Abstract
Objectives
To compare the effects of different types of physical and mental activity on self-reported sleep quality over 12 weeks in older adults with cognitive and sleep complaints.
Design
Randomized controlled trial.
Setting
General community.
Participants
Seventy-two inactive community-dwelling older adults with self-reported sleep and cognitive problems (mean age 73.3±6.1; 60% women).
Intervention
Random allocation to four arms using a two-by-two factorial design: aerobic+cognitive training, aerobic+educational DVD, stretching+cognitive training, and stretching+educational DVD arms (60 min/d, 3 d/wk for physical and mental activity for 12 weeks).
Measurements
Change in sleep quality using seven questions from the Sleep Disorders Questionnaire on the 2005–06 National Health and Nutrition Examination Survey (range 0–28, with higher scores reflecting worse sleep quality). Analyses used intention-to-treat methods.
Results
Sleep quality scores did not differ at baseline, but there was a significant difference between the study arms in change in sleep quality over time (p<.005). Mean sleep quality scores improved significantly more in the stretching+educational DVD arm (5.1 points) than in the stretching+cognitive training (1.2 points), aerobic+educational DVD (1.1 points), or aerobic+cognitive training (0.25 points) arm (all p<.05, corrected for multiple comparisons). Differences between arms were strongest for waking at night (p=.02) and taking sleep medications (p=.004).
Conclusion
Self-reported sleep quality improved significantly more with low-intensity physical and mental activities than with moderate- or high-intensity activities in older adults with self-reported cognitive and sleep difficulties. Future longer-term studies with objective sleep measures are needed to corroborate these results.
Keywords: physical activity, cognition, sleep, aging, intervention
INTRODUCTION
Poor sleep is a significant concern of older adults and is reported in 50% of individuals aged 65 and older.1 Sleep disturbances, such as difficulty falling asleep and nighttime awakenings, have been linked to depression, cognitive decline, functional impairment, and lower quality of life2–4 and are exacerbated in older adults with cognitive impairment.5–8 Traditional sleep aids commonly involve medications associated with significant side effects and falls9,10 and thus are typically not recommended for long-term use.11,12 Therefore, there is an important need for identifying safe and effective alternatives for treating disruptive sleep problems.
Exercise is a widely accepted approach to improving cardiovascular health, physical function, and mood, and recent studies have shown that exercise may also be beneficial for sleep. In older adults with chronic insomnia, moderate-intensity aerobic exercise for 16 weeks improved several self-reported measures, including sleep latency (time to fall asleep), sleep duration, daytime dysfunction (trouble staying awake), and total sleep quality.13 Objective sleep measurements using polysomnographic sleep recordings show complementary biological findings, in which older adults with mild to moderate sleep complaints spent less time in Stage 1 sleep and more time in Stage 2 sleep and had fewer nighttime awakenings after a 12-month moderate-intensity exercise program.14 Lower-intensity exercise interventions, such as yoga and weight training, have also improved self-reported sleep quality in addition to quality of life and depression in older adults.15–17 Participants reported improvements in overall sleep quality, less daytime dysfunction, and less depression. Although exercise is an affordable and accessible treatment, it is important to identify the best types of exercise for improving sleep quality.
Cognitive-behavioral therapy (CBT) and lifestyle interventions are common nonpharmacological approaches to improving sleep quality.18 Methods such as sleep restriction, mindfulness relaxation, and stimulus control therapy are frequently used to treat sleep problems19,20 and reportedly improve sleep-related features, such as sleep latency, sleep duration, and waking time.21–23 One study of group-based CBT for older adults found that, in an older population (N=86, mean age 64±6.8), a relatively younger age was a significant predictor of improvement in sleep efficiency (total sleep time/time in bed), suggesting that CBT may decrease in effectiveness with age.12 Although single behavioral approaches have produced variable results, data suggest that a combination of multiple techniques, often known as multicomponent CBT, may be maximally beneficial for enhancing sleep efficiency.22–24 No published studies have assessed the combined effects of exercise and cognitive activity on sleep quality or which regimens may be most effective for improving sleep.
This study is a secondary analysis of the Mental Activity and eXercise (MAX) Trial,25 which used a two-by-two factorial design to study the effects of exercise (aerobic intervention vs stretching control) and mental activity (cognitive training intervention vs educational DVD control) on the primary outcome of cognitive function. The goal of the current study was to use data from the MAX Trial to compare the effects of different types of exercise and mental activity on the secondary outcome of self-reported sleep quality in older adults with cognitive and sleep complaints. Based on previous literature, it was hypothesized that individuals in the aerobic groups would have the greatest improvements in self-reported sleep quality.
METHODS
Participants
Details of the MAX Trial study methods have previously been published.25 Briefly, the MAX Trial was a 12-week, single-blind, randomized controlled trial of 126 community-residing adults aged 65 and older with low activity levels (defined as engaging in aerobic exercise or intensive computer training 2 days or less per week, for 30 minutes or less per session in the past 3 months) and cognitive complaints (defined as answering yes to the question “Do you feel that your memory or thinking skills have gotten worse recently?”). All participants provided written informed consent, and the Committee on Human Research at the University of California at San Francisco and the San Francisco Veterans Affairs Medical Center Research and Development Committee approved the research protocol. The MAX Trial is registered at ClinicalTrials.gov (NCT00522899).
The current study focuses on MAX Trial participants who also had self-reported sleep problems at baseline, which was defined as rating at least one of seven sleep questions (Table 1) as occurring often (5–15 times/month) or almost always (≥16 times/month) at baseline (n=72).
Table 1.
Sleep Quality Questions
| Please indicate how often you experienced each of the following during the past month: |
| 1. Have trouble falling asleep |
| 2. Wake up during the night and have difficulty getting back to sleep |
| 3. Wake up too early in the morning no matter how many hours of sleep you had |
| 4. Feel unrested during the day, no matter how many hours of sleep you had |
| 5. Feel excessively (overly) sleepy during the day |
| 6. Do not get enough sleep |
| 7. Take sleeping pills or other medication to help you sleep |
Responses were categorized as never (0 points), rarely (≤1 time/month, 1 point), sometimes (2–4 times/month, 2 points), often (5–15 times/month, 3 points) or almost always (≥16 times/month, 4 points). A total sleep quality score was created by adding the points for each question.
Randomization, Blinding, and Group Assignment
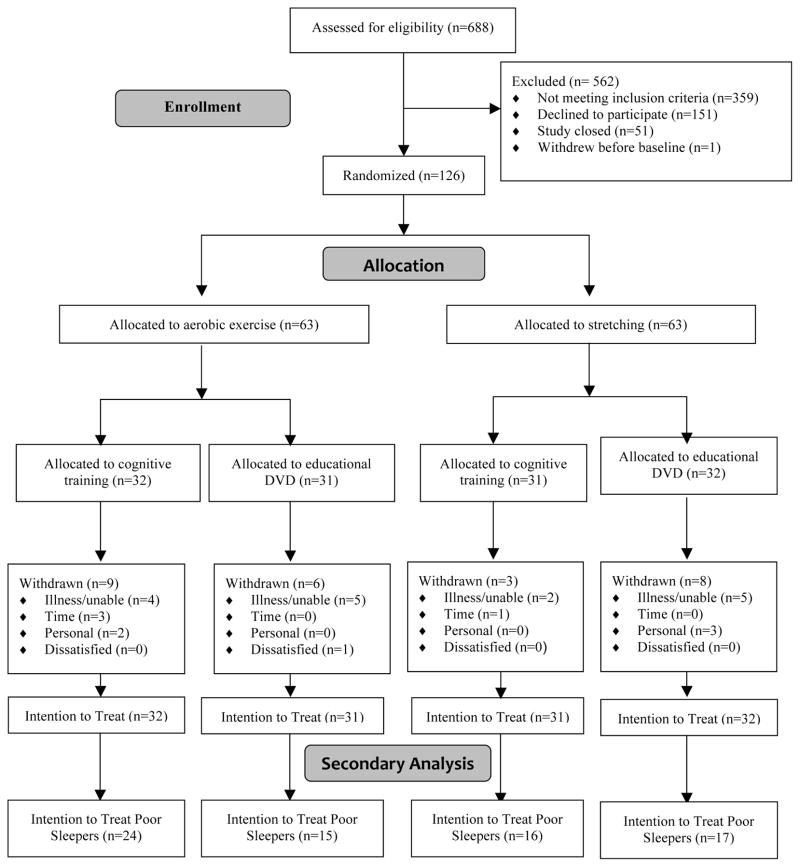
Participants were randomized in blocks of four. The randomization sequence was prepared in advance using a random number generator on a computer. Research staff members who were involved with enrollment and outcome assessment were unaware of the randomization sequence and blinded to group assignment. Study participants did not know the study hypotheses and were told that the goal of the study was to compare the effects of different physical and mental activity programs. Study participants were randomized to a class-based exercise program (aerobic activity or stretching) and a home-based cognitive engagement program (cognitive training or educational DVDs) in a two-by-two factorial design for 12 weeks (Figure 1), resulting in four arms consisting of aerobic+cognitive training, aerobic+educational DVD, stretching+cognitive training, and stretching+educational DVD. Therefore, all participants engaged in exercise and cognitive activities.
Figure 1.
Flow chart for the Mental Activity and eXercise (MAX) Trial.
Interventions
Exercise
All participants attended exercise classes designed for this study at a local YMCA for 60 minutes per day, 3 days per week for 12 weeks. The aerobic class consisted of 10 minutes of warm-up, 30 minutes of aerobic exercise (dance-based aerobics), 5 minutes of cool-down, 10 minutes of strength training, and 5 minutes of stretching and relaxation. The stretching class consisted of 10 minutes of warm-up, 30 minutes of stretching, 10 minutes of strength training (using stretch bands to train the major muscle groups), and 10 minutes of relaxation, during which participants lay on their backs and were instructed to relax different parts of their bodies. Heart rates were monitored by having participants check their wrist or neck pulse for 10 seconds at the beginning, middle, and end of class and record the values in an exercise journal, with a target peak heart rate of 60% to 75% of the maximum for the participant’s age in the aerobic arms. A certified exercise instructor with experience conducting classes for elderly adults taught all classes, which had a maximum of 12 class participants at any time. Adherence and adverse events were monitored using weekly journals and biweekly telephone check-ins, and motivational counseling was provided if adherence fell below 80%. Daily attendance was also recorded for all arms.
Cognitive Activity
All participants were provided with detailed written and in-person oral instructions regarding their assigned cognitive activities, which were performed independently at home on a computer on the days and times of their choosing for 60 minutes per day, 3 day per week for 12 weeks. Participants in the cognitive training arms played games designed to enhance the speed and accuracy of visual and auditory processing (Posit Science Corp., San Francisco, CA). Games focused on visual tasks, such as tracking the location of objects (first 6 weeks), and auditory tasks, such as matching sound pairs (second 6 weeks). Participants in the educational DVD arms watched DVDs of educational lectures on art, history, and science. After each session, participants answered approximately six paper-based, multiple-choice, or short-answer lecture-specific questions. The days and amount of time spent performing cognitive activities at home was recorded for all arms in activity journals.
Outcome
Sleep Quality
The secondary outcome measure of sleep quality was assessed using the Sleep Disorders questionnaire of the 2005–06 National Health and Nutrition Examination Survey (NHANES) (www.cdc.gov/nchs/nhanes) and has been reported in several large, population-based studies.26–30 The NHANES Sleep Disorders questionnaire is short and easy to administer and does not require feedback from a housemate. It consists of seven questions (Table 1) with a 0- to 4-point response scale (0=never, 1=rarely (≤1 times/month), 2=sometimes (2–4 times/month), 3=often (5–15 times/month), 4=almost always (≥16 times/month). A composite sleep score was created using the number of points (range 0–28) to measure total sleep quality.
Statistical Analysis
All analyses were completed using intention-to-treat (ITT) methods. Incomplete data from participants who withdrew early were addressed using the last observation carried forward method. Because the study collected two data points, this method effectively assumed that each participant who did not finish the intervention experienced no change in sleep quality over the 12-week period. Completer analyses were also performed, but because there were no differences in the results, only the ITT analyses are presented.
Baseline characteristics of participants in the four arms were compared using chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables. Change scores were calculated as postmeasurement–premeasurement—a negative change representing a decline in poor sleep quality (improved sleep). Linear regression was used to examine change in total sleep quality as a function of exercise (ignoring mental activity) and mental activity (ignoring exercise) and to assess for synergism (group-by-group interaction). ANOVA was used to compare mean change in sleep quality scores of participants in the four study arms, and unpaired t-tests were used for all pairwise comparisons, with adjustment for multiple comparisons using Holm-Bonferroni correction. Paired t-tests were used for within-arm comparisons. A plot of the standardized residuals was examined to verify that the data were normally distributed. Analyses were conducted using Stata 12 software (Stata Corp., College Station, TX).
RESULTS
The flow of subjects through the study is shown in Figure 1. Subjects were enrolled from January 2008 to September 2009, and data collection was completed in December 2009. Six hundred eighty-eight individuals asked for information about the study. Of these, 359 were ineligible, primarily because they were already at high physical activity levels; 151 refused, primarily out of concern about the time commitment; 51 contacted the researchers after enrollment had closed; and one withdrew immediately after consent, leaving 126 to be enrolled and randomized. Of these, 72 were classified as poor sleepers at baseline and were included in the current study. Seventeen (24%) subjects withdrew, with no significant differences between arms (p=.23). Six (8.3%) experienced an adverse event that was considered possibly or probably study related: three in the aerobic+cognitive training arm (1 pain, 1 fall, 1 hospitalization), two in the stretching+cognitive training arm (1 pain, 1 fall), and one in the aerobic+educational DVD arm (pain). All recovered without residual problems.
Adherence
Adherence to the exercise programs, measured according to class attendance, was 81% and did not differ between arms (p=.36). Adherence to the cognitive programs, measured according to total self-reported time spent on assigned cognitive activities, was 83% and did not differ between arms (p=.71).
Baseline characteristics
The intervention arms did not differ at baseline in terms of the demographic variables age, sex, and education (Table 2). Thirty percent of participants were from racial or ethnic minority groups. All participants performed within normative values on the modified Mini-Mental State Examination, a test of global cognition, and endorsed few depressive items on the Geriatric Depression Scale. Of all participants, 51% had hypertension, 10% had diabetes mellitus, 7% had had a heart attack, 6% had undergone bypass surgery, and 53% had a history of smoking.
Table 2.
Baseline Demographic Characteristics
| Characteristic | Aerobic Exercise + Cognitive Training, n=24 | Aerobic Exercise + Educational DVD, n=15 | Stretching + Cognitive Training, n=16 | Stretching + Educational DVD, n=17 | P-Value |
|---|---|---|---|---|---|
| Age, mean±SD | 75±6.1 | 71.2±6.2 | 71.9±5.2 | 74.3±6.3 | .18 |
| Female, % | 54 | 73 | 50 | 76 | .27 |
| Education, years, mean±SD | 16.9±1.9 | 15.2±3.3 | 17±2.1 | 16±2.1 | .61 |
| Non-Hispanic white, % | 71 | 60 | 81 | 65 | .60 |
| Modified Mini-Mental State Examination score, mean±SD | 93.8±5.9 | 94.9±5.6 | 94±4.5 | 94.2±4.8 | .94 |
| Geriatric Depression Scale score, mean±SD | 2.4±1.3 | 2.1±1.9 | 1.7±1.4 | 2.5±2.5 | .57 |
| Medical history, % | |||||
| Hypertension | 67 | 60 | 25 | 47 | .60 |
| Diabetes mellitus | 13 | 13 | 6 | 6 | .82 |
| Heart attack | 8 | 13 | 6 | 0 | .51 |
| Bypass surgery | 13 | 0 | 6 | 0 | .25 |
| Smoking history | 50 | 53 | 50 | 59 | .95 |
| Sleep quality, mean±SD (maximum 28) | 11.3±4 | 13.3±4.1 | 11.6±3.3 | 12.3±3.7 | .46 |
There were no significant group differences in baseline demographics. P-values based on analysis of variance for continuous variables and chi-square for categorical variables, with Fisher exact test used if any cell size was less than 5.
SD=standard deviation.
Sleep Quality
Main effects and group-by-group interaction
In linear regression analyses, there was a main effect of exercise on change in total sleep quality, such that individuals in the stretching arm (regardless of mental activity) improved more than those in the aerobic arm (p=.002). There was also a main effect of mental activity on change in total sleep quality, such that individuals in the educational DVD arm (regardless of exercise) improved more than those in the cognitive training arm (p=.002). A non-significant trend for a group-by-group interaction was found (p=.07), suggesting a synergistic effect in the double control arm (stretching+educational DVD).
Between-arm differences
The four study arms did not differ in total sleep quality at baseline (Table 3), although there was a significant baseline difference on one question regarding sleep medications. Seventy-one percent of the stretching+educational DVD participants endorsed taking sleep medications, versus 31% to 40% in the other three arms (all p<.05). There were no baseline differences for the other individual questions.
Table 3.
Baseline and Changes Scores of Sleep Quality
| Sleep Quality | Aerobic Exercise + Cognitive Training | Aerobic Exercise + Educational DVD | Stretching + Cognitive Training | Stretching + Educational DVD | Group by Time Interaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | P-Value | |
| 1. Falling asleep | 1.5 | 0 | 2.3 | −0.3 | 1.7 | −0.2 | 1.8 | −0.7a | .32 |
| 2. Waking at night | 1.8 | 0.2 | 2.1 | 0.1 | 2.3 | 0 | 2.1 | −0.7a | .02 |
| 3. Waking too early | 1.8 | 0 | 1.1 | 0.3 | 1.9 | −0.3 | 1.8 | −0.9a | .08 |
| 4. Feel unrested | 1.9 | 0 | 2.6 | 0.1 | 1.6 | 0.1 | 1.5 | −0.8 | .14 |
| 5. Daytime sleepiness | 1.5 | −0.1 | 1.7 | −0.7 | 2 | −0.7a | 1.3 | −0.5 | .35 |
| 6. Not enough sleep | 1.6 | −0.5 | 2.5 | −0.3 | 1.4 | −0.3 | 2.1 | −0.9a | .52 |
| 7. Take medications | 1.2 | 0.1 | 0.9 | −0.1 | 0.6 | 0.1 | 1.7 | −0.7a | .004 |
| TOTAL | 11.3 | −0.25 | 13.3 | −1.1 | 11.6 | −1.2 | 12.3 | −5.1a | .001 |
Values for each question were measured on an ordinal scale from 0 (never) to 4 (≥16 times/month). Total score is sum of individual scores. Higher scores indicate worse sleep; negative change scores reflect decline in poor sleep (i.e., improved sleep). P-values based on group by time interaction in linear regression models.
Significant change in pre/post measurements using paired t-tests (p<.05).
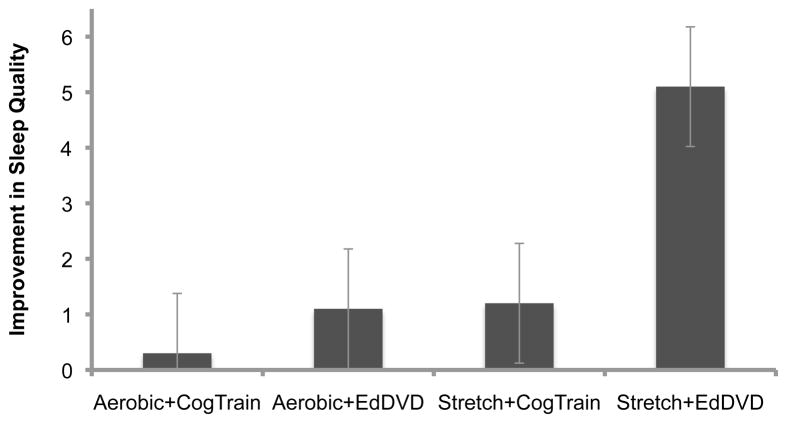
There was a significant difference in change in total sleep quality between the four study arms (F(3,68)=6.68, p=.0005). Mean improvement in self-reported sleep quality was 0.25 points in the aerobic+cognitive arm, 1.1 points in the aerobic+educational DVD arm, 1.2 points in the stretching+cognitive training arm, and 5.1 points in the stretching+educational DVD arm (Figure 2). The stretching+educational DVD arm had significantly greater improvement in overall sleep quality than the other three arms (all p<.05, corrected for multiple comparisons) (Table 3). To assess the effect of baseline sleep medications on change in total sleep quality, use of baseline sleep medications (defined as reporting any use in the past month for question 7 on the sleep quality survey) was included as a dichotomous covariate, and the results remained significant (F(3,68)=5.20, p<.001).
Figure 2. Change in total sleep quality by study arm.
There was a significant difference in change in total sleep quality between study arms (p=.0005), such that the stretching+educational DVD arm had significantly greater improvement in overall sleep quality than the other three arms (all p<.05, corrected for multiple comparisons using Holm-Bonferroni). Direction of sleep changes scores reversed so that positive change reflects improvement. Standard error bars are shown.
When evaluating the sleep quality questions individually, there was a significant between-arm difference in questions regarding waking at night (p=.02) and use of sleep medications (p=.004), with a non-significant trend in waking too early (p=.08). There was no evidence of interactions between arm and other factors, including age, sex, race or ethnicity, baseline cognitive status, and baseline functional status (data not shown).
Within-arm changes
There was a 41% within-arm improvement (5.1 points) in total sleep quality for the stretching+educational DVD arm (Figure 2) (p=.0001). Five individual questions regarding falling asleep, waking at night, waking too early, not enough sleep, and use of medications also significantly improved in this arm (all p<.05). The stretching+cognitive training arm had a significant within-group change on one question regarding daytime sleepiness. No other arms had significant within-group changes.
DISCUSSION
A combination of low-intensity exercise and cognitive activity was associated with better self-reported sleep quality in a sample of inactive community-dwelling older adults with subjective cognitive and sleep difficulties. Unexpectedly, subjective sleep quality improved in the stretching+educational DVD arm, which served as the double control group in the original MAX Trial. This was counter to the hypothesis that the aerobic arms would show the largest improvements in sleep quality. These results suggest that optimal types of exercise and cognitive activity may depend on a complex set of factors, such as the study population (level of sleep and cognitive impairment), physical activity level, and the desired outcome.
These results differ from those of other intervention trials that found a beneficial effect of aerobic exercise on sleep quality in community-residing older adults.13,14,31–33 It is possible that the frequency of the intervention (3 d/wk) was not high enough; some positive studies included greater-frequency exercise interventions (≥4 times/week).13,14,31–33 Furthermore, a longer intervention period of aerobic activity (≥12 weeks) might have produced sleep improvements, although one study observed significant improvement in self-reported sleep quality after only 14 days of light physical activity and structured social intervention.33 In another study, aerobic exercise and stretching both improved overall sleep quality and reduced use of sleep medications in sedentary, overweight postmenopausal women, although aerobic exercise was effective only if performed in the morning, suggesting there may be residual detrimental effects of aerobic exercise performed at night when sleep quality is the outcome of interest.34 Consistent with the current study’s null findings, one study showed that 12 months of exercise training increased physical fitness in older adults with poor sleep but did not improve sleep onset latency, total sleep time, sleep efficiency, or number of awakenings.38 Overall, aerobic benefits on sleep quality may depend on study populations, because participants in different studies varied in baseline physical activity, severity of sleep disturbances, and level of cognitive impairment.
The current study’s results are consistent with those of several studies of the effect of nonaerobic exercise interventions on sleep quality in healthy older adults. Two randomized controlled trials found that tai chi improved self-reported sleep quality in community-residing older adults.35,36 Similar to the current study, one of these studies compared tai chi with a traditional aerobic exercise program and found tai chi to be more beneficial for improving sleep.36 Other randomized controlled trials found that yoga improved sleep quality and perception of one’s own health40 and decreased time to sleep, increased sleep hours, and increased feeling rested in healthy elderly adults.41 Another randomized controlled trial of sedentary older adults in the community showed that qigong, a traditional Chinese exercise of eight movements, improved self-reported sleep quality over 12 weeks.42 These studies provide consistent evidence that nonaerobic exercises, a number of which are similar to the current study’s stretching regimen of slow, static movements with resistance, can improve sleep quality in older adults and that it may not be necessary to increase heart rate aerobically to experience improvement in sleep. The slower movements and full body breathing involved in stretching may have had a restorative effect.
The effect of cognitive activity on sleep quality has been given much less attention. The current study found a synergistic effect on sleep quality through a combination of stretching and educational DVDs. Previous studies of targeted cognitive-behavioral modification have shown beneficial sleep effects, but the activities differed significantly from the cognitive activities in the present study. In a recent study of individuals with chronic insomnia, problem-solving therapy involving stress and health management improved sleep quality as much as in the traditional CBT control group.37 In another recent 1-year randomized controlled trial of older adults with subjective sleep problems, an intervention of self-relaxation through progressive muscle relaxation and meditation improved self-reported sleep quality.38 It is possible that engagement in educational art, history, and science videos was cognitively stimulating yet relaxing. In contrast, another possible explanation for the findings is that older adults with subjective cognitive impairment may be more sensitive to cognitively challenging tasks. It is possible that high-intensity engagement in cognitive training tests may create anxiety, which is a negative indicator for sleep quality. Some participants in the cognitive training group expressed anxiety through personal communication with research investigators, but changes in anxiety were not assessed objectively and should be investigated further.
Strengths
A major strength of this study is that it was a randomized controlled trial with a factorial design to compare the effects of different types of exercise and cognitive activity on sleep quality. In addition, the study population was ethnically diverse and community based and included individuals with self-reported cognitive and sleep impairment. The stretching and educational DVD regimens are accessible and low cost, and most people can perform them.
Limitations
Sleep quality was not the primary outcome of the MAX Trial, so results should be interpreted as hypothesis generating. In addition, the NHANES Sleep Questionnaire is a subjective measure of sleep quality and has not been validated against objective sleep measures, such as polysomnography, or been previously used in a study population with memory complaints, although the questions have face validity because they mirror common sleep complaints of older adults. Additional studies are needed to corroborate these findings with objective or validated sleep quality measures. In addition, the study consisted of a short intervention period of 12 weeks. Although there was significant improvement in sleep quality over 12 weeks, it is not knows what effect the intervention would have had over a longer period. Also, it is possible that an aerobic exercise stimulus may have influenced known physiological mediators of sleep quality after a longer intervention period.43 The exercise classes were group based and led by a trained exercise instructor; unsupervised home-based activities may not produce the same outcomes. Also, although the stretching+educational DVD arm reported the highest baseline use of sleep medications, raising concern that regression to the mean could have affected the total sleep score, the fact that several other sleep domains changed in that arm provides reassurance. In addition, results were similar when adjusting for baseline sleep medication as a covariate, suggesting that differences in the use of baseline sleep medications do not fully explain the results.
Lastly, the study population consisted of inactive older adults, limiting generalization to other populations, although from a population perspective, the inactive older adult population is a prime target group for evidence-based physical and mental activity interventions that can promote a range of health-enhancing outcomes. Older adults who already engage in regular physical activity or who have more-severe sleep problems may not experience the same positive effects.
Implications
These results suggest that low-intensity stretching exercises and light cognitive activity may provide an effective, low-cost method for improving sleep quality in older adults with self-reported cognitive complaints and difficulty sleeping. Given that poor sleep and memory complaints are risk factors for cognitive decline and dementia, it is possible that improving sleep quality through a combination of simple programs could mitigate future cognitive decline. Additional studies are needed to determine long-term or maintenance effects of exercise and cognitive activity on sleep and cognition.
Conclusion
In this population of older adults with self-reported cognitive and sleep difficulties, self-reported sleep quality improved significantly more with low-intensity physical and mental activities than with moderate- or high-intensity activities. These results suggest that the optimal types of exercise and cognitive activity in older adults may depend on the desired outcome. In inactive older adults, a combination of low-intensity restorative activities, such as stretching and light cognitive activity, may be appropriate for improving at least short-term self-reported sleep outcomes. Future studies with objective sleep measures are needed to corroborate these findings.
Acknowledgments
The authors would like to thank the study participants and all of the research assistants, summer interns, and administrative staff who assisted with data collection, entry, and management; Ray Hanvey, BA, for serving as the instructor for the exercise classes held at the YMCA; Becky Hanvey, BA, and YMCA staff who provided logistical support with the physical exercise component of the study; and Posit Science staff who provided assistance with computer logistics.
Sponsors’ Role: The sponsors of this research had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Footnotes
Conflict of Interest: No author reported financial, personal, or potential conflict of interest relating to the information published in this article.
Financial Disclosure: This work was supported by the Alzheimer’s Association (IIRG-06–27306 & NIRP-12-259277) and the National Institutes of Health (NIH; K01-AG024069, K01-AG034175 & R01-AG026720). This work also was supported by NIH/National Center for Research Resources/University of California, San Francisco—Clinical and Translational Science Institute (KL2 RR024130). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Exercise space and equipment donated by the YMCA of San Francisco, California. Computer equipment donated by Posit Science Corp., San Francisco, California.
Author Contributions: Pa: data analysis and interpretation, manuscript drafting and revision. Goodson: conceptualization of research question, data analysis and interpretation, manuscript drafting and revision. Bloch: conceptualization of research question, data interpretation, manuscript revision. King, Yaffe: study design, data interpretation, manuscript revision. Barnes: design and implementation of MAX Trial; supervision of conceptualization of research question; manuscript drafting and revision; statistical analysis support; supervision of data collection, entry, and management.
References
- 1.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Nebes RD, Buysse DJ, Halligan EM, et al. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64B:180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochen V, Arbus C, Soto ME, et al. Sleep disorders and their impacts on healthy, dependent, and frail older adults. J Nutr Health Aging. 2009;13:322–9. doi: 10.1007/s12603-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 5.Tworoger SS, Lee S, Schernhammer ES, et al. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 6.Geda YE, Smith GE, Knopman DS, et al. De novo genesis of neuropsychiatric symptoms in mild cognitive impairment (MCI) Int Psychogeriatr. 2004;16:51–60. doi: 10.1017/s1041610204000067. [DOI] [PubMed] [Google Scholar]
- 7.Naismith SL, Rogers NL, Hickie IB, et al. Sleep well, think well: Sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23:123–130. doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- 8.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 9.Wilson NM, Hilmer SN, March LM, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc. 2011;59:875–880. doi: 10.1111/j.1532-5415.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 10.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 11.Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health. NIH State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults statement. J Clin Sleep Med. 2005;1:412–421. [PubMed] [Google Scholar]
- 13.Reid KJ, Baron KG, Lu B, et al. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11:934–940. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AC, Pruitt LA, Woo S, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63A:997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariprasad VR, Sivakumar PT, Koparde V, et al. Effects of yoga intervention on sleep and quality-of-life in elderly: A randomized controlled trial. Indian J Psychiatry. 2013;55:364–368. doi: 10.4103/0019-5545.116310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen KM, Chen MH, Lin MH, et al. Effects of yoga on sleep quality and depression in elders in assisted living facilities. J Nurs Res. 2010;18:53–61. doi: 10.1097/JNR.0b013e3181ce5189. [DOI] [PubMed] [Google Scholar]
- 17.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20:95–101. doi: 10.1093/sleep/20.2.95. [DOI] [PubMed] [Google Scholar]
- 18.Chesson AL, Jr, Anderson WM, Littner M, et al. Practice parameters for the nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 1999;22:1128–1133. doi: 10.1093/sleep/22.8.1128. [DOI] [PubMed] [Google Scholar]
- 19.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine report Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 20.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Hauri PJ, Espie CA, et al. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review Sleep. 1999;22:1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 22.McCurry SM, Logsdon RG, Teri L, et al. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22:18–27. doi: 10.1037/0882-7974.22.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell MD, Gehrman P, Perlis M, et al. Comparative effectiveness of cognitive behavioral therapy for insomnia: A systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes DE, Santos-Modesitt W, Poelke G, et al. The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173:797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandner MA, Petrov ME, Rattanaumpawan P, et al. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9:897–905. doi: 10.5664/jcsm.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandner MA, Jackson N, Gerstner JR, et al. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Gelaye B, Williams MA. Sleep characteristics and health-related quality of life among a national sample of American young adults: Assessment of possible health disparities. Qual Life Res. 2013;23:613–625. doi: 10.1007/s11136-013-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engeda J, Mezuk B, Ratliff S, et al. Association between duration and quality of sleep and the risk of pre-diabetes: Evidence from NHANES. Diabet Med. 2013;30:676–680. doi: 10.1111/dme.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ram S, Seirawan H, Kumar SK, et al. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14:63–70. doi: 10.1007/s11325-009-0281-3. [DOI] [PubMed] [Google Scholar]
- 31.King AC, Oman RF, Brassington GS, et al. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277:32–37. [PubMed] [Google Scholar]
- 32.Buman MP, Hekler EB, Bliwise DL, et al. Exercise effects on night-to-night fluctuations in self-rated sleep among older adults with sleep complaints. J Sleep Res. 2011;20:28–37. doi: 10.1111/j.1365-2869.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naylor E, Penev PD, Orbeta L, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- 34.Tworoger SS, Yasui Y, Vitiello MV, et al. Effects of a yearlong moderate-intensity exercise and a stretching intervention on sleep quality in postmenopausal women. Sleep. 2003;26:830–836. doi: 10.1093/sleep/26.7.830. [DOI] [PubMed] [Google Scholar]
- 35.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep. 2008;31:1001–1008. [PMC free article] [PubMed] [Google Scholar]
- 36.Li F, Fisher KJ, Harmer P, et al. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: A randomized controlled trial. J Am Geriatr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 37.Pech M, O’Kearney R. A randomized controlled trial of problem-solving therapy compared to cognitive therapy for the treatment of insomnia in adults. Sleep. 2013;36:739–749. doi: 10.5665/sleep.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J, Kang J, Wang P, et al. Self-relaxation training can improve sleep quality and cognitive functions in the older: A one-year randomised controlled trial. J Clin Nurs. 2013;22:1270–1280. doi: 10.1111/jocn.12096. [DOI] [PubMed] [Google Scholar]