Abstract
Neurons in the suprachiasmatic nucleus (SCN) function as part of a central timing circuit that drives daily changes in our behaviour and underlying physiology. A hallmark feature of SCN neuronal populations is that they are mostly electrically silent during the night, start to fire action potentials near dawn and then continue to generate action potentials with a slow and steady pace all day long. Sets of currents are responsible for this daily rhythm, with the strongest evidence for persistent Na+ currents, L-type Ca2+ currents, hyperpolarization-activated currents (IH), large-conductance Ca2+ activated K+ (BK) currents and fast delayed rectifier (FDR) K+ currents. These rhythms in electrical activity are crucial for the function of the circadian timing system, including the expression of clock genes, and decline with ageing and disease. This article reviews our current understanding of the ionic and molecular mechanisms that drive the rhythmic firing patterns in the SCN.
Our brains encode information through changes in the patterns and frequency of action potentials. These neural activity patterns change dramatically with the circadian cycle, so in a fundamental sense our brains behave differently as a function of the time of day. In addition, many cells within our body generate robust, synchronized rhythms in the transcription, translation and degradation of key ‘clock genes’ and their protein products through an autoregulatory loop. These rhythms have an endogenous periodicity of approximately 24 hours1,2. Moreover, our bodies are made up of a network of oscillators, each of the major organ systems (heart, liver and pancreas) with its own clockwork to regulate the transcription of genes that are important to the specific target organ3. These circadian rhythms are synchronized by central pacemaker neurons located in a small subset of cells in the CNS — known in mammals as the suprachiasmatic nucleus (SCN). In all of the animal species that have been examined so far, these pacemaker neurons exhibit circadian rhythms in spontaneous neural activity. One of their striking features is that their spontaneous activity is highest during the day, regardless of whether the species is diurnal or nocturnal.
We have a good conceptual understanding of the cell-autonomous molecular clockwork that regulates the generation of circadian rhythms in gene expression, but there is a lack of a mechanistic understanding of how this molecular feedback loop interacts with the membrane to produce physiological circadian rhythms (BOX 1). Clearly, the signals travelling to and from this molecular feedback loop must travel through the membrane, but not much is known about how the molecular feedback loop drives the rhythm in electrical membrane processes.
Box 1. Questions of coupling.
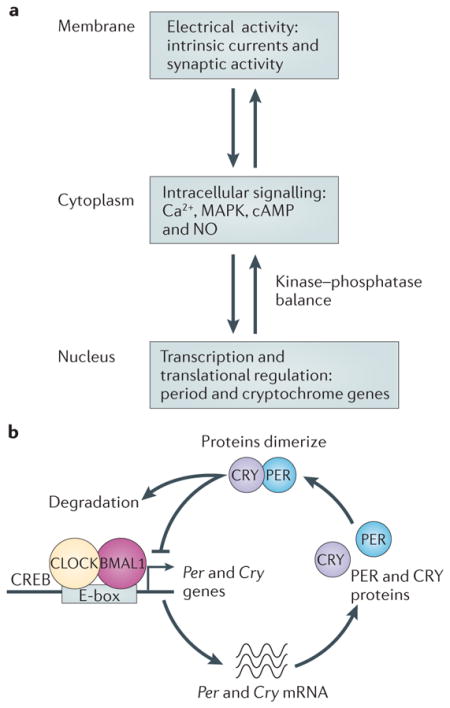
One of the major problems in the field of circadian rhythms is to understand the inter-relationships between membrane events, intracellular signalling cascades and transcriptional and translational regulation. Suprachiasmatic nucleus (SCN) neurons generate rhythms of neural activity that peak in the day. Neural activity regulates Ca2+ as well as other signalling pathways through voltage-sensitive currents and the release of neurotransmitters (see the figure, part a). In the SCN, and perhaps other neurons, many of these signalling networks — including Ca2+ and cyclic AMP, nitric oxide (NO), casein kinases and RAS-dependent mitogen-activated protein kinases (MAPKs) — are strongly rhythmic in levels and activity. The balance between the activity of kinases and phosphatases at the end of these pathways regulates the transcription and translation of genes. Within many cells in the body, a transcriptional–translational negative feedback loop drives rhythms in gene expression (see the figure, part b). At the beginning of the cycle, CLOCK–BMAL1 protein complexes bind DNA at specific promoter regions (E-box) to activate the transcription of a family of genes including the period (Per1, Per2 and Per3) genes and cryptochrome (Cry1 and Cry2) genes. The levels of the transcripts for Per and Cry genes reach their peak during the period from midday to late in the day, whereas the PER and CRY proteins peak in the early night. The PERs, CRYs and other proteins form complexes that translocate back into the nucleus and turn off the transcriptional activity driven by CLOCK–BMAL1 with a delay (owing to the time required for transcription, translation, dimerization and nuclear entry). The proteins are degraded by ubiquitylation, allowing the cycle to begin again. Thus, in its simplest form, many cells contain this molecular feedback loop that regulates the rhythmic transcription of a number of genes. Other feedback loops within the cells contribute to the precision and robustness of the core oscillation. In the nervous system, many of the genes involved in control of excitability and secretion are rhythmically regulated by this molecular feedback loop. To produce a functional cellular oscillator within SCN neurons, there must be reciprocal signalling between membrane, cytosolic and nuclear processes. CREB, cyclic AMP-responsive element (CRE)-binding protein.
In this article, I will review our present understanding of the ionic mechanisms that underlie circadian rhythms in electrical activity in the SCN, how electrical activity may regulate clock gene expression and how clock gene expression may alter electrical activity in SCN neurons. I propose the idea that a decline in neural activity in the SCN may be a crucial mechanism by which ageing and disease may weaken the circadian output and contribute to a set of symptoms that impacts human health. Part of the goal of this Review is to identify gaps in our knowledge in the hope that this will stimulate further work in this area.
The SCN circuit
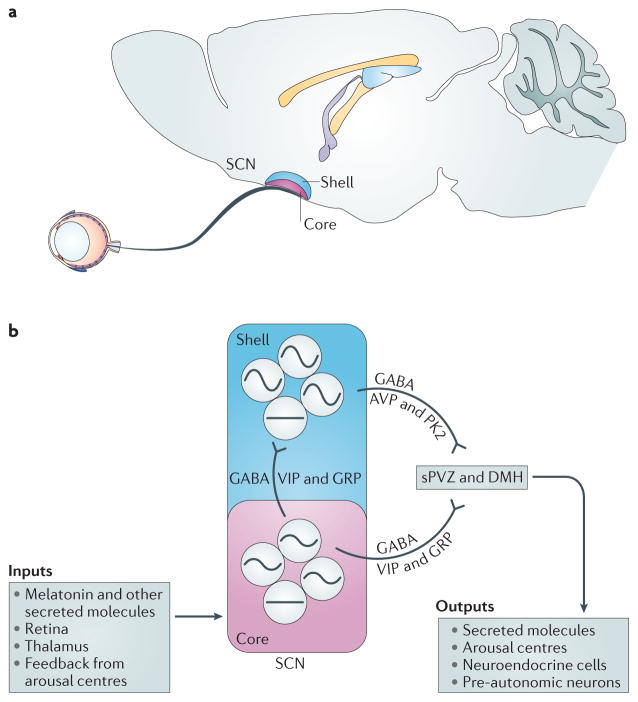
In mammals, the SCN of the hypothalamus contains the ‘master’ oscillatory network that is necessary for coordinating circadian rhythms throughout the body4–6 (FIG. 1). The SCN is a bilaterally paired nucleus made up of tightly compacted, small-diameter neurons that are located just lateral to the third ventricle, atop the optic chiasm7. Anatomical studies generally support the division of the SCN into at least two subdivisions: a ventral (core) region and a dorsal (shell) region8–10. The core neurons are thought to act as an integrator of external input, receiving information through three major pathways: the retinohypothalamic tract (RHT), the geniculohypothalamic tract from the intergeniculate leaflet of the thalamus and projections from the raphe nuclei11. Core neurons communicate this environmental information to the rest of the SCN. These sensory processing ventral cells exhibit relatively low amplitude rhythms in clock gene expression. Low amplitude rhythms may be easier to reset to environmental perturbations — a concept that has been supported by mathematical modelling studies12,13. Many of the neurons that receive retinal input within the core SCN express the neuropeptides vasoactive intestinal peptide (VIP) or gastrin-releasing peptide (GRP), as well as the neurotransmitter GABA. By contrast, neurons of the dorsal shell seem to generate robust circadian oscillations, at least at the level of gene expression14–16. The neurons in the shell express arginine vasopressin (AVP) or prokineticin 2 (PK2), as well as GABA.
Figure 1. The suprachiasmatic nucleus circuit.
a | The suprachiasmatic nucleus (SCN) in the hypothalamus is often divided into two anatomical and functional subdivisions: a ventrolateral ‘core’ and a dorsomedial ‘shell’. b | SCN core neurons are thought to integrate external input, including light information from the retinohypothalamic tract (RHT) and information from the thalamus and from midbrain structures such as the raphe nucleus. Core neurons relay this information to the rest of the SCN using GABA and vasoactive intestinal peptide (VIP) or gastrin-releasing peptide (GRP). Shell neurons use GABA and arginine vasopressin (AVP) or prokineticin 2 (PK2) to communicate with other cell populations, and at least some of the SCN shell neurons are neurosecretory cells that rhythmically release signalling molecules, including AVP, into the third ventricle. The amplitudes of the rhythms in gene expression and neural activity in core and shell neurons are relatively low and high, respectively. The outputs of core and shell SCN neurons travel mainly to other hypothalamic regions, including the subparaventricular zone (sPVZ) and the dorsal medial hypothalamus (DMH). These hypothalamic relay nuclei send projections throughout the CNS and endocrine system. Major centres in the brain that control arousal, such as the raphe nucleus, locus coeruleus, hypocretin or orexin neurons, and pars tuberalis, are rhythmically regulated through projections from the SCN.
The fact that many core projections terminate on shell neurons supports the idea that interplay between these two centres is responsible for the output of circadian information from the SCN17. The outputs of the SCN from both core and shell subpopulations travel mainly to other hypothalamic regions, including the subparaventricular zone18. These hypothalamic relay nuclei send projections throughout the nervous and endocrine systems, providing multiple pathways by which the SCN can convey temporal information to the brain and body3,19,20.
SCN neurons as intrinsic pacemakers
A unique property of central clock neurons and one that is essential to the function of the circadian timing system is the ability to generate circadian rhythms in electrical activity21–23. The SCN has been shown to generate neural activity rhythms in vivo24–26, in brain slice preparations27–30 and in SCN tissue cultures31–33. These findings are consistent with the idea that many SCN neurons are stable, self-sustained oscillators that have the intrinsic capacity to generate circadian rhythms in electrical activity. Regardless of whether an animal is diurnal or nocturnal in terms of behaviour, cells in the SCN are electrically active in the day and show circadian rhythms in firing action potentials, with peaks of around 6–10 Hz in the middle of the day30,34,35. Even when isolated from the circuit, single SCN neurons can exhibit a rhythm in firing rate, with most estimates placing the number of neurons that exhibit such a rhythm at about 60–70% of the total SCN neuron population36,37. Individual neurons do not seem to spend a full 12 hours firing action potentials; however, it is estimated that single neurons may be active for 4–6 hours38–40. In this electrically active state, the neurons do not respond strongly to excitatory stimulation but do respond to synaptic input that reduces their firing. During the night, the SCN neuron populations are electrically inactive and are most responsive to excitatory or depolarizing stimulation. Clarifying the ionic mechanisms responsible for the generation of rhythms in electrical activity in SCN neurons is an important step towards understanding the generation and output of circadian oscillations in a wide range of species, including humans.
The ionic players
SCN neurons generate action potentials in the absence of synaptic drive, and can therefore be considered endogenously active neurons. To maintain spontaneous activity, a set of intrinsic currents must interact to depolarize the cell membrane to threshold, elicit an action potential and return the membrane to negative potentials from which the next spike can be initiated. This ‘spontaneous’ firing arises from specific combinations of intrinsic membrane currents41,42. Some progress has been made in identifying the ion channels that drive spontaneous activity in the SCN. Conceptually, it can be useful to divide the ionic mechanisms: first, into currents that are responsible for providing the excitatory drive required for all spontaneously active neurons; second, into currents that translate this excitatory drive into a regular pattern of action potentials; and third, into currents that drive the membrane hyperpolarization that underlies the nightly silencing of firing (TABLE 1).
Table 1.
Ion channels and functions in the suprachiasmatic nucleus
| Function | Current | Is current rhythmic? | Channels and their genes | Rhythmic expression? | Refs |
|---|---|---|---|---|---|
| Excitatory drive | Persistent Na+ | No | Nav1.8 (SCN8A) and Nav1.9 (SCN9A) | No | 45–49 |
| L-type Ca2+ | Yes | Cav1.3 (CACNA1C and CACNA1S) | Yes | 45,47,48, 57,59 | |
| T-type Ca2+ | ? | Cav3 (CACNA1G) | Yes | 47,48,51,57,60,61 | |
| Modulation of firing and action potential width | Fast delayed rectifier (FDR) | Yes | Kv3.1 (KCNC) and Kv3.2 (KCNC2) | ? | 48,68,69 |
| A-type K+ | Yes | Kv4.1 (KCND1) and Kv4.2 (KCND2) | No | 60,70–72 | |
| Nightly silencing | Large-conductance Ca2+ activated K+ (BK) | Yes | BK (KCNMA1) | Yes | 47,48,73–75,81 |
| Two-pore K+ (K2P) | ? | TASK1 (KCNK1) and TASK2 (KCNK2) | Yes | 47,48 | |
| Others | Hyperpolarization-activated, cyclic nucleotide gated | No | HCN1 (HCN1) and HCN2 (HCN2) | No | 47,52–54, 56 |
| Na+–K+ ATPase | Yes | ? | ? | 63,64 | |
| Chloride pump | Yes | NKCC1 (SLC12A2) | Yes | 47,82,8 |
Excitatory drive to SCN neurons
During the day, SCN neurons are much more depolarized than neurons that do not show spontaneous activity. They have a resting membrane potential between −50 mV and −55 mV, which places them close to the threshold for generating an action potential (−45 mV). A subset of SCN neurons may even move to such a depolarized state (−30 mV) during the day that they cannot generate action potentials43. This relatively depolarized resting potential is the result of excitatory drive provided by multiple cation currents44–46. In dissociated SCN neurons, in which this has been investigated in most detail, the spontaneous interspike depolarization was found to be primarily attributable to Na+ currents that flow at between −60 mV and −40 mV45. SCN tissue expresses mRNA for the tetrodoxin (TTX)-sensitive voltage-gated sodium channels Nav1.1 (encoded by SCN1A) and Nav1B (encoded by SCN1B), as well as Nav1.5 (encoded by SCN5A) and Nav1.6 (encoded by SCN8A) (TABLE 1). Nav1.5 and Nav1.6 channels are likely to be responsible for the persistent Na+ current described in SCN neurons, as both are expressed in the SCN47,48. There is currently no evidence for a possible circadian regulation of persistent Na+ currents. The persistent Na+ currents in the SCN can be blocked by the neuroprotective agent riluzole46. Chronic application of riluzole prevents the expression of daily rhythms in neural activity49, indicating a role for persistent Na+ currents in the excitatory drive. However, one caveat regarding these experiments is that riluzole can also activate the two-pore K+ (K2P) channels that are expressed in the SCN (see below).
Another source of excitatory drive that is important in many pacemaking neurons is the opening of hyperpolarization- activated, cyclic nucleotide-gated (HCN) ion channels50. When these channels open, Na+ enters the neuron and K+ goes out, with the net result being a slow excitation of the membrane. Almost all SCN neurons carry a very prominent hyperpolarization-activated conductance (IH), which generates a depolarizing voltage sag in response to hyperpolarization51–54. Probably as a result of this current, SCN neurons exhibit a robust rebound excitation in response to membrane hyperpolarization. There is some evidence for a modest circadian rhythm in the magnitude of this current — it peaks during the day54 — although this rhythm was not detected in earlier studies53. Acutely blocking IH with ZD7288 did not greatly impact the firing rate of SCN neurons44,53, but sustained pharmacological block of HCN channels greatly reduced the frequency of action potentials during the day54 and curtailed circadian gene expression55. Microarray analysis indicates that the mRNAs for both the HCN2 and HCN3 subunits are present in the SCN and suggests that their expression may be rhythmic47. An immunohistochemical study found that HCN3 and HCN4 are the predominant subunits in the SCN56.
Ca2+ channels can also contribute to spontaneous activity in neurons. The genes coding for T-type (Cav3.1, Cav3.2 and Cav3.3) and L-type (Cav1.2 and Cav1.3) Ca2+ channel subunits are strongly expressed in the SCN48,57, with some evidence for rhythmic expression of transcripts47,57. Both the low voltage-activated T-type Ca2+ channels and Cav1.3 L-type Ca2+ channels exhibit the low threshold, steep voltage-dependence and low inactivation kinetics that are important for oscillations in the membrane potential42,58. Subthreshold membrane oscillations in SCN neurons have been characterized in both brain slices59 and cell cultures45. These oscillations are sensitive to the Ca2+ channel blocker nimodipine and seem to be mediated mostly by L-type Ca2+ currents. The magnitude of the L-type Ca2+ current amplitude exhibits a diurnal rhythm in the SCN, with subthreshold membrane oscillations found in the day but not the night59; however, blocking these currents had a relatively minor effect on spontaneous neuronal activity. The T-type current is also prominent in SCN neurons60,61, but present data indicate that this Ca2+ current probably mediates the response to glutamate61 rather than driving spontaneous firing during the day.
To summarize, a set of currents (persistent Na+, HCN, T- and L-type Ca2+ currents) provide the excitatory drive that is necessary for any neuron to be spontaneously active. Action potentials in the SCN are associated with a Ca2+ influx, which is presumably due to opening of T- and L-type Ca2+ channels45,62. These Ca2+ channels are also likely to play a crucial part in the regulation of Ca2+ levels in the SCN and may explain the higher levels of Ca2+ in the day compared to in the night (see below). However, broadly speaking, the excitatory drive in SCN neurons seems to be relatively constant throughout the daily cycle.
The sodium–potassium pump (Na+–K+ ATPase) is crucial for maintaining the resting membrane potential in response to this excitatory drive. The pump transports three Na+ ions out and two K+ ions into the cells using ATP hydrolysis. The pump is more active in the day than in the night63,64 and its activity may be crucial to the daily rhythm in membrane potential. It is electrogenic, adding a virtual −1 charge for every cycle of three Na+ out and two K+ in. So, if the pumps are shut off, the neural membrane could become depolarized, depending on the ratio of the two leak currents and on the typical electrogenic pump output. The use of this pump is very energy expensive and is probably responsible for the robust daily rhythm in 2-deoxyglucose use that characterizes the SCN21,65.
Translating excitatory drive into action potentials
In response to the excitatory drive, SCN neurons exhibit sustained discharge for 4–6 hours in the day without spike adaptation. Recent work suggests that the fast delayed rectifier (FDR) K+ current may allow for this type of discharge at least in other types of neurons66,67. Within the SCN, the FDR current is of particular interest because the magnitude of this current exhibits a circadian rhythm and sustained pharmacological blockade of the FDR current reduces the magnitude of the daily rhythm in firing in a brain slice preparation68. Two members of this family are expressed in the SCN: Kv3.1 (KCNC1) and Kv3.2 (KCNC2). Immunohistochemical analysis suggests a day–night difference in protein expression68, whereas in situ hybridization showed a low amplitude day to night difference in mRNA expression69. SCN neurons from mice lacking both Kcnc1 and Kcnc2 genes exhibit reduced spontaneous activity during the day and reduced NMDA-evoked excitatory responses during the night69. In addition, the width of the action potential in SCN neurons is almost double in the double knockout mice. Thus, the FDR K+ current is necessary for the circadian modulation of electrical activity in SCN neurons and represents an important part of the ionic basis for the generation of rhythmic output.
In other neurons, the subthreshold-operating A-type K+ current (IA) is mainly involved in the regulation of neuronal excitability and the timing of action potential firing. Within the SCN, this current has been described and proposed as a likely candidate to regulate the spontaneous firing rate60,70–72. The magnitude of the IA current exhibits a diurnal rhythm that peaks during the day in the dorsal region of the mouse SCN72. This rhythm is driven by a subset of SCN neurons with a larger peak current and a longer decay constant72. Importantly, this rhythm in neurons in the dorsal SCN continues in animals placed in constant darkness, providing an important demonstration of the circadian regulation of an intrinsic voltage-gated current in mammalian cells. Both in situ hybridization and Western blots have detected expression of the Shal-related family members 1 and 2 (Kv4.1 (Kcnd1) and Kv4.2 (Kcnd2)) within the SCN72 but did not see evidence for rhythmic expression of these channels.
Lastly, Ca2+-activated K+ channels (BK channels) are a major contributor to repolarization of the membrane after an action potential in the SCN. A study in which BK currents were inhibited with iberiotoxin suggests that this current may contribute 40% of the afterhyperpolarization that occurs after an action potential in the SCN73. The impact of this current on daytime firing of SCN neurons is still unclear. Iberiotoxin did not alter spike frequency during daytime73 and mice lacking the gene that encodes the pore-forming subunit of the BK channel (Kcnma1) did not show a change in firing during the day74. However, another study that focused on a subset of period 1 (Per1)-expressing SCN neurons found that blocking BK conductance with iberiotoxin can reduce daytime peak firing rate75. The Kcnma1 gene and the BK channel are rhythmically expressed in the SCN47,74,75, and the BK current is involved in the nightly hyperpolarization of the membrane (see below).
Therefore, FDR, IA and BK K+ currents all seem to have a role in the regulation of spontaneous action potential firing in SCN neurons during the day. The biophysical properties of these currents, measured in the SCN and other brain regions, suggest that these three currents are also critically involved in determining how SCN neurons respond to synaptic stimulation from other regions, particularly to photic information via the RHT.
Nightly silencing of neuronal firing
SCN neurons show a diurnal rhythm in membrane potential, such that cells are depolarized by about 6–10 mV during the day compared to the night43,76,77. This day–night difference in resting membrane potential is mediated to a large extent by a hyperpolarizing K+-dependent conductance that is active at night at resting membrane potential and inactive during the day34,77. Resting membrane potentials of neurons are mainly set by a class of two-pore domain K+ channels (K2P, TASK and TREK channels)78–80. These K+ channels are active over the whole voltage range (unlike other K+ channels) and are referred to as providing ‘leak’ or background currents80. K2P channels are encoded by the KCNK gene family. The transcripts for Kcnk1 and Kcnk2 are expressed in the SCN48, with transcripts for Kcnk1 exhibiting a robust rhythm in the SCN47. Unfortunately, specific pharmacological blockers of these channels are not available, but the rhythmic pattern of expression of K2P channels in the SCN means that they are promising candidates for driving the nightly hyperpolarization in membrane potential.
BK currents are also involved in the nightly silencing of SCN neurons. The expression of Kcnma1, the gene encoding the pore-forming subunit of the BK channel, peaks in the middle of the night75, and the relative contribution of the BK current to the outward currents is larger in the night than in the day. In addition, deletion of Kcnma1 increases night time firing in SCN neurons, although it does not completely abolish the day–night difference in firing rate74,81.
Contribution of chloride pumps
The SCN expresses at least two members of a family of cation-chloride (Cl−) cotransporters that regulate the chloride equilibrium potential (ECl−)82,83. The Na+–K+–2Cl− cotransporter NKCC1 raises intracellular Cl− levels and thus contributes to a more depolarized ECl−. The effects of NKCC1 are balanced by the other cotransporter, KCC isoform 2 (KCC2), which normally lowers intracellular Cl− levels. The balance between KCC2 and NKCC1 seems to be a critical regulator of the impact of GABA on SCN neurons83. The upregulation of NKCC1 leads to a neuron showing a depolarizing membrane response to GABA. Data indicate that the continued expression of NKCC1 in the SCN is responsible for GABA-evoked excitation83 and although this regulation varies from day to night, it is probably not important in setting the membrane potential of SCN neurons. There is some evidence that the transcript levels of the gene encoding NKCC1 (Slc12a2), but not the gene encoding KCC2 (Slc12a5), may be regulated by the circadian system47.
How is the network involved?
Although SCN neurons are circadian pacemakers that can generate rhythms in neural activity in isolation from other neurons, in vivo they are part of a circuit5,6,84 (FIG. 2). For example, SCN neurons receive a stream of GABA-mediated synaptic input that peaks at the day–night transition85. These neurons communicate through the release of peptides, and synaptic communication between cells in the SCN circuit seems to be crucial for rhythms in neural activity. For example, mice with a deletion of secretory vesicle proteins show substantial disruptions in the neural activity rhythms in the SCN86. In addition, there is compelling evidence that the neuropeptide VIP and the vasoactive intestinal polypeptide receptor 2 (VIPR2, also known as VPAC2R) may have roles in the generation of rhythms in neural activity. Extracellular recordings from cultured SCN neurons indicate that in mice lacking VIP or VIPR2, fewer neurons express rhythms in neural activity36. There is evidence that SCN neurons from Vipr2−/− mice may be chronically hyperpolarized87. The ionic mechanisms underlying this phenomenon are not yet known, and it is also not clear whether VIP acts as a transmitter or as a cofactor in the SCN. Nevertheless, the data suggest that although SCN neurons are cell-autonomous oscillators, the network plays an important part in generating the rhythms of firing and membrane potential that characterize SCN neurons.
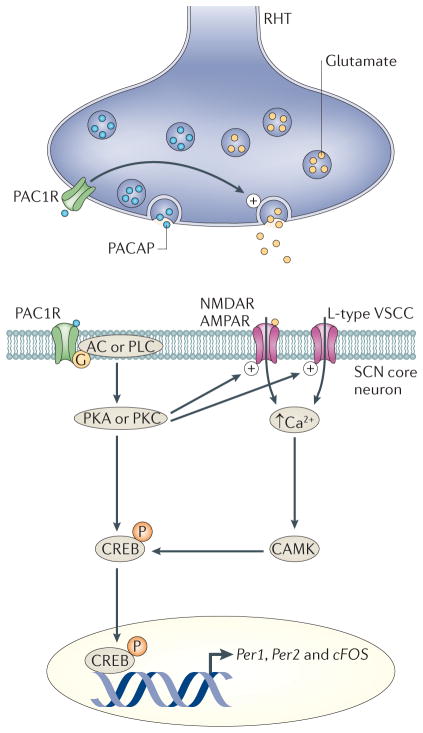
Figure 2. How light regulates the molecular clockwork in SCN neurons.
The best understood pathways by which neural activity regulates clock gene expression comes from studies that have explored how light turns on the transcription of period 1 (Per1) during the night. Melanopsin-expressing retinal ganglion cells encode ambient light and generate action potentials that travel down the retinohypothalamic tract (RHT) and innervate the suprachiasmatic nucleus (SCN). The RHT terminals release glutamate and, under certain conditions, the neuropeptide pituitary adenylate cyclase activating peptide (PACAP). The net result of RHT stimulation is an increase in firing rate and Ca2+ increase in SCN neurons through both activation of glutamate receptors (AMPA and/or NMDA receptors) and voltage-sensitive calcium currents (VSCCs). So far, all of the available evidence indicates that the PACAP type I receptor (PAC1R; also known as PACAPR1) is responsible for mediating the effects of PACAP on SCN neurons. Functionally, PACAP presynaptically enhances the release of glutamate onto SCN neurons and postsynaptically enhances the magnitude of NMDA and AMPA currents within the SCN. The increase in Ca2+ activates a number of signalling pathways that converge to alter transcriptional and/or translational regulators, including cyclic AMP-responsive element (CRE)-binding protein (CREB). Phosphorylated CREB is translocated into the nucleus where it can bind to CREs in the promoter regions of c-Fos, period 1 (Per1) and Per2, and drives transcription of these genes over the course of hours. Within the SCN, the photic regulation of c-Fos and Per1 is rapid, whereas the regulation of Per2 exhibits a slower time course. AC, adenylyl cyclase; CAMK, calcium/calmodulin-dependent protein kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C.
In summary, our understanding of the ionic mechanisms responsible for the generation of rhythms in electrical activity in SCN neurons is increasing. It is probable that a set of intrinsic voltage-sensitive currents are responsible for the circadian variation in firing rate in SCN neurons (TABLE 1). One possibility is that these cells fluctuate between two subthreshold membrane potentials, a hyperpolarized down state and a depolarized up state. During the up state (day), most SCN neurons are spontaneously active and can fire action potentials. During the down state (night), most SCN neurons are inactive owing to a membrane hyperpolarization associated with an increase in total membrane conductance77. From a circadian perspective, it is important not only to identify the currents that regulate spontaneous firing but also to understand how those currents change from day to night, to drive the rhythm in neural activity.
Activity regulates the molecular clock
For many years, the assumption was that the neural activity rhythms of the circadian system were driven — through some unknown mechanism — by the molecular clockwork that is present in single cells. However, in the past decade, a few key studies have suggested that neural activity is not only driven by the molecular clockwork but is itself required for the rhythmic expression of gene expression. An elegant study in Drosophila manipulated the expression of a non-voltage sensitive K+ channel to assess the impact of chronically hyperpolarizing, pigment dispersed factor (PDF)-expressing circadian pacemaker neurons on circadian output88. As expected, this construct electrically silenced the neurons and blocked behavioural rhythms in Drosophila. The unexpected finding was that it also blocked the rhythm in expression of the PER and timeless (TIM) proteins88. These types of observations have changed the thinking in the field for the past decade.
Other findings also suggest that membrane excitability and/or synaptic transmission may be required for the generation of molecular oscillations in SCN neurons. One of the most direct lines of evidence comes from experiments using TTX to block fast inward Na+ currents. Application of this toxin can block the expression of rhythms in behaviour21, glucose utilization89, secretion90 and, of course, electrical activity31. After TTX wash-out, the phase of each of these rhythms seems to re-emerge in phase with the prior oscillation. These results suggest that TTX does not stop the clock but blocks the expression of rhythmicity — that is, blocks circadian output — although this has not been explicitly tested and more analysis is needed to confirm that the phase is not altered by these treatments. Several groups have used optical reporters that allow real-time bioluminescence imaging to monitor oscillations at the level of single neurons. Real-time imaging of single SCN cells in a slice culture preparation from transgenic mice carrying the mPer1 promoter-driven luciferase reporter gene showed that TTX dramatically reduced both the amplitude of the molecular circadian rhythms of PER1::LUC activity and the synchrony of the SCN population23,91. Again, after wash-out of TTX, the molecular oscillation seems to re-emerge with a phase that is predicted by the bioluminescence rhythms before treatment. Interestingly, a detailed analysis of the impact of TTX on PER2::LUC rhythms recorded from cultured SCN neurons indicates that although most SCN neurons lost rhythmicity in the presence of TTX, a small minority (10 %) did not37. So, in addition to blocking circadian output, TTX may also block the expression of the core molecular clockwork within SCN neurons. But what is the underlying mechanism?
Physiological studies indicate that TTX application has three effects on SCN neuron activity. First, it stops the generation of action potentials by blocking most voltage-dependent sodium currents92. Second, through this mechanism, TTX application stops synaptic transmission, although the release of small packets of neurotransmitters still occurs. Third, in at least some SCN neurons, TTX application depolarizes the resting membrane potential through as yet unknown ionic mechanisms45,59. It is difficult to parse out the different effects of TTX to determine which of these mechanisms underlies the effect of TTX on clock gene expression. An additional consideration for the interpretation of these experiments is that TTX is the most disruptive to clock gene expression in preparations of immature neurons. Neural activity may have an organizational role in the development of the SCN circuits, which could explain why clock gene expression in adult tissue seems to be less affected by the application of TTX93.
Other studies have used pharmacological treatments to block synaptic transmission in the SCN without affecting action potential generation or membrane depolarization, and have found that these treatments alter clock gene expression. For example, treating SCN slices with botulinum toxin A or using dynasore to block exocytosis or endocytosis, respectively, compromises circadian gene expression as measured by PER2::LUC expression94. Similarly, the targeted deletion of secretory vesicle proteins receptor-type tyrosine-protein phosphatase-like N (IA2) and IA2β alters the rhythms in a variety of circadian outputs, including SCN neural activity and clock gene expression86. Most SCN neurons release GABA, and blocking synaptic transmission would be expected to block this release; however, some studies indicate that GABA is not crucial for coupling the circadian oscillations in gene expression between neurons95. Instead, as mentioned above, most data suggest that SCN neurons are coupled by peptides including VIP and its receptor VIPR2 (REFS 96,97). Mice that are deficient in VIPR2 exhibit low amplitude PER2::LUC rhythms that resemble those seen with the application of TTX98. Importantly, this loss of amplitude in the molecular clockwork in the VIPR2 knockout mice was ‘rescued’ by the addition of a medium containing high levels of K+ that broadly depolarizes the membrane. This suggests that one role of the VIPR2 may be to maintain membrane potential of SCN neurons within a range that is consistent with molecular timekeeping. In support of this model, the loss of VIPR2 results in the hyperpolarization of the membrane of SCN neurons87. So, the effect of treatments that block synaptic transmission within the SCN has to be considered in the context that they are blocking peptide signalling within this structure.
In the SCN cell population, the rhythms in Per1 and Per2 mRNA peak between circadian time 4 and circadian time 6 (REFS 99,100), whereas electrical activity peaks around circadian time 7. So, the peak of CLOCK–BMAL1 (brain and muscle ARNT-like 1) -driven transcription occurs before the highest levels of action potentials, but neural activity and CLOCK–BMAL1-driven transcription rise together in the beginning of the daily cycle. The temporal dynamics of transcriptional activation of Per1 can be optically monitored using green fluorescent protein (GFP) fluorescence in Per1::GFP mice. In these mice, there is a direct correlation between the frequency of action potentials and the level of Per1 expression in SCN neurons101,102. Specifically, Per1 mRNA expression (as measured by the GFP construct) was higher in neurons firing at 10 to 12 Hz than in cells that were electrically inactive. These experiments raise the possibility that there may be a fixed phase relationship between electrical activity and gene expression; however, there is also evidence that when the circadian system is transiently disrupted by rapid changes in the light–dark cycle, the peaks of neural activity and Per1 expression assume very different phases103. It is also not clear whether the firing rate itself or the underlying membrane depolarization has the greatest effect on Per1 expression, as a recent study reported a high GFP signal in SCN neurons that were so depolarized as to be unable to generate action potentials43. At a population level, membrane hyperpolarization that is caused by lowering the extracellular concentration of K+ in SCN cultures reversibly abolishes the rhythmic expression of Per1 and PER2 (REF. 104). Together, these studies in mammals and experiments in Drosophila88,105,106 suggest that electrical activity of SCN cells drive the molecular clockwork, and that keeping SCN cells in an appropriate voltage range may be required for the generation of circadian rhythmicity of clock gene expression at the single-cell level. One key issue that needs to be resolved is the role of development in these effects. Neural activity is crucial in development, as patterns of activity have organizational effects on the developing circuits. In addition, it is not clear whether the impact of blocking rhythmic electrical activity on gene expression is due to the loss of action potentials or the chronic hyperpolarization that will reduce Ca2+ and perhaps cyclic AMP-driven transcription.
Activity-regulated signalling pathways
Regardless of whether TTX affects action potentials or synaptic events, the effect of electrical activity of SCN neurons on clock gene expression is probably mediated, at least in part, by Ca2+. In neurons, there is a close relationship between electrical activity and Ca2+ levels107–109. SCN neurons exhibit a circadian rhythm in resting Ca2+ that can be detected by the Ca2+ sensitive fura2 indicator dye. During the peak in firing at midday, SCN neurons show resting Ca2+ levels of around 150 nM. These levels drop to about 75 nM during times of inactivity110. This rhythm is reduced, though not quite eliminated, by TTX and L-type calcium blockers. The action potential itself is one important source of Ca2+ in the SCN42, regulating Ca2+ influx into the soma through voltage-dependent activation of L-type Ca2+ channels. This was shown most clearly by recent work in which Ca2+ levels and firing of SCN neurons were measured simultaneously62. The data from this study show that driving the frequency of action potentials in the SCN neuron to 5–10Hz (daytime levels) induces a rise in somatic Ca2+ levels, and this was attenuated by the application of the L-type Ca2+ channel blocker nimodipine.
Interestingly, SCN neurons also have a rhythmically regulated pool of Ca2+ that is not driven by membrane events. Experiments in which a Ca2+-sensitive fluorescent protein cameleon was expressed in organotypic SCN cultures detected robust Ca2+ rhythms111. These rhythms were sensitive to pharmacological manipulation of ryanodine-sensitive stores of intracellular Ca2+ but not to TTX or L-type Ca2+ channel blockers. However, the Ca2+ rhythm phase precedes the neural activity rhythm and previous studies showed that the ryanodine receptor can be a potent regulator of excitability of SCN neurons112, suggesting that store-driven changes in Ca2+ are a critical part of the output pathway by which intracellular processes drive rhythms in neural activity. Future studies should determine the subcellular location of these Ca2+ pools as well as the relationship between intracellular Ca2+ and fluorescence signals in SCN neurons as the cameleon reporters are not ideal for monitoring the relatively slow changes in firing rate observed in SCN neurons113.
Regardless of the relative contribution of Ca2+ influx and release from intracellular stores, it is clear that Ca2+ oscillations are crucial in driving a robust rhythm in gene expression. For example, blocking Ca2+ influx in SCN cultures by lowering the extracellular Ca2+ concentration reversibly abolishes the rhythmic expression of Per1::luc and PER2::LUC104. Blockade of voltage-sensitive Ca2+ channels also abolishes the oscillatory patterns of Per2 and Bmal1 expression in a cell line (SCN2.2) derived from the SCN57. In Drosophila melanogaster, buffering intracellular Ca2+ in pacemaker neurons results in dose-dependent slowing of free-running behavioural and molecular rhythms114. These results strongly suggest that a transmembrane Ca2+ flux is necessary for sustained molecular rhythmicity in the SCN. Indeed, rhythms in Ca2+ levels, with the peak occurring during the day, seem to be a general feature of circadian systems, as studies in molluscs115,116, flies114 and even plants117–119 have shown.
Another crucial signalling pathway that is involved in the coupling between the membrane and clock gene expression is the cyclic AMP (cAMP) signalling pathway. Several studies have found that cAMP levels are rhythmic in the SCN55,120,121. Part of the underlying mechanism may be the rhythmic expression of the gene encoding adenylyl cyclase 1 (ADCY1) in the SCN and retina122. Peak cAMP levels in SCN tissue occur during the day55,120 and precede the peak in the neural activity rhythm. The transcriptional activity of the cAMP-responsive element (CRE) is also strongly rhythmic in the SCN55. Importantly, pharmacological blockade of adenylyl cyclase activity reduced cAMP levels, suppressed CRE expression and greatly reduced PER2::LUC expression55. Inhibition of adenylyl cyclase can also lengthen the free-running period of the Per2 rhythm, suggesting a regulatory role of this enzyme in the core clock mechanisms. Interestingly, the key cAMP-driven effector seems to be exchange protein activated by cAMP (EPAC) rather than protein kinase A (PKA). Indeed, it has been suggested that EPAC phosphorylation of CRE-binding protein (CREB) drives clock gene expression123, but the roles of firing rate and synaptically mediated events in this cytosolic oscillation are not known. However, a number of Ca2+ sensitive adenylyl cyclases are expressed in the SCN. For example, Adcy1 is expressed in the SCN48,122 and ADCY1 activity is stimulated by Ca2+ concentrations of around 150 nM124. So, the daytime peak in intracellular Ca2+ (REF. 110) would be sufficient to trigger adenylyl cyclase 1, and the crosstalk between the rhythmic electrical activity and cAMP may therefore be mediated by Ca2+. In addition, many of the synaptically evoked neurotransmitters that are expressed in the SCN, including pituitary adenylate cyclase-activating polypeptide (PACAP), VIP, GRP, AVP and serotonin are potent regulators of cAMP levels. Interestingly, a recent study showed that CRY1 can inhibit cAMP in response to G protein-coupled receptor activation, possibly through a direct physical interaction with G proteins125. Thus, electrical activity levels can alter the cAMP signalling network in the SCN through a number of mechanisms.
Besides Ca2+ and cAMP, a number of rhythmically regulated signalling molecules, including casein kinases and RAS-dependent mitogen-activated protein kinases (MAPKs), have been described in the SCN. For example, recent work suggests that protein kinase C (PKC) and receptor of activated C kinase 1 (RACK1) are integral components of the circadian clock126. These Ca2+-sensitive signalling molecules are physically recruited into the nuclear BMAL complex. Overexpression or depletion of these proteins altered the molecular clockwork, suggesting a role for PKC in the basic circadian feedback loop. It may be useful to think of these signalling pathways collectively, as a ‘cytosolic’ oscillator123. Although it is premature to throw out the molecular feedback loop concept, recent work in a variety of preparations, from cyanobacteria127 to human red blood cells128, suggests that rhythmic gene transcription is not essential for circadian timekeeping129. In neurons, cytosolic networks are regulated by neural activity, both directly and through the controlled release of neurotransmitter, and they converge to regulate the transcription factor CREB, CREB-binding protein and p300 to drive the transcription and translation of clock genes such as Per. It seems likely that the cAMP- and Ca2+-dependent activation of CREs is a necessary complement to activation of E-boxes by CLOCK::BMAL for normal circadian regulation of Per and the SCN clockwork13. Thus, dysregulated neural activity and synaptic transmission could weaken the basal Ca2+–CRE activity to a level that is insufficient to drive Per and Cry expression.
Light-evoked changes
In addition to the changes in gene expression that are driven by the daily increase in spontaneous neural activity, exposing an animal to light during the night also drives a robust increase in neural activity in the SCN25. The consequence of light exposure on the molecular clockwork varies with the phase of the daily cycle. A light stimulus during the night can induce a phase shift of the molecular oscillator, whereas it has no such effect when applied during the day. This is a central feature of entrainment, that is, the process by which circadian oscillators are synchronized to the environment. One way to consider how the membrane and the molecular clockwork are coupled is therefore by examining the signalling mechanisms through which light resets the circadian system9,10.
The cellular and molecular mechanisms by which light regulates the expression of Per1 is the subject of much analysis (FIG. 2). The melanopsin-expressing retinal ganglion cells (mRGCs) are directly light-sensitive and receive information from rods and cones130–132. These mRGCs thus encode ambient lighting133 and generate action potentials that travel down the RHT and innervate the SCN. The RHT terminals release glutamate and, under certain conditions, the neuropeptide PACAP134,135. The net result of RHT stimulation is an increase in firing rate of SCN neurons. These retinal-evoked excitatory postsynaptic responses in the SCN are mediated by AMPA136–139 and NMDA receptors140,141. PACAP142 and the metabotropic glutamate receptors143 seem to provide a mechanism to modulate the magnitude of these responses. In addition, there is a clear role for GABA regulation of the RHT input, with GABA type B (GABAB) receptors acting presynaptically to regulate the release of glutamate144,145 and GABA type A (GABAA) receptors acting postsynaptically to regulate the SCN neuron response to glutamate. Depending on the time of day and the SCN region, these GABAA receptor-mediated inputs can evoke hyperpolarizing or depolarizing responses in the SCN neuron83,146,147.
During light exposure, the firing rate of SCN neurons is greatly increased, with larger increases during the night than during the day25. Pioneering work by McMahon, Block and colleagues in molluscs has led to the development of a model that explains how the circadian system responds to photic stimulation in the night but not the day (BOX 2). Conceptually, elements of this model have begun to be applied to the SCN62,140. The basic idea is that the circadian pacemaker neurons are already electrically active during the day and that additional excitatory input from the RHT does not induce many additional action potentials. By contrast, during the night, when the neurons are electrically silent, RHT stimulation results in a large change in electrical activity and the system responds to the change in action potential frequency with a phase shift.
Box 2. Diurnal gating of light input to the circadian system in the mollusc.
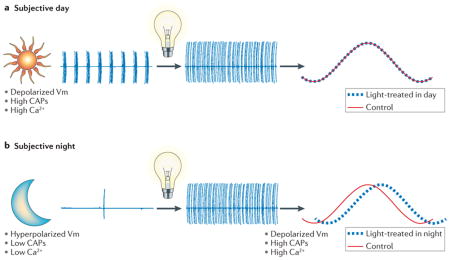
One of the fundamental features of circadian oscillators is that their response to environmental stimulation varies depending on the phase of the daily cycle when the stimuli are applied. For example, the same light treatment can produce phase shifts of the oscillator when applied during the subjective night but have no effect when applied during the subjective day. This periodic sensitivity to photic stimulation is a central feature of entrainment, that is, the process by which circadian oscillators are synchronized to the environment. What are the cellular mechanisms that underlie this periodic sensitivity to environmental stimulation? Studies on the circadian oscillators in the eye of the marine molluscs Aplysia and Bulla have led to the development of a credible model to explain this daily variation in response to photic stimulation (see the figure). In these species, specialized retinal cells known as basal retinal neurons or secondary cells show a daily rhythm in membrane potential and spontaneous neural activity116. Central to the model is the hypothesis that this circadian rhythm in membrane potential drives a rhythm in Ca2+ influx through voltage-sensitive Ca2+ channels, and that light acts to cause a Ca2+ influx in these cells115,116,234. During the day, the membrane potential (Vm) is relatively depolarized and Ca2+ enters the cell though voltage-sensitive Ca2+ channels. Light does not cause phase shifts because the intracellular Ca2+ concentration is already elevated (see the figure, part a). During the night, the membrane is relatively hyperpolarized and light-induced depolarization can cause a Ca2+ influx, leading to a shift in the phase of the rhythm (see the figure, part b). Elements of this model are now being successfully applied to the mammalian SCN25,62,140,235. CAPs, compound action potentials.
During the night, SCN neurons respond to photic stimulation transduced by mRGCs that generate action potentials of up to 20 Hz148–150. These fast synaptic responses, which can be mediated by AMPA or GABAA receptors, exhibit a short time course and move the SCN neuron into a voltage range that maximizes the contribution of the IA, FDR, and BK currents that are described above. Indeed, mice lacking the FDR current show greatly attenuated NMDA-evoked responses in the SCN69. It is likely that each of the K+ currents (IA, FDR and BK) has a major role in determining how an SCN neuron responds to RHT input throughout the daily cycle, although this has not been tested yet. This light-induced increase in neural activity drives synaptic communication with the rest of the cells in the circuit through GABA, GRP and VIP. GRP produces long-term increases (lasting hours) in excitability in SCN neurons151,152. Interestingly, the GRP-driven increases in firing rate are dependent upon the CREB pathway and activation of Per1. VIP acts presynaptically to alter GABA release153 and postsynaptically to regulate voltage-gated currents in the SCN87,96. These changes in electrical activity also trigger intracellular signalling cascades in SCN neurons.
The RHT stimulation during the night and the resulting increase in the frequency of action potentials produce a robust increase in Ca2+ in SCN neurons. In dendrites, the Ca2+ influx is probably mediated by NMDA receptors, with a major contribution from the NR2B subunit140,154, whereas in the soma the L-type voltage sensitive Ca2+ channels are the main contributor62. There is also evidence indicating that T-type Ca2+ currents are required for glutamate-induced phase shifts in the SCN neural activity rhythm in rats61. The concentration of intracellular Ca2+ in neurons is tightly controlled by various channels, pumps and buffers. In addition, ryanodine receptors have a role in mediating the effects of light- and glutamate-induced phase delays of the circadian system155 and in the regulation of the electrical activity of SCN neurons112. Thus, RHT-evoked Ca2+ influx is likely to be a major transducer of light information to the circadian system.
The signal transduction events following the influx of Ca2+ that is induced by RHT stimulation during the night are beginning to be understood and include a number of signalling pathways156–158. There is evidence for activation of MAPK–extracellular signal-regulated kinase (ERK)159–161, calmodulin162, nitrogen oxide–cyclic GMP163,164, PKC165 as well as cAMP166. The relative contributions of each pathway to regulating the molecular clockwork are not yet clear and there is evidence that the relative importance of each signalling cascade may vary between early and late night157. There is no doubt that other signalling pathways are also involved, and the role of phosphatase cascades in counter-balancing these kinase cascades has not been adequately examined in the SCN. Moreover, light stimulation causes a robust and reliable increase in the proto-oncogene c-Fos and its phosphoprotein product FOS, and many research groups have made use of Fos induction to examine the impact of light on gene expression in the SCN167,168. The kinase signalling pathways converge on CREB phosphorylation at Ser133 and Ser142 (REFS 155,169,170). CREB became phosphorylated only at times during the circadian cycle when light induces Fos expression and behavioural phase shifts of circadian rhythms169. These results suggest that circadian clock gating of light-regulated molecular responses in the SCN occurs upstream of CREB phosphorylation. Phosphorylated CREB is translocated into the nucleus, where it can bind to CREs in the promoter regions of Per1 and Per2 (REF. 171). The net result is an increase in Per1 and Per2 message that occurs over the next two hours172. This suggests that increasing the transcription of Per genes in the early night delays the molecular clockwork by postponing the normal decline in Per expression, whereas increasing the transcription of these genes in the late night speeds up the normal increase in Per expression at the beginning of the next cycle, although this has not been explicitly tested.
Several lines of evidence indicate that the phosphorylation of CREB is a crucial mediator of the effect of light on the circadian system. For example, transgenic mice in which phophorylation at Ser142 cannot take place170 or that express a CREB repressor173 show attenuated light-induced gene expression in the SCN and a reduced behavioural response to light exposure. Similarly, the use of a CRE decoy oligodeoxynucleotide in rats174 reduced both light-induced behaviour in vivo and glutamate-induced increases in Per1 mRNA and phase shifts in vitro. This evidence indicates that CRE-driven gene expression is part of the mechanism by which membrane events regulate clock gene expression in this system but it seems likely that other pathways are involved, including translational and post-translational regulation. Recent work suggests that light-evoked increases in firing also activate the mammalian target of rapamycin (mTOR) pathway and thereby regulate the translation of Per genes175. Also, PKC seems to regulate photic resetting through post-translational mechanisms that impact the stability and intracellular distribution of PER2 (REF. 165). So, like the rest of neuroscience, the circadian field has to sort through an alphabet soup of intracellular signalling cascades to explain how a light-induced increase in the firing rate of SCN neurons drives chromatin remodelling, transcriptional, translational and post-translational processes to regulate the expression of clock genes in the SCN.
The activity-dependent transcription and release of brain-derived neurotrophic factor (BDNF) has various roles in the nervous system in linking neural activity with synaptic changes that underlie circuit development and functions176,177. Previous work has raised the possibility that BDNF may be an important regulator of synaptic input into the SCN. The transcriptional repressor methyl-CpG-binding protein 2 (MECP2) is expressed in the SCN and this Bdnf regulator is phosphorylated in response to light178. Both BDNF and its high-affinity BDNF/NT3 growth factors receptor (TRKB) are expressed in the SCN93,179,180. Physiological data demonstrated that BDNF and neurotrophin receptors can enhance glutamatergic synaptic transmission within a subset of SCN neurons181, and can potentiate glutamate-induced phase shifts of the circadian rhythm of neural activity in the SCN182. Functionally, both BDNF-deficient and TRKB-deficient mice showed reduced behavioural phase shifts in response to light exposure during the subjective night183,184. The evidence is consistent with the hypothesis that BDNF may gate light input to the circadian system — that is, BDNF secreted at night may be required for light-induced phase shifts.
The molecular clock regulates activity
The best evidence that the molecular clockwork in the SCN can drive the rhythms in electrical activity — and, by extension, in behaviour and physiology — comes from several studies that have explored the impact of mutations in the core clockwork on electrical activity rhythms recorded in the SCN. The Tau (casein kinase 1ε) mutation in hamsters shortens the period of wheel-running activity and neural activity rhythms185. Similarly, homozygote Clock mutant mice are behaviourally arrhythmic, whereas heterozygote animals show lengthened behavioural rhythms, findings that are paralleled by physiological recordings from the SCN32,186. Furthermore, mutant mice lacking Cry1 and Cry2 show behavioural arrhythmicity and loss of rhythms in SCN neural activity22. These findings suggest that clock gene rhythms are translated into daily patterns of action potential activity in the SCN. Together, these studies provide clear evidence that the molecular clockwork can drive neural activity as an output.
However, very little is known about the mechanisms by which the molecular clockwork drives rhythms in neural activity (FIG. 3). The expression of the transcripts for the clock genes Per1 and Per2 peak at midday (circadian time 4–circadian time 6) in the SCN, a little before the peak in neural activity. So at dawn, when electrical activity is rising, the CLOCK–BMAL complex starts to drive the transcription of Per and Cry genes. An important issue, which has not been tested directly, is whether blocking the increase in Per or Cry transcripts alters the rise in neural activity. Application of the peptide GRP in the night produces a long-term increase in neural activity that is blocked by antisense oligodeoxynucleotides against Per1 (REF. 151). In addition, a recent study in D. melanogaster suggests that CRY can alter membrane potential through redox-based regulation of a K+ channel conductance187. Although it is too early to know whether a similar mechanism is involved in the SCN in mammals, the striking parallels between Drosophila and mammalian models certainly suggest that we should consider the possibility. In addition, the molecular clockwork is a potent regulator of transcription and there is evidence of rhythmic transcription of several ion channels, including L- and T-type Ca2+ channels, BK channels and K2P K+ channels. The genes that encode these channels could be regulated by the CLOCK–BMAL protein complex acting directly on E-box or other elements that are present in the regulatory sequences of these genes. In Drosophila, circadian rhythms in a mRNA that encodes a regulatory protein associated with BK channels have been described188,189. It is difficult to obtain clear data on the half-life of the proteins coding for ion channels in vivo. In culture, Kv1.3 channel proteins have a half-life of 1–2 hours and this duration can be regulated by TRKB190. The findings that channel-specific antisense oligodeoxynucleotides191 and RNA interference192 can produce physiological effects within 24 hours suggest that daily rhythms in transcripts for channel proteins may well be functionally important in generating daily rhythms in membrane potential.
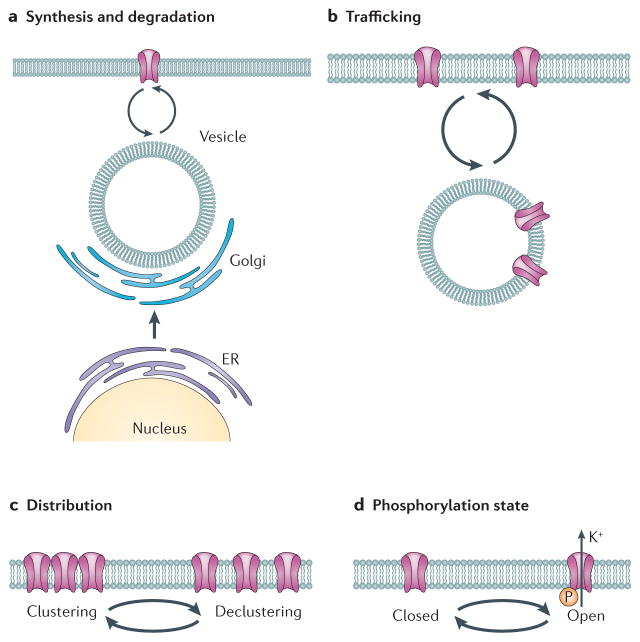
Figure 3. Possible mechanisms by which the molecular clock can regulate spontaneous neural activity in SCN neurons.
a | Ion channels and other membrane proteins are made and then transported to the membrane through the rough endoplasmic reticulum (ER) to the Golgi. The membrane proteins are transported to the membrane and removed for degradation through vesicles. There is evidence of the rhythmic transcription of several ion channels, including L- and T-type Ca2+ channels, large-conductance Ca2+ activated K+ (BK) channels and two-pore K+ (K2P) channels. Although we do not know the half-life of these channels in the SCN, direct transcription and translational regulation of membrane proteins is an obvious candidate mechanism for driving the observed rhythms in spontaneous neural activity. b | AMPA receptors and potassium channels have been shown to be rapidly inserted and removed from the membrane in response to physiological stimulation. Therefore, the daily trafficking of ion channels and associated proteins is another possible mechanism that may be responsible for the firing rate rhythms. c | The distribution of ion channels within the plasma membrane can change from day to night, and this may also contribute to changes in firing rate rhythms between day and night. d | Robust circadian rhythms in signalling pathways, including pathways involving Ca2+, cyclic AMP, phospholipase C (PLC), casein kinases and RAS-dependent mitrogen-activated protein kinases (MAPKs), have been described. These pathways are robust regulators (through phosphorylation) of ion channels and associated proteins. Circadian regulation in the phosphorylation state of ion channels through the balance between kinase and phosphotase activities is a likely mechanism that underlies the rhythm in spontaneous neural activity in SCN neurons. In summary, a diverse set of mechanisms, including changes in transcription and translation, direct phosphorylation, trafficking and distribution of ion channels (and associated proteins (not shown)) could underlie the rhythms in membrane events that characterize SCN neurons.
Another possible mechanism by which the molecular clockwork drives rhythms in neural activity involves the circadian trafficking of proteins, and there are examples of channels that can be inserted or removed from the membrane to rapidly alter synaptic function. One of the core mechanisms that is thought to underlie long-term changes in the strength of excitatory synaptic transmission involves the rapid insertion and removal of AMPA-type glutamate receptors into a postsynaptic membrane193–195. These changes in AMPA receptor localization can occur within minutes, driven by neurotransmitter regulation of the cAMP–PKA signalling pathway. There is also evidence that this type of trafficking can occur with intrinsic voltage-gated channels. One of the most interesting examples comes from work on the Kv3.1 channels. In the auditory system, the distribution of Kv3.1b can become rapidly (within 30 min) and specifically modulated after brief periods of acoustic stimulation196. This rapid modulation of the FDR current plays a part in maintaining appropriate levels of intrinsic neuronal excitability in different acoustic environments and may be the result of rapid translational regulation through the fragile X mental retardation protein 1 (FMR1)197. The Kv3 channels seem to be directly bound to kinesin 1 heavy chain198 and so can be directly targeted to the membrane to regulate excitability. We do not know if these types of rapid regulation of channel proteins occur in the SCN but it seems possible. The FDR current, Kv3.1 channels and Kcnc1 transcripts do show evidence for circadian variation. The daily insertion of the Kv3 channels into the membrane of SCN neurons would be consistent with the experimental data. Interestingly, the loss of the gene encoding FMR1 causes substantial disruption of circadian rhythms in flies199 and mice200. At present, there is no direct evidence for circadian clock regulation of the protein trafficking of ion channels.
Post-translational modifications of channels and associated auxiliary proteins are perhaps the most likely explanation for the circadian variation in the electrical activity of SCN neurons. Outside of the SCN, numerous studies have shown the importance of kinase and phosphatase activity in mediating short-term changes in current function that alter electrical excitability80,201,202. In chick photoreceptors, circadian oscillations in the expression of cone cGMP-gated channels have been described203. Within the SCN, there is clear evidence for a daily rhythm in the levels of cAMP55,120,121 and Ca2+ (REFS 110,111). The daily rhythms in cAMP- and Ca2+-associated signalling pathways would be expected to drive daily rhythms in the activity of ion channels and membrane currents. In the nervous system, many ion channels contain auxiliary proteins that modulate pore forming subunits. These proteins are attractive targets for post-translational regulation that could mediate the daily rhythm in the Ca2+ and K+ currents observed in SCN neurons. Thus, circadian patterns of phosphorylation almost certainly drive circadian rhythms in the membrane properties of SCN neurons. Future studies are needed to explore the mechanistic links between the molecular circadian oscillator and channels (including pore forming subunits and auxiliary proteins) in the membrane of SCN neurons.
Effects of ageing and disease
Disruptions in sleep–wake cycles, including decreased amplitude of rhythmic behaviours and fragmentation of sleep episodes, are commonly associated with ageing in humans and other mammals204–206. Although undoubtedly many factors contribute to these changes, a body of literature207–211 is emerging that is consistent with the hypothesis that an age-related decline in the output of the central circadian clock in the SCN may be key. In vivo multiunit recordings from the SCN of freely moving young and middle-aged (12–14-month-old) mice have shown that the day–night difference in neural activity was substantially reduced in the SCN of middle-aged mice212. Furthermore, the neural activity rhythms were clearly disrupted in the subparaventricular zone, one of the main neural outputs of the SCN. Surprisingly, the molecular clockwork in the SCN, as measured by PER2 levels, was not disrupted in middle-aged mice, suggesting that the age-related disruption in the circadian output (measured at the level of neural activity rhythms in the SCN) occurs before any disruption of the molecular clockwork.
Patients suffering from neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease and Huntington’s disease commonly exhibit sleep disorders. These patients have difficulty in sleeping at night and staying awake during the day. This dysfunction in sleep timing may not be causal to their disorder, yet these symptoms have a major impact on the quality of life of the patients and on their caregivers. Although the pathology underlying these symptoms has not yet been identified, there has been some intriguing work using mouse models of neurodegenerative diseases213–216. Most of these mouse models exhibit circadian disruption and there is at least some evidence that treatments that are designed to stabilize these rhythms can improve other, non-motor symptoms in these mice214,217. In one of the mouse models of Huntington’s disease (BACHD mice) in which the mutated human huntingtin (HTT) is expressed, the output of the circadian system, as measured by locomotor activity, heart rate and body temperature, was profoundly disrupted early in the life span216. The neural activity rhythms in the SCN, but not rhythmic PER2 expression, were also reduced in the BACHD mice. Further studies are needed to identify the underlying mechanism, but it seems that the neural activity is disrupted while the molecular clockwork keeps ticking.
Finally, infectious diseases may also be associated with disrupted neural activity rhythms within the SCN. For example, African sleeping sickness is characterized by disruption of the sleep–wake cycle218. The underlying mechanisms have been studied in rats that are infected with Trypanosoma parasites. In this model, circadian behaviours and neural activity rhythms in the SCN are disrupted219. Although there is some effect on clock gene expression in the SCN, there is a more profound impact on the gene expression rhythms in peripheral organs, such as the pituitary and the spleen220. Interestingly, these effects may be mediated by the proinflammatory cytokine interferon-γ (IFNγ), which has also been shown to alter neural activity within the SCN221. As the release of this compound is part of the response of immune cells to infections, cytokine-induced changes in SCN neural activity could be part of the mechanism underlying the loss of a coherent sleep–wake rhythm that is so characteristic of our body’s response to infectious disease.
Based on these findings, I propose that the neural activity rhythms in the SCN may be the ‘weak link’ of the circadian system. With ageing, and in models of neurogeneration, there is a weakening of the neural output despite fairly normal functioning of the molecular clock. Importantly, the maintenance of neural activity is extremely metabolically demanding. 2-deoxyglucose studies have revealed robust rhythms in energy metabolism in the SCN, with peaks during daytime21,65. Perhaps owing to this demand, neural activity may be a critically sensitive output of the SCN. It is possible that any health condition that compromises metabolism will alter SCN output through this mechanism. The disruption of circadian neural activity rhythms is likely to have profound consequences on patient health222,223. It is becoming increasingly clear that robust daily sleep–wake rhythms are essential to good health. A wide range of studies have demonstrated that disruption of the circadian system leads to a cluster of symptoms, including metabolic deficits208,224,225, cardiovascular problems226,227, difficulty in sleeping228,229 and cognitive deficits230–232. Many of these same symptoms are seen in ageing and neurodegenerative diseases. This suggests that we should put a greater emphasis on the development of pharmacological tools and behavioural interventions that can boost neural activity rhythms in the SCN in situations in which the molecular clock may still be working.
Conclusions and future directions
The neural activity patterns of SCN neurons are dynamically regulated across the circadian cycle. Even in the absence of external stimulation, these neurons exhibit a robust daily rhythm in neural activity that is high in the day and low in the night. A set of intrinsic voltage-sensitive currents is responsible for the circadian variation in firing rate of SCN neurons; these include currents that are responsible for providing the excitatory drive required for all spontaneously active neurons, currents that translate this excitatory drive into a regular pattern of action potentials and currents that, through hyperpolarizing the membrane, are responsible for the nightly silencing of firing. The role of specific currents that regulate spontaneous firing and how those currents change from day to night, to drive the rhythm in neural activity, is beginning to be understood. Unfortunately, there are a number of important gaps in our knowledge. For example, the K+ current that is turned on at night to silence firing has not yet been identified (although there are some candidate channels).
The daily increase in firing rate that starts near dawn has been assumed to be an output of the circadian system, that is, it has been assumed that the neural activity rhythms are directly driven by the molecular clockwork that occurs in single cells. There is now evidence to suggest that neural activity and synaptic activity are not just driven by the molecular clockwork but are also required for the generation of robust rhythms in gene expression. The possible signalling cascades that may be involved in this link are slowly being revealed and have led to the hypothesis that dysregulated neural activity and synaptic transmission weakens basal Ca2+–CRE activity to a level that is insufficient to drive PER and CRY expression. One key issue that needs to be resolved is the role of development in these effects. During development, patterns of neural activity have organizational effects on developing neural circuits233. Genetic manipulations that alter neural activity in the SCN throughout development need to be interpreted in consideration of the possibility of organizational effects. Another issue that needs clarification in future experiments is whether the impact of a number of experimental manipulations of neural activity on gene expression is due to a loss of action potentials or to a chronic hyperpolarization, both of which reduce Ca2+- and cAMP-driven transcription. Furthermore, many of the pharmacological and genetic manipulations that have been used to block action potentials also block synaptic transmission of crucial neuropeptides, and careful studies are required to tease apart these two mechanisms.
Our understanding of the mechanisms by which the molecular clockwork drives changes in the membrane is particularly incomplete. They may involve rhythmic transcription and/or translation of ion channels, rhythmic regulation of ion channel trafficking and distribution as well as rhythmic regulation of the channels’ open state by phosphorylation. This is an important area for future work.
Present evidence suggests that ageing and neurodegenerative diseases may have a particularly strong impact on neural activity rhythms in the SCN, although molecular rhythmicity in the SCN seems to remain relatively stable. This degradation of circadian output at the level of the SCN is likely to have profound consequences on the individual’s health222,223. If neural activity rhythms in the SCN are the ‘weak link’ of the circadian system in ageing and neurodegenerative diseases, we should be looking for interventions that boost the neural output of the circadian system for healthy ageing and management of neurodegenerative disease.
Acknowledgments
This work was supported by funding from the CHDI Foundation, the Stein–Oppenheimer Foundation and the American Heart Association. I would like to thank D. Crandall for assistance with the graphics.
Glossary
- Riluzole
Riluzole preferentially blocks tetrodotoxin-sensitive sodium channels but has been suggested to have other effects, including activating glutamate uptake and increasing potassium currents
- BMAL1
A protein that dimerizes with CLOCK to form a complex. Inside the nucleus, the protein complex binds to a site in the DNA known as the E-box, to drive transcription. The period and cryptochrome genes contain E-boxes and thus are transcriptionally activated by the complex formed of a brain and muscle ARNT-like (BMAL) protein and CLOCK, in the beginning of the daily cycle
- Period
The time that it takes for the biological oscillator to complete one cycle. In the case of circadian oscillators, the period (tau) is close to, but not equal to, 24 hours
- Phase shift
A shift in the phase of the biological oscillation. Phase (Φ) is one of the most important parameters that describe any oscillation, as it refers to the time points within the cycle. To measure the phase of the rhythm, a reliable reference point must be chosen. In the case of circadian oscillations, the onset of activity or the peak expression of a biochemical parameter are commonly used
- Entrainment
In the context of the circadian system, entrainment refers to the process by which a biological oscillator with a free-running period that is close to 24 hours is adjusted to the exact 24-hour period of the environment. When entrained, the period of the biological rhythm equals the period of the entraining stimuli and the two oscillations exhibit a stable phase relationship
- CRE decoy oligodeoxynucleotide
A synthetic DNA molecule that contains a cyclic AMP-responsive element (CRE). When expressed in a cell, the CRE decoys compete with native CRE sites in gene promoters for binding of phosphorylated CRE-binding protein (CREB). Thus, these oligodeoxynucleotides can function as competitive inhibitors of CREB binding
Footnotes
Competing interests statement
The author declares no competing financial interests.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 4.Herzog ED. Neurons and networks in daily rhythms. Nature Rev Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 5.Welsh D, Takahashi J, Kay S. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. This review focuses on what is known about how cell-autonomous oscillators are coupled within the SCN circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011;34:349–358. doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 8.Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- 9.Antle MC, Smith VM, Sterniczuk R, Yamakawa GR, Rakai BD. Physiological responses of the circadian clock to acute light exposure at night. Rev Endocr Metab Disord. 2009;10:279–291. doi: 10.1007/s11154-009-9116-6. [DOI] [PubMed] [Google Scholar]
- 10.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 11.Morin LP, Allen CN. The circadian visual system. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Lakin-Thomas PL, Brody S, Coté GG. Amplitude model for the effects of mutations and temperature on period and phase resetting of the Neurospora circadian oscillator. J Biol Rhythms. 1991;6:281–297. doi: 10.1177/074873049100600401. [DOI] [PubMed] [Google Scholar]
- 13.Pulivarthy SR, et al. Reciprocity between phase shifts and amplitude changes in the mammalian circadian clock. Proc Natl Acad Sci USA. 2007;104:20356–20361. doi: 10.1073/pnas.0708877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock–controlled genes in the suprachiasmatic nucleus. Eur J Neurosci. 2004;19:1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura W, Yamazaki S, Takasu NN, Mishima K, Block GD. Differential response of Period 1 expression within the suprachiasmatic nucleus. J Neurosci. 2005;25:5481–5487. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- 17.Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. This review provides a good introduction to the anatomy of the SCN. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 19.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz W, Gross R, Morton M. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albus H, et al. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol. 2002;12:1130–1133. doi: 10.1016/s0960-9822(02)00923-5. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi S, et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. An important study that used real-time analysis of gene expression to show synchronized, topographically patterned rhythms of clock gene transcription across hundreds of individual neurons within the SCN in organotypic slice culture. Application of TTX reduced the amplitude of single cell oscillations in SCN neurons. [DOI] [PubMed] [Google Scholar]
- 24.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer J, Watanabe K, Schaap J, Albus H, Détári L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura W, et al. In vivo monitoring of circadian timing in freely moving mice. Curr Biol. 2008;18:381–385. doi: 10.1016/j.cub.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- 28.Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- 29.Shibata S, Liou SY, Ueki S. Development of the circadian rhythm of neuronal activity in suprachiasmatic nucleus of rat hypothalamic slices. Neurosci Lett. 1983;43:231–234. doi: 10.1016/0304-3940(83)90193-3. [DOI] [PubMed] [Google Scholar]
- 30.Schaap J, Pennartz C, Meijer J. Electrophysiology of the circadian pacemaker in mammals. Chronobiol Int. 2003;20:171–188. doi: 10.1081/cbi-120019311. [DOI] [PubMed] [Google Scholar]
- 31.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 32.Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nature Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 33.Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma K. Circadian periods of single suprachiasmatic neurons in rats. Neurosci Lett. 1998;250:157–160. doi: 10.1016/s0304-3940(98)00464-9. [DOI] [PubMed] [Google Scholar]
- 34.Kuhlman S, McMahon D. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21:470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- 35.Ko G, Shi L, Ko M. Circadian regulation of ion channels and their functions. J Neurochem. 2009;110:1150–1169. doi: 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci USA. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. A very sophisticated combination of experimental and modelling work to explore the network properties of the SCN circuit. The firing rate and Per2 gene expression were measured in isolated and interconnected SCN neurons. The analysis suggests that individual SCN neurons can generate circadian oscillations but that network interactions are crucial for robust and stable oscillations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderLeest HT, et al. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 39.Brown TM, Piggins HD. Spatiotemporal heterogeneity in the electrical activity of suprachiasmatic nuclei neurons and their response to photoperiod. J Biol Rhythms. 2009;24:44–54. doi: 10.1177/0748730408327918. [DOI] [PubMed] [Google Scholar]
- 40.Meijer JH, Michel S, Vanderleest HT, Rohling JH. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur J Neurosci. 2010;32:2143–2151. doi: 10.1111/j.1460-9568.2010.07522.x. [DOI] [PubMed] [Google Scholar]
- 41.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 42.Bean BP. The action potential in mammalian central neurons. Nature Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- 43.Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. doi: 10.1126/science.1169657. During the day, SCN neurons are under strong depolarizing drive, which enables them to generate spontaneous action potentials. This intriguing study suggests that some SCN neurons are so depolarized that they cannot generate action potentials and are functionally silenced. [DOI] [PubMed] [Google Scholar]
- 44.Pennartz CM, Bierlaagh MA, Geurtsen AM. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. J Neurophysiol. 1997;78:1811–1825. doi: 10.1152/jn.1997.78.4.1811. [DOI] [PubMed] [Google Scholar]
- 45.Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. Careful analysis of the excitatory drive found in single SCN neurons in culture. The results highlight the importance of the persistent sodium influx that occurs at resting membrane potentials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kononenko NI, Shao LR, Dudek FE. Riluzole-sensitive slowly inactivating sodium current in rat suprachiasmatic nucleus neurons. J Neurophysiol. 2004;91:710–718. doi: 10.1152/jn.00770.2003. [DOI] [PubMed] [Google Scholar]
- 47.Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 48.Lein E, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 49.Kononenko NI, Honma S, Dudek FE, Honma K. On the role of calcium and potassium currents in circadian modulation of firing rate in rat suprachiasmatic nucleus neurons: multielectrode dish analysis. Neurosci Res. 2008;62:51–57. doi: 10.1016/j.neures.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 51.Akasu T, Shoji S, Hasuo H. Inward rectifier and low-threshold calcium currents contribute to the spontaneous firing mechanism in neurons of the rat suprachiasmatic nucleus. Pflugers Arch. 1993;425:109–116. doi: 10.1007/BF00374510. [DOI] [PubMed] [Google Scholar]
- 52.Jiang ZG, Nelson CS, Allen CN. Melatonin activates an outward current and inhibits Ih in rat suprachiasmatic nucleus neurons. Brain Res. 1995;687:125–132. doi: 10.1016/0006-8993(95)00478-9. [DOI] [PubMed] [Google Scholar]
- 53.de Jeu MT, Pennartz CM. Functional characterization of the H-current in SCN neurons in subjective day and night: a whole-cell patch-clamp study in acutely prepared brain slices. Brain Res. 1997;767:72–80. doi: 10.1016/s0006-8993(97)00632-x. [DOI] [PubMed] [Google Scholar]
- 54.Atkinson SE, et al. Cyclic AMP signalling controls action potential firing rate and molecular circadian pacemaking in the suprachiasmatic nucleus. J Biol Rhythms. 2011;26:210–220. doi: 10.1177/0748730411402810. [DOI] [PubMed] [Google Scholar]
- 55.O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. An important study that explores the role of cAMP signalling in the SCN. Among other findings, the authors show that the transcriptional activity of the CRE is strongly rhythmic in the SCN. Importantly, pharmacological blockade of adenylyl cyclase activity reduced cAMP levels, suppressed CRE expression and greatly reduced PER2::LUC expression. The sustained pharmacological block of HCN channels also reduced circadian gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- 57.Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J Neurosci. 2005;25:9304–9308. doi: 10.1523/JNEUROSCI.2733-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandael DH, et al. Ca(v)1.3 and BK channels for timing and regulating cell firing. Mol Neurobiol. 2010;42:185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- 59.Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- 60.Huang RC. Sodium and calcium currents in acutely dissociated neurons from rat suprachiasmatic nucleus. J Neurophysiol. 1993;70:1692–1703. doi: 10.1152/jn.1993.70.4.1692. [DOI] [PubMed] [Google Scholar]
- 61.Kim DY, et al. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci. 2005;21:1215–1222. doi: 10.1111/j.1460-9568.2005.03950.x. [DOI] [PubMed] [Google Scholar]
- 62.Irwin R, Allen C. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci. 2007;27:11748–11757. doi: 10.1523/JNEUROSCI.1840-07.2007. The work shows careful simultaneous measurements of membrane events and intracellular calcium in SCN neurons. The data from this study show that driving the frequency of action potentials in the SCN neuron to 5–10 Hz (daytime levels) induces a rise in somatic calcium levels, and that this rise is mediated by L-type calcium channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang HY, Huang RC. Diurnal modulation of the Na+/K+-ATPase and spontaneous firing in the rat retinorecipient clock neurons. J Neurophysiol. 2004;92:2295–2301. doi: 10.1152/jn.00061.2004. [DOI] [PubMed] [Google Scholar]
- 64.Wang YC, Huang RC. Effects of sodium pump activity on spontaneous firing in neurons of the rat suprachiasmatic nucleus. J Neurophysiol. 2006;96:109–118. doi: 10.1152/jn.01369.2005. [DOI] [PubMed] [Google Scholar]
- 65.Newman GC, Hospod FE, Patlak CS, Moore RY. Analysis of in vitro glucose utilization in a circadian pacemaker model. J Neurosci. 1992;12:2015–2021. doi: 10.1523/JNEUROSCI.12-06-02015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baranauskas G, Tkatch T, Nagata K, Yeh J, Surmeier D. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nature Neurosci. 2003;6:258–266. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- 67.Rudy B, McBain C. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 68.Itri J, Michel S, Vansteensel M, Meijer J, Colwell C. Fast delayed rectifier potassium current is required for circadian neural activity. Nature Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci. 2011;31:2746–2755. doi: 10.1523/JNEUROSCI.5792-10.2011. An analysis of transgenic mice lacking genes coding for the Kv3.1 and Kv3.2 channels. This provides clear evidence that the FDR current is important for both input and output of the SCN circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouskila Y, Dudek FE. A rapidly activating type of outward rectifier K+ current and A-current in rat suprachiasmatic nucleus neurones. J Physiol. 1995;488:339–350. doi: 10.1113/jphysiol.1995.sp020970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alvado L, Allen CN. Tetraethylammonium (TEA) increases the inactivation time constant of the transient K+ current in suprachiasmatic nucleus neurons. Brain Res. 2008;1221:24–29. doi: 10.1016/j.brainres.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Itri J, Vosko A, Schroeder A, Dragich J, Michel S, Colwell C. Circadian regulation of A-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–640. doi: 10.1152/jn.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meredith AL, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nature Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. An analysis of transgenic mice lacking the gene that encodes the pore-forming subunit of the BK channel. Although the expression of clock genes in the SCN was relatively normal, the authors found clear deficits in circadian behaviour and altered spontaneous firing rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 76.Pennartz CM, De Jeu MT, Geurtsen AM, Sluiter AA, Hermes ML. Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus. J Physiol. 1998;506:775–793. doi: 10.1111/j.1469-7793.1998.775bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- 78.Mathie A. Neural two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hille B. Ion Channels of Excitable Membranes. Sinauer; Sunderland, Massachusetts: 2001. [Google Scholar]
- 81.Kent J, Meredith AL. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLoS ONE. 2008;3:e3884. doi: 10.1371/journal.pone.0003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of γ-aminobutyric acid and γ-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- 83.Choi HJ, et al. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.vanderLeest HT, Rohling JH, Michel S, Meijer JH. Phase shifting capacity of the circadian pacemaker determined by the SCN neuronal network organization. PLoS ONE. 2009;4:e4976. doi: 10.1371/journal.pone.0004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SM, et al. Deletion of the secretory vesicle proteins IA-2 and IA-2β disrupts circadian rhythms of cardiovascular and physical activity. FASEB J. 2009;23:3226–3232. doi: 10.1096/fj.09-132019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pfluegers. 2006;452:7–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- 88.Nitabach M, Blau J, Holmes T. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. An elegant study in Drosophila in which the expression of a non-voltage sensitive potassium channel was manipulated to assess the impact of chronically hyperpolarizing circadian pacemaker neurons. As expected, this construct electrically silenced the neurons and blocked behavioural rhythms in Drosophila. The unexpected finding was that it also blocked the rhythm in expression of the PER and TIM proteins. [DOI] [PubMed] [Google Scholar]
- 89.Shibata S, Moore RY. Tetrodotoxin does not affect circadian rhythms in neuronal activity and metabolism in rodent suprachiasmatic nucleus in vitro. Brain Res. 1993;606:259–266. doi: 10.1016/0006-8993(93)90993-w. [DOI] [PubMed] [Google Scholar]
- 90.Earnest DJ, Digiorgio SM, Sladek CD. Effects of tetrodotoxin on the circadian pacemaker mechanism in suprachiasmatic explants in vitro. Brain Res Bull. 1991;26:677–682. doi: 10.1016/0361-9230(91)90160-l. [DOI] [PubMed] [Google Scholar]
- 91.Maywood ES, O’Neill JS, Chesham JE, Hastings MH. Minireview: the circadian clockwork of the suprachiasmatic nuclei-analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- 92.Noda M, et al. Expression of functional sodium channels from cloned cDNA. Nature. 1986;322:826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- 93.Baba K, Ono D, Honma S, Honma K. A TTX-sensitive local circuit is involved in the expression of PK2 and BDNF circadian rhythms in the mouse suprachiasmatic nucleus. Eur J Neurosci. 2008;27:909–916. doi: 10.1111/j.1460-9568.2008.06053.x. [DOI] [PubMed] [Google Scholar]
- 94.Deery MJ, et al. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Curr Biol. 2009;19:2031–2036. doi: 10.1016/j.cub.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 95.Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci USA. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci USA. 2011 Jul 25; doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maywood ES, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 99.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 100.Shigeyoshi Y, et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 101.Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr Biol. 2003;13:1538–1542. doi: 10.1016/s0960-9822(03)00560-8. [DOI] [PubMed] [Google Scholar]
- 104.Lundkvist G, Kwak Y, Davis E, Tei H, Block G. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sheeba V, Gu H, Sharma VK, O’Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi C, et al. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Regehr WG, Tank DW. Calcium dynamics in synaptically activated pyramidal cells. J Neurosci. 1992;12:4202–4223. doi: 10.1523/JNEUROSCI.12-11-04202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyakawa H, et al. Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron. 1992;9:1163–1173. doi: 10.1016/0896-6273(92)90074-n. [DOI] [PubMed] [Google Scholar]
- 109.Knopfel T, Gahwiler BH. Activity-induced elevations of intracellular calcium concentration in pyramidal and nonpyramidal cells of the CA3 region of rat hippocampal slice cultures. J Neurophysiol. 1992;68:961–963. doi: 10.1152/jn.1992.68.3.961. [DOI] [PubMed] [Google Scholar]
- 110.Colwell C. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ikeda M, et al. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 112.Aguilar-Roblero R, Mercado C, Alamilla J, Laville A, Díaz-Muñoz M. Ryanodine receptor Ca2+-release channels are an output pathway for the circadian clock in the rat suprachiasmatic nuclei. Eur J Neurosci. 2007;26:575–582. doi: 10.1111/j.1460-9568.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- 113.Pologruto TA, Yasuda R, Svoboda K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci. 2004;24:9572–9579. doi: 10.1523/JNEUROSCI.2854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci. 2007;27:12489–12499. doi: 10.1523/JNEUROSCI.3680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McMahon DG, Block GD. The Bulla ocular circadian pacemaker. J Comp Physiol. 1987;161:335–346. doi: 10.1007/BF00603959. [DOI] [PubMed] [Google Scholar]
- 116.Colwell CS, Whitmore D, Michel S, Block GD. Calcium plays a central role in phase shifting the ocular circadian pacemaker of Aplysia. J Comp Physiol A. 1994;175:415–423. doi: 10.1007/BF00199249. [DOI] [PubMed] [Google Scholar]
- 117.Johnson CH, et al. Circadian oscillation of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- 118.Wood NT, et al. The calcium rhythms of different cell types oscillate with different circadian phases. Plant Physiol. 2001;125:787–796. doi: 10.1104/pp.125.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu X, et al. Distinct light and clock modulation of cytosolic free Ca2+ oscillations and rhythmic CHLOROPHYLL A/B BINDING PROTEIN2 promoter activity in Arabidopsis. Plant Cell. 2007;19:3474–3490. doi: 10.1105/tpc.106.046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prosser RA, Gillette MU. Cyclic changes in cAMP concentration and phosphodiesterase activity in a mammalian circadian clock studied in vitro. Brain Res. 1991;568:185–192. doi: 10.1016/0006-8993(91)91396-i. [DOI] [PubMed] [Google Scholar]
- 121.Ferreyra GA, Golombek DA. Cyclic AMP and protein kinase A rhythmicity in the mammalian suprachiasmatic nuclei. Brain Res. 2000;858:33–39. doi: 10.1016/s0006-8993(99)02390-2. [DOI] [PubMed] [Google Scholar]
- 122.Fukuhara C, et al. Gating of the cAMP signaling cascade and melatonin. synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hastings MH, Maywood ES, O’Neill JS. Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol. 2008;18:R805–R815. doi: 10.1016/j.cub.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 124.Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology. 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- 125.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nature Med. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robles MS, Boyault C, Knutti D, Padmanabhan K, Weitz CJ. Identification of RACK1 and protein kinase Cα as integral components of the mammalian circadian clock. Science. 2010;327:463–466. doi: 10.1126/science.1180067. [DOI] [PubMed] [Google Scholar]
- 127.Nakajima M, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 128.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hardin PE. Essential and expendable features of the circadian timekeeping mechanism. Curr Opin Neurobiol. 2006;16:686–692. doi: 10.1016/j.conb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 130.Panda S. Multiple photopigments entrain the Mammalian circadian oscillator. Neuron. 2007;53:619–621. doi: 10.1016/j.neuron.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 131.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lall GS, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berson D, Dunn F, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 134.Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hannibal J, Brabet P, Fahrenkrug J. Mice lacking the PACAP type I receptor have impaired photic entrainment and negative masking. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2050–R2058. doi: 10.1152/ajpregu.90563.2008. [DOI] [PubMed] [Google Scholar]
- 136.Cahill GM, Menaker M. Responses of the suprachiasmatic nucleus to retinohypothalamic tract volleys in a slice preparation of the mouse hypothalamus. Brain Res. 1989;479:65–75. doi: 10.1016/0006-8993(89)91336-x. [DOI] [PubMed] [Google Scholar]
- 137.Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. J Physiol. 1991;444:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang ZG, Yang Y, Liu ZP, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J Physiol. 1997;499:141–159. doi: 10.1113/jphysiol.1997.sp021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Michel S, Itri J, Colwell CS. Excitatory mechanisms in the suprachiasmatic nucleus: the role of AMPA/KA glutamate receptors. J Neurophysiol. 2002;88:817–828. doi: 10.1152/jn.2002.88.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Colwell CS. NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system. Eur J Neurosci. 2001;13:1420–1428. doi: 10.1046/j.0953-816x.2001.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pennartz CM, Hamstra R, Geurtsen AM. Enhanced NMDA receptor activity in retinal inputs to the rat suprachiasmatic nucleus during the subjective night. J Physiol. 2001;532:181–194. doi: 10.1111/j.1469-7793.2001.0181g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci. 2006;7:15. doi: 10.1186/1471-2202-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haak LL. Metabotropic glutamate receptor modulation of glutamate responses in the suprachiasmatic nucleus. J Neurophysiol. 1999;81:1308–1317. doi: 10.1152/jn.1999.81.3.1308. [DOI] [PubMed] [Google Scholar]
- 144.Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: inhibitory synaptic mechanisms. J Physiol. 1992;458:247–260. doi: 10.1113/jphysiol.1992.sp019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen G, van den Pol AN. Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J Neurosci. 1998;18:1913–1922. doi: 10.1523/JNEUROSCI.18-05-01913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- 147.Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 149.Warren E, Allen C, Brown R, Robinson D. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tu D, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 151.Gamble KL, Allen GC, Zhou T, McMahon DG. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci. 2007;27:12078–12087. doi: 10.1523/JNEUROSCI.1109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gamble KL, Kudo T, Colwell CS, McMahon DG. Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. J Biol Rhythms. 2011;26:99–106. doi: 10.1177/0748730410396678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- 154.Wang LM, et al. Role for the NR2B subunit of the N-methyl-D-aspartate receptor in mediating light input to the circadian system. Eur J Neurosci. 2008;27:1771–1779. doi: 10.1111/j.1460-9568.2008.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cermakian N, Sassone-Corsi P. Environmental stimulus perception and control of circadian clocks. Curr Opin Neurobiol. 2002;12:359–365. doi: 10.1016/s0959-4388(02)00347-1. [DOI] [PubMed] [Google Scholar]
- 157.Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- 158.Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- 159.Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nature Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- 160.Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- 161.Butcher GQ, Lee B, Cheng HY, Obrietan K. Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J Neurosci. 2005;25:5305–5313. doi: 10.1523/JNEUROSCI.4361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yokota S, et al. Involvement of calcium-calmodulin protein kinase but not mitogen-activated protein kinase in light-induced phase delays and Per gene expression in the suprachiasmatic nucleus of the hamster. J Neurochem. 2001;77:618–627. doi: 10.1046/j.1471-4159.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 163.Ding JM, et al. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 164.Oster H, et al. cGMP-dependent protein kinase II modulates mPer1 and mPer2 gene induction and influences phase shifts of the circadian clock. Curr Biol. 2003;13:725–733. doi: 10.1016/s0960-9822(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 165.Jakubcakova V, et al. Light entrainment of the mammalian circadian clock by a PRKCA-dependent posttranslational mechanism. Neuron. 2007;54:831–843. doi: 10.1016/j.neuron.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 166.Tischkau SA, Gallman EA, Buchanan GF, Gillette MU. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci. 2000;20:7830–7837. doi: 10.1523/JNEUROSCI.20-20-07830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kornhauser JM, Ginty DD, Greenberg ME, Mayo KE, Takahashi JS. Light entrainment and activation of signal transduction pathways in the SCN. Prog Brain Res. 1996;111:133–146. doi: 10.1016/s0079-6123(08)60405-7. [DOI] [PubMed] [Google Scholar]
- 168.Rieux C, et al. Analysis of immunohistochemical label of Fos protein in the suprachiasmatic nucleus: comparison of different methods of quantification. J Biol Rhythms. 2002;17:121–136. doi: 10.1177/074873002129002410. [DOI] [PubMed] [Google Scholar]
- 169.Ginty DD, et al. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 170.Gau D, et al. Phosphorylation of CREB Ser142 regulates light-induced phase shifts of the circadian clock. Neuron. 2002;34:245–253. doi: 10.1016/s0896-6273(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 171.Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci. 2004;19:1105–1109. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee B, et al. CREB influences timing and entrainment of the SCN circadian clock. J Biol Rhythms. 2010;25:410–420. doi: 10.1177/0748730410381229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- 175.Cao R, Li A, Cho HY, Lee B, Obrietan K. Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock. J Neurosci. 2010;30:6302–6314. doi: 10.1523/JNEUROSCI.5482-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Poo MM. Neurotrophins as synaptic modulators. Nature Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 177.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liang FQ, Sohrabji F, Miranda R, Earnest B, Earnest D. Expression of brain-derived neurotrophic factor and its cognate receptor, TrkB, in the rat suprachiasmatic nucleus. Exp Neurol. 1998;151:184–193. doi: 10.1006/exnr.1998.6804. [DOI] [PubMed] [Google Scholar]
- 180.Allen GC, Earnest DJ. Overlap in the distribution of TrkB immunoreactivity and retinohypothalamic tract innvervation of the rat suprachiasmatic nucleus. Neurosci Lett. 2005;376:200–204. doi: 10.1016/j.neulet.2004.11.076. [DOI] [PubMed] [Google Scholar]
- 181.Kim YI, Choi HJ, Colwell CS. Brain-derived neurotrophic factor regulation of N-methyl-D-aspartate receptor-mediated synaptic currents in suprachiasmatic nucleus neurons. J Neurosci Res. 2006;84:1512–1520. doi: 10.1002/jnr.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Michel S, Clark JP, Ding JM, Colwell CS. Brain-derived neurotrophic factor and neurotrophin receptors modulate glutamate-induced phase shifts of the suprachiasmatic nucleus. Eur J Neurosci. 2006;24:1109–1116. doi: 10.1111/j.1460-9568.2006.04972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liang FQ, Allen G, Earnest D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci. 2000;20:2978–2987. doi: 10.1523/JNEUROSCI.20-08-02978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Allen GC, Qu X, Earnest DJ. TrkB-deficient mice show diminished phase shifts to the circadian activity rhythm in response to light. Neurosci Lett. 2005;378:150–155. doi: 10.1016/j.neulet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 185.Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 186.Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nature Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- 187.Fogle KJ, Parson KG, Dahm NA, Holmes TC. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331:1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 189.Duffield GE. DNA microarray analyses of circadian timing: the genomic basis of biological time. J Neuroendocrinol. 2003;15:991–1002. doi: 10.1046/j.1365-2826.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- 190.Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1.3) ion channel half-life and surface expression. Neuroscience. 2007;144:531–546. doi: 10.1016/j.neuroscience.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brussaard AB. Antisense oligonucleotides induce functional deletion of ligand gated ion channels in cultured neurons and brain explants. J Neurosci Methods. 1997;71:55–64. doi: 10.1016/s0165-0270(96)00126-4. [DOI] [PubMed] [Google Scholar]
- 192.Palmer ML, Fahrenkrug SC, O’Grady SM. RNA interference and ion channel physiology. Cell Biochem Biophys. 2006;46:175–191. doi: 10.1385/CBB:46:2:175. [DOI] [PubMed] [Google Scholar]
- 193.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Keifer J, Zheng Z. AMPA receptor trafficking and learning. Eur J Neurosci. 2010;32:269–277. doi: 10.1111/j.1460-9568.2010.07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nature Rev Neurosci. 2010;11:675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- 196.Strumbos JG, Polley DB, Kaczmarek LK. Specific and rapid effects of acoustic stimulation on the tonotopic distribution of Kv3.1b potassium channels in the adult rat. Neuroscience. 2010;167:567–572. doi: 10.1016/j.neuroscience.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Strumbos JG, Brown MR, Kronengold J, Polley DB, Kaczmarek LK. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J Neurosci. 2010;30:10263–10271. doi: 10.1523/JNEUROSCI.1125-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu M, Gu Y, Barry J, Gu C. Kinesin I transports tetramerized Kv3 channels through the axon initial segment via direct binding. J Neurosci. 2010;30:15987–16001. doi: 10.1523/JNEUROSCI.3565-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Inoue S, et al. A role for the Drosophila fragile X-related gene in circadian output. Curr Biol. 2002;12:1331–1335. doi: 10.1016/s0960-9822(02)01036-9. [DOI] [PubMed] [Google Scholar]
- 200.Zhang J, et al. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am J Hum Genet. 2008;83:43–52. doi: 10.1016/j.ajhg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 202.Park KS, Yang JW, Seikel E, Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology. 2008;23:49–57. doi: 10.1152/physiol.00031.2007. [DOI] [PubMed] [Google Scholar]
- 203.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 205.Van Someren EJ. Circadian rhythms and sleep in human aging. Chronobiol Int. 2000;17:233–243. doi: 10.1081/cbi-100101046. [DOI] [PubMed] [Google Scholar]
- 206.Carrier J, Bliwise DL. In: Sleep: Physiology Investigations, and Medicine. Billiard M, Kent A, editors. Kluwer Academic/Plenum; New York: 2003. pp. 297–333. [Google Scholar]
- 207.Satinoff E, et al. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol. 1993;265:R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- 208.Turek F, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695:237–239. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- 210.Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience. 2001;106:255–261. doi: 10.1016/s0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 211.Biello SM. Circadian clock resetting in the mouse changes with age. Age. 2009;31:293–303. doi: 10.1007/s11357-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nakamura TJ, et al. Age-related decline in circadian output. J Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. Recent in vivo multiunit recordings from the SCN of freely moving young and middle-aged mice have shown that the day–night difference was substantially reduced in the SCN of middle-aged mice. Surprisingly, the molecular clockwork in the SCN, as measured by PER2 levels, was not disrupted in middle-aged mice, suggesting that the age-related disruption in the circadian output occurs before any disruption of the molecular clockwork. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Morton A, et al. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pallier P, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci. 2007;27:7869–7878. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sterniczuk R, Dyck RH, Laferla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 1. Circadian changes. Brain Res. 2010;1348:139–148. doi: 10.1016/j.brainres.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 216.Kudo T, et al. Dysfunctions in circadian behavior and physiology in mouse models of Huntington’s disease. Exp Neurol. 2011;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maywood ES, et al. Disruption of peripheral circadian timekeeping in a mouse model of Huntington’s disease and its restoration by temporally scheduled feeding. J Neurosci. 2010;30:10199–10204. doi: 10.1523/JNEUROSCI.1694-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kristensson K, Nygård M, Bertini G, Bentivoglio M. African trypanosome infections of the nervous system: parasite entry and effects on sleep and synaptic functions. Prog Neurobiol. 2010;91:152–171. doi: 10.1016/j.pneurobio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 219.Lundkvist GB, et al. Altered neuronal activity rhythm and glutamate receptor expression in the suprachiasmatic nuclei of Trypanosoma brucei-infected rats. J Neuropathol Exp Neurol. 1998;57:21–29. doi: 10.1097/00005072-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 220.Lundkvist GB, et al. Clock gene expression during chronic inflammation induced by infection with Trypanosoma brucei brucei in rats. J Biol Rhythms. 2010;25:92–102. doi: 10.1177/0748730409360963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kwak Y, et al. Interferon-γ alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J Biol Rhythms. 2008;23:150–159. doi: 10.1177/0748730407313355. [DOI] [PubMed] [Google Scholar]
- 222.Hastings M, Reddy A, Maywood E. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 223.Takahashi J, Hong H, Ko C, McDearmon E. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gale JE, et al. Disruption of circadian rhythms accelerates development of diabetes through pancreatic β-cell loss and dysfunction. J Biol Rhythms. doi: 10.1177/0748730411416341. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bray M, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 227.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Reid KJ, Zee PC. Circadian rhythm disorders. Semin Neurol. 2009;29:393–405. doi: 10.1055/s-0029-1237120. [DOI] [PubMed] [Google Scholar]
- 229.Wulff K, Porcheret K, Cussans E, Foster RG. Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr Opin Genet Dev. 2009;19:237–246. doi: 10.1016/j.gde.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 230.Gerstner JR, et al. Cycling behavior and memory formation. J Neurosci. 2009;29:12824–12830. doi: 10.1523/JNEUROSCI.3353-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang L, et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1:e00012. doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Loh D, et al. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS ONE. 2010;5:e12546. doi: 10.1371/journal.pone.0012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Block GD, Khalsa SB, McMahon DG, Michel S, Guesz M. Block biological clocks in the retina: cellular mechanisms of biological timekeeping. Int Rev Cytol. 1993;146:83–144. doi: 10.1016/s0074-7696(08)60381-2. [DOI] [PubMed] [Google Scholar]
- 235.Cui LN, Dyball REJ. Synaptic input from the retina to the suprachiasmatic nucleus changes with the light-dark cycle in the Syrian hamster. J Physiol. 1996;497:485–493. doi: 10.1113/jphysiol.1996.sp021782. [DOI] [PMC free article] [PubMed] [Google Scholar]