Abstract
Stem cells hold great promise for treating cartilage degenerative diseases such as osteoarthritis (OA). The efficacy of stem cell-based therapy for cartilage repair is highly dependent on their interactions with local cells in the joint. This study aims at evaluating the interactions between osteoarthritic chondrocytes (OACs) and adipose-derived stem cells (ADSCs) using three dimensional (3D) biomimetic hydrogels. To examine the effects of cell distribution on such interactions, ADSCs and OACs were co-cultured in 3D using three co-culture models: conditioned medium (CM), bi-layered, and mixed co-culture with varying cell ratios. Furthermore, the effect of transforming growth factor (TGF)-β3 supplementation on ADSC–OAC interactions and the resulting cartilage formation was examined. Outcomes were analyzed using quantitative gene expression, cell proliferation, cartilage matrix production, and histology. TGF-β3 supplementation led to a substantial increase in cartilage matrix depositions in all groups, but had differential effects on OAC–ADSC interactions in different co-culture models. In the absence of TGF-β3, CM or bi-layered co-culture had negligible effects on gene expression or cartilage formation. With TGF-β3 supplementation, CM and bi-layered co-culture inhibited cartilage formation by both ADSCs and OACs. In contrast, a mixed co-culture with moderate OAC ratios (25% and 50%) resulted in synergistic interactions with enhanced cartilage matrix deposition and reduced catabolic marker expression. Our results suggested that the interaction between OACs and ADSCs is highly dependent on cell distribution in 3D and soluble factors, which should be taken into consideration when designing stem cell-based therapy for treating OA patients.
Introduction
Articular cartilage is a highly specialized tissue found on joint surfaces and is responsible for the load bearing, distribution, and lubrication during joint movements. Cartilage damage as a result of trauma or sports-related injuries is common among young adults.1 Owing to its limited self-repair potential, untreated cartilage damage is often irreversible and may deteriorate over time, leading to changes in mechanical loading in the joint and the early onset of osteoarthritis (OA).2 Currently, there is no effective disease-modifying treatment for OA due to the lack of understanding of the disease pathology. Common treatment options include anti-inflammatory medications, weight loss and exercise intervention, and physical therapy to provide symptomatic relief and maintain joint mobility.3,4 As cartilage continues to degenerate and progress to late-stage OA, joint replacement surgery is performed to relieve pain and restore function.
Cell-based therapy offers a promising approach to cartilage repair. Autologous chondrocyte implantation (ACI) is the most established cell-based cartilage repair procedure but suffers from several shortcomings. To obtain enough cells to repair a large chondral defect, in vitro expansion is required. Furthermore, the proliferation and tissue regeneration potential of chondrocytes declines with age and disease state5–7; ACI is, therefore, limited to treating younger patients (<55 years).8 Mesenchymal stem cells (MSCs), in particular adipose-derived stem cells (ADSCs), are an attractive cell source, as they are easily accessible, readily available in large number, and have the ability to proliferate and differentiate into mesenchymal tissues such as fat, bone, and cartilage.9,10 Various strategies have been developed to direct ADSC chondrogenesis, including induction by exogenous growth factors belonging to the transforming growth factor (TGF)-β superfamily11 and the design of scaffolds to manipulate the biochemical and mechanical properties of the cellular microenvironment.12 Although numerous studies have shown that MSCs can undergo chondrogenesis and produce cartilage tissue in vitro, their therapeutic efficacy in treating OA remains ambivalent.13–15
While MSCs hold promise for cartilage regeneration, their success in repairing osteoarthritic cartilage relies on their interaction with the local osteoarthritic chondrocytes (OACs), which remains poorly understood. Given that cell fate is largely dependent on the interactions between cells and the multi-factorial environmental cues, there is a need to understand interactions between MSCs and OACs to better predict therapeutic outcomes. Recently, co-culture studies were conducted to investigate the interactions of MSCs and OACs,16–21 but the effects of such interactions remain inconclusive. While some studies reported that MSC–OAC co-culture resulted in enhanced cartilage formation and reduced hypertrophic phenotype,20–22 other studies observed no significant effect.16,18 Most of these studies have focused on bone marrow-derived MSCs (BMSCs)19,21,22 instead of ADSCs.16,19 Furthermore, previous co-culture studies have cultured OACs on two-dimensional (2D) tissue culture plastic17 or as dense cell pellets.16 When cultured in 2D monolayer, chondrocytes would rapidly dedifferentiate into a fibroblastic-like phenotype,23 whereas cell pellet culture offers little control over cell density or distribution. To overcome these limitations, we have chosen three-dimensional (3D) hydrogel co-culture models in our study, which facilitates better control of cell density and distribution in 3D, which are important parameters in modulating cell–cell interactions.24
As such, the objective of this study was to evaluate the interactions between OACs and ADSCs in 3D using biomimetic hydrogels, and to examine the effects of cell distribution and TGF-β3 supplementation on such cell–cell interactions and the resulting cartilage formation. We hypothesized that the effect of OAC–ADSC interactions is dependent on the local concentration of paracrine factors, which may be controlled in 3D by intercellular distance and cell distribution. To test this hypothesis, we utilized three in vitro co-culture models in 3D for evaluating the interactions between OACs and ADSCs (Fig. 1): conditioned medium (CM), bi-layered co-culture, and mixed co-culture at various ratios. Using our 3D co-culture models, we have recently shown that the interaction between ADSCs and neonatal chondrocytes is highly dependent on the spatial distribution of the two cell types.24 When cultured in close proximity, ADSCs catalyzed neonatal chondrocytes to proliferate and form extensive neocartilage nodules. In this study, we aimed at evaluating whether the spatial distributions of OACs and ADSCs in 3D would modulate cell–cell interactions and the resulting cartilage formation. In addition, the effect of TGF-β3 supplementation was also examined to determine the optimal soluble factor environment for synergistic interactions to occur.
FIG. 1.
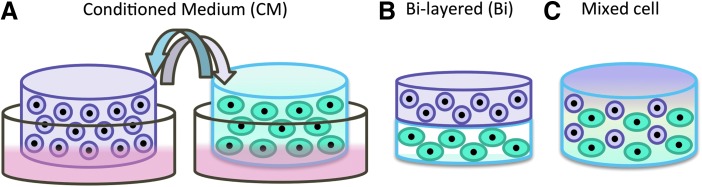
Schematics of the co-culture models. Three different in vitro co-culture models were used to examine the effects of paracrine factor concentration and intercellular distance on the interactions between adipose-derived stem cells (ADSCs) and osteoarthritic chondrocytes (OACs). (A) In the conditioned medium (CM-) co-culture, each cell type was cultured with supplementation of CM from the other cell type. (B) In the bi-layered (Bi) co-culture, ADSCs and OACs were encapsulated in two separate hydrogel layers with an acellular interface (∼250 μm) separating them. (C) In the mixed cell co-culture, ADSCs and OACs were mixed together in three dimensional (3D) at three different cell ratios (OAC:ADSC: 50C:50A, 25C:75A, and 10C:90A). In all the co-culture models, OACs and ADSCs were encapsulated 3D biomimetic hydrogels and cultured for 21 days in vitro, and two media conditions were examined: standard chondrogenic medium with or without transforming growth factor (TGF)-β3 supplementation. Color images available online at www.liebertpub.com/tea
Materials and Methods
Cell isolation and culture
Osteoarthritic chondrocytes
Cartilage tissues were collected from five OA patients (age 50–75, women) undergoing total knee replacements according to an Institutional Review Board—approved protocol by Stanford University. Macroscopically intact cartilage was dissected, and the chondrocytes were dissociated from the matrix as previously described.25 After isolation, OACs were cryopreserved and used without further passaging for all the experiments described.
Adipose-derived stem cells
Human ADSCs were isolated from excised human adipose tissue with informed consent as previously described.9 ADSCs were expanded for three passages in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 5 ng/mL basic fibroblast growth factor, 100 U/mL penicillin, and 0.1 mg/mL streptomycin.
3D hydrogel co-culture
Cells were suspended at 15×106 cells/mL in a hydrogel solution consisting of 7% weight/volume (w/v) poly(ethylene glycol diacrylate) (PEGDA, MW=5000 g/mole), 3% w/v chondroitin sulfate (CS)-methacrylate, and 0.05% w/v photoinitiator (Irgacure D 2959; Ciba Specialty Chemicals, Tarrytown, NY). To induce gelation, cell-hydrogel suspension was pipetted into a custom-made cylindrical gel mold with 75 μL volume and exposed to UV light (365 nm wavelength) at 3 mW/m2 for 5 min. To create bi-layered hydrogel, cell-hydrogel suspension (37.5 μL each) of one cell type was deposited into the cylindrical gel mold and photo-cross-linked before deposition of the next cell-hydrogel layer. An acellular hydrogel interface (10 μL) layer was included between the two cell-containing layers to prevent direct cell–cell contact between OACs and ADSCs in the bi-layered co-culture. Cell viability was examined at 24 hs postencapsulation using live/dead viability assay (Life Technologies, Grand Island, NY), which confirmed that more than 80% of the cells were viable in OAC as well as in ADSC group (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
Co-culture models
To examine the effects of cell distribution on cell fate, OACs and ADSCs were co-cultured in three different co-culture models: (1) supplementation of CM from the other cell type (Fig. 1A), (2) bi-layered co-culture confining each cell type to its own layer separated by an acellular interface (Fig. 1B), and (3) mixed co-culture of two cell types at different ratios (50C:50A, 25C:75A, and 10C:90A) (Fig. 1C). CM was collected every 2 days and diluted with an equal volume of freshly prepared chondrogenic medium. All samples were cultured in chondrogenic medium (high-glucose DMEM; Gibco, Invitrogen, Carlsbad, CA) containing 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO), 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich), 40 μg/mL proline (Sigma-Aldrich), 100 μg/mL sodium pyruvate (Gibco, Invitrogen), 100 U/mL penicillin, 0.1 mg/mL streptomycin, and ITS Premix (5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL selenious acid; BD Biosciences, San Jose, CA) with 10 ng/mL TGF-β3 (PeproTech, Rocky Hill, NJ) supplementation for 3 weeks. To evaluate the effects of TGF-β3 on cell–cell interactions, the control groups were cultured in chondrogenic medium containing all the ingredients listed earlier except for TGF-β3.
Gene expression analysis
Total RNA was extracted from cell-hydrogel constructs (n=3/group) using TRIzol (Invitrogen) and the RNeasy mini kit (Qiagen, Valencia, CA) as previously described.24 Real-time polymerase chain reaction (PCR) was performed on an Applied Biosystems 7900 Real-Time PCR system using SYBR green master mix (Applied Biosystems, Carlsbad, CA) and primers listed in Supplementary Table S1. Gene expression of chondrogenic markers, including type II collagen (COL2) and aggrecan (Agg), fibroblastic marker type I collagen (COL1), hypertrophic marker type X collagen (COLX), and catabolic markers matrix metalloproteinase (MMP)-3, MMP13, and aggrecanase ADAMTS-5, was quantified using ΔΔCt method.26 Gene expression levels were first normalized to GAPDH, a housekeeping gene. Relative fold changes in gene expression of target genes were subsequently normalized to the gene expression level in the control group, which is OAC cells cultured without TGF-β3 (OAC, −TGF-β).
Biochemical analysis
Cell-hydrogel constructs (n=3/group) were weighed wet, lyophilized, and digested in papainase solution (Worthington Biochemical, Lakewood, NJ) at 60°C for 16 h. DNA content was measured using the PicoGreen assay (Molecular Probes, Eugene, OR) with Lambda phage DNA as a standard. Sulfated glycosaminoglycan (sGAG) content was quantified using the 1,9-dimethylmethylene blue dye-binding assay with shark chondroitin sulfate (Sigma-Aldrich) as a standard.27 To determine sGAG content contributed by the cells, we subtracted sGAG content measured in the acellular hydrogels from the total sGAG content from the cell-hydrogel constructs. Collagen content was determined using acid hydrolysis followed by a reaction with p-dimethylaminobenzaldehyde and chloramine T (Sigma-Aldrich). Total collagen content was estimated by assuming 1:7.46 hydroxyproline:collagen mass ratio.28
Quantification of interaction synergy
Interaction index provides a measure of interaction synergy for gene expression (Supplementary Tables S2 and S4) or cartilage matrix production (Supplementary Tables S3 and S5). It is defined as the ratio of the measured values over the expected values of gene expression or sGAG. The expected values were calculated based on the percentage of each cell type and the measured values in the control groups,29 as shown in the following formula for sGAG:
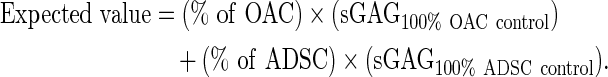 |
An interaction index of greater than one indicates positive synergy.
Histological analysis
Cell-hydrogel constructs (n=2/group) were fixed in 4% paraformaldehyde (Sigma-Aldrich) overnight and stored in 70% ethanol at 4°C until they were processed. Constructs were then embedded in paraffin and processed using standard histological procedures. To perform immunostaining, sections were first incubated in 0.1% trypsin (Gibco, Invitrogen) at 37°C for 15 min for enzymatic antigen retrieval, followed by blocking in buffer consisting of 3% bovine serum albumin (Fisher Scientific, Pittsburgh, PA) and 2% goat serum (Gibco, Invitrogen). Sections were then incubated overnight in rabbit polyclonal antibody to collagen type I, II, or X (1:100; Abcam, Cambridge, MA) at 4°C and secondary antibody (1:200, Alexa Fluor 488 goat anti-rabbit; Invitrogen) for an hour at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole mounting medium (Vectashield; Vector Laboratories, Burlingame, CA), and images were taken with a Zeiss fluorescence microscope.
Cell labeling and co-staining with collagen type II
To identify the contribution of the two cell types to cartilage production in a mixed co-culture, OACs and ADSCs were labeled with green (PKH67) and red fluorescent cell linker (PKH26; Sigma-Aldrich) following the manufacturer's protocol before encapsulation in 3D hydrogels. After 21 days of mixed co-culture, samples were fixed overnight in 4% paraformaldehyde (Sigma-Aldrich), submerged in 30% sucrose (Sigma-Aldrich) solution for 24 h, embedded in Tissue-Tek (Sakura Finetek, Torrance, CA), and frozen in liquid nitrogen. Cryosections (12 μm-thick) were washed in Dulbecco's Phosphate-Buffered Saline and collagen II, and cell nuclei were stained using the immunostaining procedures described earlier.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (Graphpad Software, San Diego, CA). One- or two-way analysis of variance and pairwise comparisons with Tukey's posthoc test were used to determine statistical significance (p<0.05). Data were represented as mean±standard deviation of at least three biological replicates.
Results
Effects of CM
In the absence of TGF-β3
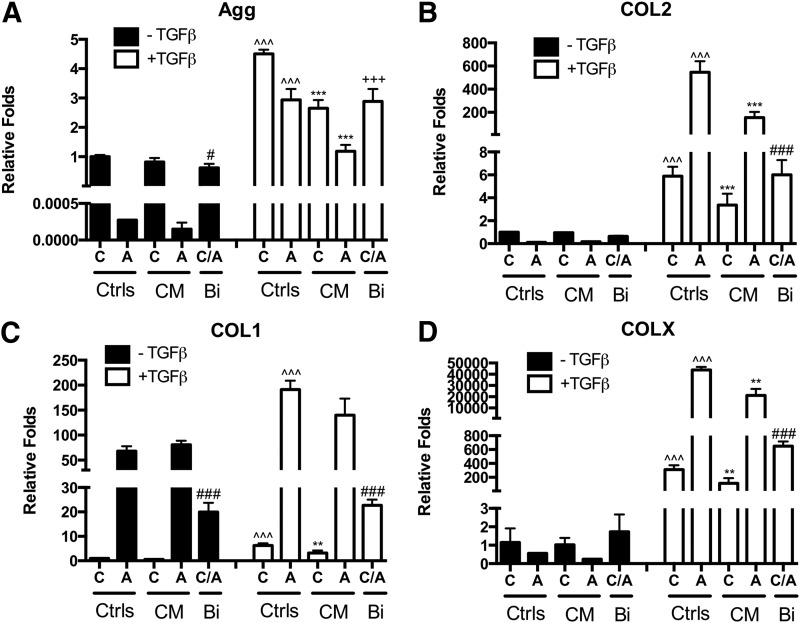
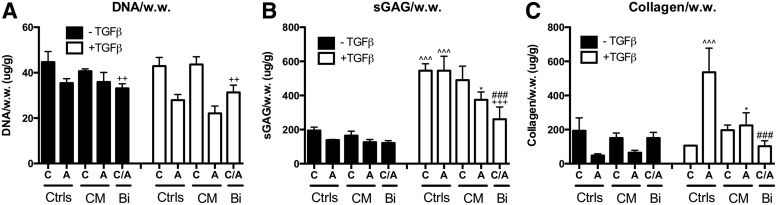
In the absence of TGF-β3, CM treatment had negligible effect on OAC and ADSC chondrogenic phenotype and cartilage matrix production. CM-treated OACs and ADSCs had comparable gene expression of chondrogenic markers Agg and COL2, COL1, and COLX compared with their respective controls (Fig. 2A–D). Consistent with gene expression analyses, biochemical analyses and immunostaining revealed that DNA, GAG, and collagen content was comparable in CM-treated OACs and ADSCs compared with their respective controls (Fig. 3), with no noticeable difference in collagen type I, II, and X deposition as demonstrated by immunostaining (data not shown).
FIG. 2.
Effects of the media condition and CM or bi-layered co-culture on gene expression. Gene expression of (A) aggrecan (Agg), (B) collagen type II (COL2), (C) collagen type I (COL1), and (D) collagen type X (COLX) in control culture (C, OACs, A, ADSC), CM, or bi-layered co-culture (Bi) after 21 days with or without TGF-β3 supplementation. Fold changes relative to OAC control without TGF-β supplementation. Bi-layered co-culture group included both OACs and ADSCs (equal initial cell number). Data presented as mean±standard deviation (SD, n=3 samples/group); * represents significance versus−TGF-β control and ^ represents significance versus−CM control of the same cell type; +and # represent significance of the bi-layered co-culture group versus OAC and ADSC under the same media condition, respectively; *p<0.05, **p<0.01, and ***p<0.001.
FIG. 3.
Effects of media condition and CM or bi-layered co-culture on cartilage matrix production. After 21 days of culture with or without TGF-β3 supplementation, (A) DNA, (B) sGAG, and (C) collagen content per wet weight were evaluated. Data presented as mean±SD (n=3 samples/group). C, OACs; A, ADSC, CM, conditioned medium; Bi, bi-layered co-culture. * Represents significance versus−TGF-β control and ^ represents significance versus−CM control of the same cell type; +and # represent significance of the bi-layered co-culture group versus OAC and ADSC under the same media condition, respectively; *p<0.05, **p<0.01, and ***p<0.001. sGAG, sulfated glycosaminoglycan.
In the presence of TGF-β3
In the presence of TGF-β3, the expression levels of Agg and COL2 expression were significantly upregulated in OAC and ADSC controls (Fig. 2A, B). Expression levels of COL1 and COLX were also significantly upregulated (Fig. 2C, D). CM treatment significantly reduced Agg, COL2, and COLX expression in OACs and ADSCs (Fig. 2A, B, D; Supplementary Table S2). COL1 expression in CM-treated OACs was also significantly lower. DNA content in CM-treated OACs and ADSCs was comparable to their respective controls. However, sGAG and collagen content in CM-treated ADSCs were significantly lower than the ADSC control (Fig. 3B, C; Supplementary Table S3).
Effects of bi-layered co-culture
In the absence of TGF-β3
Unlike CM treatment, a bi-layered co-culture enables dynamic cross-talk between OACs and ADSCs. Bi-layered co-culture did not significantly affect cartilage specific markers or cartilage matrix production in the absence of TGF-β3. Gene expression levels of Agg, COL2, COL1, and COLX (Fig. 2; Supplementary Table S2) were similar to expected levels, as they were close to the average gene expression of the OAC and ADSC controls. Collagen content was slightly higher, while DNA and sGAG per wet weight were slightly lower than the average of the control groups (Fig. 3; Supplementary Table S3). Immunostaining of COL2, COL1, and COLX showed comparable intensity in bi-layered OACs, ADSCs, and their respective control groups (data not shown).
In the presence of TGF-β3
In the presence of TGF-β3, a bi-layered co-culture resulted in an overall decrease in chondrogenic gene expression (Agg and COL2, Fig. 2A, B; Supplementary Table S2). Consistent with gene expression, sGAG and collagen content was reduced in a bi-layered co-culture (48% and 34% of the expected value, respectively) (Fig. 3; Supplementary Table S3). Immunostaining showed that ADSCs in a bi-layered co-culture had reduced collagen type II, I, and X staining compared with the control, while OACs in a bi-layered co-culture had comparable collagen staining compared with the control (data not shown).
Effects of mixed co-culture
In the absence of TGF-β3
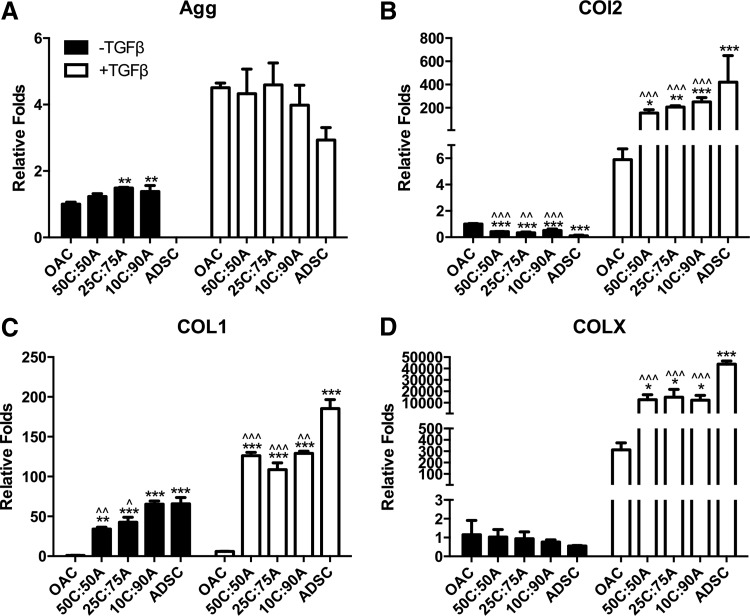
Unlike CM or a bi-layered co-culture, a mixed co-culture of OACs and ADSCs resulted in enhanced cartilage marker expression in the absence of TGF-β3. Mixed co-culture led to upregulation in Agg expression that even surpassed the OAC control (Fig. 4A; Supplementary Table S4). Agg expression peaked in mixed co-culture groups 25C:75A and 10C:90A. COL2 expression in mixed co-culture groups lay between those of the OAC and ADSC controls (Fig. 4B). COL1 expression was the lowest in OAC control and increased with an increase in ADSC ratio in mixed co-culture (Fig. 4C), whereas COLX expression was comparable in mixed co-culture and the control groups (Fig. 4D).
FIG. 4.
Effects of media condition and mixed co-culture on gene expression. Cartilage-specific gene expression of (A) Agg, (B) COL2, (C) COL1, and (D) COLX after 21 days in mixed co-culture at various cell ratios (OAC:ADSC: 50C:50A, 25C:75A, and 10C:90A) with or without TGF-β supplementation. Fold changes relative to OAC control without TGF-β supplementation at day 21. Data presented as mean±(n=3 samples/group). * and ^ represent significance versus OAC and ADSC control under the same media condition, respectively; *p<0.05, **p<0.01, and ***p<0.001.
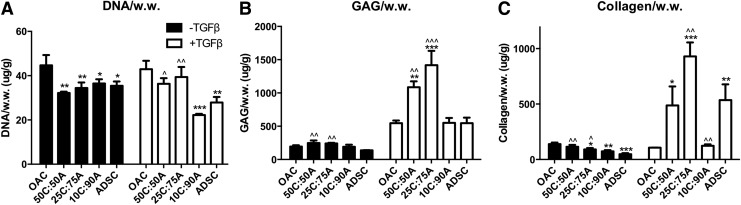
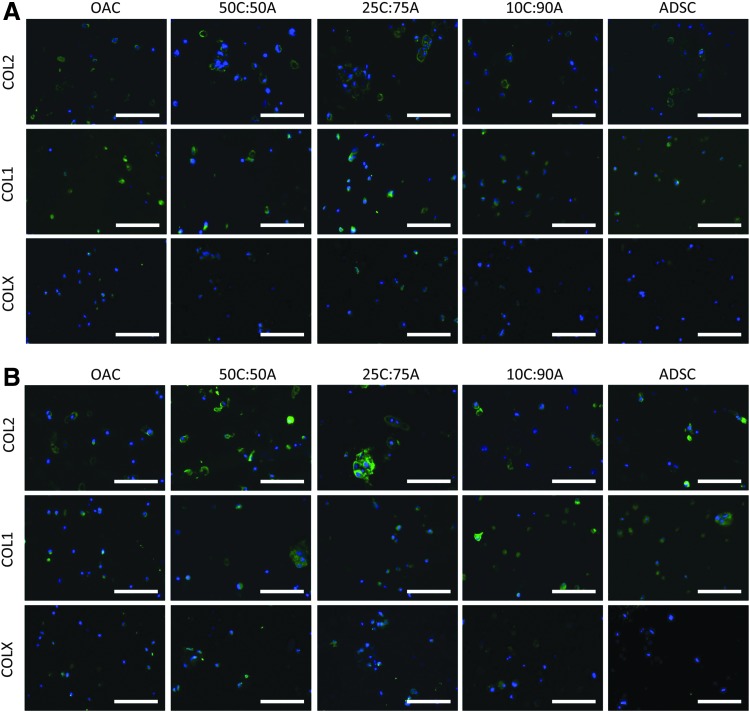
DNA content in the mixed co-culture and ADSC control was slightly lower than the OAC control. In agreement with Agg gene expression, 50C:50A and 25C:75A mixed co-culture resulted in significantly higher sGAG deposition compared with ADSC control (27% and 24% higher, respectively; Fig. 5B; Supplementary Table S4). Collagen content was the highest in the OAC control group and decreased with an increase in ADSC ratio in the mixed co-culture groups (Fig. 5C; Supplementary Table S5). Immunostaining revealed that mixed co-culture groups 50C:50A and 25C:75A resulted in slightly increased collagen II deposition (Fig. 6A). Cell labeling along with co-staining of collagen II further revealed that both cell types contributed to cartilage tissue deposition in mixed co-culture (Fig. 7A). Collagen I staining was positive, while collagen type X staining was minimal in all groups (Fig. 7A).
FIG. 5.
Effects of media condition and mixed co-culture on cartilage matrix production. (A) DNA, (B) sGAG, and (C) collagen content per wet weight in mixed co-culture at day 21 with or without TGF-β3 supplementation. Data presented as mean±SD (n=3 samples/group). * and ^ represent significance versus OAC and ADSC control under the same media condition, respectively; *p<0.05, **p<0.01, and ***p<0.001.
FIG. 6.
Cartilage formation in mixed co-culture. Immunostaining of collagen type II (top row), collagen type I (middle row), and collagen type X (bottom row after 21 days of culture in control and mixed co-culture groups in standard chondrogenic media (A) without or (B) with TGF-β3 supplementation. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
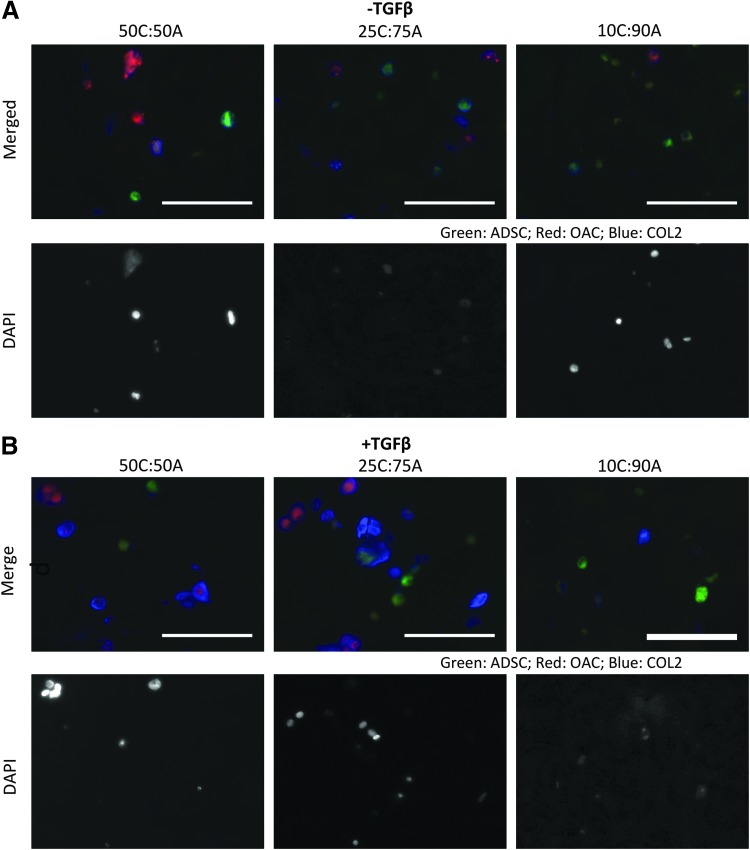
FIG. 7.
Cell labeling and co-staining of collagen type II in mixed co-culture. To identify the relative contribution of each cell type to collagen type II deposition in mixed co-culture, OACs (red) and ADSCs (green) were fluorescently labeled before encapsulation in hydrogels and co-stained with collagen type II (blue) after 21 days of mixed co-culture without (A) or with (B) TGF-β3 supplementation. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
In the presence of TGF-β3
In the presence of TGF-β3, expression levels of Agg were comparable in all the mixed co-culture groups and the OAC and ADSC controls (Fig. 4A). The expression levels of COL2, COL1, and COLX in all the mixed co-culture groups were comparable and lay between those of OAC and ADSC controls (Fig. 4B–D). On the contrary, DNA, sGAG, and collagen content in mixed co-culture was highly dependent on changes in cell ratio (Fig. 5). Interaction synergy was observed only in mixed co-culture at moderate ratios of OACs (50% and 25%). DNA content in 50C:50A and 25C:75A was comparable to the OAC control but was significantly lower in 10C:90A (Fig. 5A). Mixed co-culture with moderate ratios of OACs (50% and 25%) led to significantly higher sGAG and collagen content, peaking in the 25C:75A group (2.6-fold, Fig. 5B, C; Supplementary Fig. S2 and Supplementary Table S5). In contrast, a low ratio of OACs (10%) led to a negative interaction, with significantly lower collagen content per wet weight as well as per DNA compared with the ADSC control (Fig. 5C; Supplementary Fig. S2B). Consistent with collagen content, immunostaining showed enhanced collagen II deposition in 50C:50A and 25C:75A and a low level of staining intensity in 10C:90A (Fig. 6B). In mixed co-culture group 50C:50A, collagen II deposition was mainly contributed by OACs (red); whereas in 25C:75A, both cell types deposited collagen II, as shown by cell labeling and co-localization of collagen II immunostaining (Fig. 7B). Collagen I and X immunostaining were positive and comparable in all the mixed co-culture groups and the controls (Fig. 6B).
Catabolic gene expression in the presence of TGF-β3
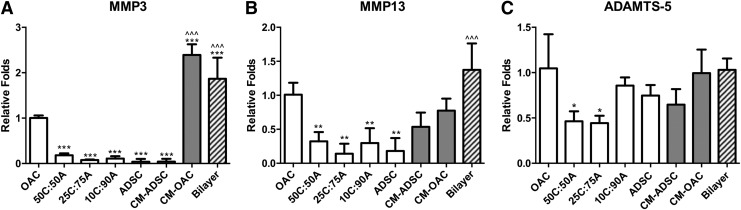
In addition to cartilage tissue-specific markers and production, we also quantified gene expression of catabolic markers, including MMP-3, MMP-13, and ADAMTS-5, in the presence of TGF-β3. When cultured alone, OACs expressed high levels of MMP-3, MMP-13, and ADAMTS-5 (Fig. 8). CM treatment led to a substantial increase in MMP-3 expression by OACs (CM-OAC, Fig. 8A). Bi-layered co-culture also resulted in significantly higher MMP-3 compared with both the OAC and ADSC controls and significantly higher MMP-13 expression compared with the ADSC control. On the contrary, mixed co-culture significantly reduced expression levels of MMP-3 and MMP-13 compared with the OAC control. As for ADAMTS-5 expression, only 50C:50A and 25C:75A exhibited significant reduction in expression (∼50%); whereas 10C:90A, CM-, and bi-layered co-culture resulted in comparable expression level as the OAC control (Fig. 8C).
FIG. 8.
Catabolic marker expression in mixed co-culture with TGF-β3 supplementation. Catabolic gene expression, including (A) matrix metalloproteinase (MMP)-3, (B) MMP-13, and (C) ADAMTS-5, after 21 days of CM, bi-layered, or mixed co-culture in standard chondrogenic medium with TGF-β3 supplementation. * and ^ represent significance versus OAC and ADSC control, respectively; *p<0.05, **p<0.01, and ***p<0.001. CM-ADSC, CM-treated ADSCs; CM-OAC, CM-treated OACs.
Discussion
Interactions between transplanted cells and the local chondrocytes in OA joint play a crucial role in the efficacy of cell-based therapy for cartilage repair. It has been reported that aging and OACs exhibit declined ability to produce cartilage extracellular matrix (ECM), as well as reduced proliferative and anabolic response to growth factor induction.30 Recent co-culture studies using healthy chondrocytes and MSCs generally demonstrated synergy,18,29,31,32 with enhanced cartilage matrix formation. However, it remains unclear how OACs and ADSCs interact, and how such interactions influence the resulting cartilage repair needs further investigation. Using a transwell co-culture model in which OACs were plated in 2D while BMSCs were encapsulated in a PEGDA hydrogel, Aung et al.17 found that BMSC chondrogenesis was enhanced drastically by OACs even in the absence of TGF-β induction, as illustrated by elevated chondrogenic gene expression and cartilage matrix production. However, CM collected from OACs led to adverse effects on BMSC chondrogenesis.17 In another study, it was found that CM from OACs contained parathyroid hormone-related protein (PTHrP), which reduced MSC hypertrophy during chondrogenesis.22 In both of these studies, however, the fate of the chondrocytes was not evaluated, which is an equally important factor in determining the success of cell-based cartilage repair.
In this study, we examined the effects of spatial organization on the interaction between OACs and ADSCs in 3D culture, and analyzed the effects of such cell–cell interactions on cell phenotype and cartilage matrix production. In particular, we evaluated cell–cell interactions using three different co-culture models in parallel, including CM, bi-layered co-culture, and CM, therefore enabling us to systematically evaluate the effect of cell distribution on the interactions between OACs and ADSCs. Our results showed that the extent of interaction and the resulting cartilage matrix formation were highly dependent on the proximity between the two cell types, and that the supplementation of exogenous TGF-β3 was required for optimal synergy to occur.
In both media conditions (±TGF-β3), mixed co-culture only, but not bi-layered co-culture or CM, led to enhanced cartilage matrix formation. In particular, two groups from our study design can be compared side by side to directly examine the effects of cell distribution on modulating OAC–ADSC interactions. The bi-layered co-culture and mixed co-culture of 50C:50A share the same ratio and number of OACs and ADSCs, and cell distribution is different in the two models. Our results showed that mixed co-culture at 50C:50A, but not bi-layered co-culture, led to enhanced cartilage matrix production (both sGAG and collagen). These results indicate that close proximity between the two cell types in mixed co-culture is required for interaction synergy to occur. In native tissue, the ECM mediates soluble signaling through the storage, binding, and presentation of soluble growth factors.33,34 Binding of cell-secreted growth factors to the ECM modulates the dynamics of autocrine and paracrine signaling, creating high local concentrations and limiting the diffusion of these factors within the ECM. Similarly, in the 3D hydrogel culture, interactions of the paracrine factors with the hydrogel matrix as well as the newly synthesized cartilage ECM may result in retention of these factors in the hydrogel construct. This may explain the differential results observed in mixed versus bi-layered or CM co-culture.
In the presence of TGF-β3 supplementation, synergistic interactions only occurred in mixed co-culture with 25–50% OACs in mixed co-culture. Cartilage matrix production, as measured by sGAG and collagen content, was significantly enhanced in groups 50C:50A and 25C:75A compared with both OAC and ADSC controls and peaked at 25C:75A. On the contrary, mixed co-culture at a low percentage of OACs (10C:90A) negatively mediated chondrogenic phenotype, cell number, and cartilage matrix synthesis. In a recent study, Bian et al.21 mixed co-cultured human BMSCs and OACs in hyaluronic acid (HA) hydrogels with TGF-β3 supplementation. Similar to our findings, they reported that OACs cultured alone showed low viability and poor cartilage matrix production. In contrast, mixed co-culture with 5–20%, but not 50% OACs, enhanced cartilage matrix content. We observed a different optimal range of cell ratio (25% and 50% OACs) for a synergistic interaction in our co-culture, which may be caused by the difference in hydrogel compositions (HA vs. CS-containing PEG). The inclusion of chondroitin sulfate in our 3D hydrogels provides a different cellular microenvironment compared with HA hydrogels, which likely modulate cell fates of both cell types and cell–cell interactions in a differential manner.
The differential results observed in different co-culture models highlighted that the extent of OAC interactions with transplanted stem cells is highly dependent on the spatial organization of the two cell types. Co-staining of collagen type II with cell labeling revealed that both cell types contributed to cartilage matrix production in mixed co-culture without TGF-β3 induction. Interestingly, under TGF-β3 induction, the relative contribution of the two cell types varied with cell ratio: While OACs were responsible for most of the collagen type II deposition in 50C:50A, both cell types deposited elevated collagen type II in 25C:75A as a result of interaction synergy. Additional experiments such as flow cytometry29 may further elucidate the fate and contribution of individual cell types in co-culture. Although mixed co-culture led to enhanced overall cartilage matrix production, immunostaining indicated that the resulting cartilage matrix also exhibited hypertrophic phenotype with increased COLX expression. Additional soluble factors such as PTHrP may be added to reduce hypertrophy.35,36
Our results showed that TGF-β3 was critical for optimal synergy between OACs and ADSCs to occur. The presence of TGF-β3 may affect the nature of OAC–ADSC interaction through several mechanisms. First, it directly affected the phenotype of the two cell types individually, which could, in turn, influence the nature of cell–cell interaction. In the controls, TGF-β3 supplementation led to chondrogenesis of ADSCs and enhanced cartilage-specific phenotype of OACs, as indicated by elevated COL2 and Agg expression as well as increased collagen and sGAG production. Such changes in cell phenotype induced by TGF-β3 supplementation may, in turn, affect paracrine factors released by the cells, therefore modulating cell–cell interactions.37 For instance, Lee et al. showed that ADSCs cultured in growth medium secreted angiogenic factors that led to chondrocyte apoptosis and reduced cartilage matrix production, and such negative effects were abolished when ADSCs were pretreated with chondrogenic medium with TGF-β supplementation.38 In another study, predifferentiation of BMSCs toward osteogenic lineage enhanced their capacity to stimulate cartilage tissue formation by chondrocytes.39 Clearly, the biochemical environment as created by media supplements can have an impact on the paracrine factor profile of stem cells. In addition, TGF-β3 may also modulate cell–cell interaction through acting synergistically with the paracrine factors secreted by the two cells. It has been shown that various growth factors such as platelet-derived growth factor, TGF-β1, and bone morphogenetic protein 7 can act synergistically to promote chondrogenesis.40 Future work is needed to elucidate the role of TGF-β3 in modulating OAC–ADSC interactions.
In addition to cartilage tissue-specific markers, anabolic markers are also crucial modulators of the overall cartilage matrix content. OA is characterized by an imbalance between chondrocyte catabolic and anabolic activities.41 In an osteoarthritic joint, elevated activities of proteolytic enzymes such as MMPs and aggrecanases led to the degradation of collagen, Agg, and other cartilage ECM components.41,42 Given that TGF-β3 led to biphasic response depending on proximity of the two cell types, we further examined the effects of co-culture on the activity of proteolytic enzymes at the gene expression level. As expected, OACs expressed high levels of MMP-3 and MMP-13. It was found that MMP-3 and MMP-13 expression was reduced in all mixed co-culture but was upregulated in CM and bi-layered co-culture. Similarly, ADAMTS-5 was reduced in mixed co-culture groups with a moderate percentage of OACs but not at a low percentage of OACs (10C:90A). The high expression of proteolytic enzyme ADAMTS-5 in 10C:90A suggested that the newly formed cartilage matrix might have been broken down rapidly, resulting in reduction in cartilage matrix synthesis per cell. In addition, a slight reduction in DNA content was also observed in 10C:90A compared with the ADSC control, suggesting an increase in cell apoptosis associated with chondrogenesis and increased proteolytic activity.43,44 In contrast, cartilage matrix content was enhanced in co-culture groups in which proteolytic enzyme expression was downregulated. Our results suggested that OAC–ADSC interaction in mixed co-culture with a moderate ratio of ADSCs (50% and 75%) had a chondroprotective effect with reduced catabolic activity and enhanced cartilage matrix content.
Overall, this study showed that interactions between ADSCs and OACs at close proximity enhanced cartilage matrix production in a 3D biomimetic hydrogel. Our results demonstrated that TGF-β3 supplementation was required for enhanced sGAG and collagen type II deposition to occur. Interaction synergy was highly dependent on the close proximity of the two cell types and peaked using 25–50% OACs in the mixed population. Nevertheless, even with the enhanced effects of TGF-β3 supplementation and mixed co-culture, the overall cartilage matrix production is still relatively low as indicated by immunostaining at day 21. A longer culture period may lead to a further increase in cartilage matrix production, and additional strategies such as gene therapy may be applied to further enhance tissue repair.45 In developing stem cell-based therapy for cartilage regeneration for OA, interactions between stem cells and the local chondrocytes should be taken into consideration in order to provide an optimal microenvironment for stem cell chondrogenesis while maintaining the phenotype or stimulate repair by native OACs.
Supplementary Material
Acknowledgments
The research was supported by Stanford–Coulter translational research grant and NSF CAREER award (CBET-1351289). The authors would like to thank Dr. Michael Keeney for valuable discussions and feedback on this article. The project was supported by NSF graduate research fellowship and DARE fellowship.
Disclosure Statement
The authors declared no conflicts of interest.
References
- 1.Cooper C., Snow S., McAlindon T., Kellingray S., Stuart B., Coggon D., et al. . Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 43,995, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Griffin T.M., and Guilak F.The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 33,195, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Hochberg M.C., Altman R.D., Brandt K.D., Clark B.M., Dieppe P.A., Griffin M.R., et al. . Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum 38,1541, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Messier S., Loeser R., Miller G., Morgan T., Rejeski W., Sevick M., et al. . Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis—the arthritis, diet, and activity promotion trial. Arthritis Rheum 50,1501, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Helmick C., Felson D., Lawrence R., Gabriel S., Hirsch R., Kwoh C., et al. . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum 58,15, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Martin J.A., and Buckwalter J.A.Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 3,257, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Barbero A., Grogan S., Schäfer D., Heberer M., Mainil-Varlet P., and Martin I.Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 12,476, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gikas P.D., Bayliss L., Bentley G., and Briggs T.W.An overview of autologous chondrocyte implantation. J Bone Joint Surg Br 91,997, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. . Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7,211, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Gimble J.M., Katz A.J., and Bunnell B.A.Adipose-derived stem cells for regenerative medicine. Circ Res 100,1249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuan R.S., Boland G., and Tuli R.Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther 5,32, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutmacher D.W.Scaffolds in tissue engineering bone and cartilage. Biomaterials 21,2529, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Frisbie D.D., Kisiday J.D., Kawcak C.E., Werpy N.M., Mcand Ilwraith C.W.Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res 27,1675, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., and Yoneda M.Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 10,199, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Orozco L., Munar A., Soler R., Alberca M., Soler F., Huguet M., et al. . Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation 95,1535, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Lee J.S., and Im G.I.Influence of chondrocytes on the chondrogenic differentiation of adipose stem cells. Tissue Eng Part A 16,3569, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Aung A., Gupta G., Majid G., and Varghese S.Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum 63,148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L., Leijten J.C., Georgi N., Post J.N., van Blitterswijk C.A., and Karperien M.Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 17,1425, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Wu L., Prins H.J., Helder M.N., van Blitterswijk C.A., and Karperien M.Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A 18,1542, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Diao H.J., Yeung C.W., Yan C.H., Chan G.C., and Chan B.P.Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regen Med 8,257, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Bian L., Zhai D.Y., Mauck R.L., and Burdick J.A.Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17,1137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer J., Dickhut A., Rickert M., and Richter W.Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 62,2696, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Benya P.D., and Shaffer J.D.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30,215, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Lai J.H., Kajiyama G., Smith R.L., Maloney W., and Yang F.Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep 3,3553 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith R.L., Lindsey D.P., Dhulipala L., Harris A.H., Goodman S.B., and Maloney W.J.Effects of intermittent hydrostatic pressure and BMP-2 on osteoarthritic human chondrocyte metabolism in vitro. J Orthop Res 29,361, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Schmittgen T.D., and Livak K.J.Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3,1101, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Farndale R.W., Buttle D.J., and Barrett A.J.Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883,173, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Stegemann H., and Stalder K.Determination of hydroxyproline. Clin Chim Acta 18,267, 1967 [DOI] [PubMed] [Google Scholar]
- 29.Acharya C., Adesida A., Zajac P., Mumme M., Riesle J., Martin I., et al. . Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol 227,88, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Loeser R.F.Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage 17,971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., Sun H., Yan D., Zhang L., Lv X., Liu T., et al. . In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials 31,9406, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Yang H., Park J., Na K., Woo D., Kwon Y., and Park K.The use of green fluorescence gene (GFP)-modified rabbit mesenchymal stem cells (rMSCs) co-cultured with chondrocytes in hydrogel constructs to reveal the chondrogenesis of MSCs. Biomaterials 30,6374, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Taipale J., and Keski-Oja J.Growth factors in the extracellular matrix. FASEB J 11,51, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Cooke M., Allon A., Cheng T., Kuo A., Kim H., Vail T., et al. . Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis Cartilage 19,1210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J., Chung U.I., Yang D., Karsenty G., Bringhurst F.R., and Kronenberg H.M.PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol 292,116, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Kim Y.J., Kim H.J., and Im G.I.PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun 373,104, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kim D.H., Yoo K.H., Choi K.S., Choi J., Choi S.Y., Yang S.E., et al. . Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine 31,119, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Lee C.S., Burnsed O.A., Raghuram V., Kalisvaart J., Boyan B.D., and Schwartz Z.Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration. Stem Cell Res Ther 3,35 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenberg A.R., Ouyang L., and Elisseeff J.H.Mesenchymal stem cell stimulation of tissue growth depends on differentiation state. Stem Cells Dev 20,405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil A.S., Sable R.B., and Kothari R.M.An update on transforming growth factor-β (TGF-β): sources, types, functions and clinical applicability for cartilage/bone healing. J Cell Physiol 226,3094, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Nesic D., Whiteside R., Brittberg M., Wendt D., Martin I., and Mainil-Varlet P.Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev 58,300, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Bondeson J., Wainwright S., Hughes C., and Caterson B.The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin Exp Rheumatol 26,139, 2008 [PubMed] [Google Scholar]
- 43.Wang C.Y., Chen L.L., Kuo P.Y., Chang J.L., Wang Y.J., and Hung S.C.Apoptosis in chondrogenesis of human mesenchymal stem cells: effect of serum and medium supplements. Apoptosis 15,439, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Dexheimer V., Frank S., and Richter W.Proliferation as a requirement for in vitro chondrogenesis of human mesenchymal stem cells. Stem Cells Dev 21,2160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nixon A.J., Haupt J.L., Frisbie D.D., Morisset S.S., McIlwraith C.W., Robbins P.D., et al. . Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene therapy 12,177, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.