSLP. This is a cytokine produced by cells in the epidermis and in the airway epithelium and is capable of directing dendritic cells toward a Th2 response, thereby providing an essential link between epithelial cell activation and allergic inflammation. It is interesting that IL-13 is able to upregulate TSLP, because it has been reported that transgenic expression of TSLP in the skin can induce an AD-like skin disorder (Yoo et al., 2005). Whether IL-13-induced AD is TSLP dependent and whether TSLP in this context helps skewing a systemic response to allergens by activating DC in favor of Th2 responses are under investigation.
IL-13 has been shown in different tissues as a profibrotic cytokine (Chiaramonte et al., 1999; Zhu et al., 1999; Kaviratne et al., 2004; Mentink-Kane et al., 2004). This study demonstrated that IL-13 is a potent stimulator of tissue remodeling in AD, at least in part, by inducing and activating TGF-β1 and by inducing TGF-β1 activating factors CCL2/MCP-1, µmP-9, and µmP-2.
VEGF is a mediator of vascular and extravascular remodeling and inflammation that enhances airway antigen sensitization and Th2 inflammation (Lee et al., 2004). IL-13 has been shown to stimulate VEGF in an acute lung injury model (Corne et al., 2000). Our studies suggest that IL-13 may contribute to skin vascular remodeling by stimulating production of VEGF in the skin, which may also exacerbate Th2 inflammation.
Both IL-4 and IL-13 are important Th2 cytokines produced primarily by CD4 + Th2 cells. The two cytokines have overlapping and distinct biological functions. Transgenic mice that expressed IL-4 in the skin developed an AD-like phenotype (Chan et al., 2001). Although the IL-4 transgenic mice and our IL-13 transgenic mice developed similar pruritus AD-like disease with increased serum IgE and IgG1, there are important differences between the two models. IL-4 transgenic mice were on a Balb/cBy genetic background and IL-4 expression was under the control of the K14 promoter constitutively. The skin rash of these mice began at 4 months of age with an incidence of 43% in 1 year. In contrast, our IL-13 transgenic mice are on a C57BL/6 background and IL-13 expression is under the control of the K5 promoter in an inducible fashion. The skin pathology of these mice begins within 2 months of induction (age 3.5 months) and with a 100% occurrence in all transgenic positive mice within 4 months of induction.
In this study we chose an inducible, instead of constitutive, transgenic system to bypass the potential effects of IL-13 on mice during development. The K5-tetracycline-controlled inducible transgenic system in this study has been successfully used to target cytokines TGF-β1 and TSLP to the skin and caused distinct dermal phenotypes (Liu et al., 2001; Yoo et al., 2005). Our tests of the inducibility and specificity showed that the IL-13 transgene can be turned on or off when desired and the clinical course of the dermal phenotype directly follows the expression of the cytokine. This transgenic system provides a flexible and precise tool for studying the initiation, progression, and resolution of chronic inflammatory diseases such as AD.
In the chronic stage of human AD the inflammation in the skin is shifted from a Th2-dominated response to a Th2/Th1-mixed response (Leung and Boguniewicz, 2003; Ong and Leung, 2006). In our model, the inflammatory response in the skin was initiated by IL-13. However, whether the inflammation will shift to a Th2/Th1-mixed response is yet to be determined.
In this study, skin-specific expression of IL-13 revealed many interesting inflammatory, immunological, and pathological features. It is recognized, however, that this system is focused on the effector functions of IL-13 in the skin. Other approaches are necessary to understand the regulation of IL-13 expression and the cell source of IL-13 in the skin, and the role of IL-13 in other models of AD.
Our transgenic study demonstrated that dermal expression of the Th2 cytokine IL-13 is sufficient to cause a chronic inflammatory response in the skin and systemic evidence of Th2 bias that remarkably resembles human AD. This model provides a good opportunity for further exploring the cellular and molecular events initiated by IL-13 in the skin. These studies will help us gain valuable insights into the pathogenesis of AD and suggest that AD may be a good target for IL-13-directed therapies.
MATERIALS AND METHODS
Animals
All procedures performed on mice were in accordance with the NIH guidelines for humane treatment of animals and were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University. Mice were housed in cages with microfilters in a specific pathogen-free environment. Different transgenic lines were first backcrossed to the C57BL/6 genetic background for at least nine generations and then crossbred to obtain desired double transgenic mice.
Generation of skin-specific inducible IL-13 transgenic mice and induction of IL-13 expression
First we generated a TRE-Tight-IL-13 transgenic mouse line. Mouse IL-13 cDNA was PCR amplified from a previous IL-13 transgenic construct (Zhu et al., 1999) using primers: 5′-CTGAATTCGGGCACCATGGCGCTCTGGGTGAC-3′ and 5′-GGTGGATCCTCATTAGAAGGGGCCGTG-3′ containing restriction enzyme sites for EcoRI/BamHI and a Kozak consensus sequence. Following EcoRI/BamHI enzyme digestion, the product was inserted into the multiple cloning site located downstream of the TRE-Tight promoter of the pTRE-Tight vector (kindly provided by Dr Andrew Farmer, Clontech). The fragment containing the TRE-Tight promoter, IL-13 cDNA, and the SV40 polyadenylation signal sequence was excised by XhoI (Figure 1a), purified, and microinjected into pronuclei as described previously (Zhu et al., 1999).
To express IL-13 specifically and inducibly in the skin, we crossbred the TRE-Tight-IL-13 mouse line with the K5-tTA mouse line; the generation and use of the K5-tTA mouse line have been described previously (Diamond et al., 2000) and produced double-transgenic K5-tTA-IL-13 mice. The breeding also produced single-transgenic mice, which were used for further breeding, and transgenic negative mice, which were used as Tg(−) littermate controls for experiments. The genotypes of the mice were determined by PCR using specific primers for K5-tTA, and TRE-Tight-IL-13. Dox was added to the drinking water (1 mgml−1) to suppress tTA and to keep the IL-13 transgene off until K5-tTA-IL-13 mice were 6 weeks old. The experiments were initiated by withdrawing Dox from the drinking water. In all experiments, Tg(−) littermate controls received the same amount of Dox or no Dox for the same length of time.
Clinical observation
Tg(+) and Tg(−) mice were examined for skin lesions and the clinical scores for disease severity were recorded as described with slight modifications (Leung et al., 1990; Matsuda et al., 1997; Chan et al., 2001; Akei et al., 2006).
Histology and immunohistochemistry evaluation
After mice were killed, lesional and nonlesional skin was excised and fixed in neutral buffered formalin at 4 °C overnight, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin (H&E), toluidine blue, and Mallory’s trichrome for histological analysis.
For immunohistological experiments, after the sectioned tissues were rehydrated, endogenous peroxidase was blocked by quenching with 3% H2O2 and methanol and the tissues were treated in a microwave oven in the presence of citrate buffer (10mM, pH 6.0) for 9 minutes (3 cycles of 3 minutes each). After preblocking with Dako blocking reagent (Dako, Carpinteria, CA) for 30 minutes, a rat antimouse major basic protein monoclonal antibody (a kind gift from Drs Nancy and James J. Lee, Mayo Clinic, Scottsdale, AZ) was applied to stain for eosinophils. Similarly, for CD4 + cells, rat anti-CD4 monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) was applied at a 1:100 dilution (1 µg ml−1) or rat anti-F4/80 antibody for evaluation of activated Langerhans cells and macrophages (Pan macrophage marker clone: BM8, 14-4801; eBioscience, San Diego, CA) at 1:100 dilution (1 µgml−1). Appropriate ABC Staining Systems were used to visualize the target proteins in the tissues (Santa Cruz Biotechnology).
Measurement of mast cell mediators
Total histamine content in the skin was measured as described previously (Schroeder and Saini, 2006). Briefly, suspensions of the skin extracts and reagents were prewarmed at 37 °C for 30 minutes. The skin extract samples in a volume of 0.4 ml were mixed with 0.1 ml of 8% HClO4 to lyse the cells for total histamine release. The cells were incubated at 37 °C for 45 minutes. PAG buffer (0.5 ml) was added to stop the reaction and the cells or debris were microcentrifuged at 1,000 r.p.m. for 10 minutes. The supernatant was analyzed for histamine using a RFA 300 rapid flow analyzer (Astoria-Pacific Int., Clackamas, OR). All the samples were tested in duplicates. β-hexosaminidase content in the skin was measured as described previously (Choi et al., 1993). Briefly, aliquots (10 µl) of the skin extracts were incubated with 10 µl of 1mm p-nitrophenyl-N-acetyl-β-d-glucosaminide in 0.1 m sodium citrate buffer (pH 4.5) at 37 °C for 1 hour. At the end of the incubation, 250 µl of buffer (0.1 m Na2CO3/0.1 m NaHCO3) was added. Absorbance was read at 410 nm. Values (mean ± SE) were expressed as the actual release (percentage of total hexosaminidase) after correction for spontaneous release (2–3%).
RNA isolation and reverse transcription–PCR
After excision, skin tissues were immediately placed in RNAlater solution (Ambion, Austin, TX), stored at −80 °C, and used later. To extract RNA, skin samples were placed in liquid nitrogen and ground with a mortar and pestle. Total RNA was then isolated with Trizol reagent (Invitrogen, Carlsbad, CA) and further purified with RNeasy Mini kit (Qiagen, Valencia, CA). Reverse transcription was performed using 0.5 µg total RNA for first-strand cDNA synthesis with SuperScript II RNase H− Reverse Transcriptase (Invitrogen) in a total volume of 20 µl. A portion of the resulting reverse-transcription product (1 µl) was used for PCR amplification. PCR conditions to amplify specific genes were 95 °C for 4 minutes for initial denaturing followed by 30 cycles of 94 °C for 1 minute, 60 °C for 1 minute, and 72 °C for 1 minute, unless specified otherwise. Primers for specific genes are listed in Table 2.
Table 2.
Primers and PCR conditions
| Moiety | Sense primer | Antisense primer | Anneal temp (°C) | Product length (bp) |
|---|---|---|---|---|
| Eotaxin | CCATCTGTCTCCCTCCACCATG | ATCCCACATCTCCTTTCATGCC | 56 | 546 |
| MCP-1 | ACCAGCCAACTCTCACTGAAGC | CAGAATTGCTTGAGGTGGTTGTG | 60 | 463 |
| MDC | CCTGGTGGCTCTCGTCCTTC | CAGGGGATGGAGGTGAGTAA | 57 | 377 |
| TARC | AGTGGAGTGTTCCAGGGATG | TTTGTGTTCGCCTGTAGTGC | 60 | 272 |
| CTACK | CTGGGTTACCAGCACAGGAT | CCTCTGGATTCCCACACACT | 60 | 139 |
| RANTES | CCCTCACCATCATCCTCACT | GGGAAGCGTATACAGGGTCA | 60 | 297 |
| TSLP | CCAGGCTACCCTGAAACTGA | CACCTCATCATGGCAGTGAC | 60 | 578 |
| β-Actin | GTGGGCCGCTCTAGGCACCAA | CTCTTTGATGTCACGCACGATTTC | 60 | 540 |
Preparation of skin protein extracts
Frozen skin tissues were placed in liquid nitrogen, crushed with a mortar and pestle and weighed. Triton X-100 0.25% (wt/vol) in phosphate-buffered saline was added to the skin powder. The homogenate was stirred at 4 °C overnight and then centrifuged at 3000 g for 15 minutes to remove debris. Supernatants were stored in small aliquots at −80 °C until assayed by ELISA. All samples were normalized to weight.
Measurement of cytokines, chemokines, and immunoglobulins
Cytokines and chemokines in the skin samples were measured using ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Serum samples in duplicates were analyzed using ELISA for total IgE (BD Biosciences, San Jose, CA), IgG1, and IgG2a (Southern Biotechnology Associates, Birmingham, AL), according to the manufacturer’s instructions.
Quantification of skin collagen
The collagen content was determined by quantifying total soluble collagen using the Sircol Collagen Assay (Biocolor, Carrickfergus, UK) according to the manufacturer’s instructions and expressed as the collagen content of the skin by weight.
Statistical analysis
All data were expressed as mean ± SEM. The significance of the variation among different groups was determined by one-way ANOVA analysis. Difference with P <0.05 was considered significant.
ACKNOWLEDGMENTS
This work was supported by NIH Grant AI55064, AAAAI Women’s Award, and AAAAI Faculty K to R Award to TZ, and NIH HL079349-01 to ZZ.
Abbreviations
- AD
atopic dermatitis
- Dox
doxycycline
- IHC
immunohistochemistry
- K5
keratin 5
- MMP-9
matrix metalloproteinase-9
- PBMC
peripheral blood mononuclear cell
- Tg
transgenic
- TGF-β1
transforming growth factor-β1
- TSLP
thymic stromal lymphopoietin
- VEGF
vascular endothelial growth factor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Akdis M, Akdis CA, Weigl L, Disch R, Blaser K. Skin-homing, CLA+ memory T cells are activated in atopic dermatitis and regulate IgE by an IL-13-dominated cytokine pattern: IgG4 counter-regulation by CLA-memory T cells. J Immunol. 1997;159:4611–4619. [PubMed] [Google Scholar]
- Akei HS, Brandt EB, Mishra A, Strait RT, Finkelman FD, Warrier MR, et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006;118:62–69. doi: 10.1016/j.jaci.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. 10. Atopic dermatitis. J Allergy Clin Immunol. 2006;117:S475–S480. doi: 10.1016/j.jaci.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–983. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi OH, Lee JH, Kassessinoff T, Cunha-Melo JR, Jones SV, Beaven MA. Antigen and carbachol mobilize calcium by similar mechanisms in a transfected mast cell line (RBL-2H3 cells) that expresses ml muscarinic receptors. J Immunol. 1993;151:5586–5595. [PubMed] [Google Scholar]
- Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest. 2000;106:783–791. doi: 10.1172/JCI9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)- 4-independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijnen D, De Bruin-Weller M, Oosting B, Lebre C, De Jong E, Bruijnzeel-Koomen C, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell-attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–340. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Horie S, Okubo Y, Hossain M, Sato E, Nomura H, Koyama S, et al. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern Med. 1997;36:179–185. doi: 10.2169/internalmedicine.36.179. [DOI] [PubMed] [Google Scholar]
- Horikawa T, Nakayama T, Hikita I, Yamada H, Fujisawa R, Bito T, et al. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int Immunol. 2002;14:767–773. doi: 10.1093/intimm/dxf044. [DOI] [PubMed] [Google Scholar]
- Jahnz-Rozyk K, Targowski T, Paluchowska E, Owczarek W, Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy. 2005;60:685–688. doi: 10.1111/j.1398-9995.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- Kaburagi Y, Shimada Y, Nagaoka T, Hasegawa M, Takehara K, Sato S. Enhanced production of CC-chemokines (RANTES, MCP-1, MIP-1alpha, MIP-1beta, and eotaxin) in patients with atopic dermatitis. Arch Dermatol Res. 2001;293:350–355. doi: 10.1007/s004030100230. [DOI] [PubMed] [Google Scholar]
- Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Boguniewicz M. Advances in allergic skin diseases. J Allergy Clin Immunol. 2003;111:S805–S812. doi: 10.1067/mai.2003.155. [DOI] [PubMed] [Google Scholar]
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Hirsch RL, Schneider L, Moody C, Takaoka R, Li SH, et al. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol. 1990;85:927–933. doi: 10.1016/0091-6749(90)90079-j. [DOI] [PubMed] [Google Scholar]
- Liu X, Alexander V, Vijayachandra K, Bhogte E, Diamond I, Glick A. Conditional epidermal expression of TGFbeta 1 blocks neonatal lethality but causes a reversible hyperplasia and alopecia. Proc Natl Acad Sci USA. 2001;98:9139–9144. doi: 10.1073/pnas.161016098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- McGirt LY, Beck LA. Innate immune defects in atopic dermatitis. J Allergy Clin Immunol. 2006;118:202–208. doi: 10.1016/j.jaci.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Mentink-Kane MM, Cheever AW, Thompson RW, Hari DM, Kabatereine NB, Vennervald BJ, et al. IL-13 receptor alpha 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci USA. 2004;101:586–590. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- Novak N, Kraft S, Bieer T. Unraveling the mission of FcepsilonR1 on antigen-presenting cells. J Allergy Clin Immunol. 2003;111:38–44. doi: 10.1067/mai.2003.2. [DOI] [PubMed] [Google Scholar]
- Ong PY, Leung DY. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006;6:384–389. doi: 10.1007/s11882-996-0008-5. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Park CW, Lee BH, Han HJ, Lee CH, Ahn HK. Tacrolimus decreases the expression of eotaxin, CCR3, RANTES and interleukin-5 in atopic dermatitis. Br J Dermatol. 2005;152:1173–1181. doi: 10.1111/j.1365-2133.2005.06474.x. [DOI] [PubMed] [Google Scholar]
- Purwar R, Werfel T, Wittmann M. IL-13-stimulated human keratinocytes preferentially attract CD4+CCR4+ T cells: possible role in atopic dermatitis. J Invest Dermatol. 2006;126:1043–1051. doi: 10.1038/sj.jid.5700085. [DOI] [PubMed] [Google Scholar]
- Schroeder JT, Saini S. Assay methods for measurement of mediators and markers of allergic inflammation. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. 7th ed. Washington, DC: ASM; 2006. pp. 964–974. [Google Scholar]
- Shimada Y, Takehara K, Sato S. Both Th2 and Th1 chemokines (TARC/ CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- Yamada H, Chihara J, Matsukura M, Yasuba H, Yudate T, Tezuka T. Elevated plasma RANTES levels in patients with atopic dermatitis. J Clin Lab Immunol. 1996;48:87–91. [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]