Abstract
Cell-based approaches have emerged as a promising therapy to achieve successful vascularization in tissue engineering. Since fibroblasts activation and migration is required for physiological events relying on angiogenesis, we hypothesize herein that different fibroblasts exhibit distinct capacity to promote capillary-like structures assembly, by mature and progenitor endothelial cells (ECs). Outgrowth endothelial cells (OECs) were isolated from human umbilical cord blood samples and characterized by immunofluorescence and imaging flow cytometry for endothelial markers. Coculture systems were established using either human umbilical vein ECs (HUVECs) or OECs with fibroblasts, being evaluated at 7, 14, and 21 days of culture. Two types of human dermal fibroblasts (HDF) were used, namely neonatal human foreskin fibroblasts-1 (HFF-1) and juvenile HDF. OECs expressed EC markers and formed capillary-like structures. HFF-1 exhibited higher expression of transglutaminase-2, while HDF exhibited a higher expression of α-smooth muscle actin (α-SMA) and podoplanin, which were not observed for HFF-1. Formation of capillary-like structures was only observed in cocultures with HDF and not with HFF-1. No significant differences were found between HDF and OECs or HUVECs cocultures. These findings suggest that HDF is a preferential cell source for promoting vascularization, either using mature or progenitor ECs, probably due to their higher expression of α-SMA and podoplanin, and increased synthesis of extracellular matrix. This work opens new research possibilities regarding the use of specific fibroblast populations cocultured with ECs, as efficient partners for vascular development in regenerative medicine strategies.
Introduction
In tissue engineering, the development of novel approaches to improve the original structural, functional, and physiological condition of a tissue is crucial. Coculture systems constitute excellent platforms to test the hypothesis behind the interaction of heterotypic cell cultures.1–3 Overall, the inability to engineer blood vessels in vitro for subsequent transplantation has been referred as the main reason for the limited clinical success of tissue engineering strategies.4–6
Over the years, different strategies have been described aiming to achieve the vascularization of an engineered tissue, including cell-based therapies, mostly based on endothelial cell (EC) transplantation, including progenitor ECs7 like outgrowth endothelial cells (OECs). These cells can be obtained by long-term differentiation of blood-derived mononuclear cells (MNCs), being a promising cell source for proangiogenic cell therapies.7–9 Coculture systems of ECs with support cells, including fibroblasts,10,11 smooth muscle cells,12,13 mesenchymal stem cells,1 and osteoblasts,14,15 among other cells, have been used as a strategy to promote vascularization,16 playing an important role regarding cellular crosstalk, namely through the production of growth factors and extracellular matrix (ECM). Fibroblasts are mesenchymal cells, being the main source of ECM components,17,18 like collagen I, fibronectin, and proteoglycans.19 Human fibroblasts are abundant in the dermis and can be easily obtained from minimally invasive skin biopsies.20,21 Considering their location within the dermis, fibroblasts are divided into papillary (superficial dermis) and reticular (deep dermis), exhibiting different characteristics in terms of cell morphology, production of ECM and growth factors, among others.22,23 Although no specific markers distinguish both types of fibroblasts, differences in gene expression patterns exist, with reticular fibroblasts exhibiting an increased expression of genes involved in cell motility and contraction, including calponin-1 and transglutaminase-2 (TG2), whereas papillary fibroblasts characteristically express genes involved in the immune response, such as netrin-1 and podoplanin (PDPN).23 Regarding vascularization, papillary fibroblasts seem to support the formation of highly branched tubular structures in vitro, while reticular fibroblasts do not.24 Understanding this process remains a challenge, since fibroblasts have the capacity to alter the mechanical extracellular microenvironment, thereby regulating vascularization processes.25 Fibroblast-derived proteins, including growth factors and matrix proteins, have been shown to modulate EC sprouting and the expansion of capillary-like networks in vitro,26–28 contributing to the role of fibroblasts as periendothelial cells in vivo.29
Thus, the hypothesis underlying herein is that, when cocultured with OECs or mature ECs, different types of fibroblasts will exert distinct influences in the assembly of capillary-like structures. Experiments using cocultures of ECs with two types of human dermal fibroblasts (HDF) in direct contact were performed; the ECM produced over time in these coculture systems was characterized and their ability to induce/support the formation of vascular-like networks was investigated. Therefore, choosing the right fibroblast-EC partners is probably a major issue in vascularization within tissue regeneration approaches.
Materials and Methods
Isolation and expansion of human OECs
Human umbilical cord blood samples were collected from healthy donors from Hospital de São João (Porto, Portugal) under informed consent, according to the Declaration of Helsinki and the local ethical committee. Human OECs were isolated by gradient centrifugation using Histopaque-1077 solution (Sigma), according to protocols already established.7 MNCs fraction was collected and cultured in type I collagen-coated six-well tissue culture plates (BD, Biosciences) in a cell density of 10×106 cells/well and cultured in microvascular endothelial cell growth medium-2 (EGM-2 MV; Lonza) supplemented with 10% (v/v) inactivated fetal bovine serum (FBS; Sigma). After 24 h, only adherent cells were further cultured. Characteristic colonies with a cobblestone-like morphology appeared after 2–3 weeks. Cells were collected and expanded at passages 2–6, characterized through immunocytochemistry, imaging flow cytometry, and for their ability to assemble into capillary-like structures in a matrigel assay.
Characterization of OECs by imaging flow cytometry
To characterize OECs through imaging flow cytometry, cells were seeded in 0.2% (w/v) gelatin-coated 25 cm2 flasks and cultured in EGM-2 MV until 90% confluence was reached. Then, cells were trypsinized, counted, fixed with 4% (v/v) of paraformaldehyde (Sigma), and permeabilized with 0.2% (v/v) Triton-X 100 (Merck). Cell suspensions were centrifuged at 1200 rpm for 5 min between every incubation and washing steps. Cells were stained against CD31 (mouse anti-human CD31, 1:100; Dako), CD34 (mouse anti-human CD34, 1:50; Dako), CD144 (mouse anti-human CD144, 1:100; BD Pharmingen), and Flk-1 (mouse anti-human Flk-1, 1: 200; Santa Cruz Biotechnology). Alexafluor 488 goat anti-mouse (1:1000; BD Pharmingen) was used as the secondary antibody. Samples were analyzed on an imaging flow cytometer ImageStream® (Amnis, EDM Millipore), acquiring at least 10,000 events at the Bioimaging Center for Biomaterials and Regenerative Therapies (b.IMAGE, INEB, Portugal). Data were analyzed using IDEAS® software (version 6.0.348; Amnis, EDM Millipore).
Assembly of OECs into capillary-like structures by matrigel assay
Growth factor reduced basement membrane matrix (GFR-Matrigel™, 200 μL/well; BD Biosciences) was added to a 24-well culture plate and incubated at 37°C for 30 min for matrigel polymerization. Then, 2×104 of OECs in 500 μL of EGM-2 MV were added per well. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere and monitored using an inverted light microscope to observe the formation of capillary-like structures, which were counted after 6, 24, and 48 h.
Culture of human umbilical vein ECs and fibroblasts
Human umbilical vein ECs (HUVECs) and juvenile HDF were kindly provided by Professor James Kirkpatrick (REPAIR-lab, University of Mainz, Germany). Neonatal human foreskin fibroblasts-1 (HFF-1) were obtained from American Type Culture Collections (ATCC). HUVECs were cultured in 0.2% (w/v) gelatin (Merck)-coated plates in endothelial cell growth medium-2 (EGM-2; Lonza) supplemented with 5% (v/v) inactivated FBS (Sigma). Both types of fibroblasts were cultured in Dulbecco's Modified Eagle Medium (DMEM; Sigma) supplemented with 15% (v/v) inactivated FBS (Sigma) and 1% (v/v) antibiotic/antimycotic solution (AB/AM; Sigma). For each experiment, HUVECs were used at passages 2–6 and fibroblasts were used at passages 8–10.
Direct contact cocultures of ECs and fibroblasts
The influence of fibroblasts (HFF-1 or HDF) in the ability of endothelial cells (HUVECs or OECs) to form capillary-like structures was evaluated in direct cocultures established with a cell ratio of 2:1 (2×104 ECs: 1×104 fibroblasts). ECs and fibroblasts were seeded at the same time onto 0.2% (w/v) gelatin-coated glass coverslips on 24-well culture plates and cultured in EGM-2 supplemented with 5% (v/v) inactivated FBS (Sigma). After 7, 14, and 21 days, cells were fixed and immunostained as described below.
Characterization of cell phenotype and ECM by immunocytochemistry
Cells were fixed with 4% (v/v) of paraformaldehyde (Sigma), permeabilized with 0.2% (v/v) Triton-X 100 (Merck), and characterized using mouse anti-human CD31 (1:100; Dako), rabbit anti-human vWF (1:8000; Dako), mouse anti-human CD34 (1:50; Dako), mouse anti-human CD144 (VE-cadherin, 1:100; BD Pharmingen), mouse anti-human Flk-1 (VEGFR2, 1:200; Santa Cruz Biotechnology), and mouse anti-human α-smooth muscle actin (α-SMA, 1:100; Dako). ECM distribution and organization was evaluated using mouse anti-human collagen I (1:2000; abcam), mouse anti-human collagen IV (1:100; Dako), mouse anti-human fibronectin (1:200; Antibody Shop), and rabbit anti-human laminin (1:1000; Sigma). Alexafluor 488 goat anti-mouse (1:1000; BD Pharmingen) and Alexafluor 555 donkey anti-rabbit (1:1000; BD Pharmingen) were used as secondary antibodies. All antibodies were diluted in 1% (w/v) bovine serum albumin (BSA; nzytech). Cell nuclei were counterstained with 1 μg/mL DAPI (Roche).
Protein extraction
Total protein extraction was performed by adding suitable amounts of RIPA buffer (RIPA buffer 10×, Millipore, diluted 1:10 in Millipore water; phosphatase inhibitor cocktail, Sigma and complete protease inhibitor cocktail, Roche) to cell pellets on ice. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay reagent kit (Pierce, Thermo Scientific) according to the manufacturer's protocol. Proteins were quantified using a microplate reader (Thermo, Electron Corporation) at 550 nm.30
Western blot analysis
Fibroblasts were characterized using mouse anti-human PDPN (1:1000; Santa Cruz Biotechnology), rabbit anti-human TG2(1:1000; Genetex), mouse anti-human α-SMA (Dako, 1:1000), and β-actin (rabbit anti-human β-actin, 1:3000; abcam) as an internal control. Goat anti-rabbit IgG HRP (1:2000; Santa Cruz Biotechnology), goat anti-mouse IgG HRP (1:2000; Santa Cruz Biotechnology), donkey anti-rabbit IgG-B (1:2000; Santa Cruz Biotechnology), and goat anti-mouse IgG-B (1:2000; abcam) were used as secondary antibodies.
Proteins were separated according to their molecular weight using SDS polyacrylamide gel electrophoresis (SDS-PAGE). Briefly, 10 μg of cell proteins were mixed with loading buffer (250 mM Tris-HCl pH 6.8, 8% SDS, 40% Glycerol, 0.04% Bromophenol blue [1:4], and dithiothreitol [DTT 1M, 1:20]), being then denatured at 99°C. Then, proteins were separated by SDS-PAGE on 8% polyacrylamide gels at 200 V (SDS-running buffer: 125 mM Tris HCl pH 8.3 and 96 mM Glycine, 0.5% SDS). Precision Plus Protein™ dual color standards (Bio-Rad) were used as protein standards. Separated proteins were transferred onto a nitrocellulose membrane (Amersham Biosciences), using a mini transfer chamber filled with transfer buffer (25 mM Trizma, 192 mM glycine, and 20% methanol) at 40 V. Subsequently, the membrane was blocked in blocking solution at room temperature, incubated with the primary antibody overnight at 4°C and then with the secondary antibody at room temperature. Antibodies were detected using enhanced chemiluminescent reagents (GE Healthcare) and the membranes were visualized using ChemiDoc™ MP System (Bio-Rad). Images were acquired and bands were quantified using Image Lab Software 4.0.1 (Bio-Rad). Each sample was assayed thrice in separate gels. Results are presented as relative protein expression normalized to signal intensity of β-actin protein.
Imaging and image quantification
Cells were characterized by immunofluorescence using a Carl Zeiss Axiovert inverted microscope. Confocal images of monocultures and cocultures were acquired on a Leica SP5 confocal microscope (CLSM, Leica TCS SP5; Leica Microsystems). Image analysis software ImageJ64 was used for quantifying the length and diameter of capillary-like structures. Capillary-like structures were counted in confocal images of cocultures and the average number of capillary-like structures was determined by dividing the number of structures by the area (mm2) of the image.
Statistical analysis
All experiments were performed in triplicate. Quantifications are expressed as mean±standard deviation. The Student's t-test was used for comparisons between two groups. One-way analysis of variance with Tukey tests was used to compare between more than two groups. A difference between experimental groups was considered significant with a confidence interval of 95%, whenever p <0.05.
Results
OECs from umbilical cord blood present an endothelial phenotype
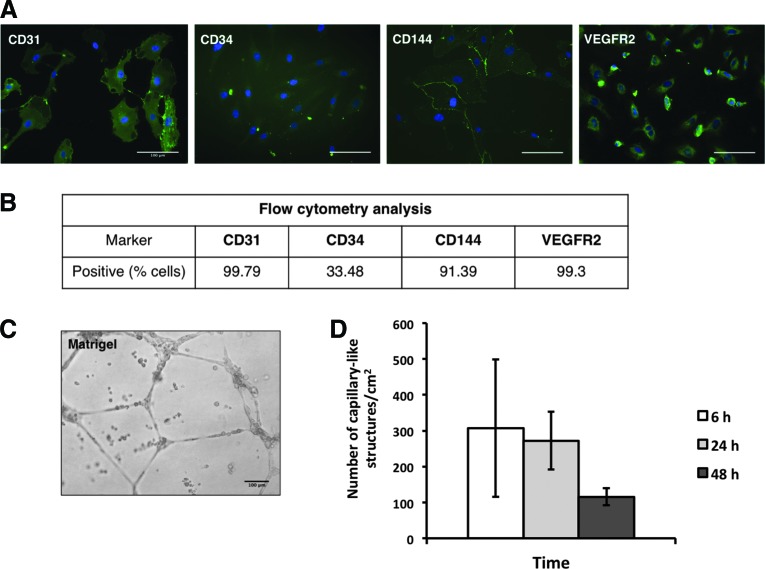
OECs appeared after 2–3 weeks as small colonies, developing a characteristic cobblestone-like morphology over time. These cells were characterized in terms of endothelial markers expression, including CD31 and CD144, in addition to VEGFR2 and CD34 (Fig. 1A), confirming their endothelial phenotype. Before establishing the coculture systems, OECs were analyzed by imaging flow cytometry for the expression of the above-mentioned markers. Figure 1B summarizes the percentage of cells expressing CD31, CD34, CD144, and VEGFR2, with over 99% of the cells being positively stained for CD31 and VEGFR2, while approximately 91% of this cell population was positive for CD144. The overall expression of CD34 was markedly lower, with only approximately 33% of this cell population staining positive for CD34. The ability of OECs to assemble into capillary-like structures was evaluated by GFR-Matrigel assay. This population of OECs was capable of forming capillary-like structures after 6 h, which were maintained at least until 48 h of culture, with a decrease in their number being observed over time (Fig. 1C, D).
FIG. 1.
Phenotypic characterization of OECs. (A) Representative images of OECs characterization by immunofluorescence analysis. OECs stained positive for CD31, CD34, CD144, and VEGFR2. Nuclei were counterstained with DAPI. Scale bars, 100 μm. (B) Percentage of cells expressing endothelial cell markers analyzed by imaging flow cytometry. The percentage of positive cells was calculated according to the gate defined for the unstained controls. (C) Representative bright field image of the formation of capillary-like structures by OECs after 24 h in matrigel. Scale bar, 100 μm. (D) Number of capillary-like structures/cm2 by OECs after 6, 24, and 48 h in matrigel (n=3). OECs, outgrowth endothelial cells. Color images available online at www.liebertpub.com/tea
Different fibroblast cell types express different markers
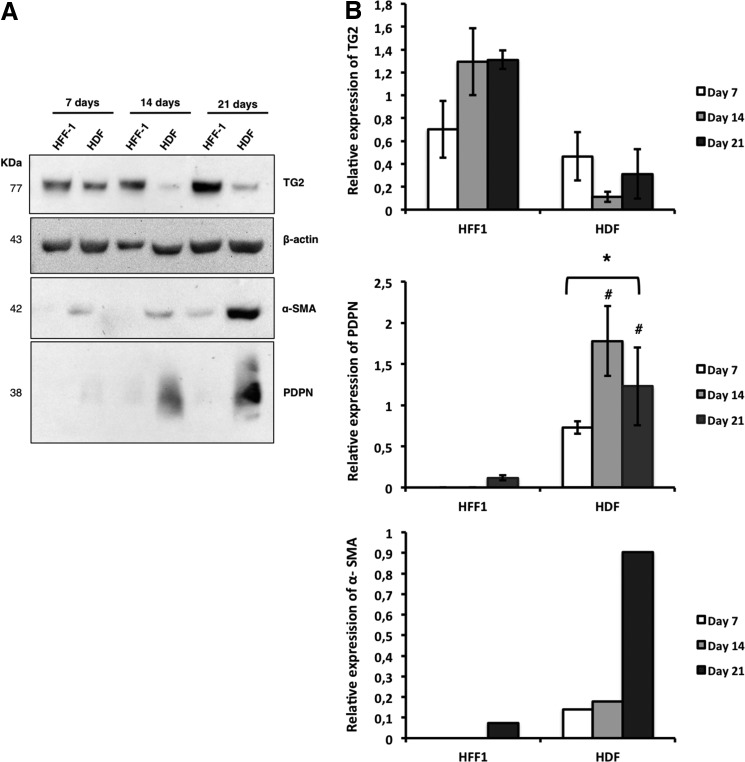
For the establishment of a coculture system, two types of HDF were used, HFF-1 and HDF, being characterized using different markers. The expression of TG2, PDPN, and α-SMA was investigated by western blot analysis in monocultures of HFF-1 and HDF after 7, 14, and 21 days in EGM-2 (Fig. 2A). Both types of fibroblasts expressed TG2 and PDPN proteins (Fig. 2B). Nonetheless, HFF-1 exhibited a higher expression of TG2 that increased along with culture time, than that observed for HDF (Fig. 2B). Conversely, HDF expressed higher amounts of PDPN, this increase being significant between days 7 and 21 (Fig. 2B).
FIG. 2.
Characterization of fibroblasts. (A) Western blot and (B) quantitative analysis of transglutaminase-2 (TG2, n=3), podoplanin (PDPN, n=3), and α-SMA (n=1) expressed by fibroblasts after 7, 14, and 21 days of culture. *Statistically significant differences (p<0.05), between time points. #Statistically significant differences (p<0.05), compared to HFF-1. α-SMA, α-smooth muscle actin.
Both fibroblasts, when cultured alone in EGM-2 culture medium, exhibited a lower expression of α-SMA after 7 days (Fig. 2B). However, after 21 days of culture, it was possible to observe an increase in the expression of α-SMA, mainly in the case of HDF (Fig. 2B).
EC assembly into capillary-like structures was only supported by HDF
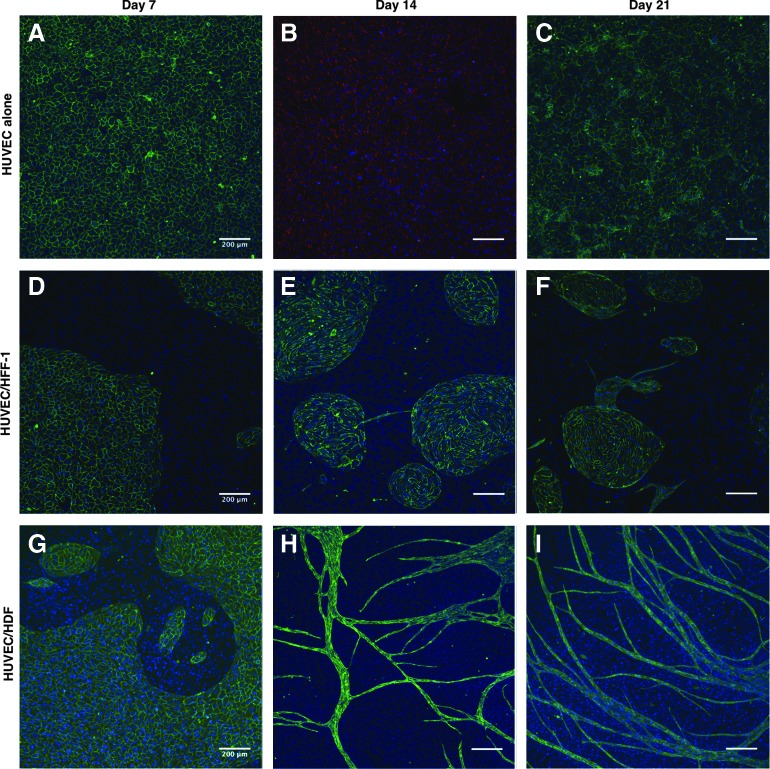
The capacity of different fibroblasts (HFF-1 and HDF) to support the formation of capillary-like structures was assessed in a coculture system with ECs (HUVECs and OECs). Figure 3 shows the behavior of HUVECs in monoculture (Control, Fig. 3A–C) and in coculture systems both with HFF-1 (Fig. 3D–F) and HDF (Fig. 3G–I). In the control condition, HUVECs stayed in a monolayer over time (Fig. 3A–C). When HFF-1 were used in the coculture system, HUVECs organized into clusters (Fig. 3D–F), although with few tubular structures appearing after 14 days (Fig. 3E) and 21 days (Fig. 3F). However, coculturing HUVECs with HDF resulted in the formation of a capillary-like network after 14 days (Fig. 3H), which was maintained at least for 21 days (Fig. 3I).
FIG. 3.
Cocultures of HUVECs with fibroblasts. Confocal images of HUVEC alone (A–C), cocultures of HUVEC/HFF-1 (D–F) and HUVEC/HDF (G–I) after 7, 14, and 21 days in EGM-2. HUVECs were stained against CD31 or vWF and nuclei were counterstained with DAPI. Scale bars, 200 μm. EGM-2, endothelial cell growth medium-2; HDF, human dermal fibroblasts; HUVECs, human umbilical vein endothelial cells; HFF-1, human foreskin fibroblasts-1. Color images available online at www.liebertpub.com/tea
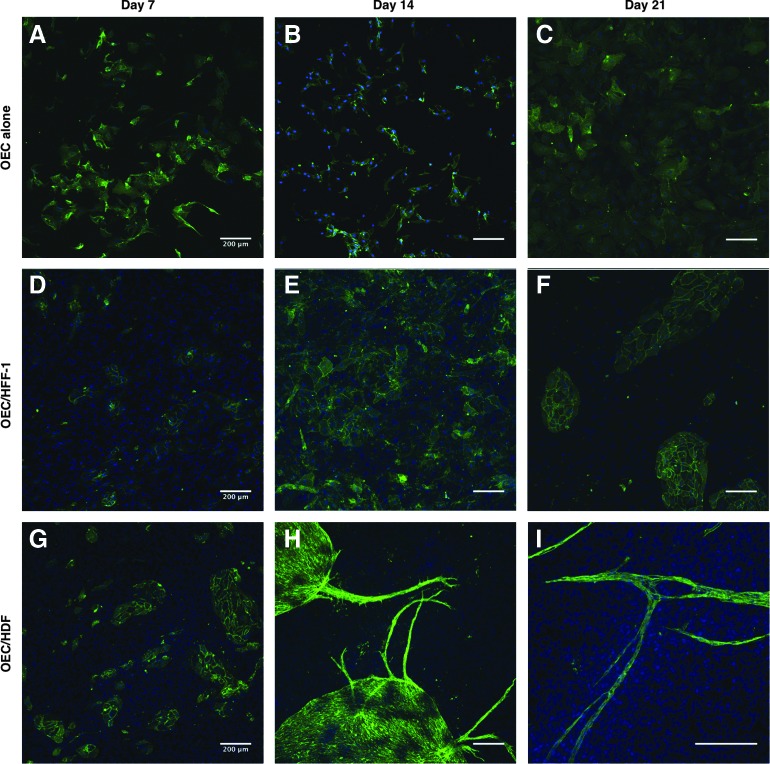
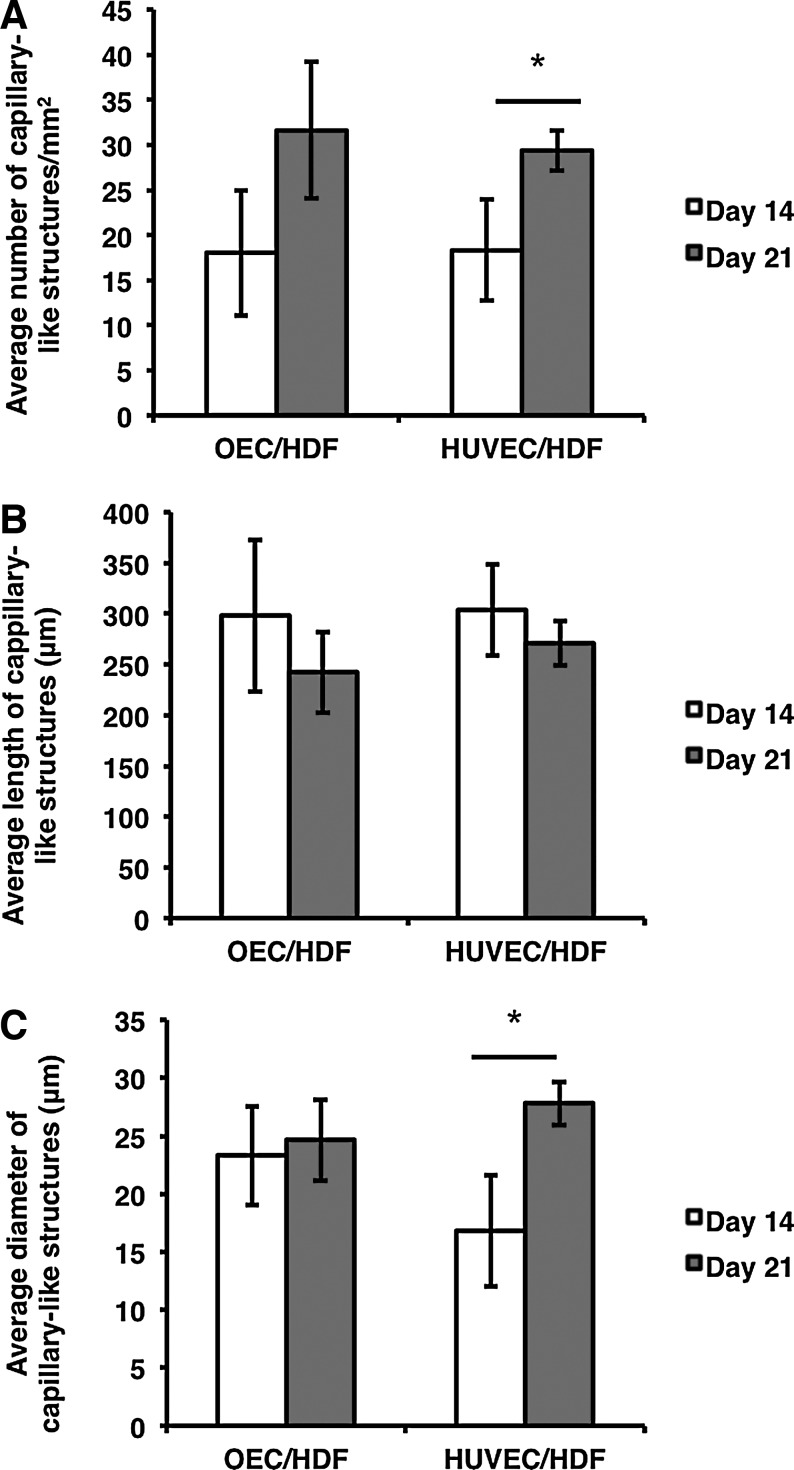
When OECs were cultured alone, they organized into a typical cell monolayer (Control, Fig. 4A–C), while organizing into clusters when cocultured with HFF-1 (Fig. 4D–F). Nevertheless, when cocultured with HDF, OECs assembled into capillary-like structures after 14 days (Fig. 4H), which were maintained after 21 days in culture (Fig. 4I). This behavior was similar to the above described for HUVECs. Moreover, the average number of capillary-like structures/mm2 was determined, and their length and diameter (Fig. 5), only in cocultures of ECs with HDF, since the formation of capillary-like structures was only observed in these systems. An increase in the number of capillary-like structures was observed between days 14 and 21, which was significantly different (p<0.05) for HUVEC/HDF coculture systems (Fig. 5A). This increase in the number of structures both in OEC/HDF and HUVEC/HDF cocultures was accompanied by a trend to a slight decrease in length (Fig. 5B), together with an increase in their diameter (Fig. 5C), again significant for HUVEC/HDF (p<0.05).
FIG. 4.
Cocultures of OECs with fibroblasts. Confocal images of OEC alone (A–C), cocultures of OEC/HFF-1 (D–F) and OEC/HDF (G–I) after 7, 14, and 21 days in EGM-2. OECs were stained against CD31 and nuclei were counterstained with DAPI. Scale bars, 200 μm. Color images available online at www.liebertpub.com/tea
FIG. 5.
Average quantifications of the number (A), length (B) and diameter (C) of capillary-like structures formed in cocultures of HUVEC/HDF and OEC/HDF, after 14 and 21 days. *Statistically significant differences (p<0.05, n=6 for HUVEC/HDF and n=4 for OEC/HDF).
ECM production by the different cells
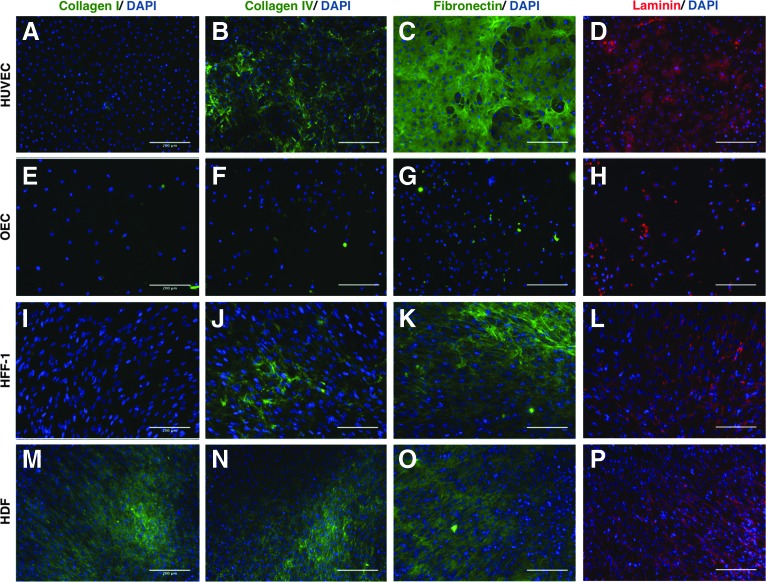
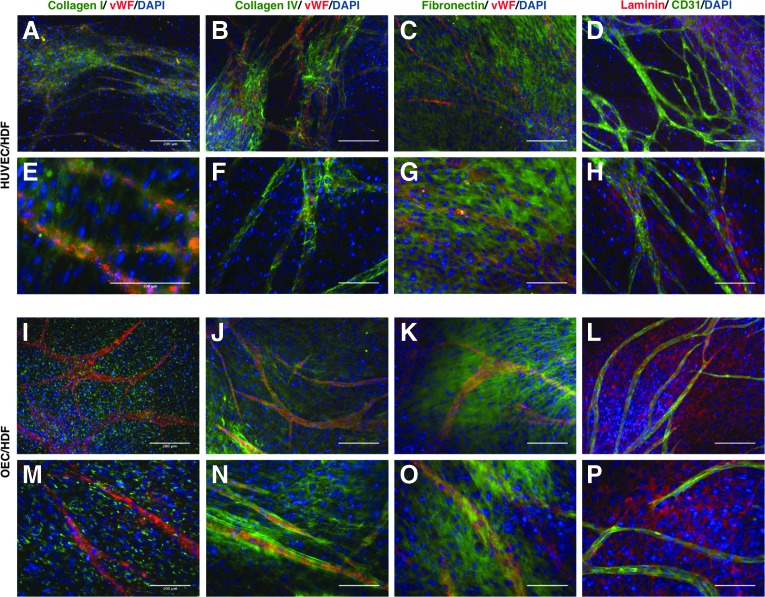
To evaluate the differences between the ECM produced by distinct types of fibroblasts and ECs, immunostainings against collagen types I and IV, fibronectin, and laminin were performed both in monocultures and in cocultures after 14 days. HUVECs secreted collagen IV, fibronectin, and laminin to the extracellular media, but not collagen I (Fig. 6A–D). In OECs, the same ECM components were not detected extracellularly (Fig. 6E–H). Both types of fibroblasts secreted collagen IV, fibronectin, and laminin to the extracellular media (Fig. 6I–P), but only HDF secreted collagen I (Fig. 6M). Collagen IV and laminin seem also to be more abundantly produced by HDF. For a better understanding about what happens with the ECM during the formation of capillary-like structures, the same staining was performed in cocultures with HDF. Collagens I and IV, laminin, and fibronectin were present in both coculture systems (Fig. 7). In HUVEC/HDF cocultures, the distribution of collagens I and IV was more heterogeneous, given that they were mainly present in areas where capillary-like structures were present (Fig. 7A, B, E, F). Magnified images more clearly show that collagens I and IV appear with a more intense staining around capillary-like structures (Fig. 7E, F) in cocultures with HUVECs, while fibronectin (Fig. 7C, G, K, O) and laminin (Fig. 7D, H, L, P) uniformly appeared distributed in both coculture systems.
FIG. 6.
ECM components produced by HUVECs (A–D), OECs (E–H), HFF-1 (I–L), and HDF (M–P) after 14 days in culture. Fluorescent microscope images of extracellular matrix components–collagen types I and IV, fibronectin, and laminin. Nuclei were counterstained with DAPI. Scale bars, 200 μm. ECM, extracellular matrix. Color images available online at www.liebertpub.com/tea
FIG. 7.
ECM in cocultures of HUVEC/HDF (A–H) and OEC/HDF (I–P) after 14 days. Fluorescent microscope images of ECM components–collagen types I and IV, fibronectin, and laminin. Endothelial cells were stained against vWF or CD31. Nuclei were counterstained with DAPI. (A–D) and (I–L), scale bars, 200 μm. (E–H) and (M–P) are magnifications; scale bars, 100 μm. Color images available online at www.liebertpub.com/tea
Discussion
Despite the controversy that still exists about terminology and exact origin, OECs appear to fulfill the main requisites for being considered a “true endothelial progenitor cell” with great potential for cellular therapies, namely (1) expressing several endothelial markers; (2) being easily obtained from circulating blood, constituting an autologous source of ECs; (3) and presenting a high expansion potential in culture due to their increased proliferation ability.
Here, OECs expressed typical endothelial markers, such as CD31, CD144, VEGFR2 and, to a lower extent, CD34, in agreement to previous descriptions of this cell population.7,9 Although CD34 is characteristically expressed by vascular ECs,31 some authors have also reported a low signal for CD34 both in HUVECs and OECs,32 which is in accordance to the fact that CD34+ ECs are enriched for biological functions related to angiogenesis and migration, whereas CD34- cells are enriched for functions related to proliferation.33 In addition, these cells displayed the ability for organizing into typical polygonal capillary-like structures at least for 48 h, according to what has been previously described.34
The use of a coculture system using ECs and fibroblasts to test biomaterials biocompatibility and their influence in in vitro angiogenesis assays is mandatory before performing in vivo studies. Since fibroblasts are known to be quite different regarding their tissue of origin and location, the main purpose of this study was to compare the behavior of two distinct fibroblast populations—HFF-1 and HDF. Despite being both derived from human foreskin, HFF-1 is neonatal and HDF of juvenile origin. To characterize both populations, different markers were evaluated. α-SMA is a known marker of fibroblast activation and myofibroblast differentiation and α-SMA-expressing fibroblasts have been shown to support capillary formation.35 TG2 belongs to a group of enzymes that catalyze post-translational modification of proteins and is involved in biological processes, such as cell death and differentiation, and matrix stabilization,36 being highly expressed in reticular fibroblasts.23 PDPN is a mucin-like transmembrane glycoprotein that has been associated to lymphangiogenesis37 and is strongly expressed by papillary fibroblasts. Although no studies relating PDPN to angiogenesis during tissue regeneration are available, different patterns of the expression of PDPN, and TG2 and α-SMA, were found herein. HFF-1 expressed higher levels of TG2, whereas PDPN and α-SMA were expressed in higher amounts in HDF, while their expression was low to none using HFF-1. Both types of fibroblasts were used to examine their capacity to influence the formation of capillary-like structures either in coculture with mature or progenitor ECs (HUVECs and OECs, respectively). HDF were found to induce to a high extent the formation of capillary-like structures, while HFF-1 failed to promote EC organization into tubular structures. Indeed, HDF promoted HUVEC assembly into a complex interconnected capillary-like network after 14 days and supported the maintenance of this network at least until day 21. In this culture system, the support of HDF resulted in a higher number and in an enlargement of the caliber of HUVEC-derived capillaries between days 14 and 21. The overexpression of α-SMA, a protein that supports vessel formation, may explain the increased capacity of HDF cells for vessel assembly. The fact that PDPN mediates invasion in thyroid cancer cells38 may as well promote the HDF angiogenic role. Similarly, decreased expression of TG2 observed in this study for HDF, is likely to destabilize the ECM, thus enhancing vascular-structure formation.
Identical to its influence in mature ECs, HDF also induced OECs to assemble into capillary-like structures for 21 days, thereby sustaining their ability to form vessels, contrary to the culture conditions of OECs alone in matrigel, where the capillary-like structures formed decreased only after 48 h (Fig. 1D). The exact mechanisms underlying the in vitro behavior of both fibroblasts in what concerns vascularization are unknown. However, an association between the expression of dermal markers and the possible role of both types of fibroblasts in vivo is likely to exist. HDF expressed markers of papillary fibroblasts (PDPN), while HFF-1 expressed markers of reticular fibroblasts (TG2). As mentioned before, it has been previously reported that, contrary to reticular fibroblasts, papillary fibroblasts appear to have a strong ability to support the formation of tubular structures in vitro.24 Considering these data together, it could be hypothesized that HFF-1 would constitute a population of reticular fibroblasts, while HDF would correspond to a population of papillary fibroblasts, with PDPN and TG2 being useful markers in this identification. However, care should be taken when extrapolating this conclusion based only on the described cell markers, mainly due to the fact that fibroblasts used here were not isolated from the same skin donor site and, consequently, are not so easily comparable. In addition, a recent work has shown that papillary fibroblasts can differentiate into reticular fibroblasts when cultured over several passages.39 Also, a noteworthy aspect is that the same authors have attributed a higher expression of α-SMA to reticular fibroblasts,23,39 whereas in the populations described here higher levels of this marker were found to be expressed by HDF. Moreover, although there is no evidence of PDPN functions in angiogenesis, TG2, in turn, has been described as a partner of endostatin, an anti-angiogenic peptide present in the ECM close to ECs.40 Although ECs are a rich source of TG2,41 the presence of TG2 produced by the HFF-1 might be one of the reasons for the observed inhibition of EC assembly into capillary-like structures, as it has already been described that the addition of exogenous TG2 blocks angiogenesis in vitro.42 In the present work, HUVECs were found to express TG2 when cultured alone for 7, 14, and 21 days (data not shown). However, expression of TG2 was not observed when formation of capillary-like structures occurred, namely in cocultures of HUVEC/HDF (data not shown), which corroborates the fact that the presence of TG2 produced by HFF-1 fibroblasts might be an inhibitor of the formation of capillary-like structures.
Nevertheless, previous studies have shown that when HFF-1 were entrapped in an artificial ECM, like modified alginate, capillary-like structures were formed and maintained during 5 days.11 In addition, when HFF-1 were used in a model of matrigel plug implantation in mice, these cells induced the ingrowth of blood vessels from the host vasculature into the plug.10 This raises the question that to what extent can a biomaterial modulate the crosstalk between cells in direct contact. Thus, more studies at the molecular level will be useful to help clarifying this issue.
Another hypothesis to explain the distinct behavior of ECs in the described coculture systems is based on distinct profiles of soluble factors or ECM components being produced by HFF-1 or HDF. Sorrell et al. seeded together papillary and reticular fibroblasts in a dish and observed a higher formation of capillary-like structures in the area where only papillary fibroblasts were present.24 This suggested that either ECM or matrix-bound molecules would be critical for the formation of capillary-like structures; otherwise the release of factors to the medium would have been sufficient to obtain a homogenous formation of tubular structures by ECs. Besides, one striking difference was observed between HUVECs and OECs concerning their release of ECM components after long-term culture. HUVECs were found to secrete collagen type IV, fibronectin and laminin to the extracellular media, whereas in OECs monocultures these proteins were only detected intracellularly.
Regarding fibroblasts, HDF secreted all investigated ECM components, primarily collagen I, which was neither secreted by HFF-1, nor HUVECs or OECs. Therefore, it can be hypothesized that the role of HDF as stimulators of the formation of vascular structures probably depends on the secretion of ECM components and mainly collagen I, which is a known angiogenesis stimulator. Collagens I and IV were present in the coculture systems analyzed, particularly in sites of capillary-like structures formation. Collagen IV, together with laminin, constitutes the basement membrane that surrounds blood vessels in vivo. In addition, fibronectin was abundantly detected in coculture systems and it is a known ECM component of developing microvessels, acting also as a scaffold for cell adhesion and migration43,44 and playing a role in the elongation of microvessels.45 All these ECM components were secreted by HDF, providing the support for capillary-like structures formation. Besides, HUVECs and OECs had a similar ability to organize into an interconnect network of tubular structures in the described coculture systems with HDF. As progenitor ECs are easily obtained, compared to mature ECs, this work shows that OECs present a promising potential to be explored in vascularization strategies aiming at tissue engineering and regeneration purposes.
Conclusions
The present results suggest that juvenile dermal fibroblasts are a preferential cell source, comparing to neonatal fibroblasts of foreskin origin, for enhancing vascularization in coculture with both mature (HUVECs) and progenitor ECs (OECs), probably due to their expression of α-SMA and papillary fibroblasts markers (podoplanin) and increased secretion of ECM components, mainly collagen type I.
Acknowledgments
This work was financed by the European Regional Development Fund (ERDF) through the Programa Operacional Factores de Competitividade—COMPETE, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia in the framework of S. Guerreiro post-doctoral grant SFRH/BPD/88745/2012, the research grants PEst-C/SAU/LA0002/2013 and Pest-OE/SAU/UI0038/2013, and co-financed by North Portugal Regional Operational Programme (ON.2–O Novo Norte) in the framework of the project SAESCTN-PIIC&DT/2011, under the National Strategic Reference Framework (NSRF).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kolbe M., Xiang Z., Dohle E., Tonak M., Kirkpatrick C.J., and Fuchs S.Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng 17,2199, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Bidarra S.J., Barrias C.C., Barbosa M.A., Soares R., Amédée J., and Granja P.L.Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res 7,186, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Grellier M., Bordenave L., and Amédée J.Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol 27,562, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Lovett M., Lee K., Edwards A., and Kaplan D.L.Vascularization strategies for tissue engineering. Tissue Eng 15,353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelps E.A., and García A.J.Engineering more than a cell: vascularization strategies in tissue engineering. Curr Opin Biotechnol 21,704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novosel E.C., Kleinhans C., and Kluger P.J.Vascularization is the key challenge in tissue engineering. Adv Drug Del Rev 65,300, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Silva E.A., Kim E.-S., Kong H.J., and Mooney D.J.Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci U S A 105,14347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs S., Motta A., Migliaresi C., and Kirkpatrick C.J.Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells as a potential source of autologous cells for endothelialization of silk fibroin biomaterials. Biomaterials 27,5399, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fuchs S., Dohle E., Kolbe M., and Kirkpatrick C.J.Outgrowth endothelial cells: sources, characteristics and potential applications in tissue engineering and regenerative medicine. Adv Biochem Eng Biotechnol 123,210, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Guerreiro S.G., Brochhausen C., Negra∼o R., Barbosa M.A., Unger R.E., Kirkpatrick C.J., Soares R., and Granja P.L.Implanted neonatal human dermal fibroblasts influence the recruitment of endothelial cells in mice. Biomatter 21,43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerreiro S.G., Oliveira M.J., Barbosa M.A., Soares R., and Granja P.L.Neonatal human dermal fibroblasts immobilized in RGD-alginate induce angiogenesis. Cell Transplant 23,945, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Foubert P., Matrone G., Souttou B., Leré-Déan C., Barateau V.R., Plouët J., Ricousse-Roussanne S.L., Lévy B.I., Silvestre J.-S., and Tobelem G.R.Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res 103,751, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Melero-Martin J.M., Khan Z.A., Picard A., Wu X., Paruchuri S., and Bischoff J.In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109,4761, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Grellier M., Ferreira-Tojais N., Bourget C., Bareille R., Guillemot F., and Amédée J.Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem 106,390, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Ghanaati S., Fuchs S., Webber M.J., Orth C., Barbeck M., Gomes M.E., Reis R.L., and Kirkpatrick C.J.Rapid vascularization of starch–poly(caprolactone) in vivo by outgrowth endothelial cells in co-culture with primary osteoblasts. J Tissue Eng Regen Med 5,136, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Costa-Almeida R., Granja P.L., Soares R., and Guerreiro S.G.Cellular strategies to promote vascularisation in tissue engineering applications. Eur Cell Mater 28,51, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Chang H.Y., Chi J.-T., Dudoit S., Bondre C., van de Rijn M., Botstein D., and Brown P.O.Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 99,12877, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri R., and Zeisberg M.Fibroblasts in cancer. Nat Rev Cancer 6,392, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Mansbridge J.N., Liu K., Pinney R.E., Patch R., Ratcliffe A., and Naughton G.K.Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab 1,265, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Wong T., McGrath J.A., and Navsaria H.The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol 156,1149, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Junker J.P., Lonnqvist S., Rakar J., Karlsson L.K., Grenegard M., and Kratz G.Differentiation of human dermal fibroblasts towards endothelial cells. Differentiation 85,67, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Sorrell J.M., and Caplan A.I.Fibroblast heterogeneity: more than skin deep. J Cell Sci 117,667, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Janson D.G., Saintigny G., van Adrichem A., Mahé C., and Ghalbzouri A.E.Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol 132,2565, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Sorrell J.M., Baber M.A., and Caplan A.I.Human dermal fibroblasts subpopulations; differential interactions with vascular endothelial cells in coculture: Nonsoluble factors in the extracellular matrix influence interactions. Wound Repair Regen 16,300, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hurley J.R., Balaji S., and Narmoneva D.A.Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol 299,444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman A.C., Nakatsu M.N., Chou W., Gershon P.D., and Hughes C.C.W.The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22,3791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthod F., Germain L., Tremblay N., and Auger F.A.Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol 207,491, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kunz-Schughart L.A., Schroeder J.A., Wondrak M., van Rey F., Lehle K., Hofstaedter F., and Wheatley D.N.Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol 290,920, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Njauw C.-N., Yuan H., Zheng L., Yao M., and Martins-Green M.Origin of periendothelial cells in microvessels derived from human microvascular endothelial cells. Int J Biochem Cell Biol 40,710, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa R., Negrão R., Valente I., Castela A.N., Duarte D., Guardão L.S., Magalhaẽs P.J., Rodrigues J.A., Guimaraẽs J.T., Gomes P., and Soares R.Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J Nat Prod 76,2047, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Fina L., Molgaard H.V., Robertson D., Bradley N.J., Monaghan P., Delia D., Sutherland D.R., Baker M.A., and Greaves M.F.Expression of the CD34 gene in vascular endothelial cells. Blood 75,2417, 1990 [PubMed] [Google Scholar]
- 32.Amini A.R., Laurencin C.T., and Nukavarapu S.P.Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res 30,1507, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Siemerink M.J., Klaassen I., Vogels I.M.C., Griffioen A.W., Noorden C.J.F.V., and Schlingemann R.O.CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis 15,151, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reagan J., Foo T., Watson J.T., Jin W., Moed B.R., and Zhang Z.Distinct phenotypes and regenerative potentials of early endothelial progenitor cells and outgrowth endothelial progenitor cells derived from umbilical cord blood. J Tissue Eng Regen Med 5,620, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Berthod F., Symes J., Tremblay N., Medin J.A., and Auger F.A.Spontaneous fibroblast-derived pericyte recruitment in a human tissue-engineered angiogenesis model in vitro. J Cell Physiol 227,2130, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Griffin M., Casadio R., and Bergamini C.M.Transglutaminases: nature's biological glues. Biochem J 368,377, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.Y., Park C., Cho Y.P., Lee E., Kim H., Yun S.H., and Yoon Y.-S.Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 122,1413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudzinska M., Gaweł D., Sikorska J., Karpinska K.M., Kiedrowski M., Stepien T., Marchlewska M., and Czarnocka B.The role of podoplanin in the biology of differentiated thyroid cancers. PLoS One 9,e96541, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janson D., Saintigny G., Mahé C., and Ghalbzouri A.E.Papillary fibroblasts differentitate into reticular fibroblasts after prolonged in vitro culture. Exp Dermatol 22,48, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Faye C., Inforzato A., Bignon M., Hartmann D.J., Muller L., Ballut L., Olsen B.R., Day A.J., and Ricard-Blum S.Transglutaminase-2: a new endostatin partner in the extracellular matrix of endothelial cells. Biochem J 427,467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korner G., Schneider D.E., Purdon M.A., and Bjornsson T.D.Bovine aortic endothelial cell transglutaminase. Enzyme characterization and regulation of activity. Biochem J 262,633, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones R.A., Kotsakis P., Johnson T.S., Chau D.Y., Ali S., Melino G., and Griffin M.Matrix changes induced by transglutaminase 2 lead to inhibition of angiogenesis and tumor growth. Cell Death Differ 13,1442, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Stoffels J.M.J., Zhao C., and Baron W.Fibronectin in tissue regeneration: timely disassembly of the scaffold is necessary to complete the build. Cell Mol Life Sci 70,4243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pankov R., and Yamada K.M.Fibronectin at a glance. J Cell Sci 115,3861, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Nicosia R., Bonanno E., and Smith M.Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J Cell Physiol 154,654, 1993 [DOI] [PubMed] [Google Scholar]